Abstract
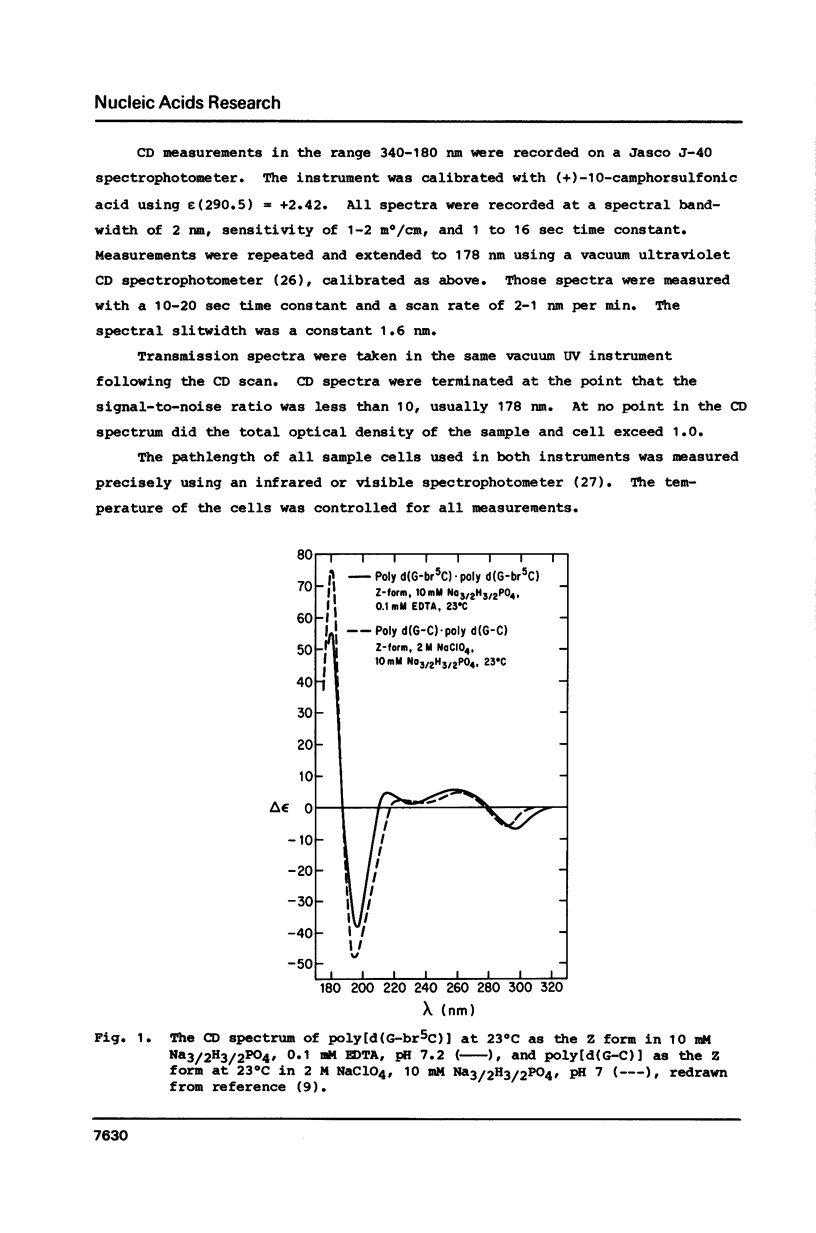
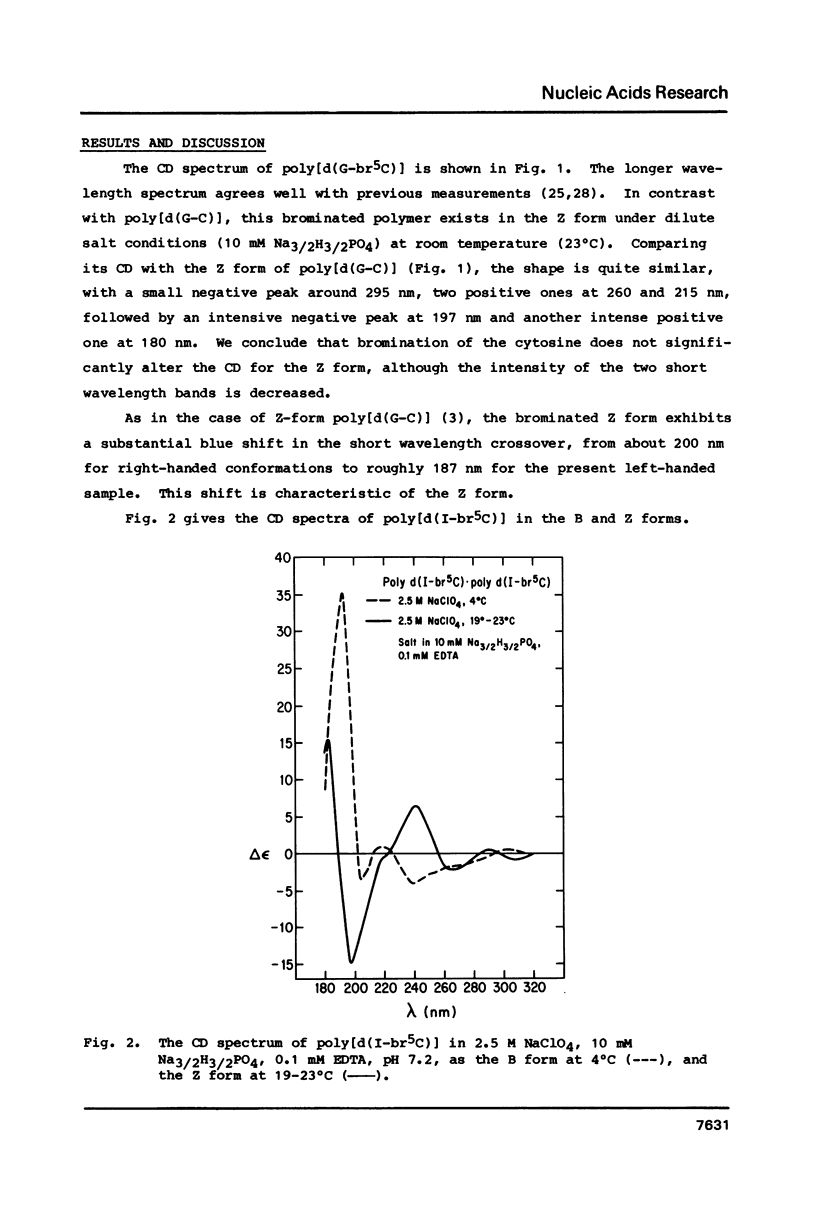
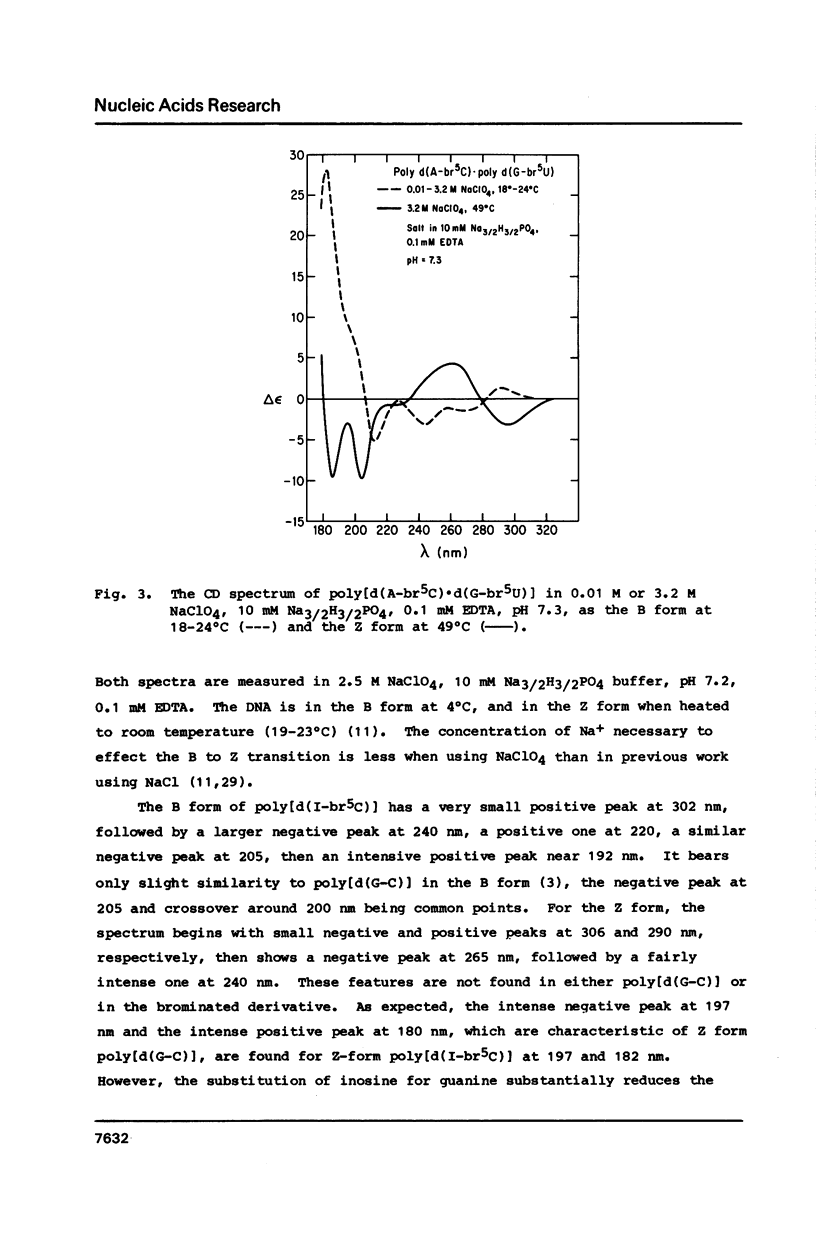
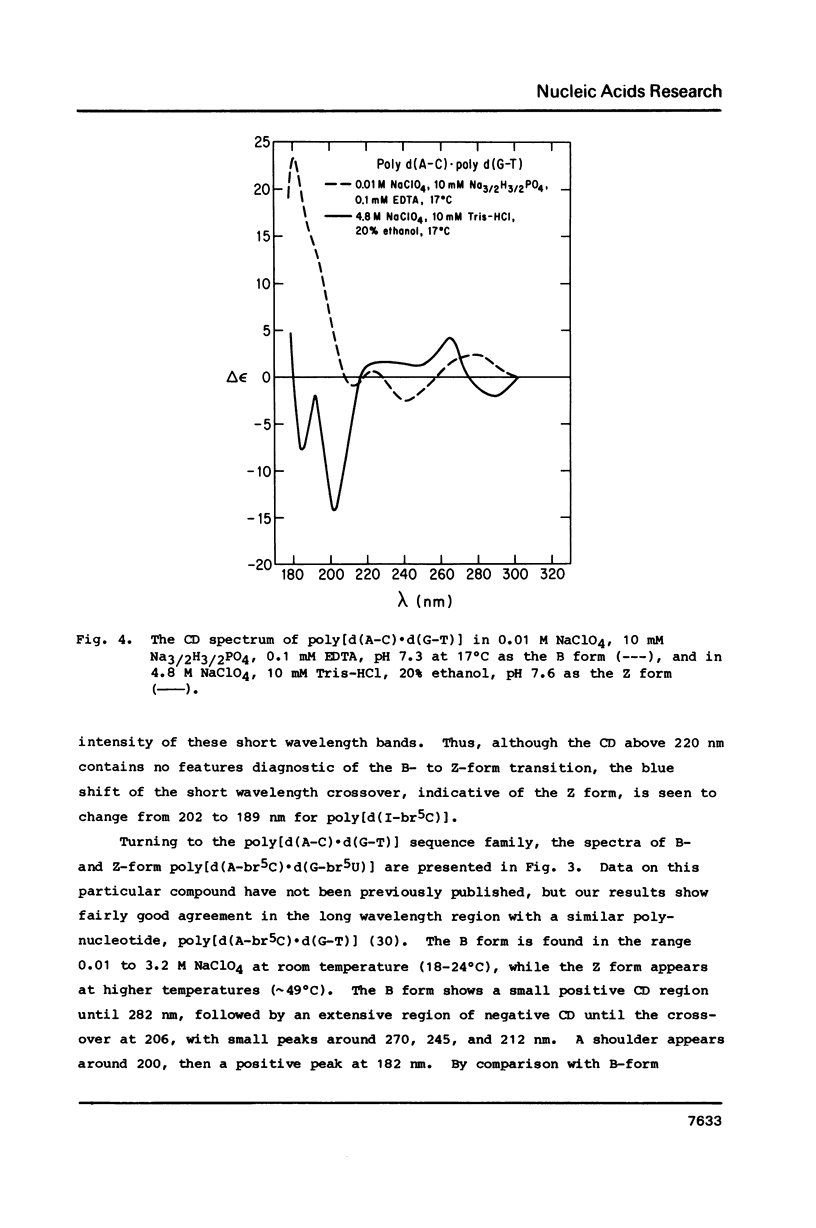
Circular dichroism spectra are extended into the vacuum UV to about 178 nm for four polydeoxynucleotides of various sequences capable of assuming the left-handed Z form. It is found that each of these polymers, including those with brominated bases and those with the four different bases, have a characteristic negative feature at short wavelengths when in the Z form. In contrast, the B form only has a positive band between 180 and 200 nm. Furthermore, a blue shift of the short wavelength crossover is diagnostic of the B- to Z-form transition for all polymers studied so far. These results confirm that poly[d(A-C).d(G-T)] can assume the Z form in solution at low concentration.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam S., Bourtayre P., Liquier J., Taillandier E. Interaction of transition metal ions with Z form poly d(A-C).poly d(G-T) and poly d(A-T) studied by I.R. spectroscopy. Nucleic Acids Res. 1986 Apr 25;14(8):3501–3513. doi: 10.1093/nar/14.8.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott S., Chandrasekaran R., Birdsall D. L., Leslie A. G., Ratliff R. L. Left-handed DNA helices. Nature. 1980 Feb 21;283(5749):743–745. doi: 10.1038/283743a0. [DOI] [PubMed] [Google Scholar]
- Evans D. H., Lee J. S., Morgan A. R., Olsen R. K. A method for the specific inhibition of poly[d(A-T)] synthesis using the A-T specific quinoxaline antibiotic TANDEM. Can J Biochem. 1982 Feb;60(2):131–136. doi: 10.1139/o82-018. [DOI] [PubMed] [Google Scholar]
- Fodor S. P., Starr P. A., Spiro T. G. Raman spectroscopic elucidation of DNA backbone conformations for poly(dG-dT).poly(dA-dC) and poly(dA-dT).poly(dA-dT) in CsF solution. Biopolymers. 1985 Aug;24(8):1493–1500. doi: 10.1002/bip.360240806. [DOI] [PubMed] [Google Scholar]
- Hartmann B., Pilet J., Ptak M., Ramstein J., Malfoy B., Leng M. The B reversible Z transition of poly(dI-br5dC).poly(dI-br5dC). A quantitative description of the Z form dynamic structure. Nucleic Acids Res. 1982 May 25;10(10):3261–3277. doi: 10.1093/nar/10.10.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins B. G., Wartell R. M., Alderfer J. L. Conformational properties of poly[d(G-T)].poly[d(C-A)] and poly[d(A-T)] in low- and high-salt solutions: NMR and laser Raman analysis. Biopolymers. 1986 May;25(5):823–849. doi: 10.1002/bip.360250507. [DOI] [PubMed] [Google Scholar]
- Johnson W. C., Jr A circular dichroism spectrometer for the vacuum ultraviolet. Rev Sci Instrum. 1971 Sep;42(9):1283–1286. doi: 10.1063/1.1685367. [DOI] [PubMed] [Google Scholar]
- Leng M. Left-handed Z-DNA. Biochim Biophys Acta. 1985 Aug 21;825(4):339–344. doi: 10.1016/0167-4781(85)90059-4. [DOI] [PubMed] [Google Scholar]
- Leslie A. G., Arnott S., Chandrasekaran R., Ratliff R. L. Polymorphism of DNA double helices. J Mol Biol. 1980 Oct 15;143(1):49–72. doi: 10.1016/0022-2836(80)90124-2. [DOI] [PubMed] [Google Scholar]
- Malfoy B., Rousseau N., Leng M. Interaction between antibodies to Z-form deoxyribonucleic acid and double-stranded polynucleotides. Biochemistry. 1982 Oct 26;21(22):5463–5467. doi: 10.1021/bi00265a013. [DOI] [PubMed] [Google Scholar]
- McIntosh L. P., Grieger I., Eckstein F., Zarling D. A., van de Sande J. H., Jovin T. M. Left-handed helical conformation of poly[d(A-m5C).d(G-T)]. Nature. 1983 Jul 7;304(5921):83–86. doi: 10.1038/304083a0. [DOI] [PubMed] [Google Scholar]
- Möller A., Nordheim A., Kozlowski S. A., Patel D. J., Rich A. Bromination stabilizes poly(dG-dC) in the Z-DNA form under low-salt conditions. Biochemistry. 1984 Jan 3;23(1):54–62. doi: 10.1021/bi00296a009. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Rich A. The sequence (dC-dA)n X (dG-dT)n forms left-handed Z-DNA in negatively supercoiled plasmids. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1821–1825. doi: 10.1073/pnas.80.7.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J., Canuel L. L., Pohl F. M. "Alternating B-DNA" conformation for the oligo(dG-dC) duplex in high-salt solution. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2508–2511. doi: 10.1073/pnas.76.6.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Riazance J. H., Baase W. A., Johnson W. C., Jr, Hall K., Cruz P., Tinoco I., Jr Evidence for Z-form RNA by vacuum UV circular dichroism. Nucleic Acids Res. 1985 Jul 11;13(13):4983–4989. doi: 10.1093/nar/13.13.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Sutherland J. C., Griffin K. P., Keck P. C., Takacs P. Z. Z-DNA: vacuum ultraviolet circular dichroism. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4801–4804. doi: 10.1073/pnas.78.8.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland J. C., Lin B. H., Mugavero J., Trunk J., Tomasz M., Santella R., Marky L., Breslauer K. J. Vacuum ultraviolet circular dichroism of double stranded nucleic acids. Photochem Photobiol. 1986 Sep;44(3):295–301. doi: 10.1111/j.1751-1097.1986.tb04667.x. [DOI] [PubMed] [Google Scholar]
- Taboury J. A., Taillandier E. Right-handed and left-handed helices of poly(dA-dC) X (dG-dT). Nucleic Acids Res. 1985 Jun 25;13(12):4469–4483. doi: 10.1093/nar/13.12.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taillandier E., Taboury J. A., Adam S., Liquier J. Left-handed helical structure of poly[d(A-C)].poly[d(G-T)] studied by infrared spectroscopy. Biochemistry. 1984 Nov 20;23(24):5703–5706. doi: 10.1021/bi00319a007. [DOI] [PubMed] [Google Scholar]
- Vorlíckovă M., Kypr J., Stokrová S., Sponar J. A Z-like form of poly(dA-dC).poly(dG-dT) in solution? Nucleic Acids Res. 1982 Feb 11;10(3):1071–1080. doi: 10.1093/nar/10.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woisard A., Fazakerley G. V. Ultrapolymorphic DNA: B, A, Z, and Z* conformations of poly(dA-dC).poly(dG-dT). Biochemistry. 1986 May 6;25(9):2672–2676. doi: 10.1021/bi00357a057. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Tymen S., Marck C., Guschlbauer W. Conformational transitions of poly(dA-dC).poly(dG-dT) induced by high salt or in ethanolic solution. Nucleic Acids Res. 1982 Feb 11;10(3):1081–1091. doi: 10.1093/nar/10.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S. B. The three-dimensional structure of DNA. Annu Rev Biochem. 1982;51:395–427. doi: 10.1146/annurev.bi.51.070182.002143. [DOI] [PubMed] [Google Scholar]



