Abstract
TRPA channels detect stimuli of different sensory modalities, including a broad spectrum of chemosensory stimuli, noxious stimuli associated with tissue damage and inflammation, mechanical stimuli, and thermal stimuli. Despite a growing understanding of potential modulators, agonists, and antagonists for these channels, the exact mechanisms of channel regulation and activation remain mostly unknown or controversial and widely debated. Relatively little is also known about the basic biophysical parameters of both native and heterologously expressed TRPA channels. Here we use conventional single channel inside-out and outside-out patch recording from the human TRPA1 channel transiently expressed in human embryonic kidney 293T cells to characterize the selectivity of the channel for inorganic mono-/divalent and organic monovalent cations in the presence of Allylisothiocyanate (AITC). We show the relative permeability of the hTRPA1 channel to inorganic cations to be: Ca2+(5.1)>Ba2+(3.5)>Mg2+(2.8)>NH4+(1.5)>Li+(1.2)>Na+(1.0)≥K+(0.98)≥Rb+(0.98)>Cs+(0.95); and to organic cations: Na+(1.0)≥Dimethylamine(0.99)>Trimethylamine(0.7)>Tetramethylammonium(0.4)>N-methyl-d-glucamine(0.1). Activation of the hTRPA1 channels by AITC appears to recruit the channels to a conformational state with an increased permeability to large organic cations. The pore of the channels in this state can be characterized as dilated by approximately 1–2.5A.
These findings provide important insight into the basic fundamental properties and function of TRPA1 channels in general and human TRPA1 channel in particular.
Keywords: Human TRPA channel, ionic permeability, selectivity, channel pore diameter
1. Introduction
There is striking similarity in the functional role of TRPA channel orthologues between evolutionary diverse species (e.g. [1] and [2]). Abundantly expressed in the somatosensory system, including in trigeminal neurons, mammalian TRPA channels detect and integrate noxious stimuli of different sensory modalities including mechanical [3], [4] and thermal [3], [5]. TRPA channels also function as broad spectrum “alarm” chemoreceptor-channels, signaling potentially harmful exposure to irritants and pungent compounds [6]. The growing list of chemical stimuli activating the channel includes isothiocyanates [7]; thiosulfinates [8]; acrolein [1] and [3]; cinnamaldehyde derivatives, eugenol, methyl salicylate, gingerol [9]; nicotine [10]; thymol and related alkyl phenols [11], as well as multiple products of oxidative stress [12] and [13]. TRPA channels can be also activated by intracellular calcium [14], [15], [16] and are subject to regulation by phosphoinositides [17] and [18], suggesting that the activity of the channels could be also under control of GPCR-mediated signaling [7], [9], [19].
Despite numerous studies of TRPA channels since their first molecular and functional identification [20], [21], [7] and the impressive number of known or suspected agonists and antagonists, the exact mechanisms of channel regulation are still unclear, and in many cases controversial and widely debated. For example, there is currently no general consensus on the role of phosphoinositides in modulating channel activity [17] and [18], on the site(s) of calcium binding [14], [16], on the proton dependent modification of channel gating ([22], [23]) or on the extent to which these channels contribute to thermo sensation [21], [15]. Moreover, relatively little is known about the basic biophysical parameters of both native and heterologously expressed TRPA channels. Relative scattered data on TRPA channel selectivity provide divergent estimates of cation permeability; e.g., estimates for the permeability ratio for calcium ions (PCa2+/PNa+) range from 0.84 [21], to ~3–4 [16], to 5.71 or 7.91, for basal and agonist-induced channel activity respectively [24].
Here, we use transient expression of the human TRPA1 (hTRPA1) channel in human embryonic kidney 293T (HEK293T) cells and conventional single channel inside-out and outside-out patch recording to characterize basic properties and the selectivity of the channel to mono- and divalent cations. We show that the channel is activated by intracellular calcium with [Ca2+]1/2 of 6µM and a cooperativity coefficient h=0.9 and that ruthenium red (RR) inhibits the hTRPA1 channel with IC50~1.8µM and Hill coefficient h~1.8. We show the relative permeability of the hTRPA1 channel in the presence of AITC to be: Ca2+(5.1)>Ba2+(3.5)>Mg2+(2.8)>NH4+(1.5)>Li+(1.2)>Na+(1.0)≥K+(0.98)≥Rb+(0.98)>Cs+(0.95). Based on the relative permeability of the channels to monovalent organic cations of different sizes (Dimethylamine, Trimethylamine, Tetramethylammonium, N-methyl-d-glucamine, Tetraethylammonium), we estimate the apparent minimal diameter of the channel pore to be approximately 7.4 Angstrom.
Activation of the hTRPA1 channels by AITC appears to recruit them to a conformational state that has an increased permeability to large organic cations. The pore of the channels in this state can be characterized as dilated by approximately 1–2.5A.
Our findings characterize and refine the basic parameters of hTRPA1 channels, providing further insight into the function of TRPA1 channels and their important roles in primary sensory transduction.
2. Materials and Methods
2.1 Heterologous expression and transient transfection
HEK293T cells were grown in HEK media [Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (MP Biomedicals, Solon, OH, USA), 2 mm l-glutamine, and 100 µg/ml penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA)] at 37°C with 5% CO2]. The hTRPA1 channel was transiently expressed in the cells from the recombinant expression plasmid pcDNA5-FRT carrying the entire protein coding region for hTRPA1 described by Doerner et al. (2007). Semi-confluent HEK293T cells in 35-mm dishes were transiently co-transfected with pcDNA5-FRT/ hTRPA1 and a separate plasmid (pXoon) carrying the coding sequence for green fluorescent protein (GFP) using 1 µg of each plasmid DNA and 6 µl Fugene 6 transfection reagent (Roche Applied Science, Indianapolis, IN, USA) following the manufacturer’s protocol. At 24 to 48 hours post-transfection, the cells were gently lifted from the dishes using 2 mM EDTA in phosphate-buffered saline, washed with HEK media, re-plated as individual cells/ small clusters in HEK media without antibiotics on 35-mm plates, and allowed to recover for at least 2 hours prior to electrophysiology.
2.2 Electrophysiology and data analysis
hTRPA1 channel activity was investigated using inside-out and outside-out patch clamp recordings. The channel unitary currents were measured with an 200B patch-clamp amplifier (Molecular Devices, Sunnyvale, CA, USA) and a digital interface (Digidata 1320A, Molecular Devices, Sunnyvale, CA, USA), lowpass filtered at 5–10 kHz, sampled at 2–20 kHz and in most cases digitally filtered at 1–1.4 kHz. Analysis of the data was carried out using pCLAMP 9.2 software (Molecular Devices, Sunnyvale, CA, USA) and SigmaPlot 10 (Systat Software Inc., San Jose, CA, USA). Channel activity was investigated at a holding potential of −50/+50 mV unless otherwise specified. The polarity of the currents/voltages is presented relative to intracellular membrane surface, in spite of the membrane patch configuration. Appropriate corrections for liquid junction potentials were made when necessary. Patch pipettes were fabricated from borosilicate capillary glass (BF150-86-10, Sutter Instrument, CA, USA) using a Flaming-Brown micropipette puller (P-87, Sutter Instrument, CA, USA). The fire polished patch pipette had a resistance 2.4–9 MOhm (5.1±0.01) when filled with standard NaCl 140mM. Only patches with seal resistance estimated higher 1 GOhms were used in the experiments.
Bath solution change was performed using a rapid solution changer with a modified tube holder, RSC-160 (Bio-Logic - Science Instruments, Claix, France). Data were recorded under continuous perfusion with the solution of interest, with different solutions applied in random order.
GFP positive HEK293T cells were visualized using an Axiovert 100 inverted microscope (Carl Zeiss, Inc., Germany) equipped with an mercury vapor compressed-arc lamp (HBO100) coupled to widefield fluorescence filter set (1114-459, Zeiss). The sample illumination time did not exceed 20 sec, to minimize the possible effects of short wavelength light on the channel activity [13].
Following modification of the Hill equation was used to fit the experimental data for calcium dependent channel activation,
| (1) |
and for Ruthenium Red (RR) dependent channel inhibition,
| (2) |
where Ps are the normalized PoN values, [X] is the agonist/antagonist concentration, [X]1/2 is the half-effective concentration, and h is the cooperativity coefficient.
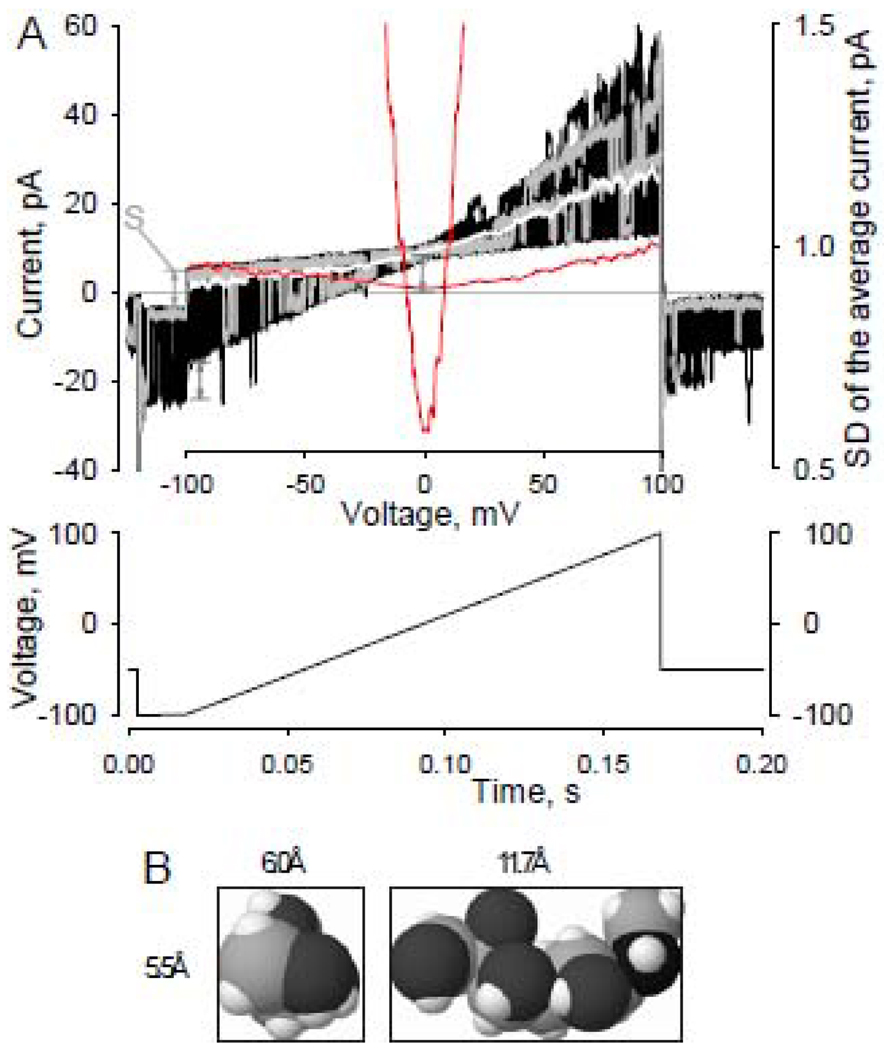
Patch current-voltage characteristics were generated using series of 15-ms step at −100 mV followed by a 150-ms voltage ramp (linear change in voltage ~0.67mV/ms) from −100 mV to +100 mV were applied from a holding potential of −50 mV (Fig.1A). The interval between sweep starts was 1s. Polarity of the protocol was changed respectively whenever holding potential was +50mV. In order to determine reversal potentials of unitary currents we typically used the following procedure provided by pCLAMP software: series of current traces (50–200 patch I–V curves) were averaged and the standard deviation (SD) for each point in the average current trace was calculated; the position of the minimum of the resulting SD curve along the voltage axis would correspond to a reversal potential value at given ionic conditions (Fig. 1A). The approach appeared to be quite effective considering little voltage dependence of the channel gating parameters (e.g. Fig. 1A, 2A). Current level shifts (S) produced by voltage ramp application and proportional to the magnitude CdV/dt (Fig. 1A) were not compensated unless noted otherwise.
Fig. 1.
A - Patch current-voltage characteristics and reversal potential estimate. Patch current-voltage characteristics (top graph, 25 black lines, two individual traces shown in grey) were generated using series of 15-ms step at −100 mV followed by a 150-ms voltage ramp (linear change in voltage ~0.67mV/ms) from −100 mV to +100 mV were applied from a holding potential of −50 mV (diagram). The interval between sweep starts was 1s. Polarity of the protocol was changed respectively whenever the holding potential was +50mV. In order to determine the reversal potentials of unitary currents we typically used the following procedure: series of current traces (50–200 patch I–V curves) were averaged (white line) and the standard deviation (SD) for each point in the average current trace was calculated (red lines); the position of the minimum of the resulting SD curve along the voltage axis corresponds to a reversal potential value at given ionic conditions. Both red lines are the same SD of the average current function. One is scaled separately (right-hand axis) to better visualize the position of the minimum of the function. Note, absolute current values can not be used to determine Vrs. Fifteen-ms step preceding voltage ramp allows visualization of the current level shifts (S) produced by voltage ramp applications and is proportional to the uncompensated capacitance of the recording configuration and voltage ramp speed (CdV/dt). Experimental conditions: Experimental conditions: outside-out patch recording; symmetrical NaCl140 solution.
B - 3-D space-filling model representation of an NMDG molecule. Dimensions of the smallest box encompassing the molecule are 5.5Å X6.0Å X11.7Å. 6.0Å was estimated as the smallest NMDG molecule diameter. Molecular size estimate of NMDG was determined by the “smallest box enclosing solute” procedure provided by HyperChem 8.0 software (Hypercube, Inc., Gainesville, FL, USA; http://www.hyper.com).
Fig. 2.
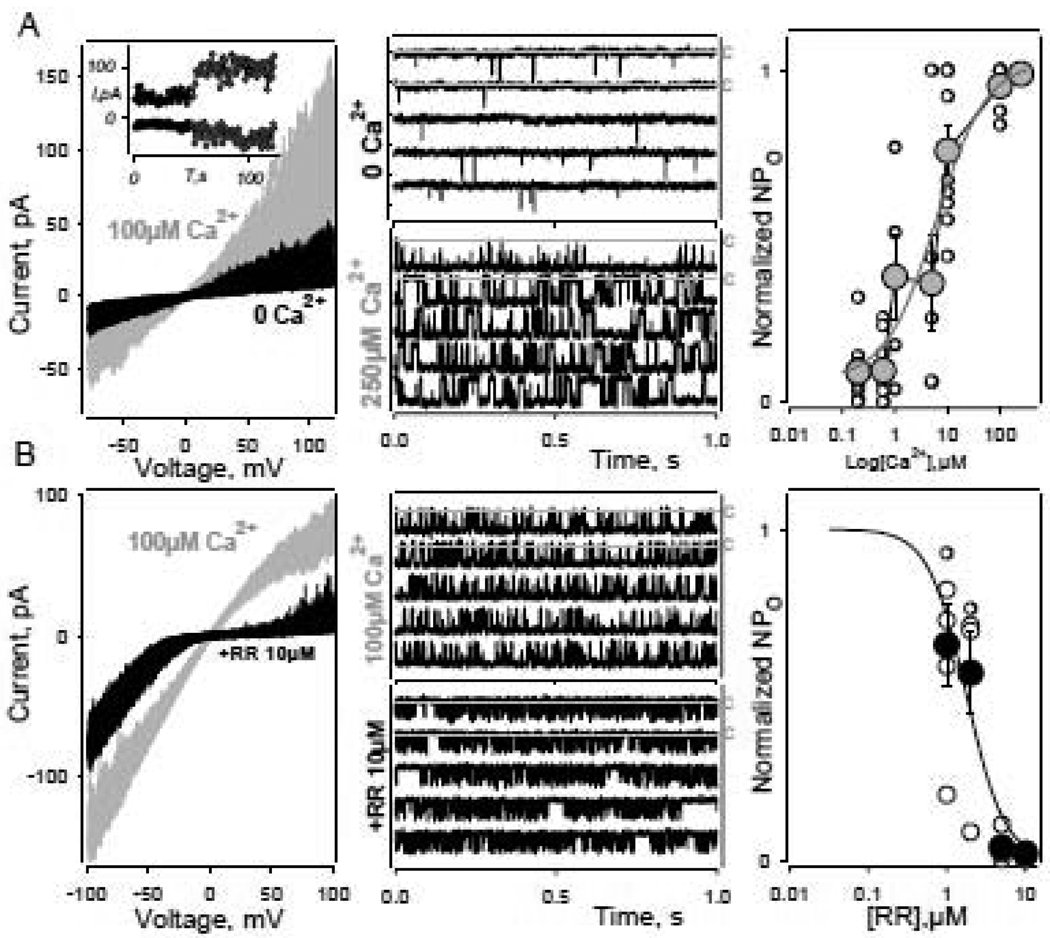
Basic pharmacological properties of heterologously expressed hTRPA1 channel. A – Activation of the hTRPA1 channel by increasing intracellular calcium concentrations in multi- (left) and single (middle) channel recordings. Note, despite great variability in calcium sensitivity of different membrane patches (n=9, right panel, empty circles), the summary parameters of [Ca2+] dependence of the channel activity (grey circles) are basically consistent with those reported earlier. Experimental data were fit by Hill equation with following parameters: [Ca2+]1/2=6.2 µM; h=0.95 (smooth grey line). Inset, the average currents at −70 and 100mV show little if any reduction of channel activity during the time course of an experiment. Experimental conditions for A: inside out patch recording; holding potential - −50mV; electrode solution - standard NaCl140mM; perfusion solution - NaCl140mM containing the different [Ca2+]free. B – Inhibition of hTRPA1 channel activity by ruthenium red (RR) applied from cytoplasmic side of membrane patch in multi- (left) and single (middle) channel recordings. Note, the RR effects were considerably voltage dependent (compare black ramp series with control ones, left panel). Right panel – summary dependence of the channel activity from [RR]. Empty circles – experimental data. Smooth black line is the result of approximation of mean values (black circles) by modified Hill equation with following parameters: IC50=1.8 µM, h=1.8, n=5. A, B, left graphs - 20 current voltage characteristics (IV curves, “ramps”) are shown for every experimental condition. Data were not filtered. Current level shift produced by voltage ramp application was compensated offline. A, B, middle panels – c marks closed states.
Experimental conditions: inside-out patch recording; holding potential - −50mV; electrode solution: NaCl140mM, Hepes 10mM, CaCl2 1µM.
The permeability ratios of monovalent cations X+ relative to Na+ (PX/PNa) was estimated based on the shift of the reversal potential (Er) upon exchanging the intra-/extra-cellular Na+ (140mM) with an solution containing an equivalent concentration of test cation X+ (see solutions) by using the Goldman-Hodgkin-Katz potential equation [25],
| (3) |
Estimation of the permeability ratios of divalent cations Y2+ (70mM) relative to Na+ (140mM) (PY/PNa) was based on the shift of the Er and calculated by using the equation [25],
| (4) |
Appropriate corrections into the equations were introduced to take into account the patch clamp recording configuration. Glucose 70mM was added to all Y2+ containing solutions to keep identical osmolarity (see solutions).
There is significant variety in published molecular diameter estimates. While this is not surprising given the nature of the question, there is often no information provided regarding the source of the estimates or how they were calculated. In this report, we determined the diameter of organic cations using standard algorithms of commonly available software rather than relying on undocumented estimates. The dimensions of Van der Waals radii scaled and energy optimized molecular models of organic cations were estimated using “smallest box enclosing solute” procedure provided by HyperChem 8.0 software (Hypercube, Inc., Gainesville, FL, USA; http://www.hyper.com). For example, based on the molecular structure of N-methyl-d-glucamine (NMDG, Fig.1B), the smallest diameter of the molecule is estimated to be 6Å. Overall, the molecular dimension estimates of the organic cations used in this report are consistent with the estimates provided by B.Hille (see e.g. [26]; D1×D2×D3,Å): Dimethylamine (DiMA, 3.4×3.9×5.6); Trimethylamine (TriMA, 3.4×5.3×5.8); Tetramethylammonium (TetMA, 5.5×5.6×5.7); N-methyl-d-glucamine (NMDG, 5.5×6×11.7); Tetraethylammonium (TEA, 5.8×6.8×8.1).
The channel pore diameter was estimated using the logarithmic form of the conventional excluded volume equation [27], [25],
| (5) |
where a is a diameter of charge carrier, and d is the channel pore diameter estimate.
Single channel current amplitudes were obtained from the multi-order Gauss distribution fitting of the appropriate all-points current histograms. The data are presented as the mean±SE of n observations. All recordings were performed at room temperature (~20–21°C).
2.3 Solutions and chemicals
The standard Na140mM solution contained (mM): 140 NaCl; 1–2 EGTA; 10 Hepes. Inorganic monovalent cation solutions contained equimolar concentration of chlorides of respective cations (mM): KCl 140; LiCl 140; RbCl 140; CsCl 140; NH4Cl 140. Divalent cation solutions contained 70mM CaCl2, MgCl2 or BaCl2; 70mM Glucose and no EGTA added. Solutions of different organic cation concentrations were prepared by appropriate substitution of [Na+] for [Organic Cation+] as noted. Following cations were used as monovalent organic cations (all – Cl−-salts): Dimethylamine; Trimethylamine; Tetramethylammonium; N-methyl-d-glucamine; Tetraethylammonium.
Solutions with different Ca2+ concentrations contained 1mM EGTA and corresponding amount of Ca2+. The free Ca2+ concentration was estimated using WebmaxC v.2.20 (http://www.stanford.edu/~cpatton/webmaxcS.htm). Solutions containing more than 10 µM Ca2+/Mg2+ were prepared without chelating agents. Ruthenium red (RR) and Allylisothiocyanate (AITC) were prepared as stock solutions of 50 mM in water and 200mM in DMSO respectively prior their dilution to the working concentrations. The pH of solutions was adjusted with NaOH or Trizma base (Sigma-Aldrich, St. Louis, MO, USA) to 7.3–7.4.
All inorganic salts were purchased from Fisher Scientific (Pittsburgh, PA, USA), except for RbCl, CsCl and NH4Cl, which were purchased from Sigma-Aldrich. All organic compounds were obtained from Sigma-Aldrich except for dextrose (D-glucose) obtained from Fisher Scientific, and DiMA and TriMA obtained from Acros Organics USA (Morris Plains, NJ, USA).
3. Results and Discussion
3.1 Basic physiological and pharmacological characterization of hTRPA1 channels
The hTRPA1 channel was heterologously expressed in HEK293T cells. A plasmid carrying the GFP gene was co-transfected to ease the identification of cells expressing the channel. We first performed physiological and pharmacological tests to establish the channel identity and functionality. All GFP positive cells possessed channels with properties consistent with those reported for TRPA1 channel. The channel was characterized by a relatively high single channel conductance of −173±2pS, as determined in symmetrical 140mM NaCl, n=41, (Fig. 2, single channel recordings); systematically occurring subconductance states with dominant levels of 63pS and 124pS, obtained by a second order Gaussian fit to the distribution of the mean subconductance level values (determined for 75 patches). It shows a slight voltage dependence of channel gating in cell-free patch clamp preparations (e.g. Fig. 2A,B, left panels). And finally, the channel recorded in cell-free membrane patches exhibited profound gradual loss in activity (rundown), possibly a consequence of the loss of intracellular soluble factor(s) that are required for channel function, e.g. inorganic polyphosphates [28], [29] and [16] (see also Fig.5). The rundown kinetics greatly varied between different patches and were dependent on the recording configuration, the presence of divalent cations, and chelator concentration, as well as on the presence of TRPA1 channel ligands. For the analysis of agonist/antagonist concentration dependence described below we did not attempt to control the channel rundown. Instead, we selected patches with channels that reached steady-state activity levels and exhibited little if any reduction of activity during the time course of an experiment (e.g. Fig. 2A, inset).
Fig. 5.
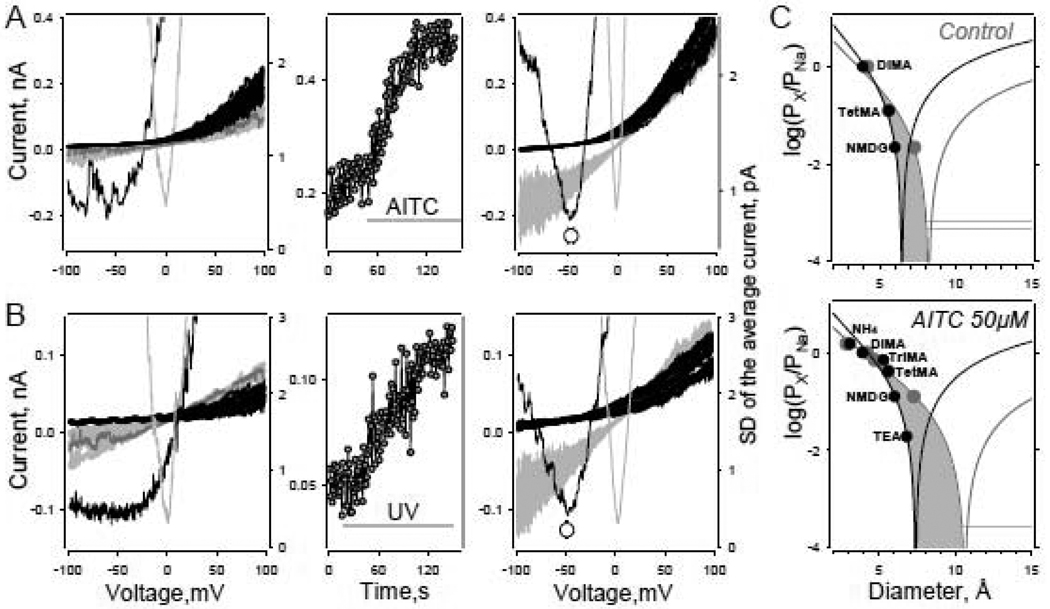
Agonist dependent change of organic cation permeability ratios. A – AITC dependent change in hTRPA1 channel permeability to NMDG+. Left – CV relationships of the patch containing hTRPA1 channels obtained before AITC application (100µM) in symmetrical NaCl140mM (grey) and in the presence of NMDG140mM (black). Number of ramps used to generate average current traces (not shown) and corresponding SD curves (parabola-shaped lines, right-hand axis) varied from 20 to 60. Note, in the presence of NMDG+, reversal potential of the channel current was undetectable. Middle – time course of the average current (within voltage range 95–100mV) of the patch before and after AITC application. Right - CV relationships of the patch obtained after incubation with AITC in symmetrical NaCl140mM (grey) and in the presence of NMDG140mM (black). Note, VrNMDG+=−48.3mV for the patch and −46.3±2.9mV on average (n=7). B – Change in hTRPA1 channel permeability to NMDG+ ions after exposure to UV light. Left – CV relationships of the patch containing hTRPA1 channels obtained before UV light application in symmetrical NaCl140mM (grey) and in the presence of NMDG140mM (black). Number of ramps used to generate average current traces (not shown) and corresponding SD curves (right-hand axis). Middle – time course of the average current (within voltage range 95–100mV) of the patch before and after UV light exposure. Right - CV relationships of the patch obtained after activation of the channels by UV light in symmetrical NaCl140mM (grey) and in the presence of NMDG140mM (black). Electrical interference seen in B originates from UV light source. Note, VrNMDG+ could be clearly determined after UV light dependent channel activation; VrNMDG+=−51mV for the patch and −49±1mV on average (n=3). Experimental conditions: Outside-out patch recording. Electrode solution – standard NaCl140mM+Na-tripolyphosphate 0.5mM. Holding potential −50mV.
C - Dependence of permeability ratios from minimal diameter (black circles) and mean diameter ((D1*D2*D3)1/3, grey circles) estimates of organic cation molecules. Lines are the approximation of the data with the simple excluded volume equation (5). Minimums of the functions indicate estimates for the channel pore diameter 6.4Å and 8.2Å in control conditions (top graph) and 7.4Å and 10.6Å in the presence of AITC (bottom graph). The permeability coefficient ratios were calculated based on ΔVrs at 140mM organic cations. The molecular dimensions were estimated using Hyperchem 8.0 software (Methods).
TRPA channels in general and the hTRPA1 channel in particular appear to be directly gated by intracellular calcium, although the exact mechanism and possible role of other factors, such as voltage or extracellular calcium concentration, in determining calcium-channel interaction require further analysis. In our experiments, elevation in cytoplasmic calcium concentration characteristically reversibly increased the channel open probability in 54 out of 60 patches (Fig. 2A). Despite considerable variability in the calcium effect among different patches (n=9, Fig. 2A, right panel, empty circles), the resulting dependence of normalized NPo values from the calcium concentration (Fig. 2A, right panel, grey circles) yields a half maximal calcium concentration [Ca2+]1/2 of 6µM with a cooperativity coefficient h=0.9. These parameters are consistent with the data obtained in whole cell experiments ([Ca2+]1/2=0.9µM, h=0.9, [14]; [Ca2+]1/2=6µM, [15]. However, in the presence of polyphosphates TRPA1 channels appear to be more sensitive to intracellular calcium, with a half maximal activation constant [Ca2+]1/2=225nM and h=1.83 [16].
Another characteristic property of TRPA1 channels is their sensitivity to Ruthenium Red (RR). Despite its apparent lack of selectivity, especially at micromolar levels, RR is commonly used to antagonize TRPA1 channels (e.g. [21], [2]). In our experiments, RR applied extracellularly or from cytoplasmic side of the membrane patches blocked the channels in a concentration dependent manner (Fig. 2B). The RR effects were reversible and clearly voltage dependent, with greater effects at positive voltages (Fig. 2B, left panel). As shown for a typical single channel inside-out recording (Fig. 2B, middle panel, holding potential, −50mV; symmetrical NaCl 140mM with 1µM [Ca2+]o and 100µM [Ca2+]i), RR decreased the open channel probability, Po, from 0.8 to 0.2, reducing the dwell-time for the channel in the open state from 6.3 to 0.8 ms. The flickery channel gating (fast channel transitions between open and closed channel stages) we observed in the presence of RR appears to be a common characteristic of RR-channel interaction (e.g. [30], [31]). Overall, as determined for 5 inside-out patch recordings at +50mV, RR inhibits the hTRPA1 channel with IC50~1.8µM and Hill coefficient h~1.8.
Although we did not pursue it in detail, the channel exhibited a relatively high level of spontaneous activity even without agonists in the cell-attached patch clamp recording mode. This activity correlated with the time of illumination of the samples with the light used to detect GFP positive HEK293T cells (see Methods for light source and filter set specifications) and could be at least partially attributed to activity of the channels due to free radical molecules formed by the exposure of cells, and even cell free membrane patches, to short wavelength light ([13], Fig. 5B). Under the given experimental conditions calcium applied from cytoplasmic side of membrane patches excised from non-transfected HEK cells did not activate any endogenous channels (n=21), indicating that the calcium dependent channel activity analyzed in our experiments can be attributed exclusively to the heterologously expressed hTRPA1 channels.
Overall these results suggest that basic properties of the human channels studied here are similar to those reported for other TRPA channels.
3.2 Estimation of hTRPA1 channel selectivity
Measurement of the selectivity of ion channels using single channel recording provides a number of advantages over experiments based on whole cell recordings. Specifically, single channel recordings allow better voltage control, leakage current control, control over possible activation of other channel types, and ionic gradient control. It also can reveal subtle effects of the ion environment on the parameters of channel gating and channel conductance, and shifts in reversal potential (ΔVr). Therefore we used single channel recording to measure the selectivity of the hTRPA1 channel pore.
The patch pipette in these experiments contained standard Na140mM solution and 50–200µM AITC unless otherwise noted. Membrane patches were first exposed to symmetrical NaCl140 solution. To obtain the reversal potential (Vr) of the channel we generated a series of current-voltage (CV) characteristics (50–200) applying a linear voltage change (ramp protocol, ~0.67mV/ms, see methods, Fig. 1). Then, the same patch was exposed to a solution in which the Na+ ions were completely replaced by one of the following cations: Li+, K+, Cs+, Rb+, Ca2+, Ba2+, or Mg2+. The different solutions were applied in a random order to detect and avoid possible irreversible effects on channel gating. A series of ramps (50–200) were used (Fig. 3) for every solution tested and averaged (the resulting curves not shown). For every data point the standard deviation (SD) was calculated using Clampfit software. The position of the minimum of the final SD curve along the voltage axis was used as a Vr of the unitary channel current in a given ion conditions. To determine the reversal potential shift (ΔVr), the Vr of the currents obtained in symmetrical Na+ conditions (VrNa+) was subtracted from the Vr obtained, then Na+ was replaced by respective cations (VrX). VrNa+ was used as a reference point for every individual patch. ΔVrXs were averaged for all patches. The dVrX means were then use to calculate the permeability ratios (PX+/PNa+ or PY2+/PNa+ for divalent cations). Overall, we obtained the following ΔVrXs (mean±SE, number of patches tested): ΔVrCa2+=−25.5±1.26, n=11; ΔVrMg2+=−16.5±1.2, n=13; ΔVrBa2+=−19.9±1.5, n=8; ΔVrK+=0.44±0.35, n=14; ΔVrCs+=1.22±0.14, n=9; ΔVrLi+=−5.83±0.49, n=6; ΔVrRb+=0.46±0.23, n=11; ΔVrNH4+=10.1±0.5, n=14. Data for ΔVrNH4+ were collected using outside-out patches. Corresponding changes were introduced into equation 3 and 4 to account for the patch recording configuration. Comparison of ΔVrXs yields statistically significant differences (p<0.05) between all ΔVrs except for ΔVrK+ vs ΔVrRb+ (p=0.09) and ΔVrMg2+ vs ΔVrBa2+ (p=0.09). Possible alterations in the channel gating parameters induced by different cations were not analyzed. All changes of the single channel conductances and Vrs were reversible.
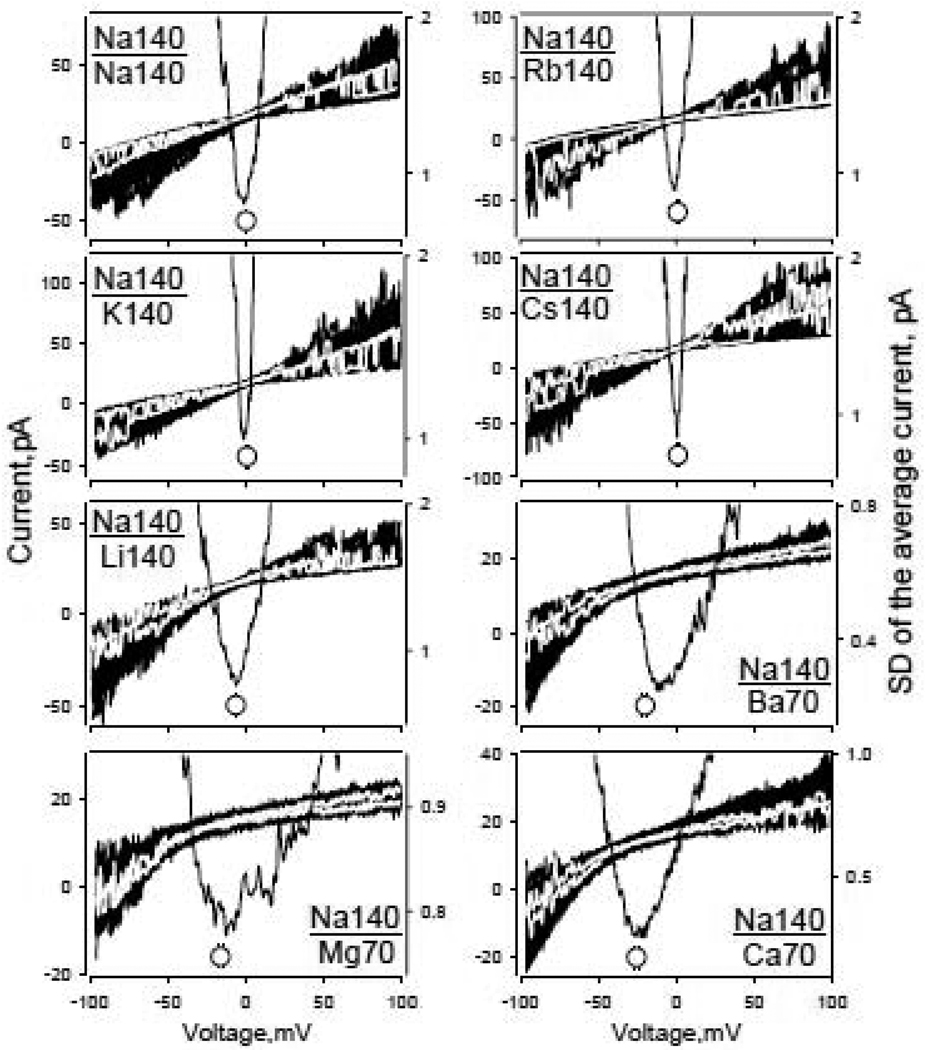
Fig. 3.
Permeation of mono- and divalent cations through heterologously expressed hTRPA1 channels. CV characteristics of the inside-out membrane patches containing hTRPA1 channels. Each graph shows at least 20 ramps superimposed. Discrete current levels correspond to current through different number of simultaneously open channels. Grey lines represent individual current traces. After obtaining 50–200 CV characteristics in symmetrical NaCl 140mM (top left graph), patches were exposed in a random order to one of the following solutions (every figure plot is labeled respectively): LiCl140mM; KCl140mM; RbCl140mM; CsCl140mM; CaCl2 70mM; MgCl2 70mM and BaCl2 70mM. Series of 50–200 ramps were obtained for every cation. Current traces containing active channels were selected and averaged. The corresponding Vrs were estimated based on position of the minimum of standard deviation (SD, parabola-shaped lines, right hand axis) for the average current trace (see methods and text for details). Empty circles are mean values of Vrs±SEM (fall within symbols, n=6–13). ΔVrXs were taken as VrNa−VrX for every individual patch recording and used to estimate permeability ratios. Corresponding corrections in polarity were introduced considering patch recording configuration. Relative permeabilities of hTRPA1 channel to monovalent cations (PX+/PNa+): Li+(1.26)>Na+(1.0)≥K+(0.98)≥Rb+(0.98)>Cs+(0.95); for divalent cations (PY2+/PNa+): Ca2+(5.1)>Ba2+(3.5)>Mg2+(2.8).
Experimental conditions: inside-out patch recording; holding potential −50mV; voltage protocol is specified in Methods; electrode solution – Standard NaCl140mM + AITC 50µM. Current level shift produced by voltage ramp application was not compensated. CV relationships for monovalent cations were obtained from the same patch. CV relationships for Na140/Mg70; Na140/Ba70 and Na140/Ca70 were recorded from the same patch. Note, Y-scales of all plots are different.
Overall the permeability ratio sequence for inorganic mono- (PX+/PNa+), including ammonium and divalent (PY2+/PNa+) cations was: Ca2+(5.1)>Ba2+(3.5)>Mg2+(2.8)>NH4+(1.5)>Li+(1.26)>Na+(1.0)≥K+(0.98)≥Rb+(0.98)>Cs+(0.95). While the sequence indicates somewhat low selectivity among cations, it is generally consistent with selectivity sequences reported for a variety of different nonselective cation channels permeable to both mono- and divalent cations (e.g. [25], [32] and [33]).
3.3 Probing the hTRPA1 channel pore with monovalent organic cations
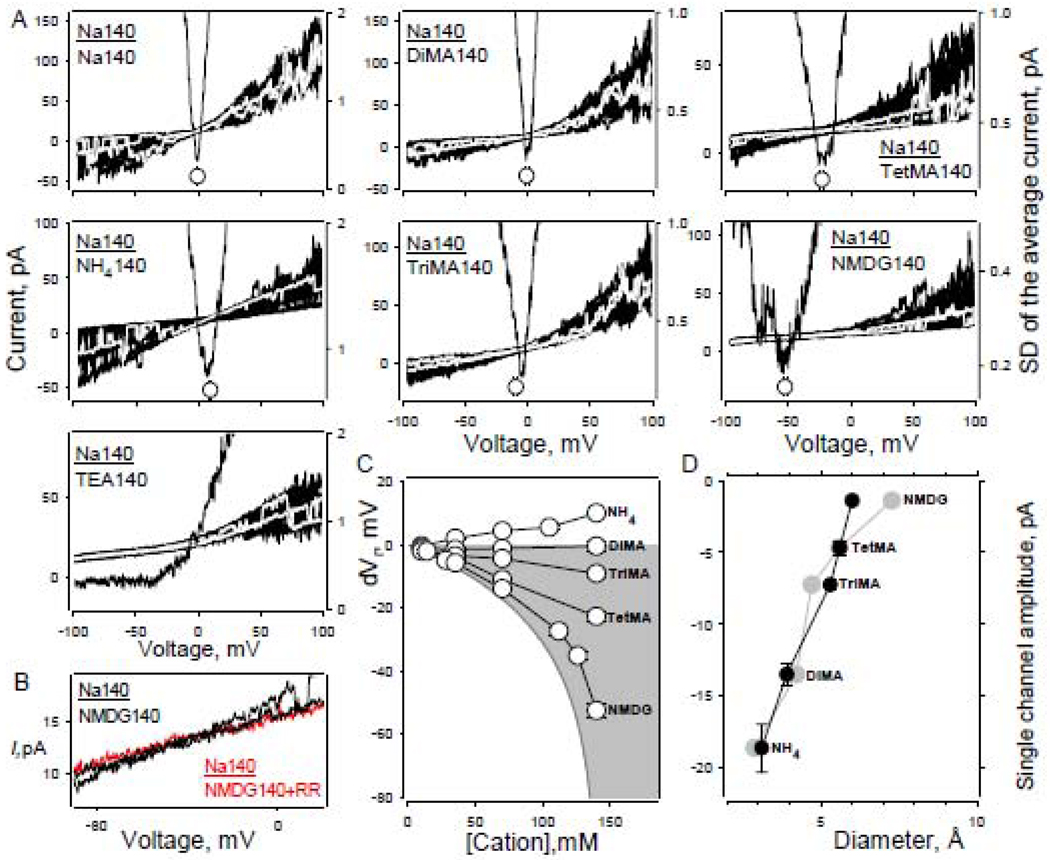
To determine the minimal apparent pore diameter of the hTRPA1 channel we used the standard approach based on the measurement of channel permeability in the presence of different size cations as charge carriers [27]. Such cations typically include organic monovalent cations (with minimal molecular diameter estimate, Å) Dimethylamine (DiMA, 3.9); Trimethylamine (TriMA, 5.3); Tetramethylammonium (TetMA, 5.6); N-methyl-d-glucamine (NMDG, 6.0); Tetraethylammonium (TEA, 6.8) and Ammonium (NH4, 3.1). The experimental paradigm described above for inorganic cations was also used to calculate the ΔVrX+s for organic cations. The experiments were performed using outside-out patch recordings with Na140-filled patch electrode solution. Every patch was initially exposed to Na140 followed by application of a series of solutions with different concentrations of various types of organic cations (Fig. 4A,C). Patch I–V curves (50–200) were generated for every ionic condition (Fig. 4A). VrX+s were estimated based on the position of the minimum of the averaged current SD curves along the voltage axis (Fig. 4A, parabolic shape lines). ΔVrX+ mean values plotted against concentrations of different cation species (Fig. 4C) clearly indicate that even relatively large organic cations, including NMDG, have partial permeability in these experimental conditions.
Fig. 4.
Probing hTRPA1 channel pore with monovalent organic cations. A – CV relationships in the presence of one of the following cations: Na+, NH4+, DiMA+, TriMA+, TetMA+, NMDG+, as indicated on the figure plots. Every plot shows at least 20 ramps superimposed (left-hand axis). Grey lines represent individual current traces. Ramps are overlapped with current SD plots (right-hand axis) where the minimum would indicate channel current Vr (see methods and text for details). Number of ramps used to generate average current traces (not shown) and corresponding SD curves varied from 50 to 200. Current level shift produced by voltage ramp application was not compensated. All recordings in A are from the same patch. Current scales are different. B – Fragments of current traces obtained in the presence of NMDG140mM before and after incubation with RR (10µM). Experimental conditions: outside-out patch recording; Holding potential −50mV; electrode solution – standard NaCl140mM+AITC50µM. C – Dependence of reversal potential shift from monovalent organic cation concentration. Smooth line circumscribing grey area depicts the behavior of ideal sodium selective conductance. Each data point is the mean of 4–14 experiments with SEM falling within symbols. Experimental conditions are as in A. D – Dependence of single hTRPA1 channel amplitude from organic cation dimensions. Black circles – minimum diameters of the cations. Grey circles – the mean diameter of the cations obtained as cube root of molecular volume {(D1*D2*D3)1/3}. Single channel amplitudes were obtained at −100mV. Electrode solution – standard NaCl140mM+AITC50µM.
Despite the greatly reduced inward component of the patch current voltage characteristics in the presence of NMDG140 (Fig. 4A,B), relatively rare channel-like events with unitary amplitude ~−1.4pA (as determined at −100mV) could be observed. These unitary currents reversed at approximately −52mV and were taken as hTRPA1 channel openings since they could be completely blocked by RR (10µM, Fig. 4B). The noticeable reduction in open channel probability suggests that perhaps a partial NMDG+ dependent channel block occurred at least at the highest concentrations of NMDG+. Overall, the reduction in single channel current amplitude correlated with the dimensions of the cation molecules (Fig. 4D, measured at −100mV and 140mM of respective cation, pA): NH4, −18.6±1.7, n=4; DiMA, −13.5±0.7, n=6; TriMA, −7.2±0.3, n=4; TetMA, −4.7±0.5, n=4; NMDG, −1.4±0.1, n=5; TEA, undetectable, n=3. With the concentration of TEA140 tested, the inward component of the unitary channel current was undetectable, suggesting that the Vr of the channel current possibly carried by TEA, if any, is below −100mV. To quantify and include TEA related data into analysis, −100mV was taken as VrTEA.
We next used the ΔVrX+s estimated in the presence of 140mM of a given cation to calculate relative permeabilities based on equation 3. The resulting values of permeability ratios (PNH4/PNa=1.49; PDiMA/PNa=0.99; PTriMA/PNa=0.7; PTetMA/PNa=0.4; PNMDG/PNa=0.1; PTEA/PNa=0.02) were approximated using the excluded volume equation (5, see methods, Fig. 5C, black symbols and line). The truncated part of the function is shown for clarity. The position of the minimum of the function indicates the minimum diameter of the channel pore, which we calculate to be ~7.4Å. The estimation based on mean diameter values obtained as the cube root of molecular volume {(D1*D2*D3)1/3}, excluding TEA, yields ~10.6 Å respectively (Fig. 5C, grey symbols and line).
As mentioned above TRPA1 channels exhibit a gradual reduction in activity even in the presence of the channel agonists. This dynamic process can be characterized by a decrease of open channel probability and was not accompanied by noticeable changes in channel pore properties such as single channel amplitude or reversal potential shifts at any given ion condition during the course of an experiment. The observed changes in the unitary current amplitudes and the Vrs at any given ion condition were reversible.
3.4 Ligand induced change of the channel organic cation permeability profile
TRPA1 channels have been recently reported to undergo agonist-induced pore dilation accompanied by changes in cation permeation through the channel pore [34], [24] and [35] in a manner similar to the dynamic selectivity phenomenon suggested for ATP-gated P2X channels [36], [37], TRPV1 channels [38], as well as some other channel types (for review: [39]). Since the recording pipette in our experiments contained AITC, the channel parameter estimates reported above apparently characterize the channel in its dilated form. In order to directly determine whether the channels demonstrate dynamic pore properties we compared parameters of the channels at rest conditions and after activation by AITC and UV light. The experiments were performed using outside-out patch recordings. The recording pipettes were filled with Na140mM standard patch solution + Na-tripolyphosphate 0.5mM to avoid the channel activity rundown. Every patch was initially exposed to Na140 followed by application of NMDG140 and NMDG140 + AITC 50–100µM or NMDG140 and NMDG140 + UV light exposure. Patch I–V curves (50–200) were generated for each of these described conditions (Fig. 5A,B). VrX+s were estimated as described above based on the position of the minimums of the averaged current SD curves along the voltage axis (Fig. 5A,B, parabolic shape lines). As can be seen for two representative patch recordings, in the control conditions the channels are characterized by relatively low channel activity. The channel currents reversed at approximately 0 mV when the patches were exposed to symmetrical NaCl 140 solution. Replacement NaCl140 with NMDG140 almost completely eliminated the channel inward current component possibly suggesting that VrNMDG lies beyond voltage range tested (Fig. 5A,B left panels). However, to quantify and include these data in our analysis, −100mV was estimated as VrNMDG in control conditions.
The ΔVrX+s estimated in the presence of 140mM of NMDG, DiMA and TetraMA were used to calculate relative permeabilities of these cations. TriMA was excluded from the experiments since we found that it could potentially serve as a hTRPA1 channel agonist at least at a concentration of 140mM. The permeability ratios (PDiMA/PNa=0.97; PTetMA/PNa=0.12; PNMDG/PNa=0.02) were then approximated using equation 5 as described above. The estimation of the minimum diameter of the channel pore based on minimum and mean molecular diameter values yields ~6.5 and 8.2 Å respectively (Fig. 5C, top). After activation of the channels (Fig. 5A,B, middle panels), VrNMDG could be clearly determined (Fig. 5A,B, right panels) and was estimated to be −48.3mV for the patch and −46.3±2.9mV on average (n=7, AITC application) and −51mV for the patch and −49±1mV on average (n=3, UV light exposure). These data are in good agreement with the VrNMDG value (52±2.3mV, n=7) obtained in the experiments described above. Thus overall comparison of the organic cation permeability ratios obtained in control conditions and in the presence of AITC suggests that hTRPA1 channels indeed undergo some rearrangements that alter the organic cation permeability profile of the channel pore. According our data AITC induced minimal channel pore diameter increases from ~6.5/8.2 to ~7.4/10.6 Å (based on minimum cation diameter/mean cation diameter estimates, Fig. 5C, top graph vs bottom graph). UV light also induced a shift in VrNMDG, suggesting that the same rearrangements may accompany channel activation by other ligands in similar experimental conditions.
In a recent paper, Karashima et al. [24] also using whole cell patch clamp recording, demonstrated that mouse TRPA1 channels can be permeable to a number of organic cations, including NMDG and predicted a “non-stimulated” (basal) channel pore diameter of ~11Å and mustard oil activated pore diameter of ~13.8Å. Interestingly, a pore of that diameter would potentially accommodate a countless variety of biologically active molecules (e.g ATP, 7.6×8.8×16.3Å; RR, 6.1×7.3×11.7Å). The considerable difference between the TRPA1 channel pore diameter approximations of our study and Karashima et al. [24] can potentially be explained by a number of different factors, but is likely primarily due to the differences in the estimated molecular dimensions of cations used for the calculations. The NMDG molecular diameter, for example, used by Karashima et al. [24] was 9Å and used here was 6Å. Indeed, a calculation combining the molecular dimensions from Karashima et al. [24] with the permeability ratios obtained in our experiments yields an apparent channel pore diameter of ~10.7 and ~14Å for “non-stimulated” and AITC activated channel respectively. This close match suggests that there may be common mechanisms controlling dynamic selectivity filters of both mouse and human versions of TRPA1 channel despite the fact that the channels share only approximately 80% amino acid sequence identity, including within regions of the predicted pore forming domains, and sometimes demonstrate striking heterogeneity in their properties (e.g. [40], [41]).
It has been shown that TRPA1 channel activation promotes permeabilization of the cell membrane to large organic compounds such as the fluorescent tracers YO-PRO [34], [35] and FM1–43 [24] (with the approximate molecular dimensions, mean diameter, Å, 8.7×7.2×17.8, 10.4 and 7.5×2.5×25.2, 7.8 respectively). The mechanism that mediates this phenomenon maybe similar to that of P2X7 receptor stimulated dye uptake ([42], [36], [43]), which remains unclear. While some reports postulate direct involvement of channel pores in the transport of these high molecular weight ions ([44], [34]), others provide abundant evidence arguing against direct permeation of the compounds through the channel pores and suggest different pathways of channel/receptor stimulated dye uptake. For example, the phenomenon is known to accompany the agonist dependent activation of broad spectrum of cation channels including TRP channels (TRPA1, TRPV1–4, TRPM8 [34], [35]) which are characterized by different biophysical properties including selectivity and channel pore dimensions. Uptake of different dye types was demonstrated to have distinct pharmacology and differential sensitivity to extra/intracellular calcium suggesting multiple dye-permeation pathways ([45], [46]). In some cases gap junction channels (e.g. pannexin1) have been proposed to serve as the dye-permeable pore forming unit of the receptor (P2X7R)/channel signaling complex ([47], [48]). Additionally, the kinetics of dye accumulation in cell cytoplasm is rather monotonic and extremely slow with time constants ranging within 16–40 minutes, and it does not correlate with the kinetic parameters of agonist dependent activation/inactivation of the channels [34], [46]. Thus receptor/channel dependent dye uptake is a complex phenomenon that perhaps can not be easily explained by an agonist evoked dramatic molecular/supramolecular reorganization that would allow housing of relatively large organic compounds within channel pores.
Overall our findings characterize and refine the basic parameters of hTRPA1 channels, providing further insight into the function of TRPA1 channels as a component of cellular signaling complexes. Additionally, the channel parameter estimates obtained in this study allow prediction of the dimensional limits of low logD compounds that could permeate the channel and pinpoint cytoplasmic targets within TRPA1 specific cells.
Research highlights.
Human TRPA1 channel weakly discriminates among both mono- and divalent cations
PX+/PNa+s are Li+(1.26)≥K+(0.98)≥Rb+(0.98)>Cs+(0.95)
PY2+/PNa+s are Ca2+(5.1)>Ba2+(3.5)>Mg2+(2.8)
Some ligands recruit the hTRPA1 channel to a state permeable to large organic cations
The pore of the channel in this state is dilated by approximately 1–2.5A
Acknowledgements
This work was supported by National Institute on Deafness and Other Communication Disorders (DC005995 and DC001655). We thank Drs. Gunter Gisselmann and Hanns Hatt for generously sharing hTRPA1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, Theobald DL, Garrity PA. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature. 2010;464 doi: 10.1038/nature08848. p.597-U155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical Nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 4.Kwan KY, Glazer JM, Corey DP, Rice FL, Stucky CL. TRPA1 Modulates Mechanotransduction in Cutaneous Sensory Neurons. Journal of Neuroscience. 2009;29:4808–4819. doi: 10.1523/JNEUROSCI.5380-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, Nilius B, Voets T. TRPA1 acts as a cold sensor in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1273–1278. doi: 10.1073/pnas.0808487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peterlin Z, Chesler A, Firestein S. A painful Trp can be a bonding experience. Neuron. 2007;53:635–638. doi: 10.1016/j.neuron.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Jordt SE, Bautista DM, Chuang HH, Mckemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 8.Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 10.Talavera K, Gees M, Karashima Y, Meseguer VM, Vanoirbeek JAJ, Damann N, Everaerts W, Benoit M, Janssens A, Vennekens R, Viana F, Nemery B, Nilius B, Voets T. Nicotine activates the chemosensory cation channel TRPA1. Nature Neuroscience. 2009;12 doi: 10.1038/nn.2379. pp. 1293-1U14. [DOI] [PubMed] [Google Scholar]
- 11.Lee SP, Buber MT, Yang Q, Cerne R, Cortes RY, Sprous DG, Bryant RW. Thymol and related alkyl phenols activate the hTRPA1 channel. British Journal of Pharmacology. 2008;153:1739–1749. doi: 10.1038/bjp.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. Journal of Neuroscience. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill K, Schaefer M. Ultraviolet light and photosensitising agents activate TRPA1 via generation of oxidative stress. Cell Calcium. 2009;45:155–164. doi: 10.1016/j.ceca.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel a1 is directly gated by calcium ions. Journal of Biological Chemistry. 2007;282:13180–13189. doi: 10.1074/jbc.M607849200. [DOI] [PubMed] [Google Scholar]
- 15.Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+ Nature Neuroscience. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]
- 16.Wang YY, Chang RB, Waters HN, Mckemy DD, Liman ER. The Nociceptor Ion Channel TRPA1 Is Potentiated and Inactivated by Permeating Calcium Ions. Journal of Biological Chemistry. 2008;283:32691–32703. doi: 10.1074/jbc.M803568200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim D, Cavanaugh EJ, Simkin D. Inhibition of transient receptor potential A1 channel by phosphatidylinositol-4,5-bisphosphate. American Journal of Physiology-Cell Physiology. 2008;295:C92–C99. doi: 10.1152/ajpcell.00023.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karashima Y, Prenen J, Meseguer V, Owsianik G, Voets T, Nilius B. Modulation of the transient receptor potential channel TRPA1 by phosphatidylinositol 4,5-biphosphate manipulators. Pflugers Archiv-European Journal of Physiology. 2008;457:77–89. doi: 10.1007/s00424-008-0493-6. [DOI] [PubMed] [Google Scholar]
- 19.Kwon Y, Kim SH, Ronderos DS, Lee Y, Akitake B, Woodward OM, Guggino WB, Smith DP, Montell C. Drosophila TRPA1 Channel Is Required to Avoid the Naturally Occurring Insect Repellent Citronellal. Current Biology. 2010;20:1672–1678. doi: 10.1016/j.cub.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaquemar D, Schenker T, Trueb B. An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. Journal of Biological Chemistry. 1999;274:7325–7333. doi: 10.1074/jbc.274.11.7325. [DOI] [PubMed] [Google Scholar]
- 21.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 22.Fujita F, Uchida K, Moriyama T, Shima A, Shibasaki K, Inada H, Sokabe T, Tominaga M. Intracellular alkalization causes pain sensation through activation of TRPA1 in mice. Journal of Clinical Investigation. 2008;118:4049–4057. doi: 10.1172/JCI35957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang YYY, Chang RB, Liman ER. TRPA1 Is a Component of the Nociceptive Response to CO2. Journal of Neuroscience. 2010;30:12958–12963. doi: 10.1523/JNEUROSCI.2715-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karashima Y, Prenen J, Talavera K, Janssens A, Voets T, Nilius B. Agonist-Induced Changes in Ca2+ Permeation through the Nociceptor Cation Channel TRPA1. Biophysical Journal. 2010;98:773–783. doi: 10.1016/j.bpj.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hille B. Ion Channels of Excitable Membrane. Sunderland: Sinauer Associates; 2001. [Google Scholar]
- 26.Villarroel A, Burnashev N, Sakmann B. Dimensions of the Narrow Portion of A Recombinant Nmda Receptor-Channel. Biophysical Journal. 1995;68:866–875. doi: 10.1016/S0006-3495(95)80263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dwyer TM, Adams DJ, Hille B. The Permeability of the Endplate Channel to Organic Cations in Frog-Muscle. Journal of General Physiology. 1980;75:469–492. doi: 10.1085/jgp.75.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim D, Cavanaugh EJ. Requirement of a soluble intracellular factor for activation of transient receptor potential A1 by pungent chemicals: Role of inorganic polyphosphates. Journal of Neuroscience. 2007;27:6500–6509. doi: 10.1523/JNEUROSCI.0623-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cavanaugh EJ, Simkin D, Kim D. Activation of transient receptor potential A1 channels by mustard oil, tetrahydrocannabinol and Ca2+ reveals different functional channel states. Neuroscience. 2008;154:1467–1476. doi: 10.1016/j.neuroscience.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 30.Ma JJ. Block by Ruthenium Red of the Ryanodine-Activated Calcium-Release Channel of Skeletal-Muscle. Journal of General Physiology. 1993;102:1031–1056. doi: 10.1085/jgp.102.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bobkov YV, Ache BW. Pharmacological properties and functional role of a TRP-related ion channel in lobster olfactory receptor neurons. Journal of Neurophysiology. 2005;93:1372–1380. doi: 10.1152/jn.00990.2004. [DOI] [PubMed] [Google Scholar]
- 32.Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiological Reviews. 2002;82:769–824. doi: 10.1152/physrev.00008.2002. [DOI] [PubMed] [Google Scholar]
- 33.Owsianik G, Talavera K, Voets T, Nilius B. Permeation and selectivity of TRP channels. Annual Review of Physiology. 2006;68:685–717. doi: 10.1146/annurev.physiol.68.040204.101406. [DOI] [PubMed] [Google Scholar]
- 34.Chen J, Kim D, Bianchi BR, Cavanaugh EJ, Faltynek CR, Kym PR, Reilly RM. Pore dilation occurs in TRPA1 but not in TRPM8 channels. Molecular Pain. 2009;5 doi: 10.1186/1744-8069-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banke TG, Chaplan SR, Wickenden AD. Dynamic changes in the TRPA1 selectivity filter lead to progressive but reversible pore dilation. American Journal of Physiology-Cell Physiology. 2010;298:C1457–C1468. doi: 10.1152/ajpcell.00489.2009. [DOI] [PubMed] [Google Scholar]
- 36.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P-2Z receptor for extracellular ATP identified as a P-2X receptor (P2X(7)) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 37.Eickhorst AN, Berson A, Cockayne D, Lester HA, Khakh BS. Control of P2X(2) channel permeability by the cytosolic domain. Journal of General Physiology. 2002;120:119–131. doi: 10.1085/jgp.20028535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung MK, Guler AD, Caterina MJ. TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nature Neuroscience. 2008;11:555–564. doi: 10.1038/nn.2102. [DOI] [PubMed] [Google Scholar]
- 39.Khakh BS, Lester HA. Dynamic selectivity filters in ion channels. Neuron. 1999;23:653–658. doi: 10.1016/s0896-6273(01)80025-8. [DOI] [PubMed] [Google Scholar]
- 40.Nagatomo K, Kubol Y. Caffeine activates mouse TRPA1 channels but suppresses human TRPA1 channels. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:17373–17378. doi: 10.1073/pnas.0809769105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu K, Samuel M, Ho M, Harrison RK, Paslay JW. NPPB structure-specifically activates TRPA1 channels. Biochemical Pharmacology. 2010;80:113–121. doi: 10.1016/j.bcp.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 42.Steinberg TH, Newman AS, Swanson JA, Silverstein SC. Atp4- Permeabilizes the Plasma-Membrane of Mouse Macrophages to Fluorescent Dyes. Journal of Biological Chemistry. 1987;262:8884–8888. [PubMed] [Google Scholar]
- 43.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- 44.Virginio C, Mackenzie A, Rassendren FA, North RA, Surprenant A. Pore dilation of neuronal P2X receptor channels. Nature Neuroscience. 1999;2:315–321. doi: 10.1038/7225. [DOI] [PubMed] [Google Scholar]
- 45.Jiang LH, Rassendren F, Mackenzie A, Zhang YH, Surprenant A, North RA. N-methyl-D-glucamine and propidium dyes utilize different permeation pathways at rat P2X(7) receptors. American Journal of Physiology-Cell Physiology. 2005;289:C1295–C1302. doi: 10.1152/ajpcell.00253.2005. [DOI] [PubMed] [Google Scholar]
- 46.Cankurtaran-Sayar S, Sayar K, Ugur M. P2X7 Receptor Activates Multiple Selective Dye-Permeation Pathways in RAW 264.7 and Human Embryonic Kidney 293 Cells. Molecular Pharmacology. 2009;76:1323–1332. doi: 10.1124/mol.109.059923. [DOI] [PubMed] [Google Scholar]
- 47.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1 beta release by the ATP-gated P2X(7) receptor. Embo Journal. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Locovei S, Scemes E, Qiu F, Spray DC, Dahl G. Pannexin1 is part of the pore forming unit of the P2X(7) receptor death complex. Febs Letters. 2007;581:483–488. doi: 10.1016/j.febslet.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]