Abstract
Background
The course of major depressive disorder is often characterized by progressing chronicity, but whether this applies to the course of self-reported psychological distress remains unclear. We examined whether the risk of self-reported psychological distress becomes progressively higher the longer the history of distress and whether prolonged history of distress modifies associations between risk markers and future distress.
Methods
Participants were British civil servants from the prospective Whitehall II cohort study (n=7934; 31.5% women, mean age 44.5 years at baseline) followed from 1985 to 2006 with repeat data collected in 7 study phases. Psychological distress was assessed with the 30-item General Health Questionnaire (GHQ). Sex, socioeconomic status, marital status, ethnicity, physical activity, alcohol consumption, smoking, and obesity were assessed as risk markers.
Results
Recurrent history of psychological distress was associated with a progressively increasing risk of future distress in a dose-response manner. Common risk markers, such as low socioeconomic status, non-White ethnicity, being single, and alcohol abstinence were stronger predictors of subsequent distress in participants with a longer history of psychological distress. Sex differences in psychological distress attenuated with prolonged distress history.
Limitations
The participants were already adults in the beginning of the study, so we could not assess the progressive chronicity of psychological distress from adolescence onwards.
Conclusions
These data suggest that self-reported psychological distress becomes more persistent over time and that a longer prior exposure to psychological distress increases sensitivity to the stressful effects of certain risk markers.
Keywords: Chronic distress, Kindling hypothesis, Longitudinal, Recurrence
Introduction
Major depressive disorder is characterized by a chronic course with a high risk of recurrence and recurring episodes predict progressively increasing risk of subsequent episodes (American-Psychiatric-Association, 2000; Hermens et al., 2004; Mitchell & Subramaniam, 2005; Mueller et al., 1999; D. A. Solomon et al., 2000). The initial onset of clinical depression is often preceded by major stressful life events, but subsequent, recurrent episodes appear to be less closely tied to such psychosocial stressors (Monroe & Harkness, 2005; Stroud, Davila, & Moyer, 2008). This may be due to recurrent depression acquiring an endogenous course that becomes increasingly independent of environmental influences over time (stress autonomy model). On the other hand, recurrent episodes of depression may heighten a person’s sensitivity to minor stressors of daily life that are encountered more frequently than major life events (stress sensitization model). (Monroe & Harkness, 2005; Post, 1992; Segal, Williams, Teasdale, & Gemar, 1996; Stroud, et al., 2008).
Self-reported mental health problems are often characterized by co-morbid symptoms of depression, anxiety and somatic complaints (Prince et al., 2007), which may not always be severe enough to meet clinical diagnostic criteria but may nevertheless cause significant impairment (Judd, Schettler, & Akiskal, 2002). Subclinical psychological distress shares some characteristics with more severe depressive disorders (Judd, et al., 2002; Kessler, Zhao, Blazer, & Swartz, 1997; Lewinsohn, Solomon, Seeley, & Zeiss, 2000); for discussion, see (Coyne, 1994; Flett, Vredenburg, & Krames, 1997; A. Solomon, Haaga, & Arnow, 2001). However, it remains unknown whether psychological distress is characterized by (a) progressively increasing chronicity over time and (b) a change in relationships with common depressogenic risk markers as a function of prior distress.
We assessed the cumulative patterns of psychological distress and their interactions with common sociodemographic and health-behavior related risk markers, including sex, socioeconomic status (SES), marital status, ethnicity, physical activity, smoking, alcohol consumption, and obesity. Such risk markers may correlate with exposure to environmental stressors or with capability of coping with such stressors and distress in general (e.g., (Hatch & Dohrenwend, 2007; McLeod & Kessler, 1990; Puhl, Andreyeva, & Brownell, 2008), so their associations with future psychological distress may also be modified by prior history of recurrent distress.
Methods
Participants
The participants were from the Whitehall II cohort study (M. Marmot & Brunner, 2005; M. G. Marmot et al., 1991). At baseline in 1985-1988, the target population was all London-based office staff (n=10308; 6895 men, 3413 women), aged 35–55 years, working in 20 civil service departments. Here we used data from all the study phases that included a measurement of psychological distress, i.e., phases 1 (1985-1988), 2 (1989-1990), 3 (1991-1993), 5 (1997-1999), 6 (2001), 7 (2002-2004), and 8 (2006). At each phase, we included participants who had data on all measurements of the GHQ questionnaire up to that phase and at the next phase (7934 participants providing a total of 35282 observations; for a detailed description of inclusion criteria, see the statistical analysis section below). Informed Ethical approval for the Whitehall II study was obtained from the University College London Medical School committee on the ethics of human research, and the participants gave informed consent.
Psychological Distress
Psychological distress was assessed using the 30-item self-administered General Health Questionnaire (GHQ; (D. P. Goldberg, 1972; D. P. Goldberg et al., 1997; Huppert & Whittington, 1995; Pevalin, 2000; Stansfeld & Marmot, 1992). The items tap into symptoms of depressive, anxiety, neurotic, and stress-related disorders experienced “over the past few weeks.” Each item was scored either 1 or 0 to indicate whether the symptom was present or not (0=not at all/no more than usual, 1=rather more than usual/much more than usual), and individuals with a total score of 5 or more as GHQ ‘cases’ (GHQ=1) and those scoring 0-4 as ‘non-cases’ (GHQ=0). This cut-off threshold was determined to be optimal in the Whitehall II cohort, with sensitivity of 73% and specificity of 78% against the Clinical Interview Schedule (Stansfeld & Marmot, 1992).
Sociodemographic and Behavior-Related Risk Markers
Sociodemographic risk markers included sex, socioeconomic status (SES; civil service occupational grade, 0=low, 1=intermediate, 2=high), ethnicity (0=white, 1=other), and marital status (0=married, 1=single, 2=divorced/separated, 3=widowed). Behavior-related measures included physical activity (weekly hours of moderate and vigorous physical activity summed together and divided into tertiles at each phase, 0=low, 1=intermediate, 2=high), smoking (0=non-smoker, 1=smoker), alcohol consumption [0=no alcohol, 1=moderate alcohol consumption (≤14 units/week in women and ≤21 units/week in men), 2=heavy alcohol consumption (>14 units/week in women and >21 units/week in men), and obesity (0=not obese, 1=obese, i.e., body mass index≥30; height and weight were measured in a medical examination).
Apart from sex and ethnicity, all covariates were coded as time-variant (reported repeatedly at each follow-up phase). Data on BMI were available only at phases 1, 3, 5, and 7, so we replaced missing data at phases 2 and 6 with data from the previous phase. Data on smoking, physical activity, and alcohol use were not available at phase 6, so missing data at these phases were imputed from data at phase 5.
Statistical Analysis
We assessed whether the cumulative score of GHQ over the first T phases, P1, P2, …, PT (exposure) predicted the probability of GHQ caseness at phase PT+1 (outcome). The creation of the exposure sum-score was carried out for each study phase up to which the participant had no missing measurements of the GHQ. For instance, a participant with a history of GHQ caseness of 0, 1, 1, 0, [missing data, MD], 1, and 1 at phases 1, 2, 3, 5, 6, 7, and 8, respectively, would have cumulative exposure scores of 0, 1, 2, 2, [MD], and [MD] at phases 1, 2, 3, 5, 6, and 7 when predicting GHQ caseness at phases 2, 3, 5, 6, 7, and 8, respectively (4 person-observations). The cumulative GHQ score ranged from 0 to 6. There were a total 7934 participants and 35282 (Table 1).
Table 1.
Descriptive Statistics for the Sample.
| Study phase | ||||||
|---|---|---|---|---|---|---|
| Phase 1 | Phase 2 | Phase 3 | Phase 5 | Phase 6 | Phase 7 | |
| Age* | 44.5 (6.1) | 48.3 (6.1) | 49.6 (6.1) | 56.0 (6.0) | 58.4 (6.0) | 61.3 (6.0) |
| Sex | ||||||
| Male | 68.5 | 69.4 | 70.9 | 71.1 | 71.3 | 71.5 |
| Female | 31.5 | 30.6 | 29.1 | 28.9 | 28.7 | 28.5 |
| SES | ||||||
| Low | 34.6 | 30.6 | 26.7 | 24.8 | 23.3 | 22.8 |
| Intermediate | 34.3 | 33.4 | 31.9 | 30.9 | 30.6 | 30.2 |
| High | 31.2 | 36.1 | 41.3 | 44.4 | 46.1 | 47.1 |
| Marital status | ||||||
| Married | 75.5 | 76.1 | 77.6 | 79.5 | 77.4 | 76.2 |
| Single | 16.0 | 15.2 | 14.3 | 11.9 | 12.9 | 12.9 |
| Divorced | 7.3 | 7.3 | 6.5 | 5.9 | 6.5 | 6.7 |
| Widow | 1.2 | 1.4 | 1.6 | 2.8 | 3.2 | 4.3 |
| Ethnicity | ||||||
| White | 91.6 | 91.9 | 93.0 | 93.5 | 93.9 | 94.2 |
| Other | 8.4 | 8.2 | 7.0 | 6.5 | 6.1 | 5.9 |
| Physical activity | ||||||
| Low | 28.1 | 30.0 | 32.0 | 32.7 | 32.0† | 33.1 |
| Intermediate | 30.8 | 33.9 | 31.2 | 33.6 | 33.7† | 33.3 |
| High | 41.1 | 36.1 | 36.8 | 33.7 | 34.3† | 33.6 |
| Alcohol consumption | ||||||
| None | 17.6 | 18.6 | 18.1 | 14.9 | 14.6† | 15.8 |
| Moderate | 67.1 | 66.6 | 66.2 | 61.2 | 61.3† | 64.9 |
| Heavy | 15.3 | 14.8 | 15.8 | 23.9 | 24.1† | 19.3 |
| Smoking | ||||||
| Non-smoker | 83.5 | 86.0 | 87.9 | 91.2 | 91.6† | 89.1 |
| Smoker | 16.5 | 14.0 | 12.1 | 8.8 | 8.4† | 10.9 |
| Obesity | ||||||
| Not obese | 93.6 | 94.1† | 91.0 | 86.3 | 86.5† | 82.2 |
| Obese | 6.4 | 5.9† | 9.0 | 13.7 | 13.5† | 17.8 |
| GHQ case at next phase | 29.5 | 22.1 | 22.1 | 21.5 | 20.0 | 17.6 |
| Cumulative GHQ score | ||||||
| 0 | 73.3 | 57.7 | 51.4 | 46.3 | 42.0 | 39.2 |
| 1 | 26.7 | 27.9 | 26.4 | 25.4 | 24.4 | 23.9 |
| 2 | 0.0 | 14.4 | 14.3 | 15.0 | 15.5 | 14.8 |
| 3 | 0.0 | 0.0 | 7.9 | 8.8 | 9.4 | 10.1 |
| 4 | 0.0 | 0.0 | 0.0 | 4.5 | 5.8 | 5.6 |
| 5 | 0.0 | 0.0 | 0.0 | 0.0 | 3.0 | 4.1 |
| 6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2.3 |
| n (participants) ‡ | 7934 | 7090 | 5896 | 5127 | 4739 | 4496 |
Note. Values are percentages of participants unless otherwise noted.
Values are means (and standard deviations).
Data imputed with data from the previous phase.
Values are numbers of participants. At any given phase PT participants were included in the study sample only if they had complete data for GHQ at all phases between P1 and PT+1.
First we examined the change in the probability of GHQ caseness at phase PT+1 as a function of cumulative GHQ sum-score at phase PT and the number of measurements of the cumulative GHQ score (i.e., the number of study phases) at phase PT. This analysis was carried out by cross-tabulating the participants by cumulative GHQ score and number of times the cumulative GHQ score was available, and plotting the proportion of GHQ cases for each group.
The association between risk markers and GHQ caseness was examined in two parts. First we examined whether the risk markers assessed at phase PT predicted GHQ score at phase PT+1 without considering the interaction between the risk markers and the cumulative GHQ score. Next, we examined the effects associated with the interaction term between the cumulative GHQ score and risk markers at phase PT in predicting GHQ caseness at phase PT+1. These analyses indicated whether the association between risk markers and future GHQ caseness was dependent on cumulative history of GHQ caseness. All models included sex, age, period effects (i.e., follow-up phase), cumulative GHQ score, and the interaction between phase and cumulative GHQ score as independent variables (data not shown). The interaction term between study phase and cumulative GHQ score was included to take into account the observation that the effect of the cumulative GHQ score on future GHQ caseness depended on the number of available measurements of the cumulative GHQ score (see below).
Statistical models were fitted using multilevel, random-intercept logistic regression (Hu, Goldberg, Hedeker, Flay, & Pentz, 1998; Twisk, 2004) in which each participant contributed cumulative GHQ score observations as described above. All models were fitted using STATA 10 statistical software (StataCorp, College Station, Texas).
Results
Table 1 shows the descriptive statistics for the sample. The bottom row tabulates the number of participants with a cumulative GHQ score included in the analyses at each phase (data on phase PT and phase PT+1. Figure 1 shows the probability of being a GHQ case at phase PT+1 according to cumulative GHQ score and number of measurements of cumulative GHQ score available at phase PT. The probability of future GHQ caseness increased in a dose-response manner with the number of times a participant had previously been a GHQ case. For example, there were 3028 participants who had 3 measurements of the cumulative GHQ available at phase 3 and had a cumulative GHQ score of 0 at that time. These participants had a 10% probability of being a GHQ case at the subsequent follow-up. In contrast, of the 466 participants with 3 measurements of the cumulative GHQ by phase 3 and a cumulative GHQ score of 3 at that point in time, 59% were GHQ cases at the next phase. The gradient was steeper with fewer measurement points, which is to be expected; the ratio of high GHQ score per measurement changes as the number of measurement points increases. For instance, a cumulative GHQ score of 3 with 3 measurement times would indicate GHQ caseness at each phase up to that point, while the cumulative score of 3 with 6 measurement times would indicate GHQ caseness only in half of the follow-up phases up to that point.
Figure 1.
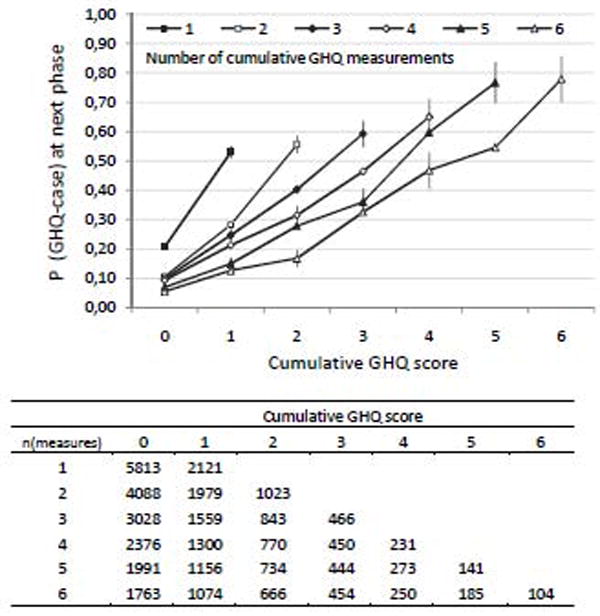
Probability (P) of being a GHQ case at Phase PT+1 according to cumulative GHQ score and number of measurements of the cumulative GHQ score available at phase PT. The error bars are 95% confidence intervals. The table below the figure shows the number of observations used in the calculation of each probability (N=7934 participants; 35282 person-observations).
Next we examined whether the risk markers assessed at phase PT predicted GHQ caseness at phase PT+1 (Table 2, Model 1). The probability of GHQ caseness was higher in women, low SES groups, in single individuals, in those of non-White ethnicity, those undertaking low levels of physical activity, the smokers and the obese. Model 2 of Table 2 shows the effect of each risk marker among participants with a cumulative GHQ score of 0 (main effect) and the proportional change in this effect associated with a one unit increase in the cumulative GHQ score (interaction effect). The interaction effect indicates whether the association between the risk markers and future GHQ depended on the prior cumulative GHQ score. For example, the main effect of sex shows that among participants with a cumulative GHQ score of 0, women have a 38% increased odds of becoming a GHQ case at the next phase, compared with men. However, the significant interaction effect indicates that this increased odds decreases by a factor of 0.95 for each unit increase in the cumulative GHQ score. For participants with a cumulative GHQ score of 6, for example, the odds ratio for women compared to men of being a GHQ case at phase 8 is only 1.01 (=1.38 × (0.95)6).
Table 2.
Predicting the Probability of GHQ Caseness at Study Phase PT+1 by Sociodemographic and Behavior-Related Risk Markers Assessed at Phase PT.
| Model 1 | Model 2 | ||
|---|---|---|---|
| Main effect† | Main effect† | Interaction effect†† | |
| Sex | |||
| Male | (reference) | (reference) | (reference) |
| Female | 1.29*** (1.21-1.37) | 1.38*** (1.27-1.50) | 0.95* (0.90-1.00) |
| SES | |||
| Low | (reference) | (reference) | (reference) |
| Intermediate | 0.92* (0.85-0.99) | 1.01 (0.91-1.12) | 0.88*** (0.83-0.94) |
| High | 0.90** (0.83-0.97) | 1.04 (0.94-1.15) | 0.83*** (0.79-0.89) |
| Marital status | |||
| Married | (reference) | (reference) | (reference) |
| Single | 1.09* (1.01-1.18) | 1.01 (0.90-1.12) | 1.09* (1.01-1.16) |
| Divorced | 0.99 (0.88-1.10) | 1.16 (1.00-1.35) | 0.87** (0.80-0.95) |
| Widow | 0.97 (0.79-1.19) | 1.14 (0.86-1.51) | 0.92 (0.80-1.05) |
| Ethnicity | |||
| White | (reference) | (reference) | (reference) |
| Other | 1.17* (1.05-1.30) | 1.03 (0.90-1.19) | 1.17** (1.06-1.29) |
| Physical activity | |||
| Low | (reference) | (reference) | (reference) |
| Intermediate | 0.87*** (0.82-0.94) | 0.93 (0.84-1.02) | 0.96 (0.90-1.01) |
| High | 0.84*** (0.78-0.90) | 0.87** (0.79-0.96) | 0.99 (0.93-1.05) |
| Alcohol consumption | |||
| None | (reference) | (reference) | (reference) |
| Moderate | 0.94 (0.87-1.02) | 1.09 (0.99-1.21) | 0.87*** (0.81-0.93) |
| Heavy | 0.91 (0.83-1.01) | 1.06 (0.93-1.21) | 0.88** (0.82-0.96) |
| Smoking | |||
| Non-smoker | (reference) | (reference) | (reference) |
| Smoker | 1.13** (1.04-1.23) | 1.13* (1.01-1.26) | 1.01 (0.94-1.09) |
| Obesity | |||
| Not obese | (reference) | (reference) | (reference) |
| Obese | 1.11* (1.01-1.23) | 1.08 (0.95-1.24) | 1.04 (0.97-1.12) |
Note:
p<.001,
p<.01,
p<.05;
All models are multilevel logistic regression models fitted separately for each risk marker and adjusted for age, sex, period, cumulative GHQ-history score, and interaction effect between period and cumulative GHQ-history score; Model 1 includes only main effects, model 2 includes main effects and interaction effects between risk markers and cumulative GHQ-history score.
The main effect estimates show odds ratios (and 95% confidence intervals) associated with a one unit change in the independent variable. In model 2, the main effect is shown for participants who have a cumulative GHQ score of 0.
There were statistically significant interactions for 5 of the 8 risk markers. The interaction effects of Table 2 are illustrated in Figure 2 and Figure 3 which plot the estimated probabilities of being a GHQ case for each risk marker according to the cumulative GHQ score. Note that these lines have a slightly different shape than the lines in Figure 1, because in Figures 2 and 3 the predictions are calculated over all measurement times combined. Low SES, non-White ethnicity, being single, and alcohol abstinence predicted future GHQ caseness more strongly in individuals with a longer history of GHQ caseness than in individuals with no such history. The nature of these GHQ-history-dependent association was similar to the main effects presented in Model 1, with one exception: compared to being married, divorce was associated with a lower risk of future GHQ caseness among individuals with a high cumulative GHQ score although among individuals with a cumulative GHQ score of 0, divorce was associated with 16% higher risk of future GHQ caseness (Model 1, Table 3).
Figure 2.
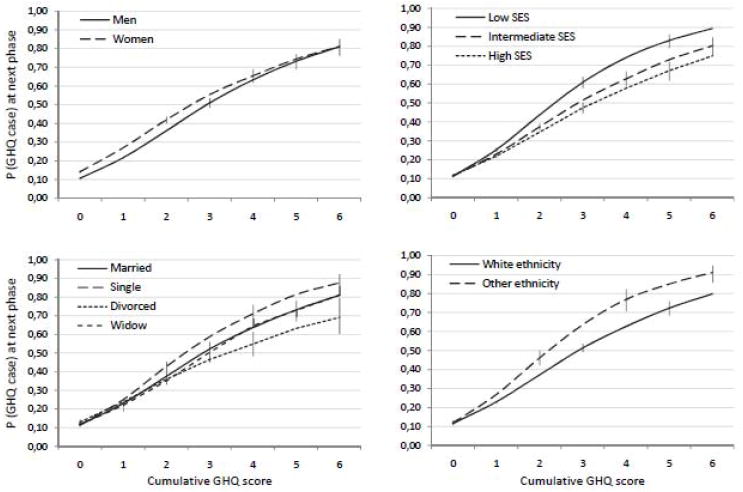
Model-predicted probability (P) of GHQ caseness at phase PT+1 as a function of sociodemographic risk markers and cumulative GHQ score at phase PT. The error bars are 95% confidence intervals. See the interaction effects of table 3 for statistical details. N=7934 participants, 35282 person-observations.
Figure 3.
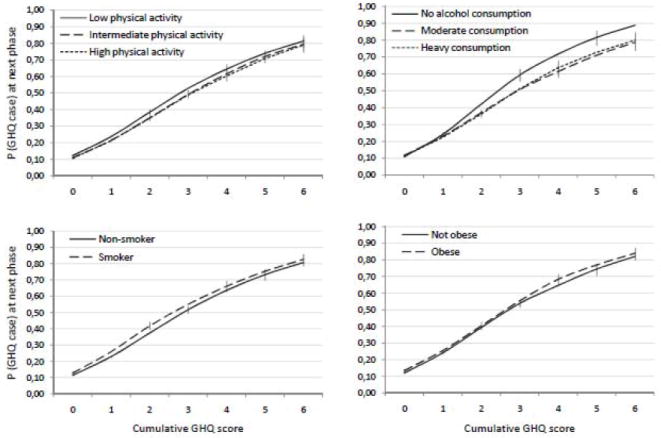
Model-predicted probability (P) of GHQ caseness at phase PT+1 as a function of health-related risk markers and cumulative GHQ score at phase PT. The error bars are 95% confidence intervals. See the interaction effects of table 3 for statistical details. N=7934 participants, 35282 person-observations.
The opposite pattern was observed for sex, as women had a higher risk of GHQ caseness than men among participants with no or only a short history of GHQ caseness but this sex difference was not observed among participants with a long history of GHQ caseness. The influence of physical activity, smoking, and obesity on the probability of being a GHQ case at the next phase was not modified by GHQ history. When all the statistically significant risk markers were mutually adjusted in a single multivariate model, all the interaction effects remained statistically significant with the exception of ethnicity (OR=1.09, CI=0.98-1.22; data not shown).
Attrition Analysis
To examine potential bias due to selective attrition, we created a dichotomous variable indicating whether the participant had data at phase PT+1 (0=data, 1=no data). We then examined whether this probability was dependent on GHQ caseness at phase PT and cumulative GHQ score at phase PT in separate models, also including sex, age, and period. Increased probability of attrition was predicted by GHQ caseness (OR=1.14, CI=1.06-1.22, p<0.001) but not by cumulative GHQ score (OR=1.01, CI=0.98-1.05, p=0.51), indicating that GHQ cases were more likely than non-cases to be lost during the follow-up. This might have led to some underestimation of the cumulative effect of prior GHQ caseness on future risk, because the number of participants with high GHQ scores was lower than it would have been without selective attrition. When the interaction effects shown in table 2 were fitted in a subsample with complete data at all of the study phases (n=4496 participants, 26976 observations), the results closely resembled the main results, with the exception of a non-significant interaction effect for ethnicity in the complete-case analysis (OR=1.09, CI=0.97-1.22; data not shown), suggesting that the interaction effects were not substantially affected by attrition bias.
Discussion
The present study has two main findings. First, a cumulative history of self-reported psychological distress strongly conditioned the likelihood of future psychological distress, such that recurrent distress became progressively more persistent over time. Thus, participants who exhibited GHQ caseness at all of 5 or 6 measurement times over an observation period of two decades had approximately an 80% probability of exhibiting GHQ caseness two years later whereas the corresponding probability was only 6% in participants with no prior distress. These findings suggest that symptoms of self-reported psychological distress exhibit a similar progressively accumulating pattern as that observed for episodes of major depressive disorder in clinical samples (D. A. Solomon, et al., 2000).
Second, a cumulative history of psychological distress modified the effects of socioeconomic and behavior-related risk markers on future distress. SES, ethnicity, marital status, and alcohol abstinence became stronger predictors of future GHQ caseness in participants with a longer history of GHQ caseness, suggesting that chronic distress may render individuals more vulnerable to risk markers and their correlates. This is analogous to the process postulated by the stress sensitization model, according to which prior episodes of depression sensitize individuals to minor stressors of daily life (Monroe & Harkness, 2005).
Comparison with Previous Studies
Most of the interaction effects between cumulative GHQ and risk markers were in the expected directions, e.g., increasing risk of GHQ caseness was associated with a combination of prior distress and low SES or ethnic minority origin. Parental SES has been shown to predict recurrent adult depression (Gilman, Kawachi, Fitzmaurice, & Buka, 2003), which is consistent with our findings. Other studies of adult SES have found no evidence of SES being related to risk of recurring episodes (Belsher & Costello, 1988; Burcusa & Iacono, 2007; Gonzales, Lewinsohn, & Clarke, 1985), but most of these studies have been carried out in relatively small and underpowered samples. Ethnic differences have been little studied in relation to recurrence.
Marriage had a protective effect against accumulating psychological distress, which was expected in light of the health benefits of being married (Stack & Eshleman, 1998). Perhaps surprisingly, however, prior history of GHQ caseness predicted future GHQ caseness less strongly in divorced participants than in married or single participants. Although divorced individuals often have poorer health, an increasing number of longitudinal studies show that becoming divorced may actually increase subjective well-being – or at least attenuate distress that has built up years before the break-up (Andress & Brockel, 2007; Booth & Amato, 1991; Gardner & Oswald, 2006). Our finding suggests that divorce may attenuate the association between past and future distress, perhaps because it represents a discontinuity in a person’s life.
Alcohol consumption was also protective, which is in agreement with a number of studies suggesting that, compared to abstinence, moderate alcohol consumption is associated with mental and other health benefits (Britton, Singh-Manoux, & Marmot, 2004; I. J. Goldberg, 2003; McDougall, Becker, Delville, Vaughan, & Acee, 2007; O’Donnell, Wardle, Dantzer, & Steptoe, 2006; Peele & Brodsky, 2000). In the present sample even heavy alcohol consumption had a beneficial effect, which contrasts with most other studies reporting an inverse J-shaped curve between alcohol consumption and mental health (O’Donnell, et al., 2006; Peele & Brodsky, 2000). As the participants worked in the British Civil Service, it is likely that the prevalence of problem drinking was lower in this cohort than seen in population-based samples.
Gender exhibited the opposite pattern to other risk markers discussed above; the increased risk of psychological distress in women compared to men was observed in participants who had no or only a short history of GHQ caseness but was absent among participants with a chronic history of GHQ caseness. The gender difference in depression and psychological distress is well-established (Boughton & Street, 2007; Davis, Matthews, & Twamley, 1999; Hyde, Mezulis, & Abramson, 2008), but the rate of depression recurrence seems to be equal in men and women (Burcusa & Iacono, 2007; Luijendijk et al., 2008). Our finding implies that the gender difference in psychological distress may diminish with increasing chronicity of distress.
The effects of physical activity, smoking, and obesity were not modified by GHQ-caseness history even though these risk markers did predict GHQ caseness in the expected manner when examined in the absence of the interaction effect with prior GHQ caseness. Thus, the moderating influence of prior psychological distress on the effect of different kinds of mental health risk markers included in our study was more apparent in relation to socio-demographic markers than behavior-related risk markers.
While our findings suggest similarities between clinical depression and self-reported psychological distress, some differences between the two need to be acknowledged. First, the recurrence of clinical depression is measured as discrete depressive episodes. We operationalized the recurrence of psychological distress using 6 repeated measurements collected over a period of 19 years. As individual differences in psychological distress exhibit moderate continuity over time, it is possible that a cumulative measure captures different levels of latent chronicity that become apparent with repeated measurements rather than the causal effects (sensitization) of prior distress on subsequent distress.
Second, the original stress autonomy and sensitization models are concerned with major life events that can trigger depressive episodes (Monroe & Harkness, 2005; Stroud, et al., 2008). We examined common sociodemographic and behavior-related health markers that are constant (sex, ethnicity) or at least moderately stable over time (e.g., marital status, obesity) and thereby cannot function as triggering events as such. Some of these risk markers might act as proxy measures of the frequency and severity with which individuals encounter major and minor life stressors and indicate their capability to deal with them. For example, ethnic minority status and low SES have been related to increased exposure to stressful environments (Hatch & Dohrenwend, 2007), which may explain their interaction effects with psychological distress history. They may also have independent effects mediated by other mechanisms besides stressful life events.
Methodological Considerations
A large sample size and repeated measurements from 7 study phases provided us with a strong longitudinal setting in which to assess the recurrent nature of psychological distress. However, there are limitations to this study. First, some of the missing covariate data had to be imputed with data from the previous study phase. This might have somewhat decreased the accuracy of the covariate data, but it is unlikely that this had a substantial effect as the imputation was applied only to one (for smoking, physical activity, and alcohol consumption) or two (for body mass index) study phases. Second, the Whitehall II sample consists mainly of white-collar civil servants and is not representative of the general population, which may limit the generalizability of the results. Third, all participants were over 30 years of age at the beginning of the study, so we could not assess the accumulation of psychological distress from adolescence when symptoms of depression and anxiety often begin to emerge (Kessler, Berglund, Demler, Jin, & Walters, 2005). Studies in younger populations are needed to further understand the timing of cumulative effects. Lastly, detailed data on diagnosed and treated clinical psychiatric disorders were unavailable, so we could not assess the influence of treatment of the recurrent course of psychological distress. At Phase 7, 3.5% of the participants reported using anti-depressive or anti-anxiety medication, suggesting that the prevalence of clinical depression or anxiety in the sample was relatively low and that treatment was unlikely to substantially bias the overall results.
Conclusions
With the limitations of our study in mind, we conclude that the recurrent course of self-reported psychological distress in non-clinical populations qualitatively resembles that of recurring episodes of major depressive disorder in patient samples. The findings also suggest that a process similar to that postulated by the stress sensitization model may generalize beyond major life events, so that chronic distress heightens individuals’ sensitivity to a range of common risk markers. These findings add evidence to support a continuum between subclinical symptoms and clinical disorders, and underscore the need for further research on the course, prognosis, and recurrence of psychological distress.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American-Psychiatric-Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: APA; 2000. [Google Scholar]
- Andress HJ, Brockel M. Income and life satisfaction after marital disruption in Germany. Journal of Marriage and the Family. 2007;69(2):500–512. [Google Scholar]
- Belsher G, Costello CG. Relapse after recovery from unipolar depression - A critical review. Psychological Bulletin. 1988;104(1):84–96. doi: 10.1037/0033-2909.104.1.84. [DOI] [PubMed] [Google Scholar]
- Booth A, Amato P. Divorce and psychological stress. Journal of Health and Social Behavior. 1991;32(4):396–407. [PubMed] [Google Scholar]
- Boughton S, Street H. Integrated review of the social and psychological gender differences in depression. Australian Psychologist. 2007;42(3):187–197. [Google Scholar]
- Britton A, Singh-Manoux A, Marmot M. Alcohol consumption and cognitive function in the Whitehall II study. American Journal of Epidemiology. 2004;160(3):240–247. doi: 10.1093/aje/kwh206. [DOI] [PubMed] [Google Scholar]
- Burcusa SL, Iacono WG. Risk for recurrence in depression. Clinical Psychology Review. 2007;27(8):959–985. doi: 10.1016/j.cpr.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne JC. Self-reported distress - Analog or ersatz depression. Psychological Bulletin. 1994;116(1):29–45. doi: 10.1037/0033-2909.116.1.29. [DOI] [PubMed] [Google Scholar]
- Davis MC, Matthews KA, Twamley EW. Is life more difficult on mars or venus? A meta-analytic review of sex differences ln major and minor life events. Annals of Behavioral Medicine. 1999;21(1):83–97. doi: 10.1007/BF02895038. [DOI] [PubMed] [Google Scholar]
- Flett GL, Vredenburg K, Krames L. The continuity of depression in clinical and nonclinical samples. Psychological Bulletin. 1997;121(3):395–416. doi: 10.1037/0033-2909.121.3.395. [DOI] [PubMed] [Google Scholar]
- Gardner J, Oswald AJ. Do divorcing couples become happier by breaking up? Journal of the Royal Statistical Society Series a-Statistics in Society. 2006;169:319–336. [Google Scholar]
- Gilman SE, Kawachi I, Fitzmaurice GM, Buka SL. Socio-economic status, family disruption and residential stability in childhood: relation to onset, recurrence and remission of major depression. Psychological Medicine. 2003;33(8):1341–1355. doi: 10.1017/s0033291703008377. [DOI] [PubMed] [Google Scholar]
- Goldberg DP. Detecting Pychiatric Illness by Questionnaire. London: Oxford University Press; 1972. [Google Scholar]
- Goldberg DP, Gater R, Sartorius N, Ustun TB, Piccinelli M, Gureje O, et al. The validity of two versions of the GHQ in the WHO study of mental illness in general health care. Psychological Medicine. 1997;27(1):191–197. doi: 10.1017/s0033291796004242. [DOI] [PubMed] [Google Scholar]
- Goldberg IJ. To drink or not to drink? New England Journal of Medicine. 2003;348(2):163–164. doi: 10.1056/NEJMe020163. [DOI] [PubMed] [Google Scholar]
- Gonzales LR, Lewinsohn PM, Clarke GN. Longitudinal follow-up of unipolar depressives - An investigation of predictors of relapse. Journal of Consulting and Clinical Psychology. 1985;53(4):461–469. doi: 10.1037//0022-006x.53.4.461. [DOI] [PubMed] [Google Scholar]
- Hatch SL, Dohrenwend BP. Distribution of traumatic and other stressful life events by race/ethnicity, gender, SES and age: A review of the research. American Journal of Community Psychology. 2007;40(3-4):313–332. doi: 10.1007/s10464-007-9134-z. [DOI] [PubMed] [Google Scholar]
- Hermens MLM, van Hout HPJ, Terluin B, van der Windt D, Beekman ATF, van Dyck R, et al. The prognosis of minor depression in the general population: a systematic review. General Hospital Psychiatry. 2004;26(6):453–462. doi: 10.1016/j.genhosppsych.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Hu FB, Goldberg J, Hedeker D, Flay BR, Pentz MA. Comparison of population-averaged and subject-specific approaches for analyzing repeated binary outcomes. American Journal of Epidemiology. 1998;147(7):694–703. doi: 10.1093/oxfordjournals.aje.a009511. [DOI] [PubMed] [Google Scholar]
- Huppert FA, Whittington JE. SYMPTOMS OF PSYCHOLOGICAL DISTRESS PREDICT 7-YEAR MORTALITY. Psychological Medicine. 1995;25(5):1073–1086. doi: 10.1017/s0033291700037569. [DOI] [PubMed] [Google Scholar]
- Hyde JS, Mezulis AH, Abramson LY. The ABCs of depression: Integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychological Review. 2008;115(2):291–313. doi: 10.1037/0033-295X.115.2.291. [DOI] [PubMed] [Google Scholar]
- Judd LL, Schettler PJ, Akiskal HS. The prevalence, clinical relevance, and public health significance of subthreshold depressions. Psychiatric Clinics of North America. 2002;25(4):685–+. doi: 10.1016/s0193-953x(02)00026-6. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Walters EE. Lifetime prevalence and age-of-onset distributions’ of DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Zhao SY, Blazer DG, Swartz M. Prevalence, correlates, and course of minor depression and major depression in the national comorbidity survey. Journal of Affective Disorders. 1997;45(1-2):19–30. doi: 10.1016/s0165-0327(97)00056-6. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Solomon A, Seeley JR, Zeiss A. Clinical implications of “subthreshold” depressive symptoms. Journal of Abnormal Psychology. 2000;109(2):345–351. [PubMed] [Google Scholar]
- Luijendijk HJ, van den Berg JF, Dekker M, van Tuijl HR, Otte W, Smit F, et al. Incidence and Recurrence of Late-Life Depression. Archives of General Psychiatry. 2008;65(12):1394–1401. doi: 10.1001/archpsyc.65.12.1394. [DOI] [PubMed] [Google Scholar]
- Marmot M, Brunner E. Cohort profile: The Whitehall II study. International Journal of Epidemiology. 2005;34(2):251–256. doi: 10.1093/ije/dyh372. [DOI] [PubMed] [Google Scholar]
- Marmot MG, Smith GD, Stansfeld S, Patel C, North F, Head J, et al. Health inequalities among British civil-servants - The Whitehall II study. Lancet. 1991;337(8754):1387–1393. doi: 10.1016/0140-6736(91)93068-k. [DOI] [PubMed] [Google Scholar]
- McDougall GJ, Becker H, Delville CL, Vaughan PW, Acee TW. Alcohol use and older adults: A little goes a long way. International Journal on Disability and Human Development. 2007;6(4):431–440. doi: 10.1901/jaba.2007.6-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod JD, Kessler RC. Socioeconomic-status differences in vulnerability to undesirable life events. Journal of Health and Social Behavior. 1990;31(2):162–172. [PubMed] [Google Scholar]
- Mitchell AJ, Subramaniam H. Prognosis of depression in old age compared to middle age: A systematic review of comparative studies. American Journal of Psychiatry. 2005;162(9):1588–1601. doi: 10.1176/appi.ajp.162.9.1588. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Harkness KL. Life stress, the “Kindling” hypothesis, and the recurrence of depression: Considerations from a life stress perspective. Psychological Review. 2005;112(2):417–445. doi: 10.1037/0033-295X.112.2.417. [DOI] [PubMed] [Google Scholar]
- Mueller TI, Leon AC, Keller MB, Solomon DA, Endicott J, Coryell W, et al. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. American Journal of Psychiatry. 1999;156(7):1000–1006. doi: 10.1176/ajp.156.7.1000. [DOI] [PubMed] [Google Scholar]
- O’Donnell K, Wardle J, Dantzer C, Steptoe A. Alcohol consumption and symptoms of depression in young adults from 20 countries. Journal of Studies on Alcohol. 2006;67(6):837–840. doi: 10.15288/jsa.2006.67.837. [DOI] [PubMed] [Google Scholar]
- Peele S, Brodsky A. Exploring psychological benefits associated with moderate alcohol use: a necessary corrective to assessments of drinking outcomes? Drug and Alcohol Dependence. 2000;60(3):221–247. doi: 10.1016/s0376-8716(00)00112-5. [DOI] [PubMed] [Google Scholar]
- Pevalin DJ. Multiple applications of the GHQ-12 in a general population sample: an investigation of long-term retest effects. Social Psychiatry and Psychiatric Epidemiology. 2000;35(11):508–512. doi: 10.1007/s001270050272. [DOI] [PubMed] [Google Scholar]
- Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. American Journal of Psychiatry. 1992;149(8):999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- Prince M, Patel V, Saxena S, Maj M, Maselko J, Phillips MR, et al. Global mental health 1 - No health without mental health. Lancet. 2007;370(9590):859–877. doi: 10.1016/S0140-6736(07)61238-0. [DOI] [PubMed] [Google Scholar]
- Puhl RM, Andreyeva T, Brownell KD. Perceptions of weight discrimination: prevalence and comparison to race and gender discrimination in America. International Journal of Obesity. 2008;32(6):992–1000. doi: 10.1038/ijo.2008.22. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Williams JM, Teasdale JD, Gemar M. A cognitive science perspective on kindling and episode sensitization in recurrent affective disorder. Psychological Medicine. 1996;26(2):371–380. doi: 10.1017/s0033291700034760. [DOI] [PubMed] [Google Scholar]
- Solomon A, Haaga DAF, Arnow BA. Is clinical depression distinct from subthreshold depressive symptoms? A review of the continuity issue in depression research. Journal of Nervous and Mental Disease. 2001;189(8):498–506. doi: 10.1097/00005053-200108000-00002. [DOI] [PubMed] [Google Scholar]
- Solomon DA, Keller MB, Leon AC, Mueller TI, Lavori PW, Shea T, et al. Multiple recurrences of major depressive disorder. American Journal of Psychiatry. 2000;157(2):229–233. doi: 10.1176/appi.ajp.157.2.229. [DOI] [PubMed] [Google Scholar]
- Stack S, Eshleman JR. Marital status and happiness: A 17-nation study. Journal of Marriage and the Family. 1998;60(2):527–536. [Google Scholar]
- Stansfeld SA, Marmot MG. Social class and minor psychiatric disorder in British civil servants - A validated screening survey using the General Health Questionnaire. Psychological Medicine. 1992;22(3):739–749. doi: 10.1017/s0033291700038186. [DOI] [PubMed] [Google Scholar]
- Stroud CB, Davila J, Moyer A. The relationship between stress and depression in first onsets versus recurrences: A meta-analytic review. Journal of Abnormal Psychology. 2008;117(1):206–213. doi: 10.1037/0021-843X.117.1.206. [DOI] [PubMed] [Google Scholar]
- Twisk JWR. Longitudinal data analysis. A comparison between generalized estimating equations and random coefficient analysis. European Journal of Epidemiology. 2004;19(8):769–776. doi: 10.1023/b:ejep.0000036572.00663.f2. [DOI] [PubMed] [Google Scholar]


