Abstract
The long-term maintenance of memory CD4 T cells promotes protective immunity against future pathogen re-infection. As a site rich in survival cytokines, the bone marrow is proposed to be a critical niche for the survival of memory CD4 T cells. We demonstrate that endogenous, polyclonal Ag-specific CD4 T cells rapidly enter and are recovered long-term from the bone marrow following intravenous infection with Listeria monocytogenes. β1 integrin-deficient CD4 T cells also populate the bone marrow early following an infection, but their numbers in this site rapidly decline. This decline was not caused by increased death of T cells lacking β1 integrin but rather by reduced retention in the bone marrow after the primary immune response. The loss of memory CD4 T cells from the bone marrow does not lead to a loss of the predominant source of memory CD4 T cells in the spleen or the ability to mount a memory response. Thus, β1 integrin-dependent maintenance of memory CD4 T cells in the bone marrow is not required for long-term CD4 T cell memory.
INTRODUCTION
The maintenance of Ag-specific T cell memory against previously encountered pathogens is a defining aspect of the adaptive immune system. Following antigen challenge, activated T cells distribute to lymphoid (spleen and lymph nodes) and non-lymphoid peripheral tissues in the body (1, 2). Peripheral tissues that are in direct contact with the environment, such as the lungs and skin, serve as the initial barrier against invading pathogens. T cells at these peripheral sites are predicted to enact their protective functions and provide surveillance against pathogen invasion. Accumulation of T cells in the bone marrow is also observed following infection. However, the function of these cells and the benefits they may receive from residing in this location are not completely understood.
The bone marrow is a unique site that shares overlapping properties with both primary/secondary lymphoid organs and peripheral non-lymphoid sites (3). T cells in the bone marrow are involved in promoting memory antibody responses (4, 5), hematopoietic development (6), anti-viral (7) and anti-tumor responses (8–10). Additionally, the bone marrow microenvironment has been proposed to support memory T cell maintenance (11), as bone marrow resident memory CD8 T cells demonstrate an active phenotype and a higher basal rate of proliferation than their lymphoid counterparts (12–14). IL-15 and IL-7 present in this site are predicted to promote CD8 T cell longevity by driving homeostatic division. Thus, the bone marrow may function as a survival niche where CD8 T cells are maintained in reserve until needed to respond to a secondary encounter with a pathogen (3, 15).
Memory CD4 T cells are also found in the bone marrow and co-localize with IL-7 producing stromal cells that express the α4β1 integrin ligand VCAM-1 (16, 17). As IL-7 is the main cytokine required for CD4 T cell survival (18, 19), the bone marrow is also predicted to function as a survival niche for memory CD4 T cells (3, 17). The CD4 T cells recovered from the bone marrow express low levels of CD62L and CCR7, an effector memory-like phenotype (5, 17). One group has demonstrated that these cells have a lower proliferation rate (17). Thus, enhanced survival in the bone marrow may be mediated by IL-7-induced survival signaling rather than homeostatic division. In that system, the bone marrow was demonstrated to be the predominant site of memory CD4 T cell residence. In contrast, others have demonstrated that memory CD4 T cells can localize to the bone marrow following an infection, but predominantly reside in the lymph nodes and spleen (20). The specific role of the bone marrow as a re-circulatory survival pit-stop for the maintenance of systemic CD4 T cell memory was not explored. Additionally, after bacterial infection, the kinetics of CD4 T cell migration to the bone marrow and the adhesion molecule requirements for maintenance in this location have not been investigated.
Binding of the α4β1 integrin to VCAM-1 promotes firm arrest and the subsequent entry of T cells into the bone marrow (15, 21). A function for the αLβ2 (LFA-1) integrin in bone marrow entry has also been shown, particularly when the α4β1/VCAM-1 interaction is inhibited (21–23). Another β1 integrin family member, α1β1, is induced following T cell activation and is thought to be critical for T cell retention in non-lymphoid tissues, such as the lung (24–26). The binding of T cell expressed α1β1 integrin to tissue collagen may also provide signals that promote T cell survival (27). As VCAM-1 is expressed on stromal cells that produce IL-7 in the bone marrow (28), α4β1 may also promote CD4 T cell survival in the bone marrow. However, the exact function that β1 integrins play in CD4 T cell maintenance in this unique site remains poorly defined.
Here, we find that endogenous Ag-specific CD4 T cells rapidly enter the bone marrow following i.v. bacterial infection. We show that memory CD4 T cell maintenance in the bone marrow is dependent upon expression of the β1 integrin subunit. The reduced number of CD4 T cells in the bone marrow in the absence of β1 integrin is not related to altered survival, but primarily driven by reduced retention in this site. In our system, the bone marrow is not required for the systemic maintenance of CD4 T cell memory or for a rapid response to secondary antigen challenge.
MATERIALS AND METHODS
Mice
Mice with a “floxed” β1 integrin gene and the CD4-Cre transgene (β1−/−) mice were produced as previously described (29). β7 integrin-deficient mice (Jackson Laboratory, Bar Harbor, ME) were bred with β1−/− mice to make β1β7-deficient (β1β7−/−) mice. CD4-Cre+ mice were used as wild-type (wt)2 controls. All mice were on the C57BL/6 background. All experimental protocols involving the use of mice were approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
Listeria infections, peptide:MHC class II tetramer enrichments, and cell staining
Infection with Listeria monocytogenes and peptide:MHCII-tetramer enrichment were performed as previously described (29). Mice were infected i.v. with 1 × 107 CFUs of 2W1S-peptide expressing ActA-deficient L. monocytogenes (A−Lm-2W1S). Single cell suspensions were made from spleens and bilateral hind limb (femur and tibia) bone marrow. The enriched 2W1S-specfic population was stained with the previously reported cocktail of antibodies in order to identify the 2W1S-specific CD4 T cells. Antibodies against β1 integrin (HMβ1.1-PB), LFA-1 (M17/4-FITC), and CCR7 (4B12-PE) were used in some experiments (eBioscience, San Diego, CA). P-selectin Fc chimera protein (1 µg/sample) (R&D Systems, Minneapolis, MN) followed by anti-human IgG Fc (eBioscience) was used to stain for functional PSGL-1 on T cells. All samples were collected on a BD LSRII flow cytometer (BD Biosciences, San Jose, CA) and analyzed with FlowJo software (Tree Star, Ashland, OR). Cell counts were obtained using AccuCheck Counting Beads (Invitrogen Carlsbad, CA). Hind limb bone marrow cell numbers were multiplied by five to obtain total body bone marrow cell numbers (29). For rechallenge, 50 µg of purified 2W1S-peptide (GenScript, Piscataway, NJ) and 5 µg LPS (Sigma Aldrich, St. Louis, MO) was injected i.v.
FLICA staining for active caspases 3 & 7
Carboxyfluorescein-FLICA reagent was used per manufacturer’s instructions for visualization of active caspases 3 & 7 (Immunochemistry Tech. LLC, Bloomington, MN). Tetramer staining for FLICA stained samples was performed at 4°C, which decreased background staining. FLICA solution (30X) was added with standard antibody cocktail for 1 hr at 37°C. Samples were then washed twice with FLICA wash buffer and resuspended in 200 µl of 0.1 µg/ml DAPI in FLICA staining buffer for 20 min at room temperature. DAPI+ cells were excluded from the analysis.
Short-term in vivo cohoming assay
Spleen cells from mice infected 5 or 20 days previously with A−Lm-2W1S were enriched for CD4 T cells by negative selection using MACS LS columns (Miltenyi Biotec, Auburn, CA). Cells were then labeled with 0.25 mM Cell Tracker Green (CTG) or 2 mM Cell Tracker Orange (CTO) and mixed 1:1 prior to i.v. injection into host mice (5–10 × 106 cells/mouse). Host mice were uninfected, day 5 or day 20 post-infection with A−Lm-2W1S. A sample of the input mixture was taken to determine the number of injected cells. Spleens and bone marrow were harvested at 2 and 18 hours post-cell transfer. Day 5 transfers were processed as indicated above for peptide:MHCII-tetramer enrichment and day 20 transfers were processed similarly but tetramer staining was not performed.
Statistical analysis
GraphPad Prism software (La Jolla, CA) was used for all statistical analysis.
RESULTS
CD4 T cells rapidly enter the bone marrow following i.v. infection but are not maintained in the absence of β1 integrin
We utilized a recently described peptide:MHCII tetramer approach (30) to track the localization of an endogenous, polyclonal Ag-specific CD4 T cell population in wild-type (wt) mice following an i.v. infection with Act-A deficient Listeria monocytogenes expressing the 2W1S peptide variant of peptide 52–68 from the I-E α-chain (A−Lm-2W1S) (20, 29). This attenuated pathogen is rapidly cleared, thus allowing us to track the endogenous CD4 T cell response in the absence of chronic infection.
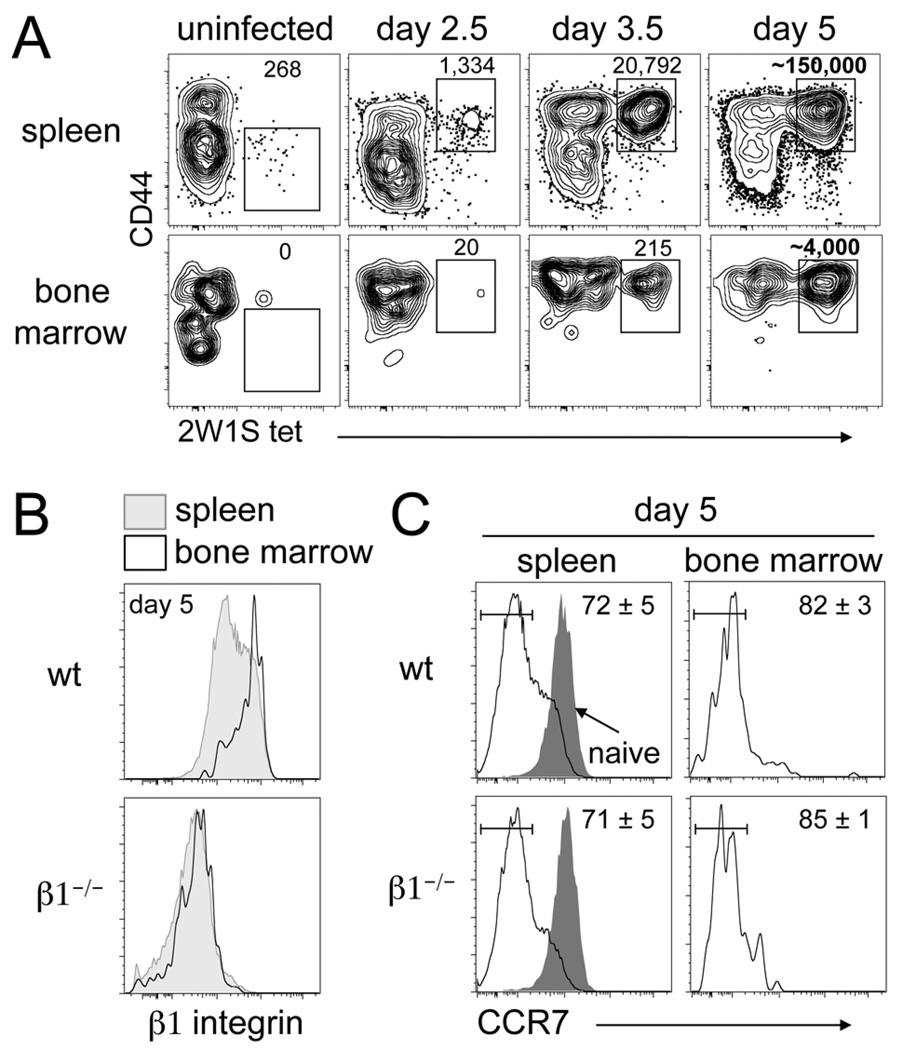
In uninfected mice, several hundred 2W1S-specific CD44low CD4 T cells are present in the spleen, but 2W1S-specific CD44low CD4 T cells were rarely recovered from bone marrow (Fig. 1A). This is in agreement with previous studies showing the preferential colonization of the bone marrow by a CD44high T cell population (5, 20). By day 2.5 post-infection, a population of 2W1S-specific CD44high CD4 T cells is observed in the spleen, but is essentially absent in the bone marrow. By day 3.5, we observe an expanded population of 2W1S-specific CD44high CD4 T cells in the spleen and also begin to recover these cells from the bone marrow. These findings are consistent with the activation and proliferation of naïve CD4 T cells in the spleen and their subsequent migration into the bone marrow. By the peak of the response (day 5), 2W1S-specific CD4 T cells have massively expanded in the spleen and we recover a large population from the bone marrow (Fig. 1A). Whole body mouse perfusion via the left ventricle did not alter the number of 2W1S-specific CD4 T cells recovered from the bone marrow (data not shown). Thus, endogenous Ag-specific CD4 T cells rapidly enter the bone marrow following i.v. bacterial infection.
Figure 1. CD4 T cells rapidly enter the bone marrow following i.v bacterial infection.
(A) Tracking the 2W1S-specific population following i.v. infection with A−Lm-2W1S in wt mice. Numbers indicate calculated cell number from gate (day 5 are averages, n=9). (B) Spleen and bone marrow histograms of β1 integrin expression on 2W1S-specific CD44high CD4 T cells day 5 post-infection from wt and β1−/− mice. (C) Spleen and bone marrow histograms of CCR7 expression on 2W1S-specific CD44high CD4 T cells day 5 post-infection from wt and β1−/− mice. Gray histogram represents naïve CD44low CD4 T cells from the spleen. Numbers indicate percent of cells in the drawn gate (n=3, mean +/− s.d.).
Migration into the bone marrow at steady-state is largely dependent on the interaction between T cell expressed α4β1 integrin and its vascular ligand VCAM-1 (15, 21). At day 5, 2W1S-specific CD44high CD4 T cells recovered from the bone marrow are enriched for those expressing the highest levels of β1 integrin compared to the 2W1S-specific population in the spleen (Fig. 1B). To determine if the entry of these cells is dependent on β1 integrin, we tracked the 2W1S-specific response in mice lacking β1 on their T cells (β1−/− mice) (29). We have previously reported similar numbers of 2W1S-specific CD4 T cells from the spleens of wt and β1−/− mice at all sampled time points following A−Lm-2W1S infection (29). At day 5 following infection, CD4 T cells were found in the bone marrow of β1−/− mice and as expected these cells lacked expression of β1 integrin (Fig. 1B). Thus, the CD4 T cells entering the bone marrow are not enriched for those that may have escaped β1 integrin deletion as has been previously reported in another system (31). The majority of 2W1S-specific CD4 T cells in the spleen at day 5 are CCR7low in both wt and β1−/− mice compared to naïve CD4 T cells (Fig. 1C). CD4 T cells recovered from the bone marrow are even further enriched for the CCR7low phenotype.
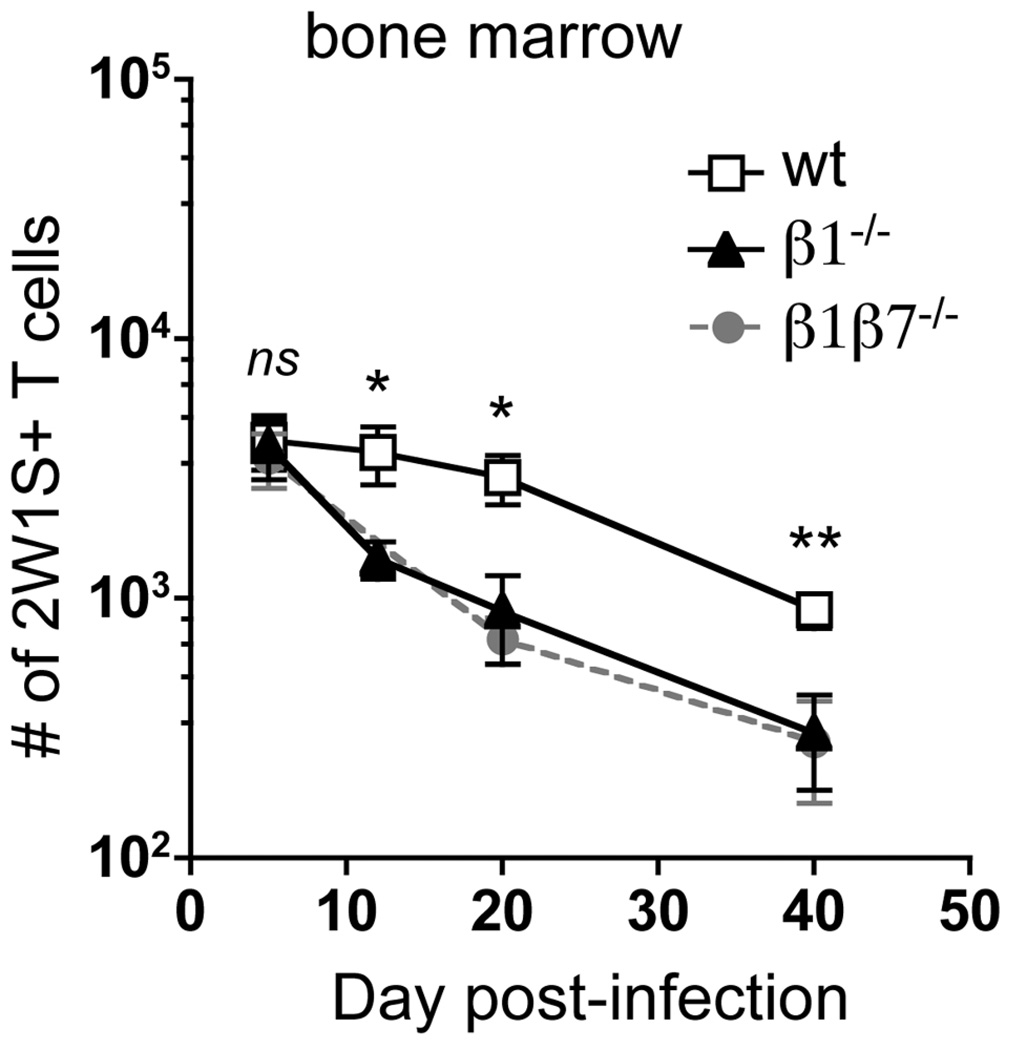
Unexpectedly, in the absence of β1 integrin, similar numbers of 2W1S-specific CD4 T cells were recovered from the bone marrow at day 5 (Fig. 2). The ability of β1−/− CD4 T cells to enter the bone marrow is not related to their enhanced expression of α4β7 (29), as CD4 T cells lacking both β1 and β7 integrins (β1β7−/−) also have similar CD4 T cell numbers in the bone marrow at day 5 (Fig. 2). Thus, the entry of recently activated CD4 T cells to the bone marrow can occur in a β1 integrin and β7 integrin-independent manner.
Figure 2. Maintenance, but not entry, of CD4 T cells in the bone marrow is dependent on β1 integrin.
2W1S-specific CD44high CD4 T cells recovered from the bone marrow of wt, β1−/−, and β1β7−/− mice following A−Lm-2W1S infection. Data are from ten experiments (n=3–11, mean +/– s.e.m.). (ns) p > 0.05, *p < 0.05, **p=0.009 compared to wt, two-tailed unpaired t test.
Interestingly, β1−/− T cells are lost from the bone marrow during the contraction phase (day 5–20) of the immune response and are present in statistically lower numbers in the bone marrow than wt cells out to day 40 post-infection (Fig. 2). Since β1β7−/− CD4 T cells are also lost from the bone marrow, enhanced homing to the gut of β1−/− cells (29) is unlikely to account for the T cell loss from the bone marrow. In summary, following a bacterial infection, the absence of β1 integrin does not alter the early accumulation of CD4 T cells in the bone marrow, but alters their steady-state maintenance at this site.
Cell death does not account for the loss of CD4 T cells from the bone marrow after the peak of the response
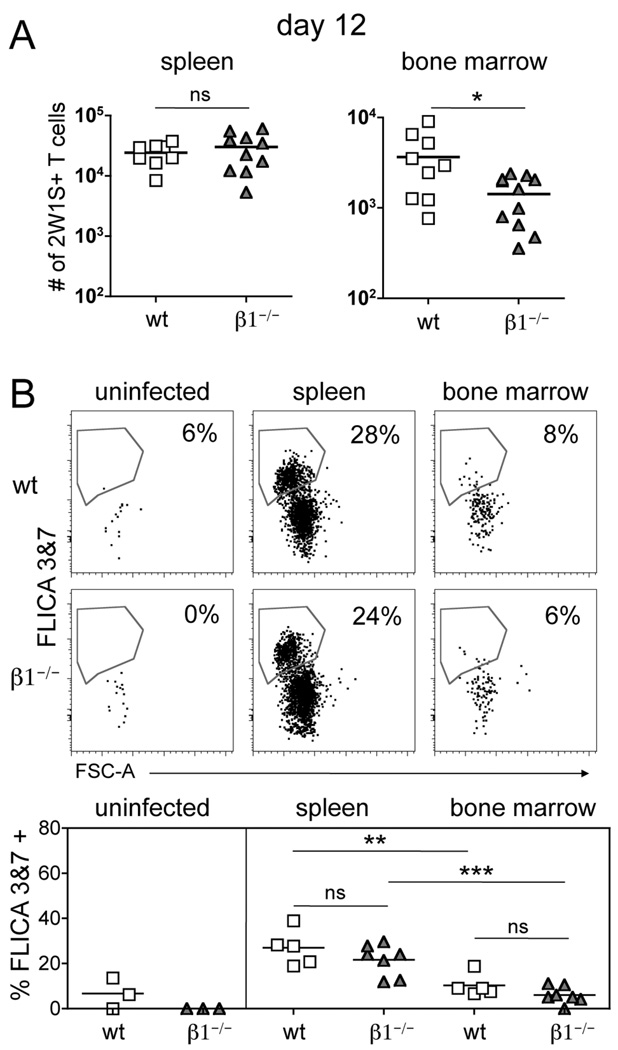
Integrins may provide pro-survival signals to memory T cells in non-lymphoid tissue (24, 27) and facilitate T cell localization to IL-7-producing, VCAM-1+ stromal cells in the bone marrow (28). To determine if the loss of CD4 T cells from the bone marrow of β1−/− mice could be accounted for by increased rates of apoptosis, we assessed activation of the effector caspases 3 & 7 in 2W1S-specific CD4 T cells during the contraction phase. These caspases are downstream of both the intrinsic and extrinsic apoptotic pathways in T cells (32). At day 12, the total 2W1S-specific CD4 T cell numbers in the spleen were identical between wt and β1−/− mice whereas the bone marrow of β1−/− mice contained significantly less CD4 T cells (Fig. 3A). To assess the percentage of cells undergoing apoptosis, we stained 2W1S-specific CD4 T cells using a fluorescent reagent that specifically binds and inhibits active caspases 3 & 7 (33). As expected, naïve 2W1S-specific CD4 T cells from the spleen of uninfected mice have little staining for active caspases 3 & 7 (Fig. 3B). Robust activation of caspases 3 & 7 was observed in ~25% of wt and β1−/− 2W1S-specific CD4 T cells in the spleen at day 12 post-infection (Fig. 3B). Importantly, the percentage of 2W1S-specific CD4 T cells in the bone marrow of β1−/− mice undergoing apoptosis was similar to that of wt mice (Fig. 3B). The percentage of DAPI+ cells was also equivalent between wt and β1−/− mice (data not shown), indicating our analysis is not missing cells that have already undergone apoptosis.
Figure 3. Loss of CD4 T cells from the bone marrow is not related to increased cell death.
(A) Number of 2W1S-specific CD44high CD4 T cells recovered from the spleen and bone marrow 12 days post-infection with A−Lm-2W1S in wt and β1−/− mice (mean). * p < 0.05, two-tailed unpaired t test. (B) Staining for active caspases 3 & 7 (FLICA 3&7) in 2W1S-specific CD44low CD4 T cells from the spleen of uninfected mice or 2W1S-specific CD44high CD4 T cells from the spleen and bone marrow 12 days post-infection. Bottom graphs show replicates (mean). (ns) p > 0.05, ** p<0.01, *** p<0.001, one-way ANOVA, followed by Tukey’s multiple comparison test.
Interestingly, the percentage of 2W1S-specific CD4 T cells in the bone marrow undergoing apoptosis was significantly less than the apoptotic percentage of 2W1S-specific CD4 T cells from the spleen in both wt and β1−/− mice (Fig. 3B). Overall, the loss of β1−/− CD4 T cells from the bone marrow between days 5 and 20 is not accounted for by increased cell death.
β1 integrin is not required for bone marrow localization at day 5 but is required at day 20
T cells can emigrate out of the bone marrow and enter into other lymphoid organs and bones (34, 35). Additionally, connecting the blood circulation (parabiosis) of an immune mouse and an uninfected mouse rapidly results in the equilibration of bone marrow memory T cells between mice (36). These findings predict that memory T cells continually enter and exit the bone marrow rather than take up long-term residence, as is seen in the intestinal epithelium (36, 37). Thus, loss of CD4 T cells from the bone marrow of β1−/− mice may relate to an altered ability of cells lacking β1 integrin to enter into and/or be retained in the bone marrow after the peak of the immune response.
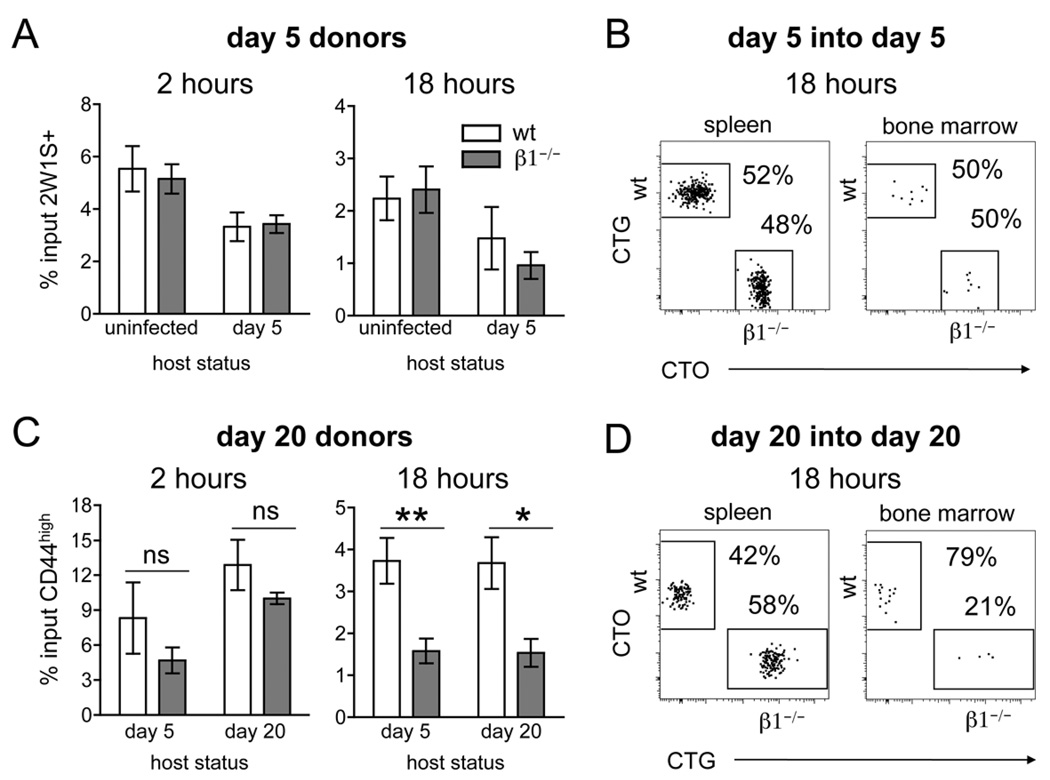
To determine the entry and retention properties of recently activated (day 5) versus early memory T cells (day 20), we isolated 2W1S-specific wt and β1−/− CD4 T cells following infection and performed co-transfers into various hosts. First, we transferred recently activated (day 5) wt and β1−/− 2W1S-specific CD4 T cells into recipient mice infected 5 days earlier with A−Lm-2W1S. Infected hosts were used in order to replicate the inflammatory milieu present during infection and also to provide an antigen source. The bone marrow was harvested at 2 and 18 hours post-transfer as relative measures of entry and retention, respectively (15). At 2 and 18 hours post-transfer, the percentage of input wt and β1−/− 2W1S-specific CD4 T cells localizing to the bone marrow of day 5 hosts was similar (Fig. 4A). As a control, we demonstrate that an equivalent percentage of transferred wt and β1−/− 2W1S-specific CD4 T cells are observed in the spleen of these same mice at 18 hours post-transfer (Fig. 4B). These results reproduce the findings from infections of intact mice, where similar numbers of wt and β1−/− 2W1S-specific CD4 T cells are recovered in the spleen and bone marrow at day 5 post-infection (Fig. 2) (29). In order to separate T cell intrinsic homing capabilities from environmental alterations that may occur during an acute infection, we also co-transferred cells into uninfected hosts. Interestingly, an equivalent percentage of wt and β1−/− 2W1S-specific CD4 T cells were recovered at 2 and 18 hours (Fig. 4A). Overall, at day 5 post-infection, wt and β1−/− CD4 T cells have similar entry into and retention in the bone marrow with or without the presence of infection.
Figure 4. Loss of CD4 T cells from the bone marrow is due to decreased ability to recirculate.
(A) Bar graphs showing the percentage of transferred 2W1S-specific CD44high CD4 T cells recovered from the bone marrow of host mice at 2 and 18 hours post-transfer. Percentage of input was calculated by dividing recovered cell number by transferred cell number (mean +/− s.e.m., n=4–6). (B) Representative dot plots of spleen and bone marrow of mice infected 5 days prior to co-transfer of day 5 post-infection wt and β1−/− 2W1S-specific CD44high CD4 T cells. Transferred cells are distinguished from the host population by labeling with CTG (wt) and CTO (β1−/−). (C) Bar graphs showing the percentage of transferred CD44high CD4 T cells recovered from the bone marrow of host mice at 2 and 18 hours post-transfer (n=4–5, mean +/− s.e.m.). * p=0.02, ** p=0.008, two-tailed unpaired t test. (D) Representative dot plots of spleen and bone marrow of host mice infected 20 days prior to co-transfer of day 20 post-infection wt and β1−/− CD44high CD4 T cells. Transferred cells are distinguished from the host population by labeling with CTO (wt) and CTG (β1−/−). The 2W1S-specific host population (negative for CTO and CTG) has been excluded from all dot plots.
By day 20 post-infection, the population of 2W1S-specific CD4 T cells in the spleen of wt and β1−/− mice has contracted to ~20,000 cells (29). At this time point, we observe a significantly decreased number of CD4 T cells in the bone marrow of β1−/− mice (Fig. 2). Due to the small number of 2W1S-specific CD4 T cells at day 20, we were unable to obtain enough Ag-specific early memory CD4 T cells from donor mice to exclusively track the migration of the 2W1S-specific population to the bone marrow following transfer. Thus, we monitored the localization of the bulk population of CD44high CD4 T cells (including 2W1S-specific cells) at day 20 post-infection. At 2 hours post-transfer, there was a modest reduction in the percentage of day 20 post-infection β1−/− CD4 T cells recovered from the bone marrow of day 20 hosts that was not statistically significant (Fig. 4C). Similar findings were observed when day 20 cells were transferred into day 5 post-infection hosts (Fig. 4C). These results reveal little to no reduction in the entry of day 20 β1−/− CD4 T cells into the bone marrow.
By 18 hours post-transfer, there is a notable reduction in the percentage of β1−/− CD4 T cells recovered from the bone marrow (Fig. 4C). To determine if the reduced presence of β1−/− CD4 T cells in the bone marrow was related to changes in the bone marrow versus T cell intrinsic changes, we transferred day 20 cells into mice infected 5 days prior. These experiments also demonstrate a reduced percentage of β1−/− CD4 T cells localized to the bone marrow by 18 hours post-transfer (Fig. 4C). Of note, equivalent percentages of wt and β1−/− CD44high CD4 T cells were recovered from the spleen after co-transfer into day 20 hosts even when the number of β1−/− CD44high CD4 T cells in the bone marrow was significantly reduced (Fig. 4D). These findings replicate the loss of β1−/− 2W1S-specific CD4 T cells observed in the bone marrow 20 days following infection (Fig. 2). Overall, our results suggest that the loss of CD4 T cells from the bone marrow of β1−/− mice after an acute infection is due primarily to reduced retention of β1−/− CD4 T cells during the memory phase.
Altered homing molecule expression on CD4 T cells early and late after infection
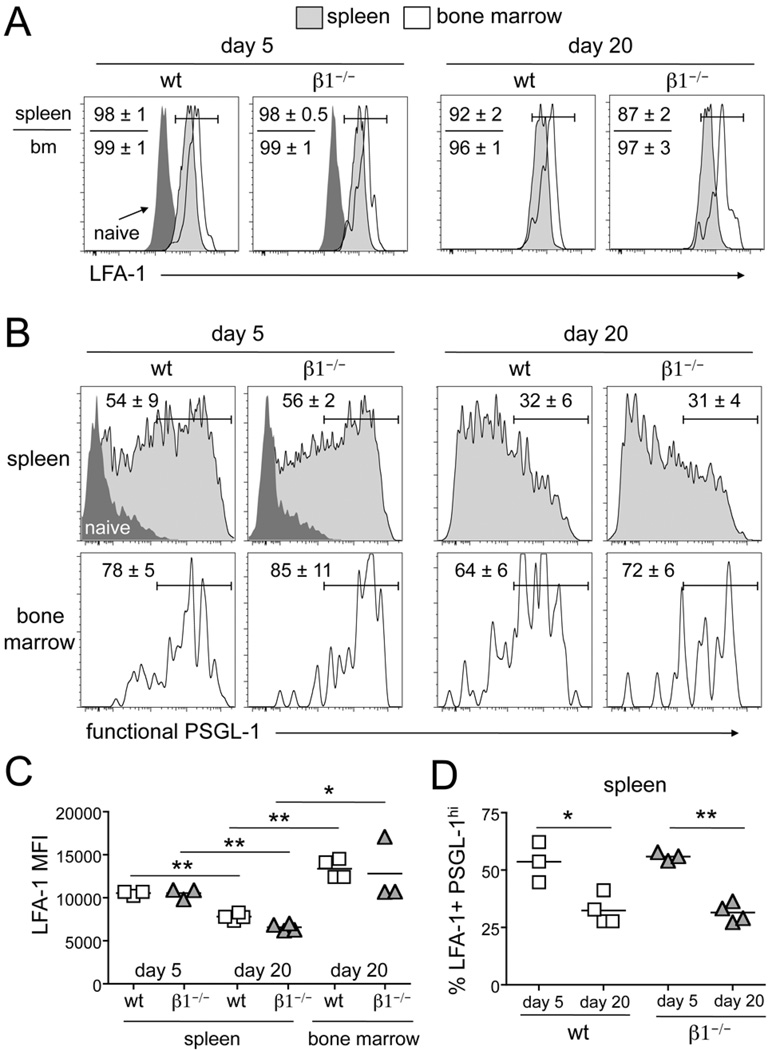
T cell activation quickly results in altered cell surface expression of multiple co-stimulatory and homing receptors (38). The interaction of appropriately glycosylated, functional P-selectin glycoprotein ligand-1 (PSGL-1) with P- and E-selectin results in the initial slowing and rolling of T cells on the bone marrow sinusoid (15, 39). This is followed by firm adhesion to the vessel wall, which is mediated primarily by α4β1/VCAM-1 at steady-state. αLβ2 (LFA-1) integrin binding to vascular ICAM-1 can also play a secondary role for entry into the bone marrow when α4β1 or VCAM-1 function has been compromised (21–23). We show that β1 integrin expression is upregulated following activation and this is maintained on the majority of wt 2W1S-specific CD4 T cells (Fig. 1B) (29). At day 5, the responding wt and β1−/− CD4 T cells in the spleen have also all upregulated expression of LFA-1 compared to naïve CD4 T cells (Fig. 5A). All of the CD4 T cells recovered from the bone marrow of wt and β1−/− mice also have similar high levels of LFA-1. On CD4 T cells in the spleen, LFA-1 expression significantly decreases by day 20 (Fig. 5A and 5C). However, CD4 T cells recovered from the bone marrow at day 20 remain enriched for the highest LFA-1 expression in both wt and β1−/− mice (Fig. 5A and 5C). Early after an infection, ~55% of the cells in the spleen are also high for functional PSGL-1 (Fig 5B and 5D). The population of T cells recovered from the bone marrow of wt and β1−/− mice are enriched for high expression of functional PSGL-1 (Fig. 5B). By day 20, only ~30% of the cells remaining in the spleen display this phenotype, yet the majority of cells recovered from the bone marrow remain high for functional PSGL-1 (Fig. 5B and 5D). Thus, early after an infection, β1 integrin, LFA-1, and functional PSGL-1 are all seen on a large population of activated cells in the spleen and the cells recovered from the bone marrow are enriched for this population. By day 20, cells from the spleen express lower amounts of both LFA-1 and functional PSGL-1 and in the absence of β1 integrin, these same cells have reduced localization to the bone marrow. Taken together, these findings suggest a compensatory redundancy in homing molecule expression early following an infection but a reduced level of redundancy by day 20.
Figure 5. Expression of LFA-1 and functional PSGL-1 is decreased between day 5 and 20 post-infection.
2W1S-specific CD44high CD4 T cells from the spleen and bone marrow of wt and β1−/− mice following i.v. infection with A−Lm-2W1S stained for (A) LFA-1 or (B) functional PSGL-1. Dark gray histograms represent staining of CD44low CD4 T cells (naïve) from the spleen. Numbers indicate percent of cells in the drawn gate (n=3–4, mean +/− s.d.). (C) Median Fluorescence intensity (MFI) of LFA-1 staining on PSGL-1hi 2W1S-specific CD44highCD4 T cells from the spleen and bone marrow of wt and β1−/− mice following i.v. infection with A−Lm-2W1S. (D) Percentage of LFA-1+ PSGL-1hi 2W1S-specific CD44high CD4 T cells from the spleen and bone marrow of wt and β1−/− mice following i.v. infection with A−Lm-2W1S. Bar represents the mean. * p<0.02, ** p<0.0002, two-tailed unpaired t test.
Memory CD4 T cell retention in the bone marrow is not required for global memory survival or rapid memory response
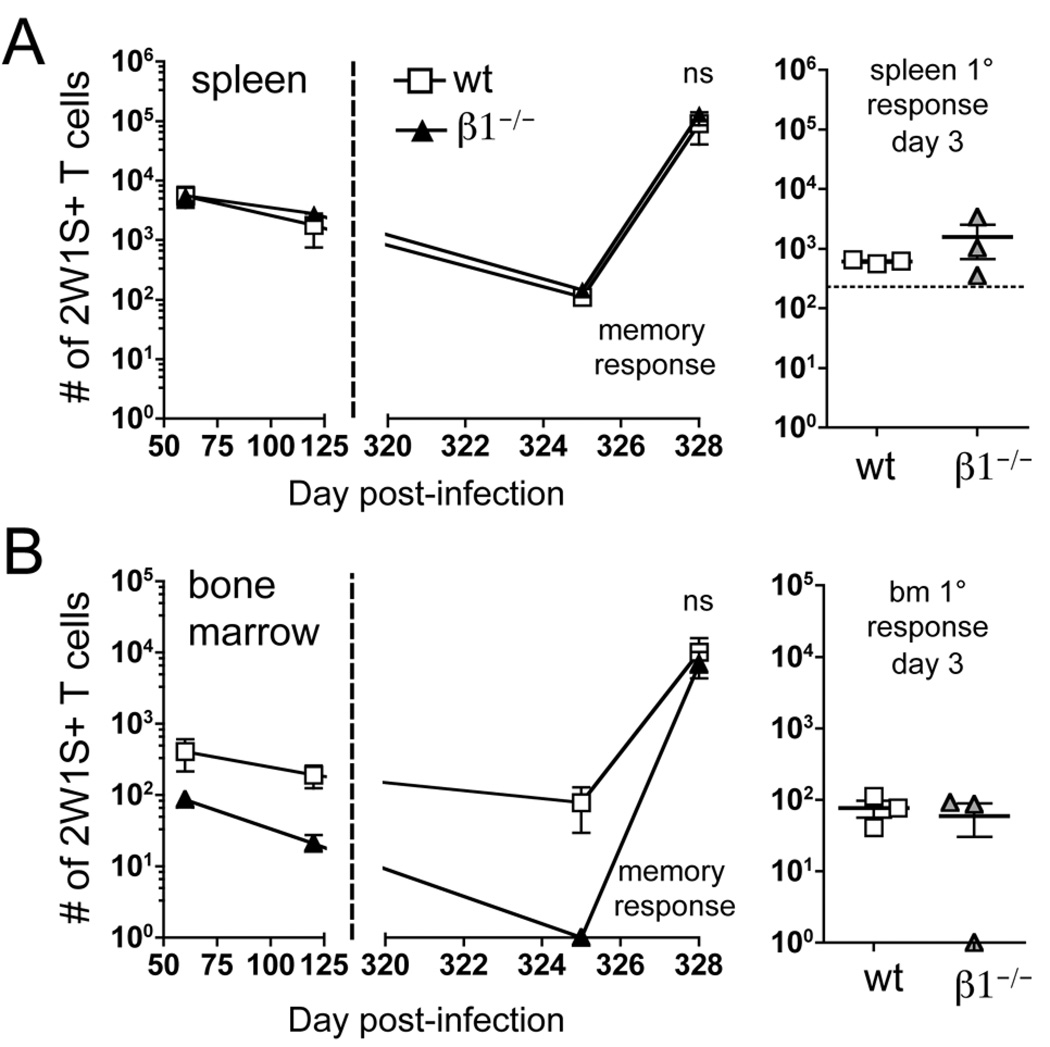
As the bone marrow is considered a “survival niche” for memory CD4 T cells (17), we hypothesized that decreased localization to the bone marrow during the memory phase would result in a systemic loss of CD4 T cell memory. Surprisingly, this general loss of 2W1S-specific CD44high CD4 T cells was not observed in our system, as cell numbers in the spleen are equivalent in wt and β1−/− mice out to day 325 (Fig. 6A). Additionally, the number of 2W1S-specific CD4 T cells recovered from the spleen was greater at all time points than those recovered from the bone marrow (Fig. 6A and 6B). At the latest time point collected, no 2W1S-specific CD4 T cells could be recovered from the bone marrow of β1−/− mice, whereas wt mice contain ~100 cells (Fig. 6B). Of note, in one wt mouse, no 2W1S-specific CD4 T cells were recovered from the bone marrow but the spleen contained normal numbers of memory cells. Thus, even in the absence of memory CD4 T cells in the bone marrow, memory CD4 T cells are maintained in the spleen.
Figure 6. Retention of CD4 T cells in the bone marrow is not required for survival or response of memory CD4 T cells.
2W1S-specific CD44high CD4 T cells recovered from the spleen (A) and bone marrow (B) of wt and β1−/− mice following i.v. infection with A−Lm-2W1S. At day 325 post-infection, mice were re-challenged with 2W1S-LPS. Spleen and bone marrow were harvested three days following re-challenge (n=5, mean+/− s.e.m.). The primary (1°) response of naïve (uninfected) mice to 2W1S-LPS was also characterized three days following challenge. Horizontal dotted lines on graphs indicate the average number of 2W1S-specific CD44low CD4 T cells in naïve mice. (ns) p > 0.05, two-tailed unpaired t test.
To test the ability of day 325 memory mice to respond to antigen re-challenge, we immunized them with 2W1S-peptide and LPS (2W1S-LPS). The secondary proliferative response in the spleen at day 3 post-immunization was 150 times larger than the primary response from naïve mice challenged with 2W1S-LPS (Fig. 6A). This indicates that the observed 2W1S-specific CD4 T cell proliferation is a memory recall response. Furthermore, there were comparable numbers of 2W1S-specific CD4 T cells in the spleen following secondary challenge of wt and β1−/− mice (Fig. 6A). Interestingly, three days post-immunization, 2W1S-specific cell numbers in the bone marrow equilibrate between wt and β1−/− mice (Fig. 6B). As observed in the primary response, this is consistent with the activation of 2W1S-specific CD4 T cells in the spleen and subsequent migration of these cells into the bone marrow. As seen in the spleen, there were a much larger number of CD4 T cells recovered from the bone marrow following a memory response than primary response (Fig. 6B). These results indicate that the bone marrow is not required to maintain long-term, antigen responsive CD4 T cell memory following i.v. bacterial infection.
DISCUSSION
The long-term survival of Ag-specific memory CD4 T cells provides enhanced protection against previously encountered pathogens (40). As a site rich in survival cytokines, the bone marrow is predicted to be a critical ‘survial niche’ for memory T cells (3, 11, 15, 17). However, it is unknown if the entry of memory CD4 T cells into the bone marrow is required for their long-term survival. In this study, we use a peptide:MHC class II tetramer enrichment technique to examine the role of β1 integrin in the maintenance of endogenous, polyclonal Ag-specific CD4 T cells in the bone marrow following bacterial infection. We demonstrate that CD4 T cells lacking β1 integrin enter the bone marrow early following an infection but are not maintained there long-term. This lack of Ag-specific CD4 T cell maintenance in the bone marrow does not result in a systemic loss of CD4 T cell memory or a reduced ability to mount a secondary response. These findings support the idea that the localization of CD4 T cells to the bone marrow is not required for the long-term maintenance of CD4 T cell memory.
We observed comparable numbers of wt and β1−/− Ag-specific CD4 T cells in the bone marrow at early time points after a primary Listeria infection. Co-homing assays of day 5 post-infection cells into day 5 post-infection hosts confirmed that localization of recently activated CD4 T cells to the bone marrow was independent of β1 integrin expression. Similar findings were also obtained when day 5 cells were transferred into uninfected hosts. This revealed that potential infection-induced alterations in the bone marrow microenvironment or the presence of antigen in the bone marrow is not required for β1 integrin-independent localization of recently activated CD4 T cells to the bone marrow. Our results are consistent with the previously reported promiscuous distribution of recently activated CD4 T cells to normal and inflamed tissue without the presence of antigen (1). Thus, the initial entry of β1−/− T cells into the bone marrow is mediated by cell intrinsic factors rather than systemic changes that occur during acute infection.
Our observation that β1 integrin expression is not required for the entry of recently activated CD4 T cells into the bone marrow is surprising, as previous studies have reported that the binding of the α4β1 integrin to VCAM-1 promotes firm arrest and subsequent entry of T cells into the bone marrow (21). However, T cell migration into the bone marrow also involves the function of other adhesion molecules, such as PSGL-1, which promotes T cell rolling (15, 21). In addition, LFA-1 integrin plays a minor role in bone marrow entry that becomes more prominent when the α4β1/VCAM-1 interaction is compromised (21–23). Of note, the vast majority of systemic Ag-specific CD4 T cells responding to Listeria infection express high amounts of LFA-1 and functional PSGL-1. Furthermore, the CD4 T cells recovered from the bone marrow are enriched for this population. Thus, the high level expression of functional PSGL-1 and LFA-1 on Ag-specific CD4 T cells early after infection may allow for efficient bone marrow entry in the absence of β1 integrin expression. Alternatively, the enhanced expression of α4β7 on β1−/− CD4 T cells might compensate for the absence of β1 integrin (29). α4β7 integrin-mediated compensation could occur either through direct binding to VCAM-1 (41) or binding to the α4β7 ligand MAdCAM-1, which can be unregulated on the bone marrow vasculature during inflammation (42). Our data argues against either of these possibilities, as β1−/− CD4 T cells additionally lacking β7 integrin (β1β7−/−) enter the bone marrow in similar numbers as wt cells at day 5.
Previous work has demonstrated that T cells emigrate from and recirculate back to the bone marrow (34, 35). Parabiosis studies also predict that T cells recirculate through the bone marrow (36) rather than take up long-term residence, as can be seen in the intestine (37). Steady-state maintenance of CD4 T cells in the bone marrow is thus highly related to the rates of cell entry and exit. In our system, by day 12 post-infection, the number of 2W1S-specific CD4 T cells in the bone marrow of β1−/− mice is ~60% reduced compared to wt controls. This decreased number of Ag-specific CD4 T cells in the bone marrow of β1−/− mice continues to decline and becomes ~90% reduced compared to wt by day 120. In an attempt to determine the relative contributions of entry and retention to the gradual loss of β1−/− CD4 T cells from the bone marrow, we co-transferred wt and β1−/− CD4 T cells 20 days following an infection into day 20 hosts. There was a modest reduction in the localization of β1−/− CD4 T cells in the bone marrow at 2 hours post-transfer, but this finding did not reach statistical significance (p>0.05). Although these cells express lower levels of LFA-1 and functional PSGL-1 than at day 5 post-infection, high level expression of these molecules is still observed and may provide some functional redundancy. These findings predict that 20 days after an infection, CD4 T cells lacking β1−/− would only be minimally impaired in their ability to enter into the bone marrow. However, by 18 hours post-transfer there is a significant reduction in the maintenance of β1−/− CD4 T cells in the bone marrow. This is also observed when day 20 cells are transferred into day 5 hosts. Thus, this effect is likely cell intrinsic as it is not overcome by potential alterations in the bone marrow vasculature or stroma that may occur with an acute infection. Together, our results suggest that the rapid decline of β1−/− CD4 T cells in the bone marrow following bacterial infection is due predominantly to impaired retention. However, as transferred T cells are continuously entering and exiting the bone marrow, the modest reduction in the entry of β1−/− CD4 T cells observed at 2 hours may, over time, also contribute to the loss of T cell accumulation in the bone marrow.
In addition to a reduction in retention of cells, another possible mechanism for the reduced maintenance of β1−/− CD4 T cells in the bone marrow is increased cell death. Signals from β1 integrins have been proposed to promote the survival of memory T cells in non-lymphoid tissue (24, 26, 27). In addition, β1 integrin may be important for the localization of CD4 T cells to VCAM-1+ stromal cells producing IL-7 in the bone marrow (16, 17). Staining for effector caspases 3 & 7 revealed no differences between wt and β1−/− CD4 T cells in either the bone marrow or the spleen. In both wt and β1−/− mice, 2W1S-specific CD4 T cells did demonstrate significantly reduced caspase activity in bone marrow compared to the spleen. This reduced caspase activity is consistent with the idea of the bone marrow as a T cell ‘survival niche’. As both wt and β1−/− CD4 T cells demonstrate higher viability in the bone marrow, this suggests that access to survival factors in the bone marrow is independent of β1 integrin expression. This is a surprising result, as the IL-7 producing stromal cells express the β1 integrin ligand VCAM-1 (28). Another possibility is that the most viable population of 2W1S-specific CD4 T cells from the spleen are the cells that migrate to the bone marrow. Our experimental results do not allow us to distinguish between these two possibilities. Overall, our results suggest that cell death is not a major factor reducing the abundance of β1−/− CD4 T cells in the bone marrow.
In many systems, β1 integrins function as T cell retention molecules in peripheral tissue (38). Maintenance of memory T cells in peripheral tissue is predicted to provide rapid protection against pathogen reinfection. The collagen binding integrin α1β1 is critical for T cell retention in the lung following influenza infection (24, 25). In this system, lung resident α1β1+ effector-memory CD4 cells are the major IFN-γ-secreting, rapid responders during a secondary infection (26). In humans, the collagen binding integrin α1β1 is a marker of similar TH1 phenotype CCR7low effector-memory CD4 T cells (43). Additionally, skin resident effector-memory CD4 T cells specifically express α1β1, which is involved in their retention in the epidermis (44). In our system, the majority of the CCR7low effector-memory like CD4 T cells are maintained in the spleen and lymphoid organs (20). The CD4 T cells we recover from the bone marrow are enriched for this CCR7low effector-memory like population compared to the spleen. This CCR7low effector-memory like population expresses high levels of both β1 and LFA-1, consistent with the characterization of effector-memory T cells in humans (45). By day 20, CD4 T cells expressing a CCR7low effector-memory like phenotype remain the predominant population in the bone marrow (data not shown), suggesting that this population is particularly efficient at localizing to this site. This also corresponds to the population of CD4 T cells reported to localize to the bone marrow in other systems (5, 17). Bone marrow resident CD4 T cells express high levels of the collagen binding integrin α2β1 (17), but a role for α1β1 as a retention molecule in the bone marrow has not been excluded.
For naïve CD4 T cell homeostasis, stromal components of the lymph nodes and spleen are thought to be the major source of the survival cytokine IL-7 (46). In contrast, the bone marrow has recently been proposed to be the major site for memory CD4 T cell maintenance and IL-7 survival signaling (17). The bone marrow has unique properties that situate it somewhere between peripheral tissue and lymphoid tissue (3). Unlike peripheral tissue, where the presence of antigen is thought to drive retention (47), localization to the bone marrow can occur with or without local antigen (3, 16). Thus, local immunosurveillance may not completely explain the presence of CD4 T cells in the bone marrow and its function as a survival reservoir has been gaining support (3, 15). If the bone marrow is the major source of IL-7 for memory CD4 T cells, we expected to observe a global loss of CD4 T cell memory over-time in the β1−/− mice. Surprisingly, the decreased ability of β1−/− CD4 T cells to be maintained in the bone marrow does not result in a systemic decrease in CD4 T cell memory or loss of a rapid proliferative response to antigen re-challenge. At nearly one year post-infection, when we are unable to detect β1−/− memory CD4 T cells in the bone marrow using our peptide:MHC class II tetramer enrichment technique, the secondary response in the spleen and bone marrow is comparable to wt mice. In our system, the spleen remains the predominant site of memory CD4 T cell localization, although the total percentage of CD4 T cells maintained in the bone marrow compared to the spleen in wt mice does increase over time. These results are consistent with other recently published work utilizing this system (20). The ability to maintain long-term CD4 T cell memory in the presence of decreased bone marrow localization is seemingly in contrast to other work using adoptive transfer systems (17). The reasons for this discrepancy are unclear but may relate to the analysis of polyclonal versus TCR transgenic T cells, or the route/method of antigen challenge.
In summary, we have demonstrated an important function for β1 integrin in the long-term maintenance of memory CD4 T cells in the bone marrow. Our findings reveal that β1 integrin expression is critical for steady-state maintenance of Ag-specific CD4 T cells in the bone marrow during the memory phase. Although we demonstrate significantly reduced numbers of CD4 T cells maintained in the bone marrow of β1−/− mice during the memory phase, this does not result in a global decrease in CD4 T cell memory survival or proliferative response upon re-challenge. Our results suggest that survival signals produced by sources other than the bone marrow, such as the stromal compartment of the spleen and lymph nodes (46), may be sufficient for long-term memory CD4 T cell survival.
ACKNOWLEDGMENTS
We thank Dr. M. Jenkins, Dr. M. Pepper, and A. Pagán for use of reagents and critical input, Dr. J. Mitchell and Dr. B. Burbach for discussion, and T. Lee, L. Yang and H. Nguyen for mouse genotyping and colony maintenance.
Footnotes
This work is supported by NIH grants AI031126 (Y.S.), NIH grant F30 DK082139 (C.C.D.) and NIH MSTP grant T32 GM008244 (C.C.D.). Y.S. is supported in part by the Harry Kay Chair in Biomedical Research at the University of Minnesota.
Abbreviations. CTG. Cell Tracker Green; CTO, Cell Tracker Orange; wt: wild-type
REFERENCES
- 1.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 2.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 3.Di Rosa F. T-lymphocyte interaction with stromal, bone and hematopoietic cells in the bone marrow. Immunol. Cell Biol. 2009;87:20–29. doi: 10.1038/icb.2008.84. [DOI] [PubMed] [Google Scholar]
- 4.Benner R, Meima F, van der Meulen GM. Antibody formation in mouse bone marrow. II. Evidence for a memory-dependent phenomenon. Cell. Immunol. 1974;13:95–106. doi: 10.1016/0008-8749(74)90230-5. [DOI] [PubMed] [Google Scholar]
- 5.Price PW, Cerny J. Characterization of CD4+ T cells in mouse bone marrow. I. Increased activated/memory phenotype and altered TCR Vβ repertoire. Eur. J. Immunol. 1999;29:1051–1056. doi: 10.1002/(SICI)1521-4141(199903)29:03<1051::AID-IMMU1051>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 6.Monteiro JP, Benjamin A, Costa ES, Barcinski MA, Bonomo A. Normal hematopoiesis is maintained by activated bone marrow CD4+ T cells. Blood. 2005;105:1484–1491. doi: 10.1182/blood-2004-07-2856. [DOI] [PubMed] [Google Scholar]
- 7.Letsch A, Knoedler M, Na IK, Kern F, Asemissen AM, Keilholz U, Loesch M, Thiel E, Volk HD, Scheibenbogen C. CMV-specific central memory T cells reside in bone marrow. Eur. J. Immunol. 2007;37:3063–3068. doi: 10.1002/eji.200636930. [DOI] [PubMed] [Google Scholar]
- 8.Feuerer M, Beckhove P, Bai L, Solomayer EF, Bastert G, Diel IJ, Pedain C, Oberniedermayr M, Schirrmacher V, Umansky V. Therapy of human tumors in NOD/SCID mice with patient-derived reactivated memory T cells from bone marrow. Nat. Med. 2001;7:452–458. doi: 10.1038/86523. [DOI] [PubMed] [Google Scholar]
- 9.Feuerer M, Rocha M, Bai L, Umansky V, Solomayer EF, Bastert G, Diel IJ, Schirrmacher V. Enrichment of memory T cells and other profound immunological changes in the bone marrow from untreated breast cancer patients. Int. J. Cancer. 2001;92:96–105. [PubMed] [Google Scholar]
- 10.Choi C, Witzens M, Bucur M, Feuerer M, Sommerfeldt N, Trojan A, Ho A, Schirrmacher V, Goldschmidt H, Beckhove P. Enrichment of functional CD8 memory T cells specific for MUC1 in bone marrow of patients with multiple myeloma. Blood. 2005;105:2132–2134. doi: 10.1182/blood-2004-01-0366. [DOI] [PubMed] [Google Scholar]
- 11.Di Rosa F, Pabst R. The bone marrow: a nest for migratory memory T cells. Trends Immunol. 2005;26:360–366. doi: 10.1016/j.it.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Di Rosa F, Santoni A. Bone marrow CD8 T cells are in a different activation state than those in lymphoid periphery. Eur. J. Immunol. 2002;32:1873–1880. doi: 10.1002/1521-4141(200207)32:7<1873::AID-IMMU1873>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 13.Parretta E, Cassese G, Barba P, Santoni A, Guardiola J, Di RF. CD8 cell division maintaining cytotoxic memory occurs predominantly in the bone marrow. J. Immunol. 2005;174:7654–7664. doi: 10.4049/jimmunol.174.12.7654. [DOI] [PubMed] [Google Scholar]
- 14.Becker TC, Coley SM, Wherry EJ, Ahmed R. Bone marrow is a preferred site for homeostatic proliferation of memory CD8 T cells. J. Immunol. 2005;174:1269–1273. doi: 10.4049/jimmunol.174.3.1269. [DOI] [PubMed] [Google Scholar]
- 15.Mazo IB, Honczarenko M, Leung H, Cavanagh LL, Bonasio R, Weninger W, Engelke K, Xia L, McEver RP, Koni PA, Silberstein LE, von Andrian UH. Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity. 2005;22:259–270. doi: 10.1016/j.immuni.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Nemoto Y, Kanai T, Makita S, Okamoto R, Totsuka T, Takeda K, Watanabe M. Bone marrow retaining colitogenic CD4+ T cells may be a pathogenic reservoir for chronic colitis. Gastroenterology. 2007;132:176–189. doi: 10.1053/j.gastro.2006.10.035. [DOI] [PubMed] [Google Scholar]
- 17.Tokoyoda K, Zehentmeier S, Hegazy AN, Albrecht I, Grun JR, Lohning M, Radbruch A. Professional memory CD4 + T lymphocytes preferentially reside and rest in the bone marrow. Immunity. 2009;5:721–730. doi: 10.1016/j.immuni.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J. Exp. Med. 2003;198:1807–1815. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. J. Exp. Med. 2003;198:1797–1806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pepper M, Linehan JL, Pagan AJ, Zell T, Dileepan T, Cleary PP, Jenkins MK. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat. Immunol. 2010;11:83–89. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, Flavell RA. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J. Exp. Med. 2001;193:741–754. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berlin-Rufenach C, Otto F, Mathies M, Westermann J, Owen MJ, Hamann A, Hogg N. Lymphocyte migration in lymphocyte function-associated antigen (LFA)-1-deficient mice. J. Exp. Med. 1999;189:1467–1478. doi: 10.1084/jem.189.9.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papayannopoulou T, Priestley GV, Nakamoto B, Zafiropoulos V, Scott LM. Molecular pathways in bone marrow homing: dominant role of α4β1 over β2-integrins and selectins. Blood. 2001;98:2403–2411. doi: 10.1182/blood.v98.8.2403. [DOI] [PubMed] [Google Scholar]
- 24.Ray SJ, Franki SN, Pierce RH, Dimitrova S, Koteliansky V, Sprague AG, Doherty PC, de Fougerolles AR, Topham DJ. The collagen binding α1β1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity. 2004;20:167–179. doi: 10.1016/s1074-7613(04)00021-4. [DOI] [PubMed] [Google Scholar]
- 25.Richter M, Ray SJ, Chapman TJ, Austin SJ, Rebhahn J, Mosmann TR, Gardner H, Kotelianski V, deFougerolles AR, Topham DJ. Collagen distribution and expression of collagen-binding α1β1 (VLA-1) and α2β1 (VLA-2) integrins on CD4 and CD8 T cells during influenza infection. J. Immunol. 2007;178:4506–4516. doi: 10.4049/jimmunol.178.7.4506. [DOI] [PubMed] [Google Scholar]
- 26.Chapman TJ, Topham DJ. Identification of a unique population of tissue-memory CD4+ T cells in the airways after influenza infection that is dependent on the integrin VLA-1. J. Immunol. 2010;184:3841–3849. doi: 10.4049/jimmunol.0902281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richter MV, Topham DJ. The α1β1 integrin and TNF receptor II protect airway CD8+ effector T cells from apoptosis during influenza infection. J. Immunol. 2007;179:5054–5063. doi: 10.4049/jimmunol.179.8.5054. [DOI] [PubMed] [Google Scholar]
- 28.Tokoyoda K, Hauser AE, Nakayama T, Radbruch A. Organization of immunological memory by bone marrow stroma. Nat. Rev. Immunol. 2010;10:193–200. doi: 10.1038/nri2727. [DOI] [PubMed] [Google Scholar]
- 29.DeNucci CC, Pagan AJ, Mitchell JS, Shimizu Y. Control of α4β7 integrin expression and CD4 T cell homing by the β1 integrin subunit. J. Immunol. 2010;184:2458–2467. doi: 10.4049/jimmunol.0902407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moon JJ, Chu HH, Hataye J, Pagan AJ, Pepper M, McLachlan JB, Zell T, Jenkins MK. Tracking epitope-specific T cells. Nat. Protoc. 2009;7:565–581. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bauer M, Brakebusch C, Coisne C, Sixt M, Wekerle H, Engelhardt B, Fassler R. β1 integrins differentially control extravasation of inflammatory cell subsets into the CNS during autoimmunity. Proc. Natl. Acad. Sci. USA. 2009;106:1920–1925. doi: 10.1073/pnas.0808909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat. Rev. Immunol. 2007;7:532–542. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- 33.Ekert PG, Silke J, Vaux DL. Caspase inhibitors. Cell Death Differ. 1999;6:1081–1086. doi: 10.1038/sj.cdd.4400594. [DOI] [PubMed] [Google Scholar]
- 34.Pabst R, Kaatz M, Westermann J. In situ labelling of bone marrow lymphocytes with fluorescein isothiocyanate for lymphocyte migration studies in pigs. Scand. J. Haematol. 1983;31:267–274. doi: 10.1111/j.1600-0609.1983.tb00651.x. [DOI] [PubMed] [Google Scholar]
- 35.Pabst R, Miyasaka M, Dudler L. Numbers and phenotype of lymphocytes emigrating from sheep bone marrow after in situ labelling with fluorescein isothiocyanate. Immunology. 1986;59:217–222. [PMC free article] [PubMed] [Google Scholar]
- 36.Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, Lefrancois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20:551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 37.Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS, Fraser KA, Webby RJ, Brinkmann V, Butcher EC, Newell KA, Ahmed R. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 2010;207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeNucci CC, Mitchell JS, Shimizu Y. Integrin function in T-cell homing to lymphoid and nonlymphoid sites: getting there and staying there. Crit. Rev. Immunol. 2009;29:87–109. doi: 10.1615/critrevimmunol.v29.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carlow DA, Gossens K, Naus S, Veerman KM, Seo W, Ziltener HJ. PSGL-1 function in immunity and steady state homeostasis. Immunol. Rev. 2009;230:75–96. doi: 10.1111/j.1600-065X.2009.00797.x. [DOI] [PubMed] [Google Scholar]
- 40.MacLeod MK, Kappler JW, Marrack P. Memory CD4 T cells: generation, reactivation and re-assignment. Immunology. 2010;130:10–15. doi: 10.1111/j.1365-2567.2010.03260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruegg C, Postigo AA, Sikorski EE, Butcher EC, Pytela R, Erle DJ. Role of integrin α4β7/α4βP in lymphocyte adherence to fibronectin and VCAM-1 and in homotypic cell clustering. J. Cell Biol. 1992;117:179–189. doi: 10.1083/jcb.117.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katayama Y, Hidalgo A, Peired A, Frenette PS. Integrin α4β7 and its counterreceptor MAdCAM-1 contribute to hematopoietic progenitor recruitment into bone marrow following transplantation. Blood. 2004;104:2020–2026. doi: 10.1182/blood-2003-12-4157. [DOI] [PubMed] [Google Scholar]
- 43.Goldstein I, Ben-Horin S, Li J, Bank I, Jiang H, Chess L. Expression of the α1β1 integrin, VLA-1, marks a distinct subset of human CD4+ memory T cells. J. Clin. Invest. 2003;112:1444–1454. doi: 10.1172/JCI19607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conrad C, Boyman O, Tonel G, Tun-Kyi A, Laggner U, de FA, Kotelianski V, Gardner H, Nestle FO. α1β1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat. Med. 2007;13:836–842. doi: 10.1038/nm1605. [DOI] [PubMed] [Google Scholar]
- 45.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 46.Link A, Vogt TK, Favre S, Britschgi MR, cha-Orbea H, Hinz B, Cyster JG, Luther SA. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat. Immunol. 2007;8:1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 47.Reinhardt RL, Bullard DC, Weaver CT, Jenkins MK. Preferential accumulation of antigen-specific effector CD4 T cells at an antigen injection site involves CD62E-dependent migration but not local proliferation. J. Exp. Med. 2003;197:751–762. doi: 10.1084/jem.20021690. [DOI] [PMC free article] [PubMed] [Google Scholar]