Abstract
We examined interactions between children’s physiological activity across two systems, the hypothalamic-pituitary-adrenal (HPA)-axis and the parasympathetic nervous system (PNS), as predictors of child-reported internalizing symptoms (depression, anxiety). HPA activity was indexed by baseline salivary cortisol, and PNS activity was indexed by baseline respiratory sinus arrhythmia (RSA). Study 1 consisted of 57 children (54% girls; M age = 8.81 years ±.34), and Study 2 included 219 children (51% girls; M age = 9.31 years ±.79). Cortisol interacted with RSA to explain unique variance in children’s internalizing symptoms. Across the two studies, children with higher cortisol levels in conjunction with higher RSA levels tended to exhibit the lowest levels of depression and anxiety symptoms. Findings demonstrate that contemporaneous consideration of physiological activity across multiple systems can advance understanding of internalizing symptoms in children.
Keywords: cortisol, respiratory sinus arrhythmia, hypothalamic-pituitary-adrenal-axis, parasympathetic nervous system, depression, anxiety
1. Introduction
Internalizing symptoms are common among children and may forecast persistent psychological distress [1]. To illuminate the development of internalizing disorders, it is critical to investigate vulnerability and protective factors. The developmental psychopathology framework advocates multi-level approaches that include physiological measurement to advance understanding of adjustment [2]. Individual differences in children’s physiological functioning are well-documented correlates of internalizing problems [3]. However, research has only recently considered interactions between physiological systems as predictors of children’s adjustment, despite the fact that physiological systems do not operate independently.
We examined children’s internalizing symptoms in relation to interactions between indices of two biologically distinct, yet interrelated systems involved in physiological activity and regulation: The parasympathetic nervous system (PNS) and the hypothalamic-pituitary-adrenal (HPA)-axis. PNS activity was indexed by basal respiratory sinus arrhythmia (RSA), and HPA activity was indexed by afternoon basal cortisol levels (cortisol). Whereas several studies have investigated interactions between sympathetic nervous system (SNS) activity and cortisol (e.g., [4]), no studies have reported interactions between PNS activity and cortisol as predictors of children’s adjustment. Basal (i.e., resting) RSA is an important and commonly used index of PNS activity. Baseline RSA reflects the activity of the PNS at rest and perhaps the ability to sustain attention, regulate emotion, and engage in social communication[5, 6]. Lower basal RSA is associated with a wide range of internalizing problems[3] and interacts with individual and family risk to exacerbate child maladjustment [7]. Similarly, basal cortisol is an important and frequently used measure of HPA activity[8] and higher levels are associated with internalizing symptoms [9, 10].
Since basal RSA [11] and cortisol [12] have been associated individually with children’s internalizing symptoms, assessing their conjoint or interactive contributions is an important next step.
1.1. Parasympathetic activity and RSA
The PNS is the branch of the autonomic nervous system (ANS) that has an inhibitory influence at the cardiovascular level and helps to maintain homeostasis [5, 13]. The vagal nerve is the efferent source of parasympathetic influence on the heart [14]. Parasympathetic-vagal influence is also afferent, carrying information back to the central nervous system (CNS), particularly to the brain stem (medulla) and emotion centers of the brain (e.g., amygdala and hypothalamus) [15]. PNS-vagal influence on the heart is commonly measured via RSA, the natural ebb and flow of heart rate that accompanies inhalation and exhalation [16].
Higher resting PNS activity is a marker of emotion regulatory abilities and the potential to engage adaptively with the environment [5]. Consistent with this conceptualization, lower resting RSA has been viewed as a nonspecific indicator and risk factor for several forms of psychopathology. Empirical evidence supports relations between lower basal RSA and higher Internalizing (depressive and anxiety) symptoms in children[11, 17]
1.2. HPA-axis activity and cortisol
The HPA-axis is a component of the neuroendocrine stress response system and the biological “fight or flight” response, which contrast with the “rest and digest” functions of the PNS [6]. The HPA response is cued by the limbic system and hypothalamus, which stimulates the pituitary gland to release adrenocorticotropic hormones that activate cortisol production by the adrenal gland. The end-product of this process, cortisol, can be assessed through salivary analysis [18]. Resting Cortisol levels show a diurnal pattern that peak shortly after waking and are lowest in the evening [19]. Because individual differences in basal cortisol are more pronounced during the afternoon [20], we examined children’s cortisol in the afternoon and focus our review primarily on studies in which cortisol was examined in the afternoon or evening.
Excessively low or high basal cortisol levels have been linked with stress and psychological maladjustment [19, 21]. Generally, lower basal cortisol levels are associated with increased externalizing problems [22], whereas higher basal cortisol levels are associated with increased internalizing symptoms. Of relevance, children’s and adolescents’ depressive symptoms are related to higher afternoon or evening cortisol levels [23]. Findings linking resting cortisol and anxiety in children are somewhat less consistent, yet results tend to support positive associations between the two [12].
1.3. Multiple systems approach
Although studies have investigated relations between either resting RSA or cortisol and children’s internalizing symptoms, physiological systems do not operate in isolation [21]. Both physiological systems (HPA, PNS) are governed by the hypothalamus and its reciprocal connections to limbic and lower order structures (e.g., the brain stem; [24, 25]). Just as the PNS and the sympathetic nervous system (SNS) tend to be inversely activated, the PNS and HPA tend to be inversely coordinated, such that higher resting RSA is linked with lower resting cortisol levels and vice versa [6, 26]. The functional integration of these stress response systems in the CNS is quite complex (see [25], for an excellent review).
A small number of studies have investigated interactions between physiological systems to predict child adjustment. For example, the association between SNS activity and externalizing outcomes varied as a function of individual differences in children’s basal RSA [27, 28]. Some studies have investigated interactions between SNS activity indices and cortisol (indicated by resting levels[4] and reactivity [29]) as predictors of child outcomes. The highest levels of internalizing symptoms were predicted among children with higher levels of cortisol in conjunction with higher levels of either skin conductance or salivary alpha-amylase [4]. Although these studies did not examine interactions between indices of PNS and HPA activity, they highlight the potential significance of this research avenue.
1.4. The current study
We examined interactions between basal cortisol and RSA levels as predictors of children’s anxiety and depression symptoms. Literature findings generally support negative associations between RSA and internalizing symptoms and positive associations between cortisol and internalizing symptoms (especially depression). Thus, we expected the highest levels of internalizing symptoms among children with lower RSA in conjunction with higher cortisol levels. We used two diverse and independent samples of children in middle childhood to examine our research question. Child-reported symptoms of anxiety and depression were assessed to explore possible differences in physiological correlates and interactions. For a conservative test of hypotheses, we controlled for potential confounds including demographic characteristics (i.e., child age, gender, ethnicity, SES), externalizing symptoms, and timing of cortisol assessment.
2. Materials and methods
2.1. Participants
Children were recruited for two independent studies from a southeastern US public school system. Study 1 participants were 57 third-graders (54% girls; M age = 8.81 years; SD = .34) who had cortisol data from the first wave of a larger study of 176 (same as those who participated in El-Sheikh et al.[30]. Based on information provided by schools, we contacted families who fit our inclusion criteria: 29% participated. Inclusion criteria served to reduce potential confounds and included children living in a two-parent household, having good physical health based on parent report during an initial screening phone interview (i.e., no chronic or acute physical illness), and no diagnoses of mental retardation, learning disabilities, Attention Deficit-Hyperactivity Disorder, or a history of diagnosed sleep problems); depression and anxiety symptoms or diagnoses were not exclusion criteria. Families represented the complete range of possible economic status (SES; M =38.56,SD = 11.47, Range =18 – 63) backgrounds [31] and ranged from level 1 to 5 [level 1or 2 (unskilled or semiskilled laborers; 23% ), level 3 (skilled labor; 31%), and level 4 or 5 (professional; 46%)] and income [5% (≤ $20,000), 24%($20,000 – $35,000), 30% ($35,000 – $50,000), 26% $50,000–$75,000), 15%( ≥> $75,000)] levels. Educational attainment of mothers and fathers also varied widely: Mothers’ education (12% - partial high school, 30% - high school, 36% - partial college, 18% - college graduation, 4%-graduate professional training); Fathers’ education (11%-partial high school, 46%-high school, 25%-partial college, 14%-college graduation, 4%-graduate professional training ). Families represented the ethnic composition of the community: 32% African- and 68% European-American. We oversampled to include African and European Americans across a wide range of SES.
Study 2 utilized similar recruitment strategies and inclusion/exclusion criteria as in Study 1; 37% of the families who qualified for participation did so. Study 2 participants were 219 second- and third-graders from the second wave of a larger longitudinal study [32]. Children in the sample (51% girls) had a mean age of 9.31 years (SD = .79). The sample was diverse in relation to ethnicity (36% African- and 64% European-Americans) and SES ([level 1or 2 (unskilled or semiskilled laborers; 24%), level 3 (skilled labor; 34%), and level 4 or 5 (professional; 42%)]; M = 37.73, SD = 10.03, Range 19 – 66; [31]). Family income was as follows: 16% ≤ $20,000, 24% $20,000 – 35,000, 21% $35,000–50,000, 21% $50,000–75,000, and 18% ≥ $75,000). Education level of mothers was as follows: 7% - partial high school, 29% - high school, 45% - partial college, 13% - college graduation, and 6%-graduate professional training). Fathers’ education: 10% - partial high school, 31% - high school, 41% - partial college, 14% - college graduation, and 4% -graduate professional training).
2.2. Procedure
Study 1 and 2 procedures are identical unless otherwise specified. Children and their parents visited our University-based laboratory; studies were approved by the University’s Institutional Review Board for the protection of human subjects. Informed consent and assent forms were obtained. Saliva samples were collected 20 minutes following arrival at the lab [average start time was 3:15 p.m. (SD = 2:57) for Study1 and 1:08 p.m. (SD = 3:06 hrs) for Study 2]. Children then participated in a psychophysiological session to assess their basal RSA.
2.3. RSA data acquisition and reduction
RSA reflects rhythmic fluctuations in heart period that accompany inhalation and exhalation [33]. RSA was assessed following the standard procedure by Berntson et al. [13] whereby two electrocardiography (ECG) electrodes were placed on each rib cage at a distance of 10–15 cm below the armpits. A third electrode was placed in the center of the chest to ground the signal. Data were collected by equipment and software from the James Long Company including the Snap-Master Data Acquisition System (HEM, Corporation, Southfield, MI); see [34] for more detail about data collection and reduction.
R-waves were identified in the ECG with an automated algorithm, and an interactive graphical program was used for manual correction of missing or misidentified R-waves. The R-wave times were then converted to inter-beat intervals (IBIs) and resampled into equal time intervals of 125 ms. The peak-to-valley method was used to quantify RSA [13]. All units are reported in seconds. Baseline RSA (RSA-B1) was obtained for 3-min after a 10-min adaptation period following saliva collection; the child was seated quietly and asked to relax after the physiological sensors were attached. The second baseline (3 min) was obtained after 10 min from the end of the first baseline in Study 1, and after 6 min for Study 2. RSA-B1 and -B2 were highly correlated in Studies 1(r = .80, p < .001) and 2 (r = .87, p < .001). For parsimony, and consistent with the literature at large, we report analyses based on the initial baseline. However, we also conducted analyses based on RSA-B2; we comment on the one finding in which results varied slightly between Baseline 1 and Baseline 2 (i.e., results are similar when either baseline is used in analyses unless otherwise indicated).
2.4. Determination of salivary cortisol
Saliva sampling was conducted using the passive drool technique in which children are asked to imagine chewing their favorite food and then gently drooling through a drinking straw into a collection vial (see[35]). Saliva samples were placed in the freezer immediately upon collection, and frozen to −20°C. Samples were stored frozen until they were shipped overnight on dry ice to the Behavioral Endocrinology Laboratory at Pennsylvania State University. The samples were stored at −80°C until assay. For testing the saliva, these samples were then brought to room temperature and centrifuged at 3,000 rpm for 15 minutes, following which the clear top-phase of the sample was pipetted into appropriate test tubes. The saliva samples were assayed for salivary cortisol with a highly-sensitive enzyme immunoassay US FDA (510k) cleared for use as an in vitro diagnostic measure of adrenal function (Salimetrics, State College, PA). The test used 25 μl of saliva (for singlet determinations). Further, the test had a lower limit of sensitivity of .007 μg/dl, and a range of sensitivity from .007 to 1.8 μg/dl, and average intra-and inter-assay coefficients of variation of less than 5% and 10%.
2.5. Study 1 internalizing symptoms
Internalizing symptoms include behaviors such as withdrawal, anxiety, inhibition, and depression ([36]; depression and anxiety were examined in our studies. Children completed the well-established Children’s Depression Inventory (CDI; [37]) and the Revised Children’s Manifest Anxiety Scale (RCMAS; [38]) via an interview with a researcher. The CDI assesses overall levels of depressive symptoms and has acceptable reliability and discriminant and concurrent validity [37]. Example CDI items include: 1) “I am sad once in a while, I am sad many times, or I am sad all the time”; 2) “Things bother me all the time, things bother me many times, or things bother me once in a while.” The participant was asked to select one of three possible options per item; items are rated on a scale from 0 (Absence of symptom) to 2 (Definite symptom) with a total scale of 27 items (alpha= .89). In the present study, CDI scores ranged from 0 to 22 (skewness statistic = 1.13). The RCMAS is frequently used to measure child anxiety and has demonstrated test-retest reliability [39] and concurrent validity [40]. Example RCMAS items include “I worry a lot of the time” or “I am afraid of a lot of things”. The participant was asked to rate each item on a “Yes/No” response scale. The total RCMAS score had 28 items assessing physiological anxiety, social concerns, and worry. Internal consistency was high (α = .90). RCMAS scores ranged from 0 to 26 (skewness statistic = .65). Four percent of children had clinically significant levels of depression on the CDI (score ≥ 20), and 4% had moderately problematic levels of anxiety on the RCMAS (T score ≥ 60).
2.6. Study 2 internalizing symptoms
Children completed the CDI, RCMAS, and the Trauma Symptoms Checklist for Children (TSCC) subscales of Depression and Anxiety via interview. Alphas for the CDI and RCMAS were .85 and .86, respectively. CDI scores ranged from 0 to 32, and RCMAS scores ranged from 0 to 26; skewness statistics were .38 and 1.72, respectively. The TSCC 9-item Depression subscale measured the extent to which children experience feelings of sadness, unhappiness, depressive cognitions, etc., and the 9-item Anxiety subscale reflected the extent to which children experience generalized anxiety, hyperarousal, worry, and fears. Example items of the TSCC Depression scale include: 1) “Feeling lonely”; 2) “Feeling sad or unhappy” and example items from the Anxiety subscale include: 1) “Feeling afraid that something bad might happen”; 2) “Feeling nervous or jumpy inside.” Each item is rated according to its frequency of occurrence using a four point scale ranging from 0 (“never”) to 3 (“almost all of the time”). Both subscales have established reliability and validity[41]; alpha with the present sample is .81 for Depression and .79 for Anxiety. Depression T-scores ranged from 32 to 83, and Anxiety T-scores ranged from 32 to 86; skewness statistics were 1.47 and .85, respectively. Seven percent and 4% of children had clinically significant levels of CDI- or TSCC-based (T score ≥ 65) depression symptoms, respectively; 5% and 6% of children had moderately problematic/clinical levels of anxiety symptoms indexed by the RCMAS or TSCC, respectively.
2.7. Control variables
Age, gender, ethnicity, SES, start time of saliva (i.e., cortisol) collection, and externalizing behavior were controlled. Externalizing behavior problems refer to a “series of behavior problems that are manifested in children’s outward behavior” ([36], p. 93) and include “destructive behavior, hostile defiance, impulsivity, temper tantrums, and low frustration tolerance” ([42], pg. 24). Mothers provided demographic information; information about the family’s SES was collected using mothers’ reports on the Hollingshead Index [31]. The SES raw score is a continuous variable derived from mothers’ and fathers’ education and occupation and was highly correlated with measures of income in Study1 (r = .50, p < .001) and 2 (r = .59, p < .001). Mothers also reported on children’s externalizing behaviors using the Personality Inventory for Children-2 (PIC2; [43]). The externalizing problem scale included measures of aggression, impulsivity, disruptive behavior, delinquency, and noncompliance. The PIC2 has well-established reliability and validity [43]; α = .85 and .88 for Study 1 and 2, respectively.
2.8. Plan of analysis
Path analyses were conducted using Mplus software, version 5.21 [44], which utilizes Full Information Maximum Likelihood (FIML) to handle missing data. All path models were tested as nested models with the controls added in the first step; the substantive predictors (cortisol and RSA) were added next, followed by the interaction among the substantive predictors in the third and final step of the nested model (Cortisol X RSA). The Δχ2 test was used to assess the significance of change in model fit as predictors were added. A separate model was conducted for each outcome: CDI depression, RCMAS anxiety, in Study 1, and CDI depression, RCMAS anxiety, and TSCC anxiety and depression in Study 2, leading to a total of 6 and 8 models in Studies 1 and 2, respectively. Separate analyses were conducted for each anxiety or depression measure for a better elucidation of their relations with RSA and cortisol. Similarly, research questions were examined independently with RSA during each baseline for replication testing1.
All control variables and substantive predictors were mean-centered prior to model fitting [45]. Cortisol was negatively skewed in both studies, and a log10 transformation was conducted. In Study 2, RSA-B1 and RSA-B2 were moderately positively skewed, and a square root transformation was conducted; when the deviation from normality is pronounced, a logarithmic transformation (versus a square root transform) is more effective [46].
We used the recommended approach for identifying outliers (multiple screening approaches including bivariate scatterplots, standardized residuals, extreme standardized values in predictors, and extreme mahalanobis distance, a measure of multivariate outliers based on the distance of a case from the centroid of the remaining cases; [46]). No outliers were found in Study 1; six outliers were found in Study 2 and corresponding values were deleted. Consistent with standard practice for assessing interactions, each significant interaction was further explored using the Aiken and West approach [45] of plotting regression lines linking cortisol and internalizing symptoms at high values (i.e., +1 SD) and low values (i.e., −1 SD) of RSA. Preacher, Curran, and Bauer’s [47] interaction utility was used to plot the interactions using estimates obtained from the fitted models. The significance of slopes was also tested by calculating the t-statistic from the estimate and standard error of the slope.
2.9 Missing values analysis
Although analyses were performed with Mplus version 5.2, which handles missing data with Full Information Maximum Likelihood ( FIML), we also performed additional preliminary analysis of missing data to examine whether the data were Missing Completely at Random (MCAR). A prerequisite for any analyses involving FIML is that it should be MCAR; when data are MCAR, the nature of missing values does not relate to the data values. The SPSS version 17 Missing Values Analysis and Analysis of Missing Pattern procedures were used to examine the nature of missingness. Study 1 had no missing data; hence, all missing data procedures were only examined for Study 2. Twenty six percent (57 of 219 cases) had missing data on at least one variable. The percent of missing data across each variable ranged from 0.9% to 11.9%; children’s age, gender, and ethnicity (0.9%) had the lowest percentage of missing data, and RSA-B1 (11.9%) and RSA-B2 (11%) had the highest percent of missing data. Based on Little’s MCAR test (χ2 = 187.18; df = 192, p = .58), results indicate that the data are missing completely at random and analyses involving FIML are appropriate.
3. Study 1 results
3.1. Preliminary analyses
Means and standard deviations of key Study 1 variables are presented in Table 1, and correlations are presented in Table 2. There were no significant correlations between either cortisol or RSA and internalizing outcomes. Cortisol was negatively associated with RSA. Anxiety was negatively associated with time of saliva collection; this effect was controlled in the analyses. Few associations were reported between the controls and key study variables. There was no association between cortisol and time of saliva collection. Age had a marginal negative association with cortisol (r = −.26, p < .10) and RCMAS Anxiety (r = −.23, p < .10). Socioeconomic status (SES) was negatively associated with cortisol (r = −.27, p < .05) and time of cortisol collection (min) was positively associated with RCMAS Anxiety (r = .24, p < .10).
Table 1.
Means and Standard Deviations among Study 1 and 2 Variables
| Study 1 | Study2 | |||
|---|---|---|---|---|
| M | SD | M | SD | |
| Cortisol (μg/dl) | 0.15 | 0.14 | 0.09 | 0.07 |
| RSA-B1 (seconds) | 0.13 | 0.06 | 0.16 | 0.08 |
| RSA-B2 (seconds) | 0.12 | 0.07 | 0.15 | 0.08 |
| Depression (CDI) | 6.07 | 5.66 | 7.15 | 6.67 |
| Anxiety (RCMAS) | 9.56 | 6.87 | 9.84 | 7.14 |
| Anxiety (TSCC)a | - | - | 47.38 | 11.10 |
| Depression (TSCC)a | - | - | 43.33 | 9.77 |
Note. RSA Respiratory Sinus Arrhythmia; CDI Child Depression Inventory; RCMAS Revised Child Manifest Anxiety Checklist; TSCC Trauma Symptoms Checklist for Children;
The TSCC questionnaire was only used in study 2.
Table 2.
Correlations among Study 1 Variables
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| 1. Cortisol | -- | |||
| 2. RSA-B1 | −.31* | -- | ||
| 3. RSA-B2 | −.26† | .80*** | -- | |
| 4. Depression (CDI) | .18 | .09 | −.05 | -- |
| 5. Anxiety (RCMAS) | .11 | −.04 | −.17 | .69** |
Note. N = 57.
RSA Respiratory Sinus Arrhythmia; CDI Child Depression Inventory; RCMAS Revised Child Manifest Anxiety Checklist
p < .10;
p ≤ .05;
p < .01;
p < .001.
3.2. Cortisol x RSA interactions as predictors of internalizing symptoms
In Table 3, we report the results of the path analytic models for Study 1; all interactions reported pertain to RSA-B1 and the reader should assume that analyses using RSA-B2 yielded the same pattern of results unless otherwise indicated. As shown in Table 3, RSA and cortisol were not significant main effects, yet interacted to predict depression and anxiety symptoms.
Table 3.
Interactions between Cortisol and RSA as Moderators of Internalizing Symptoms
| Step and Variables | R2 | Δ R2 | β | X2(df) | Δ X2 (Δdf) | |
|---|---|---|---|---|---|---|
|
Depressive Symptoms (CDI)-Study 1 | ||||||
| Step 1: | Control variables | .151 | .151 | 9.117(4) | ||
| Step 2: | Cortisol | .166 | .015 | .151 | 8.014(2) | 1.103(2) |
| RSA | .101 | |||||
| Step 3: | Cortisol X RSA | .292 | .126 | −.402** | .073(1) | 7.941(1)** 9.044(3)* |
|
Anxiety Symptoms (RCMAS)-Study 1 | ||||||
| Step 1: | Control variables | .196 | .196 | 11.470(4) | ||
| Step 2: | Cortisol | .204 | .008 | −.072 | 10.852(2) | 0.618(2) |
| RSA | −.099 | |||||
| Step 3: | Cortisol X RSA | .344 | .140 | −.433*** | .073(1) | 10.779(1)** 11.397(3)** |
|
Depressive Symptoms (CDI)-Study 2 | ||||||
| Step 1: | Control variables | .029 | .029 | 6.172(4) | ||
| Step 2: | Cortisol | .041 | .012 | −.038 | 3.996(2) | 2.176(2) |
| RSA | −.102 | |||||
| Step 3: | Cortisol X RSA | .058 | .017 | −.135† | .875(1) | 3.121(1) † 5.297(3) |
|
Anxiety Symptoms (TSCC)-Study 2 | ||||||
| Step 1: | Control variables | .030 | .030 | 8.858(4) | ||
| Step 2: | Cortisol | .055 | .025 | −.079 | 4.173(2) | 4.685(2) |
| RSA-B2 | −.133† | |||||
| Step 3: | Cortisol X RSA-B2 | .077 | .022 | −.144† | .829(1) | 3.344(1) † 8.029(3)* |
Note. In the first path analytic model, child age, gender, ethnicity, SES, start time of cortisol intake, and externalizing behavior (collectively referred to as control variables) were entered. In the second model, main effects were entered: cortisol and RSA. For each model, the ΔX2 (Δdf) for step 3 was calculated based on the initial model as well as the previous model. Unit of measurement for salivary cortisol is μg/dl and the unit of measurement of RSA is seconds. RSA Respiratory Sinus Arrhythmia; CDI Child Depression Inventory; RCMAS Revised Child Manifest Anxiety Checklist; TSCC Trauma Symptoms Checklist for Children.
p < .10;
p ≤ .05;
p < .01;
p < .001.
3.2.1. Depression symptoms
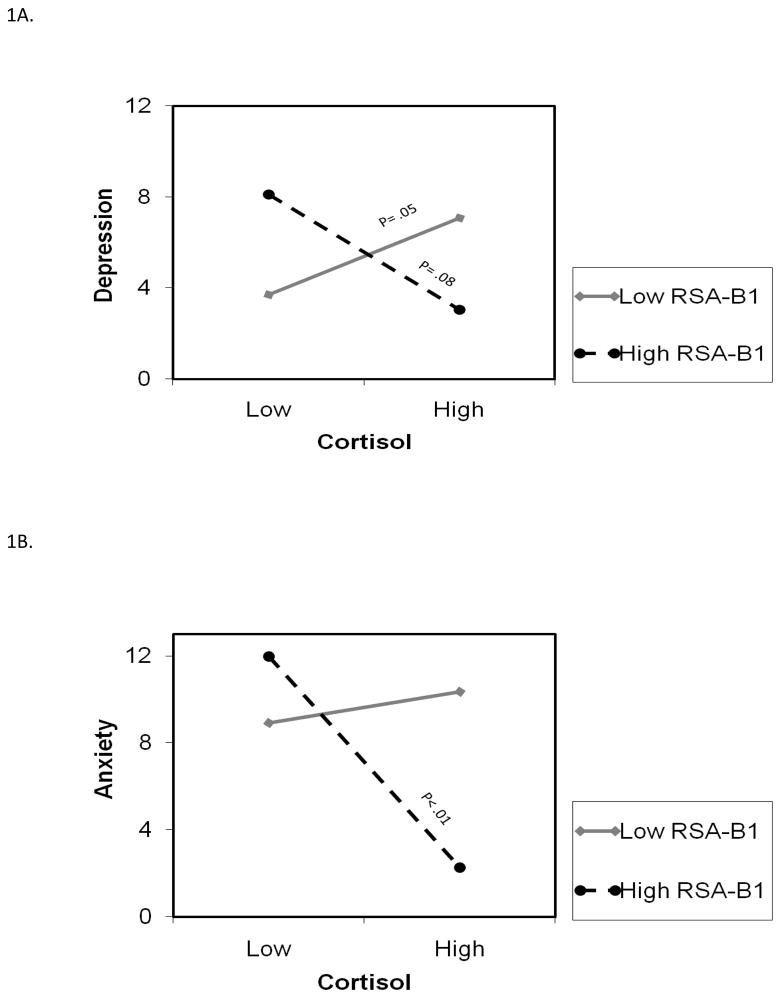
After accounting for control variables and main effects, cortisol interacted with RSA and accounted for 13% of the unique variance in depressive symptoms (total model R2 was .29; Table 3). This interaction is depicted in Figure 1A. Recall that High and Low levels of either RSA or cortisol refer to +/− 1SD from the mean, with corresponding values of .29 and .01 for cortisol (μg/dl) and .19 (sec) and .07 for RSA. Figure 1A shows the positive and significant association between cortisol levels and depression symptoms for children with lower RSA. The highest levels of symptoms were predicted for children with either (a) lower cortisol levels in conjunction with higher RSA, or (b) higher cortisol levels accompanied by lower RSA levels.
Figure 1.
Figure 1A. RSA-B1 moderates the relation between cortisol and children’s depressive symptoms (Study1). High and Low values for either cortisol or RSA represent values at +/− 1 SD from the Mean, with corresponding values of .29 and .01 for cortisol (μg/dl) and .19 (sec) and .07 for RSA.
Figure 1B. RSA-B1 moderates the relation between cortisol and children’s anxiety symptoms (Study1). High and Low values for either cortisol or RSA represent values at +/− 1 SD from the Mean, with corresponding values of .29 and .01 for cortisol (μg/dl) and .19 (sec) and .07 for RSA.
3.2.2. Anxiety symptoms
The interaction between cortisol and RSA accounted for 14% of unique variance in children’s anxiety symptoms (see Table 3); total model variance explained was 34%. As shown in Figure 1B, there was a significant negative association between cortisol levels and anxiety symptoms for children with higher levels of RSA. For children with higher levels of cortisol, anxiety symptoms were substantially lower for children with higher versus lower levels of RSA.
4. Study 2 results
4.1. Preliminary analysis
In Table 1, we present the means and SDs for Study 2 variables; correlations are presented in Table 4. Cortisol was not associated with RSA, depression, or anxiety symptoms. RSA was negatively associated with anxiety and depressive symptoms. Further, cortisol was related to time of saliva collection (r = −.18, p < .05); the latter was controlled in all analyses. Associations were also noted between the controls and main study indicators. Age was associated with CDI Depression (r = −.12, p < .10), RCMAS (r = −.17, p < .05) and TSCC anxiety (r = −.12, p < .10) symptoms. Further, gender was related to cortisol levels (r = .16, p < .05), with higher levels observed for boys. Ethnicity was associated with RSA (r = .16, p < .05), indicating higher levels for European Americans. Externalizing Behavior was associated higher RCMAS Anxiety (r = .14, p < .05).
Table 4.
Correlations among Study 2 Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. Cortisol | -- | ||||||
| 2. RSA-B1 | .05 | -- | |||||
| 3. RSA-B2 | .07 | .87*** | -- | ||||
| 4. Depression (CDI) | .00 | −.12† | −.07 | -- | |||
| 5. Anxiety (RCMAS) | −.04 | −.17* | −.15* | .44** | -- | ||
| 6. Anxiety (TSCC) | −.09 | −.17* | −.13† | .49** | .62** | -- | |
| 7. Depression (TSCC) | −.06 | −.13† | −.10 | .51** | .54** | .65** | -- |
Note. N = 219.
RSA Respiratory Sinus Arrhythmia; CDI Child Depression Inventory; RCMAS Revised Child Manifest Anxiety Checklist; TSCC Trauma Symptoms Checklist for Children
p < .10;
p ≤ .05;
p < .01;
p < .001
4.2. Cortisol x RSA interactions as statistical predictors of internalizing symptoms
Results for the path analytic models for the interactions are reported in Table 3. Although no main effects were found, cortisol and RSA-B interacted to predict children’s adjustment.
4.2.1. Depression symptoms
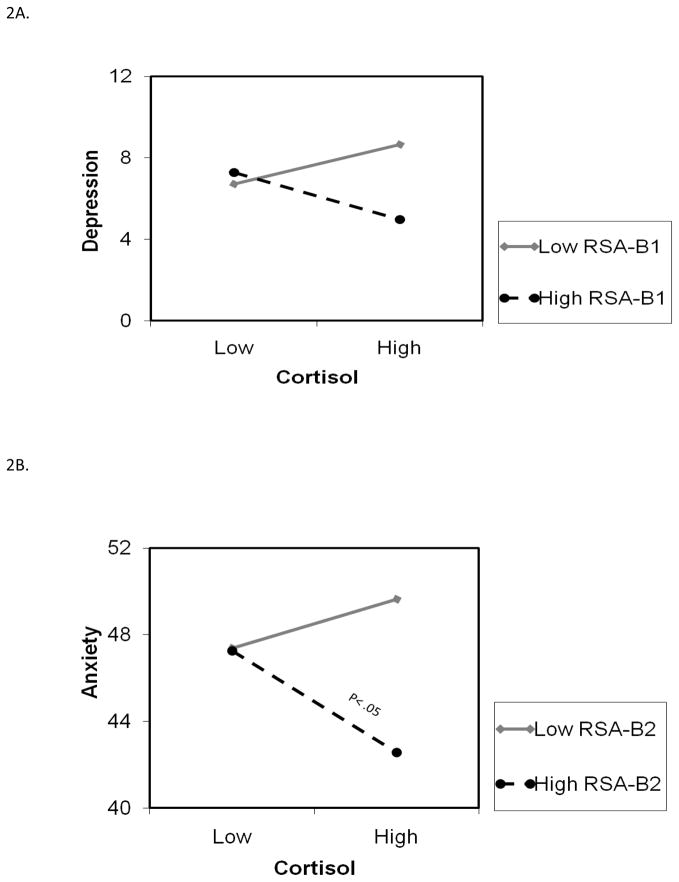
Cortisol and RSA interacted to predict CDI depression symptoms marginally, and accounted for 2% of unique variance; the total model variance was 6% (see Table 3). As shown in Figure 2, the lowest levels of depression symptoms were observed for children with higher levels of both cortisol and RSA. Conversely, the highest levels of depression symptoms were found for children with higher levels of cortisol in conjunction with lower levels of RSA. Cortisol levels (μg/dl) indicative of High and Low values on the graph correspond with .16 and .02, respectively; RSA levels are .24 and .08 at +1 and −1 SDs, respectively. Analyses with RSA-B2 yielded a statistically significant interaction effect with cortisol, and the pattern of effects was almost identical to that observed in Figure 2.
Figure 2.
Figure 2A. RSA-B1 moderates the relation between cortisol and children’s depressive symptoms (Study2). High and Low values for either cortisol or RSA represent values at +/− 1 SD from the Mean. Cortisol levels (μg/dl) indicative of High and Low values on the graph correspond with .16 and .02, respectively; RSA levels are .24 and .08 at +1 and -1 SDs, respectively.
Figure 2B. RSA-B2 moderates the relation between cortisol and children’s anxiety symptoms (Study2). High and Low values for either cortisol or RSA represent values at +/− 1 SD from the Mean. Cortisol levels (μg/dl) indicative of High and Low values on the graph correspond with .16 and .02, respectively; RSA levels are .24 and .08 at +1 and -1 SDs, respectively.
4.2.2. Anxiety symptoms
While effects were not significant when RSA-B1 was used in analyses, cortisol interacted with RSA-B2 and marginally predicted TSCC anxiety symptoms, accounting for 2% of the unique variance in TSCC anxiety symptoms; the total model variance explained was 8% (refer to Table 3). As shown in Figure 2B, there was a significantly negative association between cortisol and anxiety symptoms at higher levels of RSA. The lowest levels of anxiety symptoms were predicted for children who had higher levels of both cortisol and RSA-B2. Conversely, higher anxiety levels were predicted for children with higher cortisol levels in conjunction with lower RSA-B2.
5. Discussion
We investigated interactions between resting levels of cortisol and RSA as predictors of children’s anxiety and depression symptoms. Physiological systems do not operate independently and assessments of activity across multiple stress response systems, including interactions between systems, are critical for understanding children’s adaptation [2]. Interactions between cortisol and RSA consistently predicted children’s internalizing symptoms, such that children with higher baseline cortisol and higher baseline RSA reported the lowest levels of anxiety and depression.
The present findings suggest that investigating interactions between indices of HPA axis and PNS activity may explain more variance in internalizing symptoms than these systems explain alone or additively. The HPA axis and PNS are coordinated via the Central Autonomic Network (e.g., CAN including hypothalamic, amygdala, and prefrontal components), which regulates both PNS activity (directly) and HPA activity (via the amygdala; [6]). Although there are individual differences in the extent to which the PNS and HPA are inversely coordinated, PNS activation generally appears to inhibit sympathetic-excitatory stress responses, including cortisol excretion, through a negative feedback mechanism. Indeed, higher resting heart rate variability has been linked with lower HPA activity in adults [6]. Likewise, Blair et al. [26] found that children with higher RSA had lower basal cortisol. Basal cortisol and RSA were also inversely correlated in Study 1.
Interaction effects revealed greater variability in internalizing symptoms at higher levels of baseline cortisol compared to lower levels of baseline cortisol. At higher levels of baseline cortisol, the level of internalizing symptoms varied by baseline RSA. In partial support of hypotheses, higher RSA (compared to lower RSA) was generally protective against internalizing symptoms among children with elevated cortisol, but not protective against internalizing symptoms among children with lower cortisol. Indeed, children with lower cortisol and higher RSA (not consistent with hypotheses) and children with higher cortisol and lower RSA (consistent with hypotheses) reported relatively high levels of internalizing symptoms.
Higher cortisol and lower RSA should reflect the highest levels of general physiological arousal (e.g., heart rate), a common symptom of internalizing symptoms, especially anxiety (e.g., [48]). Physiological arousal may operate as an internal cue of threat that draws attention inward and exacerbates self-focused fears among anxious children, or that diminishes perceived control among depressed children [49]. This physiological pattern may also reflect a mismatch between high arousal (e.g., high cortisol) and poor emotion regulation (e.g., low RSA). Physiological over-arousal and dysregulation are not only symptoms of internalizing problems, but may also exacerbate self-focused fears and diminish feelings of control and competence. Results of the present study parallel Blair et al’s., [26] finding of higher cortisol levels and lower RSA levels among preschool children with both high approach and high avoidance motivations, which itself is consistent with the anxious-solitary profile often associated with psychosocial maladjustment [51].
In contrast to expectations, children with lower baseline cortisol and higher baseline RSA did not report the lowest levels of internalizing symptoms. Rather, children with higher cortisol in conjunction with higher RSA levels reported the lowest levels of depression and anxiety. It is not clear why higher RSA was particularly protective against internalizing symptoms in the context of higher cortisol rather than lower cortisol. Further research is necessary to replicate and explain these findings. Given that the association between cortisol levels and maladjustment may be nonlinear (hypo- and hyper-cortisol levels have been linked with maladjustment) [19], it would be informative to consider whether the lowest internalizing symptoms are observed among children with high RSA and moderate (rather than high or low) cortisol levels. Including assessments of subjective emotional and cognitive processes would also help explicate relations between these physiological parameters and internalizing symptoms.
Several study limitations warrant consideration. Cortisol level was assessed based on a single measurement 20 minutes after the children’s arrival in the lab, which may not constitute a true resting baseline. Cortisol levels follow a diurnal rhythm, typically reaching highest levels in the early morning and declining during the day. As such, multiple measurements throughout the day would provide a more reliable estimate of HPA activity. Most saliva assessments were conducted in the afternoon, although the variability of time of saliva collection ranged from morning to early evening across both studies. (i.e., 8:00 a.m. to 5:00 p.m.). To address this, the time of saliva collection was controlled in all analyses to limit the influence of time on the association between cortisol levels and internalizing symptoms. Furthermore, the limitations were counterbalanced by some replication of interactions across two independent samples. It is widely acknowledged that replication of interaction effects, especially with the same pattern of findings, is unusual if the results are due to chance. Thus, even though complete replication of findings across the two samples was not attained, the fact that cortisol and RSA interacted in similar ways in the prediction of depression symptoms across the two studies strengthens the confidence in findings.
Several additional design limitations could be addressed by future research. For example, the two-parent, community sample may not generalize to other family arrangements or clinical populations. In addition, although we suggested that higher RSA may “protect” against the risk for internalizing symptoms typically associated with elevated cortisol, the cross-sectional design of the present study cannot establish directionality or causality among variables. Testing hypotheses of the current study with longitudinal data would help clarify the direction of effects among study variables. Finally, it would be interesting to examine the interaction between cortisol and RSA as a predictor of other forms of behavioral or psychological maladjustment, such as externalizing behaviors.
Despite these limitations, the present study provides new evidence that the association linking baseline cortisol levels with internalizing symptoms may be conditional upon baseline RSA (or vice versa). Notably, results suggest that a physiological index of adaptive regulation (i.e., RSA) may protect against elevated internalizing symptoms in the context of a well-documented physiological risk factors for internalizing symptoms (i.e., higher cortisol levels).
Acknowledgments
This research was supported in part by an Alabama Agricultural Experiment Station/Lindsey Foundation Grant no. ALA080-001, a National Institute of Health Grant R01-HD046795, and National Science Foundation Grants 0339115. We are grateful to the students and laboratory staff of our research laboratory, especially Lori Staton and Bridget Wingo for their assistance with data collection. We also thank school personnel, and the children and parents who participated.
Footnotes
Disclosure
No conflicts of interest.
We also examined the interactions between cortisol and RSA-B using an averaged measure of RSA-B1 and RSA-B2. The results of these analyses were similar to those reported in the text reflecting separate examinations of RSA-B1 and RSA-B2. Specifically, in Study 1, there was a significant interaction between cortisol and RSA-B for depression and anxiety. In Study 2, there was a significant interaction between cortisol and RSA-B for depression as the outcome, and a marginally significant interaction for anxiety. Follow up examinations of these analyses revealed that in Study 1, the association between cortisol and depression was marginally negatively associated at high RSA and marginally positive at low levels of RSA; there was a negative association between cortisol and anxiety at high RSA. In Study 2, there was a negative association between cortisol and anxiety and depression at high levels of RSA. The graphs illustrated that patterns of effects are similar when the two baseline RSA measures were composited.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. 2003;60:837–44. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- 2.Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children’s behavior: Advantages of a multisystem approach. J Dev Behav Pediatr. 2002;23:102–13. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Beauchaine T. Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Dev Psychopathol. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- 4.El-Sheikh M, Erath SA, Buckhalt JA, Granger DA, Mize J. Cortisol and children’s adjustment: The moderating role of sympathetic nervous system activity. J Abnorm Child Psychol. 2008;36:601–11. doi: 10.1007/s10802-007-9204-6. [DOI] [PubMed] [Google Scholar]
- 5.Porges SW. The polyvagal perspective. Biol Psychol. 2007;74:116–43. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thayer JF, Sternberg E. Beyond heart rate variability: Vagal regulation of allostatic systems. Ann N Y Acad Sci. 2006;1088:361–72. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- 7.El-Sheikh M, Erath SA, Keller PS. Children’s sleep and adjustment: the moderating role of vagal regulation. J Sleep Res. 2007;16:396–405. doi: 10.1111/j.1365-2869.2007.00618.x. [DOI] [PubMed] [Google Scholar]
- 8.Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Dev Psychopathol. 2001;13:515–38. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- 9.Smider NA, Essex MJ, Kalin NH, Buss KA, Klein MH, Davidson RJ, Goldsmith HH. Salivary cortisol as a predictor of socioemotional adjustment during kindergarten: A prospective study. Child Dev. 2002;73:75–92. doi: 10.1111/1467-8624.00393. [DOI] [PubMed] [Google Scholar]
- 10.Goodyer IM, Park RJ, Netherton CM, Herbery J. Possible role of cortisol and dehydroepiandrosterone in human development and psychopathology. The British Journal of Psychiatry. 2001;179:243–9. doi: 10.1192/bjp.179.3.243. [DOI] [PubMed] [Google Scholar]
- 11.Calkins SD, Blandon AY, Williford AP, Keane SP. Biological, behavioral, and relational levels of resilience in the context of risk for early childhood behavior problems. Dev Psychopathol. 2007;19:675–700. doi: 10.1017/S095457940700034X. [DOI] [PubMed] [Google Scholar]
- 12.Van den Bergh BRH, Van Calster B, Puissant SP, Van Huffel S. Self-reported symptoms of depressed mood, trait anxiety and aggressive behavior in post-pubertal adolescents: Associations with diurnal cortisol profiles. Horm Behav. 2008;54:253–7. doi: 10.1016/j.yhbeh.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Berntson GG, Bigge JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–48. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 14.Levy MN, Warner MR. Parasympathetic effects of cardiac function. In: Armour JA, Ardell JL, editors. Neurocardiology. New York: Oxford University Press; 1994. pp. 77–94. [Google Scholar]
- 15.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Autonomic Neuroscience: Basic and Clinical. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 16.Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biol Psychol. 2007;74:263–85. doi: 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Hinnant JB, El-Sheikh M. Children’s Externalizing and Internalizing Symptoms over Time: The Role of Individual Differences in Patterns of RSA Responding. J Abnorm Child Psychol. 2009;37:1049–61. doi: 10.1007/s10802-009-9341-1. [DOI] [PubMed] [Google Scholar]
- 18.Granger DA, Schwartz EB, Booth A, Arentz M. Salivary testosterone determination in studies of child health and development. Horm Behav. 1999;35:18–27. doi: 10.1006/hbeh.1998.1492. [DOI] [PubMed] [Google Scholar]
- 19.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 20.Stone AA, Schwartz JE, Smyth J, Kirschbaum C, Cohen S, Hellhammer D, et al. Individual differences in the diurnal cycle of salivary free cortisol: A replication of flattened cycles for some individuals. Psychoneuroendocrinology. 2001;26:295–306. doi: 10.1016/s0306-4530(00)00057-3. [DOI] [PubMed] [Google Scholar]
- 21.Lopez NL, Vazquez DM, Olson SL. An integrative approach to the neurophysiological substrates of social withdrawal and aggression. Dev Psychopathol. 2004;16:69–93. doi: 10.1017/s0954579404044414. [DOI] [PubMed] [Google Scholar]
- 22.Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Dev Psychopathol. 2005;17:167–84. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- 23.Van den Bergh BRH, Van Calster B. Diurnal cortisol profiles and evening cortisol in post-pubertal adolescents scoring high on the Children’s Depression Inventory. Psychoneuroendocrinology. 2009;34:791–4. doi: 10.1016/j.psyneuen.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Bao o-M, Meynen G, Swaab DF. The stress system in depression and neurodegeneraton: Focus on the human hypothalamus. Brain Research Reviews. 2008;57:531–53. doi: 10.1016/j.brainresrev.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blair C, Peters R, Granger D. Physiological and neuropsychological correlates of approach/withdrawal tendencies in preschool: Further examination of the behavioral inhibition system/behavioral activation system scales for young children. Dev Psychobiol. 2004;45:113–24. doi: 10.1002/dev.20022. [DOI] [PubMed] [Google Scholar]
- 27.Gordis EB, Feres N, Olezeski CL, Rabkin AL, Trickett PK. Skin conductance reactivity and respiratory sinus arrhythmia among maltreated and comparison youth: Relations with aggressive behavior. J Pediatr Psychol. 2010;35:547–58. doi: 10.1093/jpepsy/jsp113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keller PS, El-Sheikh M. Salivary alpha-amylase as a longitudinal predictor of children’s externalizing symptoms: Respiratory sinus arrhythmia as a moderator of effects. Psychoneuroendocrinology. 2009;34:633–43. doi: 10.1016/j.psyneuen.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 29.Gordis EB, Granger DA, Susman EJ, Trickett PK. Asymmetry between salivary cortisol and α-amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31:976–87. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 30.El-Sheikh M, Buckhalt JA, Keller PS, Granger DA. Children’s Objective and Subjective Sleep Disruptions: Links With Afternoon Cortisol Levels. Health Psychol. 2008;27:26–33. doi: 10.1037/0278-6133.27.1.26. [DOI] [PubMed] [Google Scholar]
- 31.Hollingshead AB. Four factor index of social status. 1975. [Google Scholar]
- 32.El-Sheikh M, Keiley MK, Hinnant JB. Developmental trajectories of skin conductance level in middle childhood: Sex, race, and externalizing behavior problems as predictors of growth. Biol Psychol. 2010;83:116–24. doi: 10.1016/j.biopsycho.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grossman P, Karemaker J, Wieling W. Prediction of tonic parasympathetic cardiac control using respiratory sinus arrhythmia: The need for respiratory control. Psychophysiology. 1991;28:201–16. doi: 10.1111/j.1469-8986.1991.tb00412.x. [DOI] [PubMed] [Google Scholar]
- 34.El-Sheikh M. Stability of respiratory sinus arrhythmia in children and young adolescents: A longitudinal examination. Dev Psychobiol. 2005;46:66–74. doi: 10.1002/dev.20036. [DOI] [PubMed] [Google Scholar]
- 35.Granger DA, Kivlighan KT, Fortunato C, Harmon AG, Hibel LC, Schwartz EB, Whembolua GL. Integration of salivary biomarkers into developmental and behaviorally-oriented research: Problems and solutions for collecting specimens. Physiol Behav. 2007;92:583–90. doi: 10.1016/j.physbeh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Liu J. Childhood Externalizing Behavior: Theory and Implications. J Child Adolesc Psychiatr Nurs. 2004;17:93–103. doi: 10.1111/j.1744-6171.2004.tb00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kovacs M. The Children’s Depression Inventory (CDI): A self-rated depression scale for school-aged youngsters. University of Pittsburg School of Medicine; 1983. [Google Scholar]
- 38.Reynolds CR, Richmond BO. What I think and feel: A revised measure of children’s manifest anxiety. J Abnorm Child Psychol. 1978;6:271–80. doi: 10.1007/BF00919131. [DOI] [PubMed] [Google Scholar]
- 39.Wisniewski JJ, Mulick JA, Genshaft JL, Coury DL. Test-retest reliability of the Revised Children’s Manifest Anxiety Scale. Percept Mot Skills. 1987;65:67–70. doi: 10.2466/pms.1987.65.1.67. [DOI] [PubMed] [Google Scholar]
- 40.James EM, Reynolds CR, Dunbar J. Self-report instruments. In: Ollendick TH, King NJ, Yule W, editors. International handbook of phobic and anxiety disorders in children and adolescents. New York: Springer; 1994. pp. 317–30. [Google Scholar]
- 41.Briere J. Trauma Sympton Checklist for Children (TSCC): Professional Manual. Odessa, FL: Psychological Assessment Resources; 1996. [Google Scholar]
- 42.Denham SA, Workman E, Cole PM, Weissbrod C, Kendziora KT, Zahn Waxler C. Prediction of externalizing behavior problems from early to middle childhood: The role of parental socialization and emotion expression. Dev Psychopathol. 2000;12:23–45. doi: 10.1017/s0954579400001024. [DOI] [PubMed] [Google Scholar]
- 43.Lachar D, Gruber CP. Personality Inventory for Children. 2. Los Angeles, CA: Western Psychological Services; 2001. (PIC-2) [Google Scholar]
- 44.Muthen LK, Muthen BO. Mplus User’s Guide. 5. Los Angeles, CA: Muthen & Muthen; 2007. [Google Scholar]
- 45.Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- 46.Tabachnick BG, Fidell LS. Using Multivariate Statistics. 5. Boston, MA: Allyn & Bacon; 2007. [Google Scholar]
- 47.Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31:437–48. [Google Scholar]
- 48.Weems CF, Zakem AH, Costa NM, Cannon MF, Watts SE. Physiological response and childhood anxiety: Association with symptoms of anxiety disorders and cognitive bias. J Clin Child Adolesc Psychol. 2005;34:712–23. doi: 10.1207/s15374424jccp3404_13. [DOI] [PubMed] [Google Scholar]
- 49.Vasey MW, Daleiden EL. Information-processing pathways to cognitive interference in childhood. In: Sarason IG, Pierce GR, Sarason BR, editors. Cognitive interference: Theories, methods, and findings. Hillsdale, NJ: Erlbaum; 1996. pp. 117–38. [Google Scholar]
- 50.Hinnant JB, El-Sheikh M. Children’s externalizing and internalizing symptoms over time: The role of individual differences in patterns of RSA responding. J Abnorm Child Psychol. 2009;37:1049–61. doi: 10.1007/s10802-009-9341-1. [DOI] [PubMed] [Google Scholar]
- 51.Gazelle H, Rudolph KD. Moving toward and moving away from the world: Social approach and avoidance trajectories in anxious solitary youth. Child Dev. 2004;75:829–49. doi: 10.1111/j.1467-8624.2004.00709.x. [DOI] [PubMed] [Google Scholar]