Abstract
Context
The Brief Pain Inventory (BPI) is a frequently used instrument designed to assess the patient-reported outcome of pain. The majority of factor analytic studies have found a two-factor (i.e., pain intensity and pain interference) structure for this instrument; however, since the BPI was developed with an a priori hypothesis of the relationship among its items, it follows that construct validity investigations should utilize confirmatory factor analysis (CFA).
Objectives
The purpose of this work is to establish the construct validity of the BPI using a CFA framework and demonstrate factorial invariance using a range of demographic variables.
Methods
A retrospective CFA was completed in a sample of individuals diagnosed with HIV/AIDS and cancer (n = 364; 63% male; age 21-92 years, M = 51.80). A baseline one-factor model was compared against two-factor and three-factor models (i.e., pain intensity, activity interference, and affective interference) that were developed based on the hypothetical design of the instrument.
Results
Fit indices for the three-factor model were statistically superior when compared to the one-factor model and marginally better in comparison to the two-factor model. This three-factor structure was found to be invariant across disease, age, and ethnicity groups.
Conclusion
The results of this study provide evidence to support a three-factor representation of the BPI, as well as the originally hypothesized two-factor structure. Such findings will begin to provide clinical trialists, pharmaceutical sponsors, and regulators with confidence in the psychometric properties of this instrument when considering its inclusion in clinical research.
Keywords: Factor analysis, psychometrics, pain, reproducibility of results, affective symptoms
Introduction
The clinical importance of pain as a primary patient-reported outcome (PRO) endpoint in registration track research has been well established by trialists and pharmaceutical sponsors (1-3). The frequently used short form of the Brief Pain Inventory (BPI), and particularly the BPI's single “pain at its worst in the last 24 hours” item, has become the preferred measure to assess pain PRO endpoints in the regulatory setting in the United States (4). In a recent review of the psychometric properties of the BPI with respect to the United States Food and Drug Administration (FDA) Guidance for Industry on the Use of PRO Measures in Medical Development to Support Labeling Claims (5), we found that most FDA key recommendations for conceptual framework, content validity, reliability, and the ability to detect clinically meaningful score changes were satisfied (4). However, several issues remain regarding the construct validity of this commonly used instrument.
Construct validity testing was incorporated as part of BPI development. Items were designed with the intention of assessing pain intensity and pain interference (6-8). Through the use of exploratory factor analysis (EFA), the test developers replicated this two-factor pain model in a series of BPI studies in patients of various disease types, as well as a wide range of BPI language translations (9-20). As an alternative to the two-factor BPI representation of pain, the test developers subsequently proposed that the pain interference factor can be further divided into affective interference and activity interference sub-components (21-22). EFA is typically used for the investigation of construct validity in cases where the relationships amongst variables are unknown or ambiguous (23).
A commonly used method (24-25) to investigate construct validity is confirmatory factor analysis (CFA). Like EFA, CFA is a tool that a researcher can use to attempt to reduce the overall number of observed variables into latent factors based on commonalities within the data. CFA differs from EFA in that it assists in the reduction of measurement error and allows for the comparison of alternatively proposed a priori models at the latent factor level (26). CFA can also be used to statistically compare the factor structure of two or more groups (e.g., different disease conditions). The use of CFA to investigate the construct validity of hypothesis-based testing instruments adds a level of statistical precision and can assist in the development of abbreviated forms of an instrument or confirmation of its possible sub-domains.
Therefore, we examined an existing dataset in order to evaluate the construct validity of this instrument using CFA. We hypothesized that the two-factor representation would be replicated in this analysis. To further investigate the construct validity of the BPI, a three-factor model (i.e., pain intensity, activity interference, affective interference) proposed by the original test authors (21-22) was used as an alternative model to compare against the two-factor structure to determine which best represents the data.
Methods
Participants
The study sample consists of a retrospective review of 364 patients diagnosed either with HIV/AIDS or cancer who were receiving treatment at Calvary Hospital or Memorial Sloan-Kettering Cancer Center (MSKCC) in New York City between January 1999 and August 2004 (27-29). Patients were eligible to participate if they were newly referred to the Palliative Care Service at MSKCC or newly admitted to Calvary Hospital, 18 years of age or older, English-speaking, had no history of a psychotic mental disorder, scored a 20 or higher on the Mini-Mental State Examination, and experienced a daily “pain at its worst in the last 24-hours” score greater than 3 on the BPI. Informed consent was obtained from each patient. All ethical guidelines were followed as required for conducting human research. The study was approved by the Institutional Review Boards of Calvary Hospital and MSKCC.
Materials
Brief Pain Inventory (6-8)
The BPI is an 11-item questionnaire that consists of four 0-to-10 numeric rating scale (NRS) items asking patients to rate their pain at its “worst in the last 24-hours,” least in the last 24-hours,” “average,” and “now,” with a 0 indicating “no pain” and 10 representing “pain as bad as you could imagine.” The remaining seven BPI items probe the degree to which pain interferes with general activity, mood, walking ability, normal work, relations with other people, sleep, and enjoyment of life, again using a 0-to-10 NRS. For these interference items, 0 represents “does not interfere” and 10 indicates “interferes completely.”
Procedure
The BPI was completed by all patients in the sample at baseline. Due to the advanced disease progression in the present sample, the “normal work” interference item was not included in this version of the BPI.
Statistical Analysis
Confirmatory factor analysis (CFA) was used to investigate construct validity of the BPI. Several fit indices were selected in order to test which CFA model best represents the present dataset: root-mean-squared error of approximation (RMSEA) (30), comparative fit index (CFI) (31)), chi-square, and change in chi-square given the change in degrees of freedom between models. RMSEA is a measure of the average of the residual variance and covariance; good models have RMSEA values that are at or less than 0.08 (32). CFI is an index that fall between 0 and 1, with values greater than 0.90 considered to be indicators of good fitting models (32). When comparing models, a lower chi-square value indicates a better fit, given an equal number of degrees of freedom.
Based upon the hypothetical underlying constructs for the BPI (7, 21), three models were developed to represent the best fit for the overall data. Model 1 was a one factor model used as a baseline comparison against the other models. Model 2 was a two factor model with pain severity and pain interference treated as latent factors. For Model 3, pain severity was again treated as a latent factor, with pain interference treated as two separate factors of activity interference (i.e., general activity, walking ability) and affective interference (i.e., mood, relations with other people, sleep, enjoyment of life). Amos Version 16 (33) was used for all analyses.
Results
The dataset consisted of 364 male (n = 227) and female (n = 137) patients ages 21-92 years (M = 51.78, standard deviation [SD] = 13.69) with advanced HIV/AIDS (n = 209) or cancer (n = 155) diagnoses who were receiving treatment as part of palliative care (27-29). Characteristics are displayed in Table 1. The patient sample consisted largely of Caucasian (38.5%) and African-American (41.8%) individuals, with a smaller proportion identifying themselves as Hispanic (13.5%) or “other” (6.3%).
Table 1. Patient Characteristics.
| Characteristic | No. of Patients (n=364) | % | |
|---|---|---|---|
| Age range (yrs) | |||
| Mean | 51.8 | ||
| Median | 49.0 | ||
| 21-39 | 66 | 18.1 | |
| 40-49 | 119 | 32.7 | |
| 50-59 | 84 | 23.1 | |
| 60-69 | 45 | 12.4 | |
| >70 | 50 | 13.7 | |
| Gender | |||
| Female | 137 | 37.6 | |
| Disease Type | |||
| HIV/AIDS | 209 | 57.4 | |
| Cancer | 155 | 42.6 | |
| Race/Ethnicity | |||
| African-American | 152 | 41.8 | |
| White Hispanic | 49 | 13.5 | |
| White Non-Hispanic | 140 | 38.5 | |
| Other | 23 | 6.2 |
Table 2 is a display of the means, SDs, and Pearson correlation coefficients among the ten items of the BPI. The present dataset satisfied all CFA requirements for normality, multicollinearity, residual values, and multivariate outliers (34).
Table 2. Correlation Coefficients, Means, and Standard Deviations for Outcome Measures.
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Pain at its Worst in the last 24 Hours | 1.00 | |||||||||
| 2. Pain at its Least in the last 24 Hours | 0.33a | 1.00 | ||||||||
| 3. Pain on Average | 0.55a | 0.54a | 1.00 | |||||||
| 4. Pain Right Now | 0.41a | 0.61a | 0.58a | 1.00 | ||||||
| 5. Interference with General Activity | 0.33a | 0.36a | 0.29a | 0.24a | 1.00 | |||||
| 6. Interference with Mood | 0.36a | 0.21a | 0.22a | 0.16a | 0.57a | 1.00 | ||||
| 7. Interference with Walking Ability | 0.25a | 0.26a | 0.29a | 0.21a | 0.55a | 0.41a | 1.00 | |||
| 8. Interference with Relations/Other People | 0.17a | 0.10 | 0.10 | 0.14a | 0.41a | 0.56a | 0.34a | 1.00 | ||
| 9. Interference with Sleep | 0.37a | 0.28a | 0.33a | 0.18a | 0.47a | 0.52a | 0.39a | 0.36a | 1.00 | |
| 10. Interference with Enjoyment of Life | 0.37a | 0.25a | 0.22a | 0.18a | 0.62a | 0.59a | 0.51a | 0.51a | 0.53a | 1.00 |
| Mean | 7.78 | 2.23 | 5.39 | 3.90 | 6.21 | 6.03 | 5.64 | 4.09 | 6.01 | 6.16 |
| SD | 2.18 | 2.44 | 2.45 | 3.01 | 3.55 | 3.46 | 3.88 | 3.72 | 3.69 | 3.65 |
SD = standard deviation.
Indicates correlation statistically significant P < 0.05.
According to the fit indices (Table 3), Model 2 was a significant improvement over Model 1. Model 2 had a lower RMSEA value (0.081), a higher CFI value (0.941), and a significant change in chi-square given the change in degrees of freedom when compared to Model 1 (χ2(1) = 267.20, P < 0.05). Model 3 was statistically superior to Model 2 in terms of RMSEA (0.075), CFI (0.953), and change in chi-square given the degrees of freedom values (χ2(2) = 9.53, P < 0.05). From these results, Model 3 was selected as the best fit for the data (Figure 1), with Model 2 treated as a suitable alternative representation in this sample. Standardized factor loadings for all models are displayed in Table 4.
Table 3. Fit Indices for Confirmatory Factor Models in Overall Sample.
| RMSEA | 90% CI | CFI | df | χ2 | χ2/df | P | |
|---|---|---|---|---|---|---|---|
| Model 1 | 0.165 | 0.151, 0.181 | 0.750 | 35 | 382.89 | ||
| Model 2 | 0.081 | 0.065, 0.098 | 0.941 | 34 | 115.69 | 267.20 | P < 0.05 |
| Model 3 | 0.075 | 0.058, 0.092 | 0.953 | 32 | 96.64 | 9.53 | P < 0.05 |
RMSEA = Root Mean Squared Error of Approximation; CI = confidence interval; CFI = Comparative Fit Index.
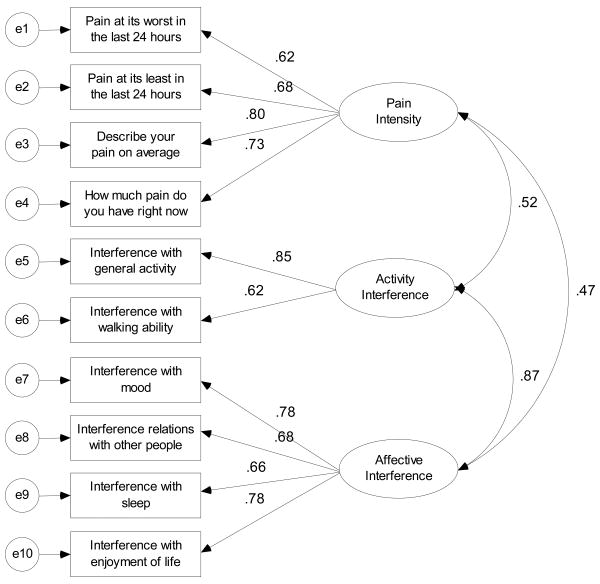
Figure 1. Confirmatory Factor Model for the 3-Factor Solution.
Table 4. Standardized Factor Loadings and Factor Correlations by Model and Latent Construct (n = 364).
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Measure | Unitary | Intensity | Interference | Intensity | Activity Interference | Affective Interference |
| Pain at its Worst in the last 24 Hours | 0.53 (-)a | 0.62 (-) | 0.62 (-) | |||
| Pain at its Least in the last 24 Hours | 0.37 (0.13) | 0.68 (0.12) | 0.68 (0.12) | |||
| Pain on Average | 0.50 (0.14) | 0.80 (0.13) | 0.80 (0.12) | |||
| Pain Right Now | 0.45 (0.16) | 0.73 (0.15) | 0.73 (0.15) | |||
| Interference with General Activity | 0.77 (0.25) | 0.77 (-) | 0.85 (-) | |||
| Interference with Mood | 0.74 (0.24) | 0.58 (0.08) | 0.78 (-) | |||
| Interference with Walking Ability | 0.58 (0.24) | 0.65 (0.07) | 0.62 (0.07) | |||
| Interference with Relations/Other People | 0.63 (0.24) | 0.77 (0.07) | 0.66 (0.07) | |||
| Interference with Sleep | 0.66 (0.24) | 0.67 (0.07) | 0.68 (0.07) | |||
| Interference with Enjoyment of Life | 0.75 (0.25) | 0.78 (0.07) | 0.78 (0.07) | |||
| Factor Correlations (with standard errors) | ||||||
| Factor 1: Pain Intensity | 0.51 (0.05) | 0.52 (0.06) | 0.47 (0.06) | |||
| Factor 2: Activity Interference | 0.87 (0.07) | |||||
Standardized factor coefficients and the standard errors of the coefficients. Entries marked with (-) were constrained at a raw factor coefficient of 1.0 and thus yielded no standard error estimates.
Multi-Group Analysis
A multi-group structural analysis was used in order to investigate whether the three factors from the CFA were invariant across disease type (i.e., AIDS/HIV or cancer), age, and ethnicity groups (35). Age was treated as a binary variable, with the overall sample divided into those above (n = 206) or below (n = 158) the mean of 51.78. Ethnicity was also treated as a binary variable, with the sample was coded as Caucasian (n = 140) or non-Caucasian (n = 224).
For each of these three multi-group analyses, a model was fit that simultaneously imposed constraints on all of the factor loadings and covariances. Placing these constraints forced the values to be equal across groups. This model was then compared to a baseline model where none of the factor loadings were constrained. In this analysis, a lack of observed statistical differences between the constrained and unconstrained models is an indicator of factorial invariance. Table 5 is a display of the fit indices for the multi-group analyses. Since for each of the analyses (i.e., disease type, age, and ethnicity) there were no significant differences between the models in chi-square value, given the change in degrees of freedom, the three factor solution from the CFA was invariant.
Table 5. Multi-Group Analysis Fit Indices by Disease Type, Age, and Ethnicity Groups by Factor Loading Constraints for the Three-Factor Solution.
| RMSEA | 90% CI | CFI | df | χ2 | χ2/df | P | |
|---|---|---|---|---|---|---|---|
| Analysis - Disease | |||||||
| No Constraints | 0.059 | 0.046, 0.072 | 0.939 | 64 | 143.90 | ||
| All Constrained | 0.053 | 0.040, 0.065 | 0.944 | 74 | 148.09 | 0.42 | P > 0.05 |
| Analysis - Age | |||||||
| No Constraints | 0.055 | 0.042, 0.068 | 0.945 | 64 | 135.12 | ||
| All Constrained | 0.050 | 0.038, 0.063 | 0.948 | 74 | 141.50 | 0.64 | P > 0.05 |
| Analysis - Ethnicity | |||||||
| No Constraints | 0.059 | 0.033, 0.081 | 0.957 | 64 | 123.16 | ||
| All Constrained | 0.045 | 0.032, 0.058 | 0.960 | 74 | 128.34 | 0.52 | P > 0.05 |
RMSEA = Root Mean Squared Error of Approximation; CI = confidence interval; CFI = Comparative Fit Index.
Discussion
The recent release of the final FDA Guidance for Industry on the Use of PRO Measures in Medical Product Development to Support Labeling Claims has reinforced the need for clinical trialists, pharmaceutical sponsors, and regulators to utilize psychometrically sound assessment instruments (5). In light of a recent review of the measurement properties of the commonly used Brief Pain Inventory, it was prudent to establish the construct validity of this instrument using a confirmatory method as opposed to previously employed exploratory techniques (4). The present study investigated the construct validity of the BPI in a heterogeneous patient sample using confirmatory factor analysis. Our results support the originally hypothesized two-factor structure (i.e., pain intensity and pain interference), as well as an alternatively suggested (21-22) three-factor representation (i.e., pain intensity, activity interference, and affective interference) of this pain assessment instrument. Both the two-factor and three-factor models are superior to a one-factor model and were found to be consistent across disease type, age, and ethnicity groups. The three-factor model does exhibit a marginally better fit than the two-factor model, although arguments in favor of each could be made based solely on our quantitative results. However, from a qualitative perspective, our results support the inclusion of a third factor when analyzing the BPI, affective interference, which may be beneficial when evaluating study results or patient populations.
In fact, the emergence of the three-factor model as a representation of the BPI has several implications. Scoring the BPI using a three-factor framework would allow for flexibility in the assessment of changes in affective and activity interference in varied populations where a focus on this dichotomy is regularly observed. Monitoring differences in these types of pain interference may help clinicians in their interpretation of pain palliation in their patients. For example, in populations of patients with metastatic cancer leading to bone pain, the importance of activity interference versus affective interference would lead to different conclusions about appropriate clinical interventions, where clinicians might focus on interventions to minimize affective interference to enhance patients' abilities to interact with family and friends at the end of life. Alternatively, in an acute pain context such as in patients following fractures, a clinician may be more focused on activity rather than affective interference due to the transient nature of the injury, and the importance of patients' adherence with physical therapy or rehabilitation activities. In instances where a researcher is interested in reducing the patient response burden while also obtaining all clinically relevant information on pain, it may be possible to reduce the BPI to three separate items that address pain intensity, activity interference and affective interference. It may also be feasible to administer the identified factors of the BPI as separate sets of items, depending on the needs and research interests of a particular clinician. Finally, it may be useful to examine differential levels of the three identified factors to enhance understanding of response shift phenomena (36), where prospective improvements in affective interference may precede improvement in pain intensity or activity interference. Further research would be necessary to investigate the equivalency of a shorter BPI form with its original instrument.
Our findings support the use of either the two-factor or three-factor model for scoring the BPI. The two factor model (pain intensity and pain interference) of the BPI that has been established in a number of previous studies using exploratory factor analysis (9-20) and has been replicated here. In populations where a distinction between activity and affective interference is not applicable or feasible, the two-factor structure can still be employed. However, when additional nuance is sought, or distinction between activity and affective interference is useful, the three-factor model would be reasonable and justifiable to use.
There are a number of limitations of this study. The omission of the “normal work” interference item in this sample may limit generalizability, but the nature of advanced disease progression in these patients would have likely limited meaningful information from this item. A follow-up analysis in a dataset which includes the “normal work” item is currently underway. The present analysis was conducted based on a sample of patients with HIV/AIDS and cancer, which may limit the clinical application of these results in other patient populations, including populations facing less advanced disease, although it should be pointed out that this study is the first to look at the construct validity of the BPI across disease types rather than by individual disease. In the future, studies in other disease groups could be used to confirm these results.
The psychometric evaluation of an assessment instrument is an important consideration when determining which measurement tools are included as part of a research study. The results of the present analysis provide further evidence for the use of the BPI as a pain assessment instrument. This use of confirmatory factor analysis to support a two or three-factor structure of the BPI is intended to provide clinical trialists, pharmaceutical sponsors, and regulators with greater confidence when making clinical interpretations based upon scores from this instrument.
Acknowledgments
This project was supported by a National Institutes of Health Research Training Grant (T32 CA009461-25), as well as grants from the National Institute of Nursing Research (NR-05183 W. Breitbart, P.I.) and National Institute of Mental Health (MH R01-57629 W. Breitbart, P.I.).
The authors wish to thank Raymond Baser and Drs. Colin Begg, Katherine Panageas, and Bryce Reeve for their helpful feedback and suggestions.
Footnotes
Disclosures: There are no financial relationships associated with these results that may reflect a conflict of interest or be perceived to reflect a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cella D, Nichol MB, Eton D, Nelson JB, Mulani P. Estimating clinically meaningful changes for the Functional Assessment of Cancer Therapy - Prostate: results from a clinical trial of patients with metastatic hormone-refractory prostate cancer. Value Health. 2009;12:124–129. doi: 10.1111/j.1524-4733.2008.00409.x. [DOI] [PubMed] [Google Scholar]
- 2.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 3.Turk DC, Dworkin RH, McDermott MP, et al. Analyzing multiple endpoints in clinical trials of pain treatments: IMMPACT recommendations. Pain. 2008;139:485–493. doi: 10.1016/j.pain.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson TM, Mendoza TR, Sit L, et al. The Brief Pain Inventory and its “pain at its worst in the last 24 hours” item: clinical trial endpoint considerations. Pain Medicine. doi: 10.1111/j.1526-4637.2009.00774.x. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Food and Drug Administration. Guidance for industry. Patient-reported outcome measures: use in medical development to support labeling claims. [December 9, 2009];2009 doi: 10.1186/1477-7525-4-79. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf. [DOI] [PMC free article] [PubMed]
- 6.Cleeland CS. Research in cancer pain What we know and what we need to know. Cancer. 1991;67:823–827. doi: 10.1002/1097-0142(19910201)67:3+<823::aid-cncr2820671412>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 7.Daut RL, Cleeland CS. The prevalence and severity of pain in cancer. Cancer. 1982;50:1913–1918. doi: 10.1002/1097-0142(19821101)50:9<1913::aid-cncr2820500944>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 8.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17:197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- 9.Badia X, Muriel C, Gracia A, et al. Validación española del cuestionario Brief Pain Inventory en pacientes con dolor de causa neoplásica. Med Clin (Barc) 2003;120:52–59. doi: 10.1016/s0025-7753(03)73601-x. [DOI] [PubMed] [Google Scholar]
- 10.Caraceni A, Mendoza TR, Mencaglia E, et al. A validation study of an Italian version of the Brief Pain Inventory (Breve Questionario Per La Valutazione Del Dolore) Pain. 1996;65:87–92. doi: 10.1016/0304-3959(95)00156-5. [DOI] [PubMed] [Google Scholar]
- 11.Cleeland CS, Ladinsky JL, Serlin RC, Nugyen CT. Multidimensional measurement of cancer pain: comparison of U.S and Vietnamese patients. J Pain Symptom Manage. 1988;3:23–27. doi: 10.1016/0885-3924(88)90134-0. [DOI] [PubMed] [Google Scholar]
- 12.Ger LP, Ho ST, Sun WZ, Wang MS, Cleeland CS. Validation of the Brief Pain Inventory in a Taiwanese population. J Pain. 1999;9:105–121. doi: 10.1016/s0885-3924(99)00087-1. [DOI] [PubMed] [Google Scholar]
- 13.Kalyadina SA, Ionova TI, Ivanova MO, et al. Russian Brief Pain Inventory: validation and application in cancer pain. J Pain Symptom Manage. 2008;35:95–102. doi: 10.1016/j.jpainsymman.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 14.Klepstad P, Loge JH, Borchgrevink PC, et al. The Norwegian Brief Pain Inventory questionnaire: translation and validation in cancer pain patients. J Pain Symptom Manage. 2002;24:517–525. doi: 10.1016/s0885-3924(02)00526-2. [DOI] [PubMed] [Google Scholar]
- 15.Mystakidou K, Mendoza T, Tsilika E, et al. Greek Brief Pain Inventory: validation and utility in cancer pain. Oncology. 2001;60:35–42. doi: 10.1159/000055294. [DOI] [PubMed] [Google Scholar]
- 16.Radbruch L, Loick G, Kiencke P, et al. Validation of the German version of the Brief Pain Inventory. J Pain Symptom Manage. 1999;18:180–187. doi: 10.1016/s0885-3924(99)00064-0. [DOI] [PubMed] [Google Scholar]
- 17.Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61:277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 18.Uki J, Mendoza T, Cleeland CS, Nakamura Y, Takeda F. A brief cancer pain assessment tool in Japanese: the utility of the Japanese Brief Pain Inventory – BPI-J. J Pain Symptom Manage. 1998;16:364–373. doi: 10.1016/s0885-3924(98)00098-0. [DOI] [PubMed] [Google Scholar]
- 19.Wang XS, Mendoza TR, Gao SZ, Cleeland CS. The Chinese version of the Brief Pain Inventory (BPI-C): its development and use in a study of cancer pain. Pain. 1996;67:407–416. doi: 10.1016/0304-3959(96)03147-8. [DOI] [PubMed] [Google Scholar]
- 20.Yun YH, Mendoza TR, Heo DS, et al. Development of a cancer pain assessment tool in Korea: a validation of a Korean version of the Brief Pain Inventory. Oncology. 2004;66:439–444. doi: 10.1159/000079497. [DOI] [PubMed] [Google Scholar]
- 21.Cleeland CS, Nakamura Y, Mendoza TR, et al. Dimensions of the impact of cancer pain in a four country sample: new information from multidimensional scaling. Pain. 1996;67:267–273. doi: 10.1016/0304-3959(96)03131-4. [DOI] [PubMed] [Google Scholar]
- 22.Saxena A, Mendoza T, Cleeland CS. The assessment of cancer pain in North India: the validation of the Hindi Brief Pain Inventory – BPI-H. J Pain Symptom Manage. 1999;18:27–41. doi: 10.1016/s0885-3924(98)00104-3. [DOI] [PubMed] [Google Scholar]
- 23.Brown TA. Confirmatory factor analysis for applied research. New York: Guilford; 2006. [Google Scholar]
- 24.Boelen PA, van den Hout MA, van den Bout J. The factor structure of posttraumatic stress disorder symptoms among bereaved individuals: aconfirmatory factor analysis study. J Anxiety Disord. 2008;22:1377–1383. doi: 10.1016/j.janxdis.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Fournier-Vicente S, Larigauderie P, Gaonac'h D. More dissociations and interactiosn with central executive functioning: a comprehensive latent-variable analysis. Acta Psychol. 2008;129:32–48. doi: 10.1016/j.actpsy.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 26.McArdle JJ. Current directions in structural factor analysis. Curr Dir Psychol Sci. 1996;5:11–18. [Google Scholar]
- 27.Olden M, Rosenfeld B, Pessin H, Breitbart W. Measuring depression at the end of life: is the Hamiliton Depression Rating Scale a valid instrument? Assessment. 2009;16:43–54. doi: 10.1177/1073191108320415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McClain CS, Rosenfeld B, Breitbart W. Effect of spiritual well-being on end-of-life dispair in terminally-ill cancer patients. Lancet. 2003;361:1603–1607. doi: 10.1016/S0140-6736(03)13310-7. [DOI] [PubMed] [Google Scholar]
- 29.Rosenfeld B, Breitbart W, Gibson C, et al. Desire for hastened death among patients with advanced AIDS. Psychosomatics. 2006;47:504–512. doi: 10.1176/appi.psy.47.6.504. [DOI] [PubMed] [Google Scholar]
- 30.Browne MW, Cudeck R. Alternative ways of assessing model fit. Newbury Park, CA: Sage; 1993. [Google Scholar]
- 31.Bentler PM. Comparative fit indixes in structural models. Psychol Bull. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- 32.Hu L, Bentler PM. Cutoff criteria for fit indices in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- 33.Amos [computer program], Version 16. Chicago: SmallWaters Corp; 2007. [Google Scholar]
- 34.Schreiber JB, Stage FK, King J, Nora A, Barlow EA. Reporting structural equation modeling and confirmatory factor analysis results: a review. J Educ Res. 2006;99:323–337. [Google Scholar]
- 35.Dolan CV, Molenaar PC. Testing specific hypotheses concerning latent group differences in multi-group covariance structure analysis with structured means. Multivariate Behav Res. 1994;29:203–222. doi: 10.1207/s15327906mbr2903_1. [DOI] [PubMed] [Google Scholar]
- 36.Sprangers MA, Schwartz CE. Integrating response shift into health-related quality of life research: a theoretical model. Soc Sci Med. 1999;48:1507–1515. doi: 10.1016/s0277-9536(99)00045-3. [DOI] [PubMed] [Google Scholar]