Abstract
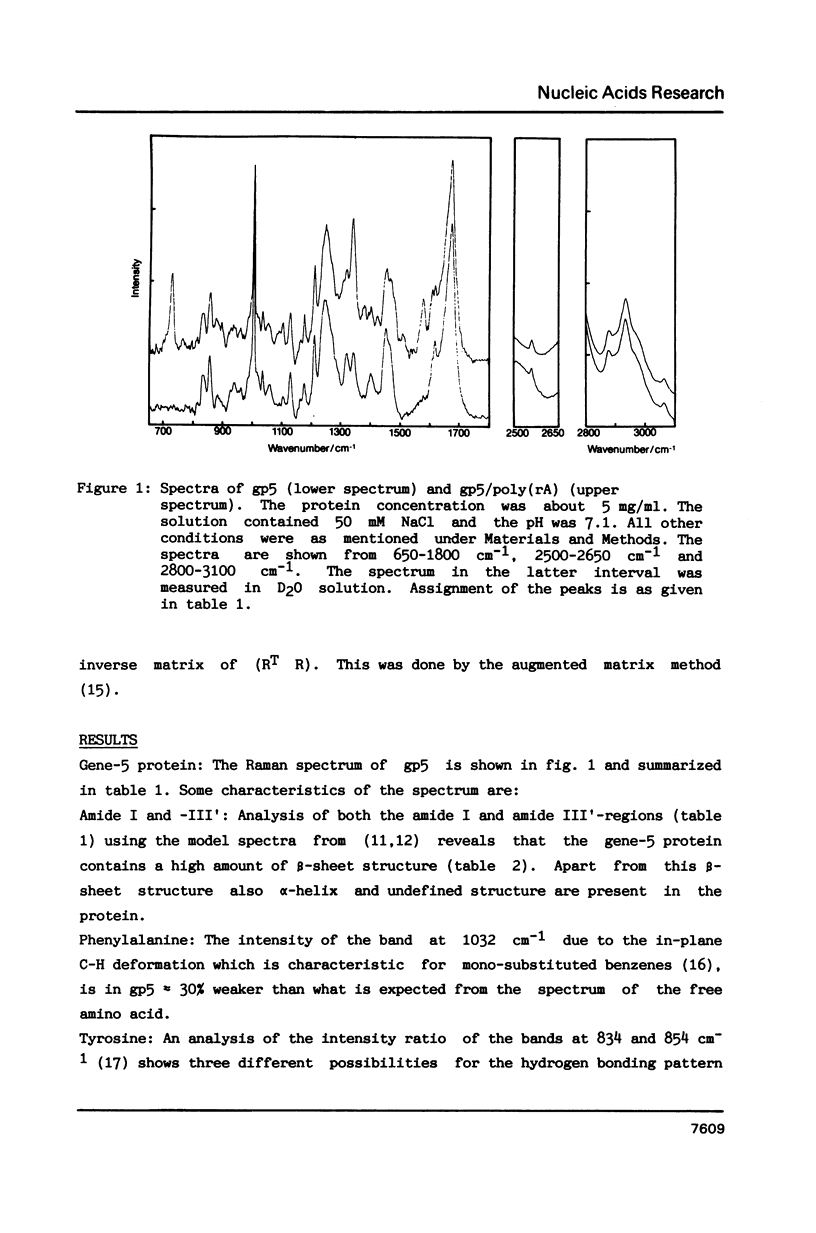
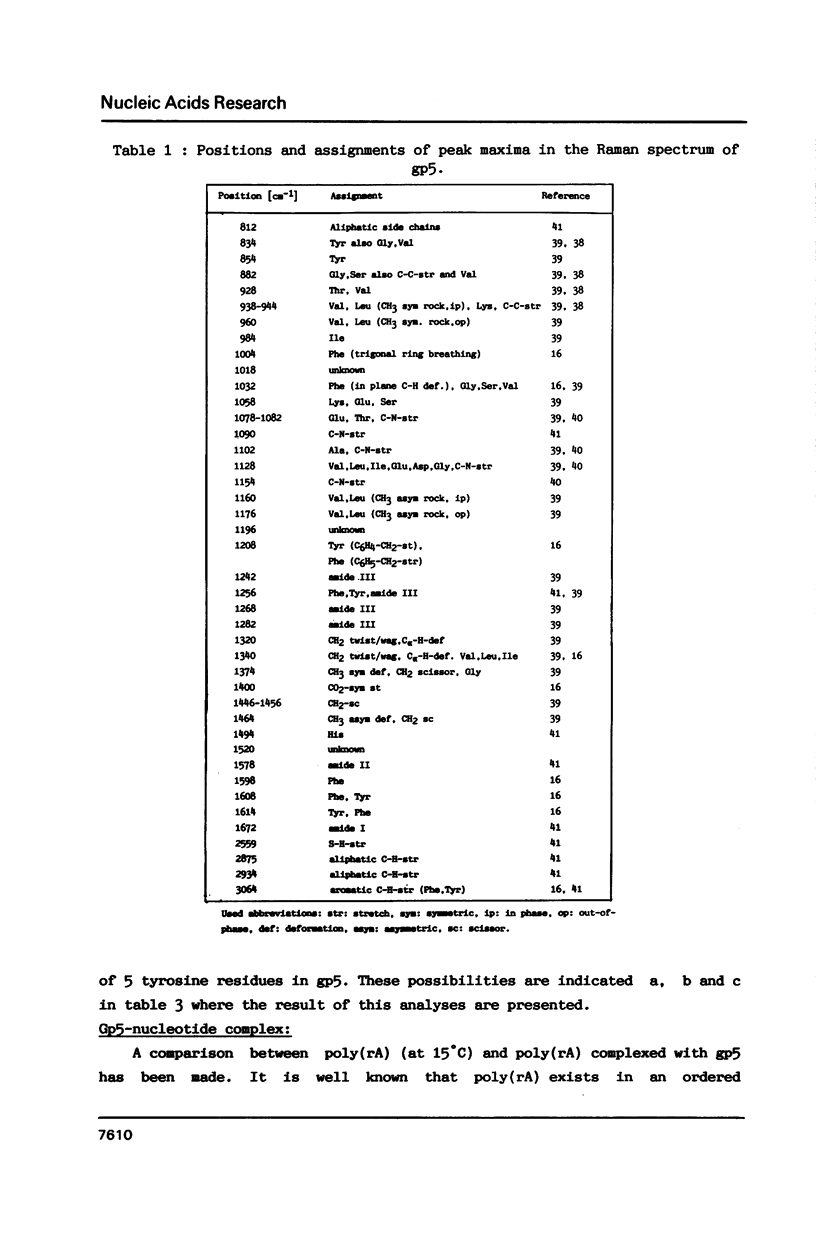
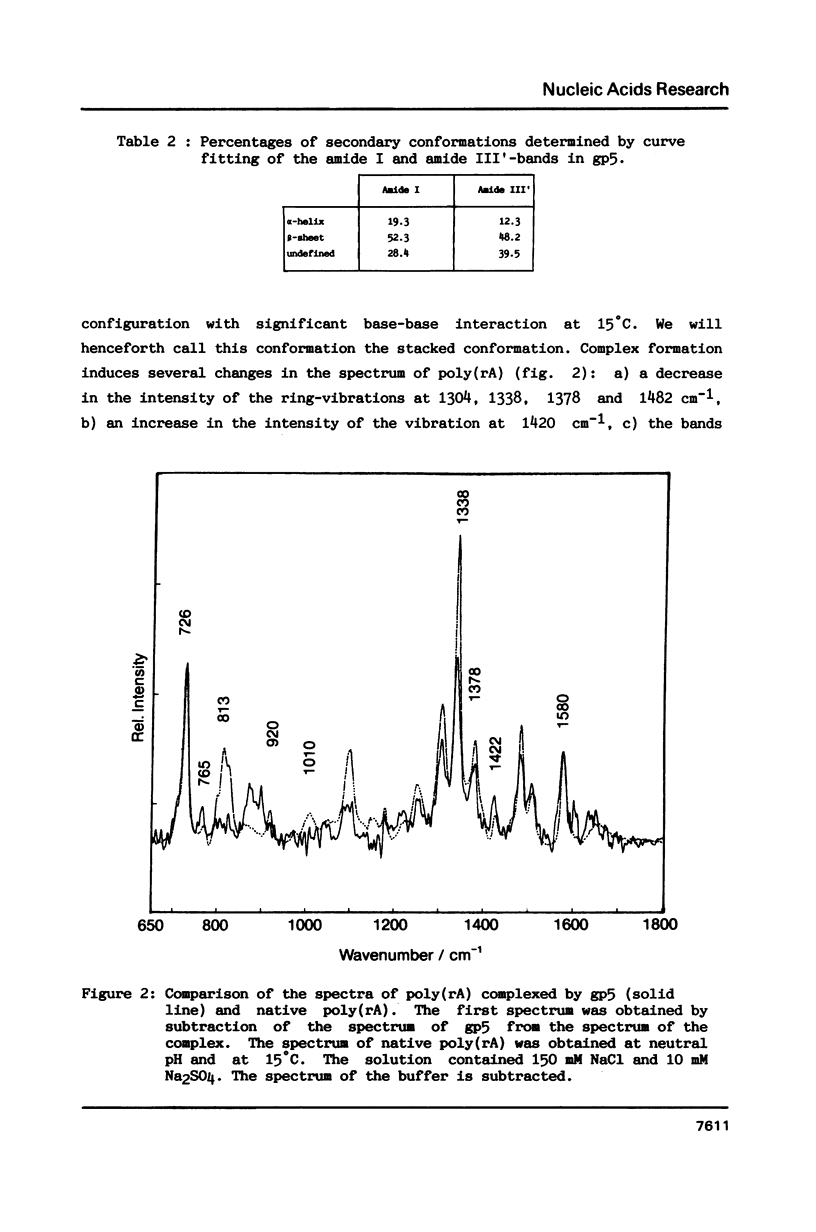
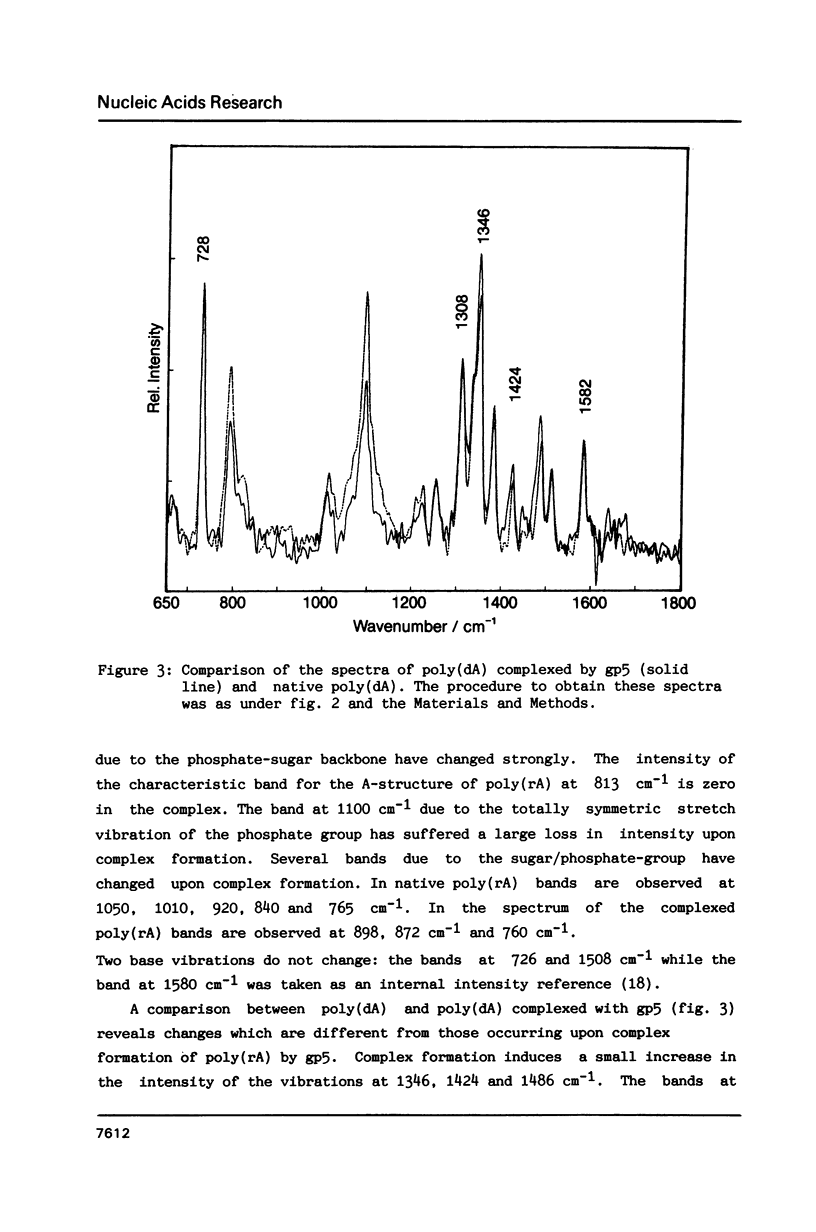
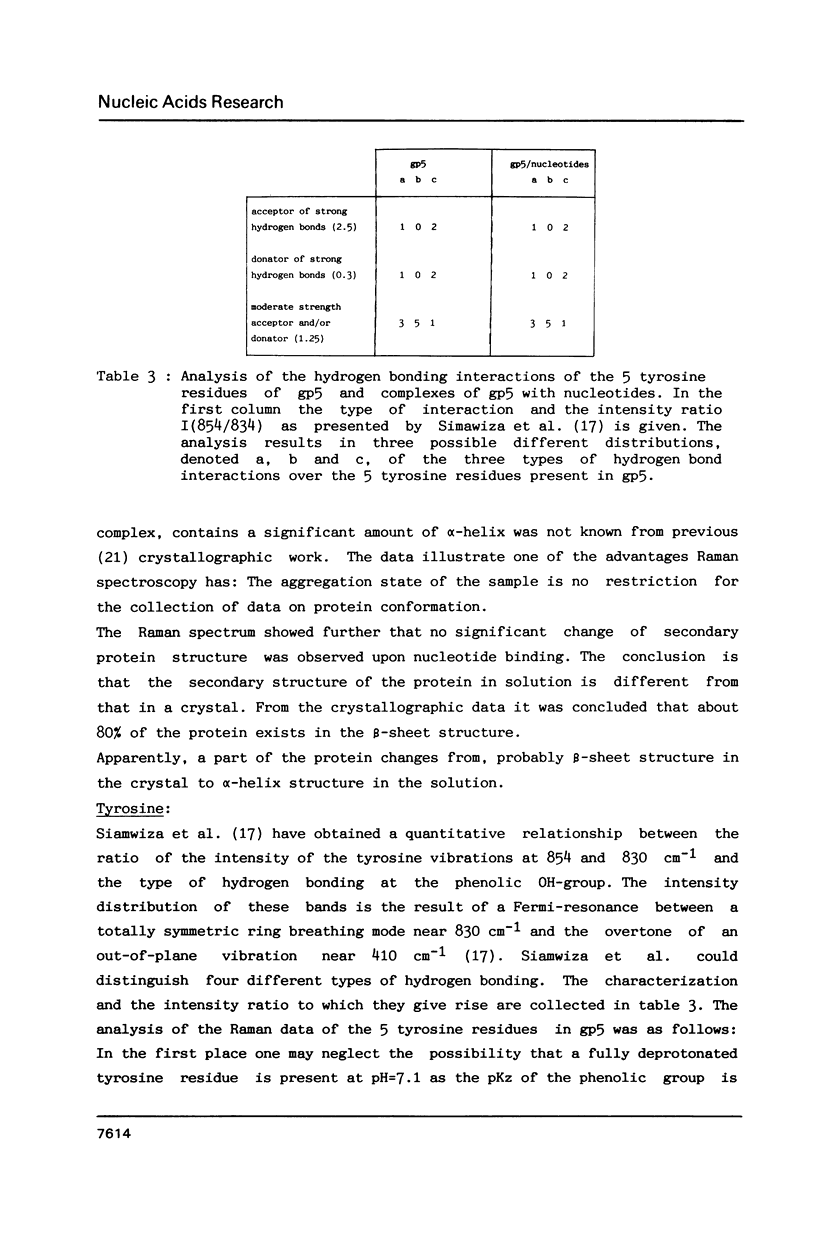
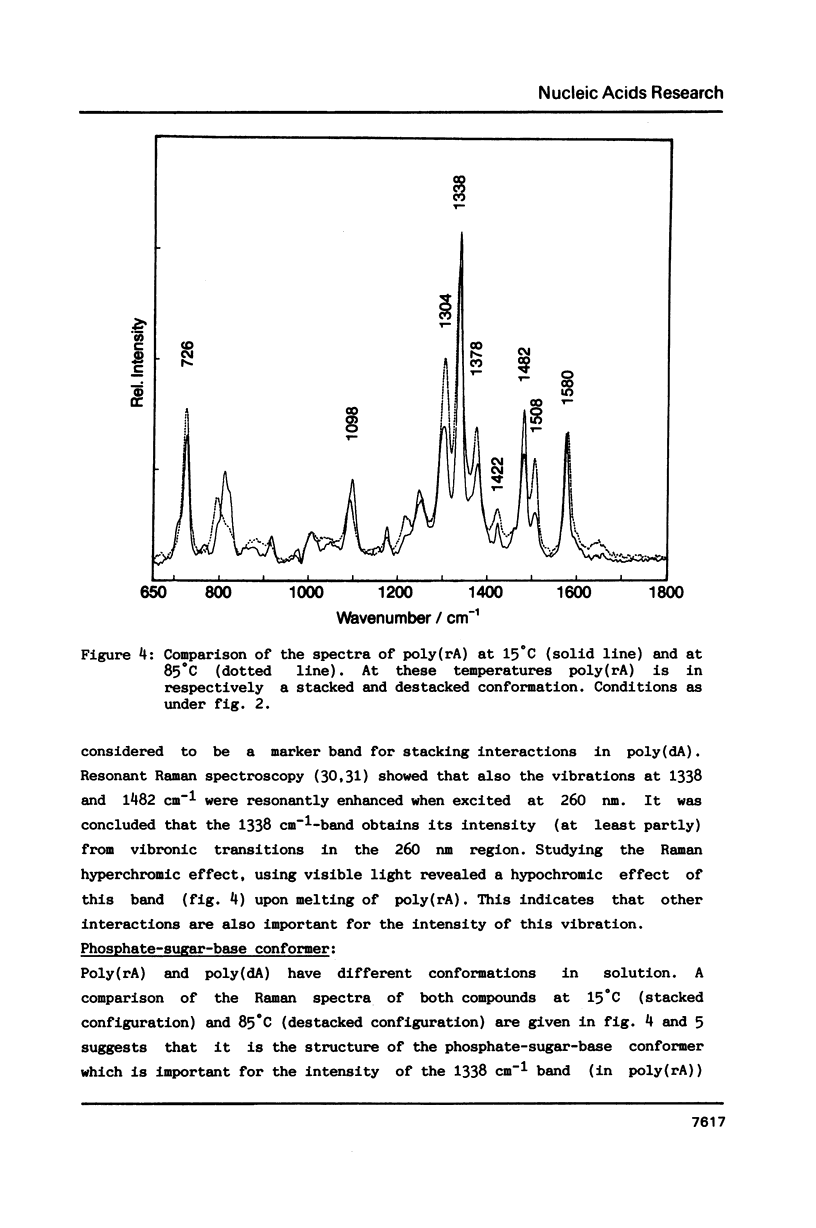
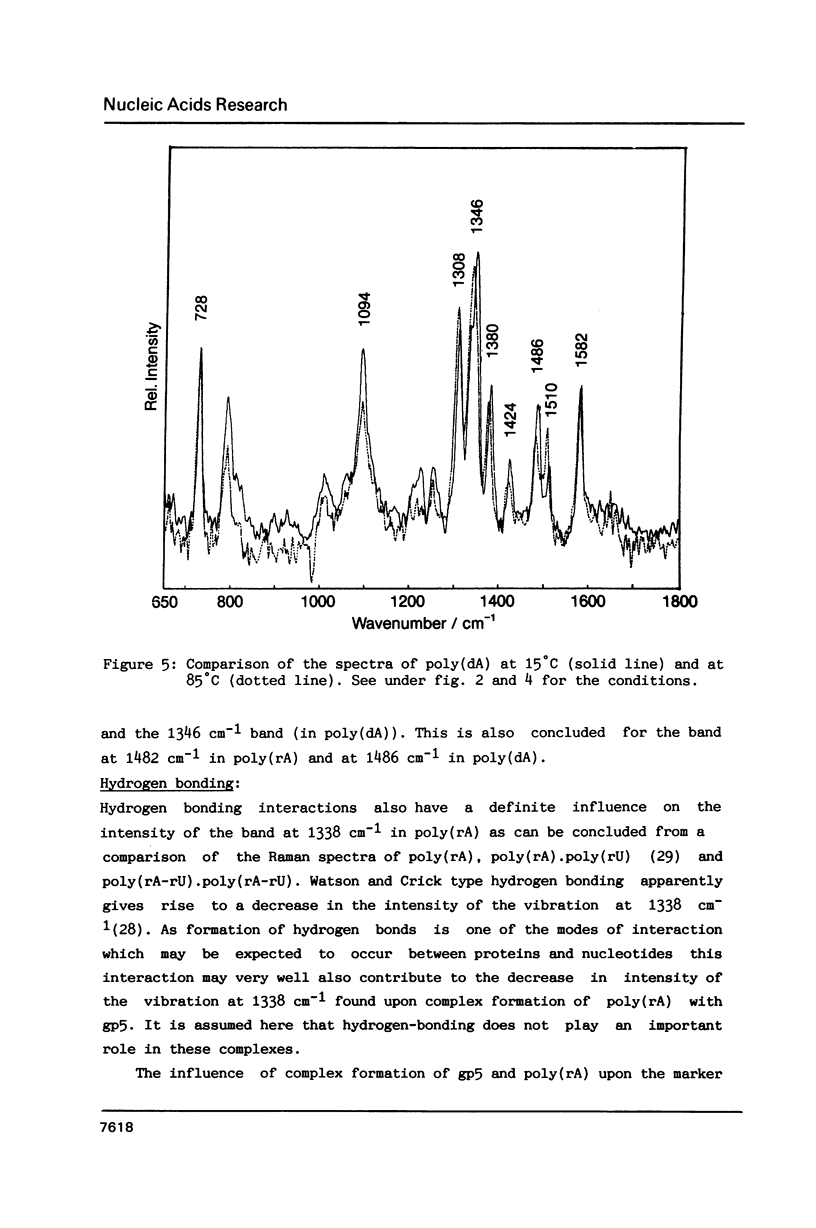
Raman spectra of gp5 and complexes of gp5 with poly(rA) and poly(dA) have been determined and analysed. From a fit of the amide I-band with model spectra it follows that the secondary structure of gp5 contains 52% beta-sheet, 28% undefined conformation and 19% alpha-helix. The band at 1032 cm-1 due to phenylalanine has an anomalous intensity both in the spectra of the complexes and the free protein. This possibly indicates a stacked structure present in the protein. Binding of gp5 to poly(rA) and poly(dA) influences the intensity of bands near 1338 and 1480 cm-1 which are considered to be marker-bands for the phosphate-sugar-base conformer. A change in conformation of the nucleotides is also reflected by vibrations originating in the phosphate- and sugar-residues of the backbone. In the spectrum of complexed poly(rA) the intensity of the conformation sensitive band at 813 cm-1, which is due to the phosphodiester group, is zero. It seems that gp5 forces poly(rA) and poly(dA) to a similar conformation. A marker band for stacking interaction in poly(rA) indicates that stacking interactions in the complex have increased.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B., Frey L., Delius H. Isolation and characterization of gene 5 protein of filamentous bacterial viruses. J Mol Biol. 1972 Jul 14;68(1):139–152. doi: 10.1016/0022-2836(72)90269-0. [DOI] [PubMed] [Google Scholar]
- Alma N. C., Harmsen B. J., Hilbers C. W., van der Marel G., van Boom J. H. 500 MHz 1H NMR study of the role of lysines and arginines in the binding of gene-5 protein to oligoadenylic acids. FEBS Lett. 1981 Nov 30;135(1):15–20. doi: 10.1016/0014-5793(81)80934-9. [DOI] [PubMed] [Google Scholar]
- Alma N. C., Harmsen B. J., Hull W. E., van der Marel G., van Boom J. H., Hilbers C. W. Double-resonance experiments at 500 MHz on gene-5 protein and its complex with octadeoxyriboadenylic acid. Biochemistry. 1981 Jul 21;20(15):4419–4428. doi: 10.1021/bi00518a029. [DOI] [PubMed] [Google Scholar]
- Anderson R. A., Coleman J. E. Physiochemical properties of DNA binding proteins: gene 32 protein of T4 and Escherichia coli unwinding protein. Biochemistry. 1975 Dec 16;14(25):5485–5491. doi: 10.1021/bi00696a017. [DOI] [PubMed] [Google Scholar]
- Chen M. C., Lord R. C., Mendelsohn R. Laser-excited Raman spectroscopy of biomolecules. V. Conformational changes associated with the chemical denaturation of lysozyme. J Am Chem Soc. 1974 May 15;96(10):3038–3042. doi: 10.1021/ja00817a003. [DOI] [PubMed] [Google Scholar]
- Cuypers T., van der Ouderaa F. J., de Jong W. W. The amino acid sequence of gene 5 protein of bacteriophage M 13. Biochem Biophys Res Commun. 1974 Jul 24;59(2):557–563. doi: 10.1016/s0006-291x(74)80016-1. [DOI] [PubMed] [Google Scholar]
- Fish S. R., Hartman K. A., Stubbs G. J., Thomas G. J., Jr Structural studies of tobacco mosaic virus and its components by laser Raman spectroscopy. Biochemistry. 1981 Dec 22;20(26):7449–7457. doi: 10.1021/bi00529a019. [DOI] [PubMed] [Google Scholar]
- Garssen G. J., Hilbers C. W., Schoenmakers J. G., van Boom J. H. Studies on DNA unwinding. Proton and phosphorus nuclear-magnetic-resonance studies of gene V protein from bacteriophage M13, interacting with d(pC-G-C-G). Eur J Biochem. 1977 Dec;81(3):453–463. doi: 10.1111/j.1432-1033.1977.tb11970.x. [DOI] [PubMed] [Google Scholar]
- Jollès B., Laigle A., Chinsky L., Turpin P. Y. The poly dA strand of poly dA.poly dT adopts an A-form in solution: a UV resonance Raman study. Nucleic Acids Res. 1985 Mar 25;13(6):2075–2085. doi: 10.1093/nar/13.6.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafleur L., Rice J., Thomas G. J., Jr Raman studies of nucleic acids. VII. Poly A-poly U and poly G-poly C. Biopolymers. 1972;11(12):2423–2437. doi: 10.1002/bip.1972.360111205. [DOI] [PubMed] [Google Scholar]
- Lippert J. L., Tyminski D., Desmeules P. J. Determination of the secondary structure of proteins by laser Raman spectroscopy. J Am Chem Soc. 1976 Oct 27;98(22):7075–7080. doi: 10.1021/ja00438a057. [DOI] [PubMed] [Google Scholar]
- Lord R. C., Yu N. T. Laser-excited Raman spectroscopy of biomolecules. I. Native lysozyme and its constituent amino acids. J Mol Biol. 1970 Jun 14;50(2):509–524. doi: 10.1016/0022-2836(70)90208-1. [DOI] [PubMed] [Google Scholar]
- McPherson A., Jurnak F. A., Wang A. H., Molineux I., Rich A. Structure at 2.3 A resolution of the gene 5 product of bacteriophage fd: a DNA unwinding protein. J Mol Biol. 1979 Nov 5;134(3):379–400. doi: 10.1016/0022-2836(79)90359-0. [DOI] [PubMed] [Google Scholar]
- McPherson A., Jurnak F., Wang A., Kolpak F., Rich A., Molineux I., Fitzgerald P. The structure of a DNA unwinding protein and its complexes with oligodeoxynucleotides by x-ray diffraction. Biophys J. 1980 Oct;32(1):155–173. doi: 10.1016/S0006-3495(80)84931-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson A., Wang A. H., Jurnak F. A., Molineux I., Kolpak F., Rich A. X-ray diffraction studies on crystalline complexes of the gene 5 DNA-unwinding protein with deoxyoligonucleotides. J Biol Chem. 1980 Apr 10;255(7):3174–3177. [PubMed] [Google Scholar]
- Medeiros G. C., Thomas G. J., Jr Raman studies of nucleic acids. IV. Vibrational specra and associative interactions of aqueous inosine derivatives. Biochim Biophys Acta. 1971 Oct;247(3):449–462. [PubMed] [Google Scholar]
- Morikawa K., Tsuboi M., Takahashi S., Kyogoku Y., Mitsui Y., Iitaka Y., Thomas G. J., Jr The vibrational spectra and structure of poly(rA-rU)-poly(rA-rU). Biopolymers. 1973 Apr;12(4):799–816. doi: 10.1002/bip.1973.360120409. [DOI] [PubMed] [Google Scholar]
- Paradiso P. R., Nakashima Y., Konigsberg W. Photochemical cross-linking of protein . nucleic acid complexes. The attachment of the fd gene 5 protein to fd DNA. J Biol Chem. 1979 Jun 10;254(11):4739–4744. [PubMed] [Google Scholar]
- Pratt D., Laws P., Griffith J. Complex of bacteriophage M13 single-stranded DNA and gene 5 protein. J Mol Biol. 1974 Feb 5;82(4):425–439. doi: 10.1016/0022-2836(74)90239-3. [DOI] [PubMed] [Google Scholar]
- Prescott B., Steinmetz W., Thomas G. J., Jr Characterization of DNA structures by laser Raman spectroscopy. Biopolymers. 1984 Feb;23(2):235–256. doi: 10.1002/bip.360230206. [DOI] [PubMed] [Google Scholar]
- Scheerhagen M. A., Blok J., van Grondelle R. The conformation of the complex of the helix destabilizing protein GP32 of bacteriophage T4 and single stranded DNA. J Biomol Struct Dyn. 1985 Feb;2(4):821–829. doi: 10.1080/07391102.1985.10506326. [DOI] [PubMed] [Google Scholar]
- Siamwiza M. N., Lord R. C., Chen M. C., Takamatsu T., Harada I., Matsuura H., Shimanouchi T. Interpretation of the doublet at 850 and 830 cm-1 in the Raman spectra of tyrosyl residues in proteins and certain model compounds. Biochemistry. 1975 Nov 4;14(22):4870–4876. doi: 10.1021/bi00693a014. [DOI] [PubMed] [Google Scholar]
- Small E. W., Peticolas W. L. Conformational dependence of the Raman scattering intensities from polynucleotides. 3. Order-disorder changes in helical structures. Biopolymers. 1971;10(8):1377–1418. doi: 10.1002/bip.360100811. [DOI] [PubMed] [Google Scholar]
- Small E. W., Peticolas W. L. Conformational dependence of the Raman scattering intensities from polynucleotides. Biopolymers. 1971;10(1):69–88. doi: 10.1002/bip.360100107. [DOI] [PubMed] [Google Scholar]
- Thomas G. J., Jr, Prescott B., Day L. A. Structure similarity, difference and variability in the filamentous viruses fd, If1, IKe, Pf1 and Xf. Investigation by laser Raman spectroscopy. J Mol Biol. 1983 Apr 5;165(2):321–356. doi: 10.1016/s0022-2836(83)80260-5. [DOI] [PubMed] [Google Scholar]
- Thomas G. J., Jr, Prescott B., McDonald-Ordzie P. E., Hartman K. A. Studies of virus structure by laser-Raman spectroscopy. II. MS2 phage, MS2 capsids and MS2 RNA in aqueous solutions. J Mol Biol. 1976 Mar 25;102(1):103–124. doi: 10.1016/0022-2836(76)90076-0. [DOI] [PubMed] [Google Scholar]
- Torbet J., Gray D. M., Gray C. W., Marvin D. A., Siegrist H. Structure of the fd DNA--gene 5 protein complex in solution. A neutron small-angle scattering study. J Mol Biol. 1981 Mar 5;146(3):305–320. doi: 10.1016/0022-2836(81)90390-9. [DOI] [PubMed] [Google Scholar]
- Williams R. W., Cutrera T., Dunker A. K., Peticolas W. L. The estimation of protein secondary structure by laser Raman. Spectroscopy from the amide III' intensity distribution. FEBS Lett. 1980 Jun 30;115(2):306–308. doi: 10.1016/0014-5793(80)81193-8. [DOI] [PubMed] [Google Scholar]
- Williams R. W. Estimation of protein secondary structure from the laser Raman amide I spectrum. J Mol Biol. 1983 Jun 5;166(4):581–603. doi: 10.1016/s0022-2836(83)80285-x. [DOI] [PubMed] [Google Scholar]



