Abstract
Rats raised in an enriched condition (EC) are less sensitive to the locomotor effects of stimulant drugs than rats raised in an impoverished condition (IC). Methylphenidate (MPD), a primary pharmacotherapy for attention-deficit/hyperactivity disorder, has abuse potential. This study determined whether environmental enrichment differentially altered the effects of MPD on locomotor activity and dopamine (DA) transporter (DAT) function. Acute and repeated MPD (3 or 10 mg/kg, s.c.) increased locomotion in EC, IC and social condition (SC) rats; however, EC rats showed a blunted response to repeated MPD (3 mg/kg). The maximal velocity (Vmax) of [3H]DA uptake in the presence of the combination of phorbol 12-myristate 13-acetate, a protein kinase C (PKC) activator and okadaic acid, a protein phosphatase inhibitor was decreased in EC and IC rats by 68% and 40%, respectively, indicating that DAT in prefrontal cortex (PFC) is more sensitive to PKC-mediated down-regulation in EC rats. Acute MPD (10 mg/kg) administration decreased the Vmax of [3H]DA uptake in PFC and striatum in EC rats, but not in IC rats. Furthermore, [3H]WIN 35,428 binding density was decreased in PFC of EC and IC rats, and in striatum of EC rats given repeated MPD (10 mg/kg). These results demonstrate that environmental enrichment modulates DAT dynamics in PFC. However, since the change in DAT function was observed only following the high dose of MPH (10 mg/kg), the attenuated locomotor response to repeated MPD (3 mg/kg) in EC rats is not likely due to a specific DAT alteration in the brain regions examined.
Keywords: dopamine transporter, environmental enrichment, methylphenidate, prefrontal cortex, protein kinase C, behavioral sensitization
1. Introduction
Preclinical animal models have shown that environmental factors are important determinants of individual differences in drug abuse vulnerability. Rats raised in an enriched condition (EC) consisting of exposure to novel objects and social cohorts during development exhibit reduced basal locomotor activity compared to rats raised in an impoverished condition (IC) with no access to novel objects or social cohorts [1–3]. EC rats are also more sensitive to the acute locomotor and rewarding properties of amphetamine, yet show decreased amphetamine-induced sensitization and amphetamine self-administration relative to IC rats [1, 4–6]. These changes in sensitivity to the abuse-related effects of amphetamine and other drugs are mediated, at least in part, by differential modulation of dopamine (DA) activity produced by these rearing conditions [2, 5, 7, 8].
In addition to drug abuse vulnerability, environmental conditions also can modulate individual differences in impulsivity, which has been linked to drug abuse [9–15]. Although environmental enrichment increased impulsivity relative to isolation rearing in one report [15], two other studies found that EC rats were less impulsive than IC rats [11, 14]. A study by Perry et al. [11] also demonstrated that d-amphetamine increased impulsive choice in EC rats, yet decreased impulsive choice in IC rats. However, impulsivity is a multifaceted construct [13, 14, 16], and it is possible that environmental enrichment may alter distinct aspects of impulsivity differently. In the study by Hellemans et al. [15], enrichment rearing did not alter motor impulsivity on the go/no-go task, yet decreased impulsivity on a delayed reinforcement task. Thus, it appears that rearing conditions can modulate baseline levels of some, but not all, forms of impulsivity, as well as the effects of psychostimulants on impulsive choice. Overall, individuals with attention-deficit hyperactivity disorder (ADHD) tend to show greater levels of impulsivity, which are typically reduced by administration of stimulant medications [17–19]; specifically, it has been argued that deficient response inhibition is a particularly important aspect of impulsivity that contributes to the elevated rates of substance abuse in this population [20]. Based on differences in the ability of stimulants to reduce impulsivity between EC and IC rats, similar to the effects of these drugs in ADHD patients, further preclinical investigation of the neurobiology of these drug effects in rats reared in different environments is warranted.
Methylphenidate (MPD) is a widely used and effective treatment for ADHD [21, 22]. MPD produces effects typical of psychostimulant drugs, including abuse liability [23, 24]. MPD acts primarily as an indirect DA agonist by binding to the extracellular domain of the DA transporter (DAT) to block DA uptake into the presynaptic terminal [25], resulting in an increase in the concentration of extracellular DA [26–28]. This effect is dependent upon ongoing neuronal activity, as reduction of extracellular calcium or administration of tetrodotoxin blocks the MPD-induced increase in DA efflux [29]. This mechanism is in contrast to amphetamine-type drugs that release DA directly by acting as substrates for uptake through the DAT [30]. Once in the terminal, these drugs inhibit monoamine oxidase and interact directly with vesicular-bound DA to promote DA release [30, 31]. There is, however, evidence that MPD is able to alter the intracellular distribution of readily-releasable DA, based on results showing that MPD alters the distribution of vesicles from synaptosomal membranes to the cytoplasm [32, 33].
As is the case with most psychostimulants, acute administration of MPD produces hyperactivity in rodents that is augmented with repeated administration [34–37]. Previous studies show that acute administration of 2.5 mg/kg of MPD increases locomotor activity [38, 39] and induces behavioral sensitization with repeated administration [40, 41] in standard-reared rats. Our previous work using standard-reared rats has shown also that repeated administration of 3 mg/kg of MPD produces significant sensitization in adolescent rats, whereas repeated administration of 10 mg/kg of MPD induces sensitization in adult rats [42]. Interestingly, the prefrontal cortex (PFC) appears to be involved in MPD-induced sensitization, as lesioning the PFC blocks induction of sensitization to MPD [41]. This region is also known to play a prominent role in the pathology of ADHD, and is an important mediator of the therapeutic actions of MPD [34–37]. Specifically, clinically relevant doses of MPD that enhance cognitive function in rats preferentially enhance DA and norepinephrine (NE) efflux in PFC relative to limbic structures [43]. Since DA reuptake in PFC is accomplished by both DAT and the norepinephrine transporter (NET) [44, 45], it is possible that therapeutic efficacy and/or the sensitization-inducing effects of MPD are linked to alterations in NE as well as DA transmission in PFC.
Similarly, the ability of environment enrichment during development to reduce drug self-administration and impulsivity is linked to altered DAT function and expression in PFC [2, 46]. For instance, EC rats have a lower maximal velocity of [3H]DA uptake and DAT cell surface expression in medial PFC compared to IC rats [2, 46]. While the exact biochemical mechanisms underlying these differences are not clear, the dynamic regulation of DAT function is under the control of complex processes involving phosphorylation, protein-protein interaction, substrate pretreatment, and interactions with presynaptic receptors [47]. Many of these processes are regulated by protein kinase C (PKC), as acute PKC activation decreases DAT function and cell surface expression [48–50], leading to reduced DA uptake. In contrast, several studies also reported that down-regulation of DAT function is under a PKC-independent mechanism [51, 52]. Collectively, these findings highlight the important role of PFC in both the therapeutic and abuse-related properties of MPD. Therefore, the present study sought to determine whether a relation exists between environment-induced changes in sensitivity to the behavioral effects of MPD and DAT expression/function in PFC.
2. Materials and Methods
2.1. Animals
Male Sprague-Dawley rats (21 days of age) were obtained from Harlan Laboratories (Indianapolis, IN) and were housed with free access to food and water in a colony room in the Division of Laboratory Animal Resources at the University of Kentucky or the University of South Carolina. Rats were maintained on a 12-h light: dark cycle (lights on at 07:00 AM). All animal handling procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Kentucky and the University of South Carolina, and were performed in accordance with the 1996 version of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.3. Environmental enrichment
Upon arrival, rats were assigned randomly to EC or IC conditions using previously published methods [2]. EC rats were group-housed (8–12 per cage) in a large metal cage (120 cm length × 60 cm width × 45 cm height). Fourteen hard, non-chewable plastic objects were placed randomly in the large cage. Each day, EC rats were handled while one half of the objects were replaced with new plastic objects, and the remaining plastic objects were rearranged to enhance novelty. In contrast, IC rats were housed individually in wire mesh hanging cages (25 cm length × 18 cm width × 17 cm height) with wire mesh floor, solid metal sides, back and top; in addition, IC rats were not handled. In a follow-up experiment, another group of rats was raised in a social control condition (SC) where rats were raised with a single cohort (i.e., 2 rats per cage) but had no exposure to novel objects. SC rats were housed in pairs in a clear polycarbonate maternity cage (43 cm length × 20 cm width × 20 cm height) with wire rack top. The SC condition was chosen because it conforms to the typical housing conditions indicated in the NIH Guide for the 1996 version of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Rats were maintained in their respective home environments until the start of the behavioral and neurochemical experiments, which commenced when rats were at least 53 days of age.
2.4. Drug administration
EC, IC and SC rats were administered MPD hydrochloride (Sigma-Aldrich, 3 or 10 mg/kg, s.c.) or saline (1 ml/kg, s.c.) once daily for a total of 10 injections. MPD was prepared in 0.9% NaCl (saline) and administered in a volume of 1 ml/kg body weight. Dose was expressed as the weight of the salt.
2.5. Locomotor activity
Locomotor activity was recorded using two automated Digiscan animal activity monitoring systems (AccuScan Instruments, Columbus, OH, USA). Each system consisted of 6 clear chambers (42 cm length × 42 cm width × 30 cm height). The outer walls of each chamber were made opaque by attaching sheets of white plastic to their outer surface. Each chamber incorporated a horizontal 16 × 16 grid of photo beam sensors (beams were spaced 2.5 cm apart and 7.0 cm above the chamber floor), and was connected to a personal computer operating Digipro System software (v. 1.40, AccuScan Instruments). Horizontal activity was measured as photo beam interruptions and converted to distance traveled (cm).
For experiment 1, beginning at 55 days of age, EC and IC rats (n=6 per group), with the exception of saline-treated IC rats (n=5), were assigned to each treatment group. The locomotor experiment assigned rats to a 2 (EC vs. IC) × 3 (0 vs. 3 vs. 10 mg/kg of MPD) factorial design. On the day immediately prior to the start of the experiment, rats were habituated to the locomotor apparatus during a single 60-min session; no injections were given on this day. Subsequently, on the next 10 consecutive days (Days 1–10), rats were given injections of saline or MPD (3 or 10 mg/kg) once per day, and activity was recorded for 60 min immediately after each injection. Once the 10-day repeated treatment phase was completed, rats remained in their home cage for a 14-day drug-free period (Days 11–24). Then, over the next two days, all rats were challenged with saline (Day 25) and subsequently 10 mg/kg of MPD (Day 26).
For experiment 2, SC rats (n=8 per group) were given either saline or MPD (3 or 10 mg/kg) using the same procedure for treatment and behavioral testing described in experiment 1.
2.6. [3H]DA Uptake Assay
To determine whether DA uptake in PFC of EC and IC rats was mediated by activation of PKC, EC rats (n=12) and IC (n=12) rats at 53–60 days of age were used; 6 rats from each condition were exposed to PMCA plus OA and 6 rats were control. To determine acute effects of MPD (3 or 10 mg/kg) on DAT function in EC and IC rats, separate groups of EC (n=18) and IC (n=18) rats were used, with 6 rats from each rearing condition used at each MPH dose (0, 3 or 10 mg/kg); all rats were killed 60 min after injection. The kinetic parameters (Vmax and Km) of synaptosomal [3H]DA (3,4-ethyl-2 [N-3H] dihydroxyphenylethylamine; specific activity, 31 Ci/mmol, PerkinElmer Life Sciences) uptake in PFC and striatum were determined using a previously published method [2]. Brain regions from each rat were homogenized immediately in 20 ml of ice-cold sucrose solution (0.32 M sucrose and 5 mM sodium bicarbonate, pH 7.4) with 16 passes of a Teflon pestle homogenizer. Homogenates were centrifuged at 2,000 g for 10 min at 4 °C, and resulting supernatants were centrifuged at 20,000 g for 15 min at 4 °C. Resulting pellets from PFC and striatum were resuspended in 4.8 and 3.0 ml, respectively, of ice-cold assay buffer (125 mM NaCl, 5 mM KCl, 1.5 mM MgSO4, 1.25 mM CaCl2, 1.5 mM KH2PO4, 10 mM glucose, 25 mM HEPES, 0.1 mM EDTA, 0.1 mM pargyline and 0.1 mM L-ascorbic acid, saturated with 95% O2/5% CO2, pH 7.4). Nonspecific [3H]DA uptake in PFC and striatum was determined in the presence of 10 μM nomifensine. Kinetic analysis of [3H]DA uptake by DAT in the PFC was assessed in the presence of desipramine (1 μM) and paroxetine (5 nM) to prevent [3H]DA uptake into NE- and serotonin-containing nerve terminals, respectively, thereby isolating uptake of DA by DAT [53]. PFC and striatal synaptosomes, containing ~150 μg protein/100 μl and ~50 μg protein/30 μl, respectively, were incubated in a metabolic shaker for 5 min at 34 °C and then incubated for 10 min at 34 °C after adding 1 of 10 [3H]DA concentrations (0.1 nM – 5 μM) in a 500 μl total volume. Amounts of protein for PFC and striatal synaptosomes were chosen based on our previous report showing DAT density is greater in striatum than in PFC [2].
To determine whether the diminished DAT function in PFC from EC rats was mediated by a phosphorylation-dependent mechanism, kinetic analysis of [3H]DA uptake into PFC synaptosomes was measured in the absence or presence of PKC activator, phorbol 12 myristate 13 acetate (PMA, 10 μM) and protein phosphatase inhibitor, okadaic acid (OA, 10 μM). The concentrations of PMA and OA were chosen based on previous reports [54]. This assay was conducted for 15 min at 34 °C prior to analysis of [3H]DA uptake as described previously [54].
Incubation was terminated by the addition of 3 ml of ice-cold assay buffer, followed by immediate filtration through Whatman GF/B glass fiber filters (presoaked with 1 mM pyrocatechol for 3 h). Filters were washed 3 times with 3 ml ice-cold buffer containing pyrocatechol using a Brandel cell harvester (Model MP-43RS; Biochemical Research and Development Laboratories Inc., Gaithersburg, MD). Radioactivity was determined by liquid scintillation spectrometry (Model B1600TR, Perkin-Elmer Life Sciences, Downers Grove, IL). Protein concentrations were determined with bovine serum albumin as the standard [55]. Kinetic parameters (Vmax and Km) were determined using the commercially available GraphPad Prism 4.0 program (GraphPad Software, Inc., San Diego, CA).
2.7. [3H]WIN 35,428 Binding Assay
After the completion of the behavioral study, EC, IC and SC rats previously injected with MPD (3 or 10 mg/kg) or saline once daily for 10 days were maintained in their home cages for a 14-day drug-free period (Days 11–24). All rats were then challenged with saline on Day 25 or MPD (10 mg/kg) on Day 26 and brains were removed immediately after the last challenge injection. [3H]WIN 35,428 ((−)-3β-(4-fluorophentl)-tropan-2β-carboxylic acid methyl ester tartrate; specific activity, 85 Ci/mmol, PerkinElmer Life Sciences) binding was determined using a previously described method [56]. Brain regions were homogenized in 80 vol (w/v) of ice-cold sodium-phosphate buffer (2.1 mM NaH2PO4, 7.3 mM Na2HPO4, 320 mM sucrose, pH 7.4) with 7 passes of a Teflon pestle homogenizer. Homogenates were centrifuged at 40,000 g for 20 min at 4°C, and resulting pellets were washed twice by resuspension in ice-cold buffer and centrifuged at 40,000 g at 4°C for 20 min. Final pellets from PFC and striatum were resuspended in fresh buffer at a concentration of 10 mg/ml original wet weight and homogenized using a Polytron homogenizer (Brinkman Instruments Co., Westbury, NY; setting 5 for 15 s). Saturation binding assays were conducted in duplicate in a final volume of 250 μl for PFC and 500 μl for striatum, containing crude membrane (185–200 μg protein/200 μl and 35–40 μg/100 μl for PFC and striatum, respectively). PFC and striatal crude membrane, containing ~200 μg protein/200 μl and ~40 μg protein/100 μl, respectively, were incubated with 7 concentrations of [3H]WIN 35,428 (0.1 – 30 nM) on ice for 2 h. Nonspecific binding was determined in the presence of 10 μM cocaine. Assays were terminated by rapid filtration onto Whatman GF/B glass fiber filters, presoaked for 2 h with assay buffer containing 0.5% polyethylenimine. Filters were washed 3 times with 4 ml of ice-cold assay buffer. Radioactivity remaining on the filters was determined using liquid scintillation spectrometry (Model B1600TR, Perkin-Elmer Life Sciences, Downers Grove, IL).
2.8. Statistical Analyses
Locomotor activity data are presented as means ± SEM distance traveled (cm) and were analyzed by mixed-factor analyses of variance (ANOVA) with rearing condition (EC or IC) and treatment (saline or MPD dose) as between-group factors; data from Experiment 2 using SC rats were analyzed in a separate ANOVA. Day and/or time served as within-subjects factors for analysis of changes in sensitivity to MPD-induced hyperactivity. Where appropriate, Tukey’s post hoc tests were performed. For the neurochemical assays, differences in the parameters (Vmax, Bmax, Km and Kd) between EC and IC rats for kinetic parameters of [3H]DA uptake and [3H]WIN 35,428 binding in respective brain regions were analyzed using unpaired Student’s t-tests. Log transformed Km and Kd values were used for statistical analyses. Differences were considered statistically significant at p < 0.05.
3. Results
3.1. Effects of MPD on locomotor activity
3.1.1. Experiment 1
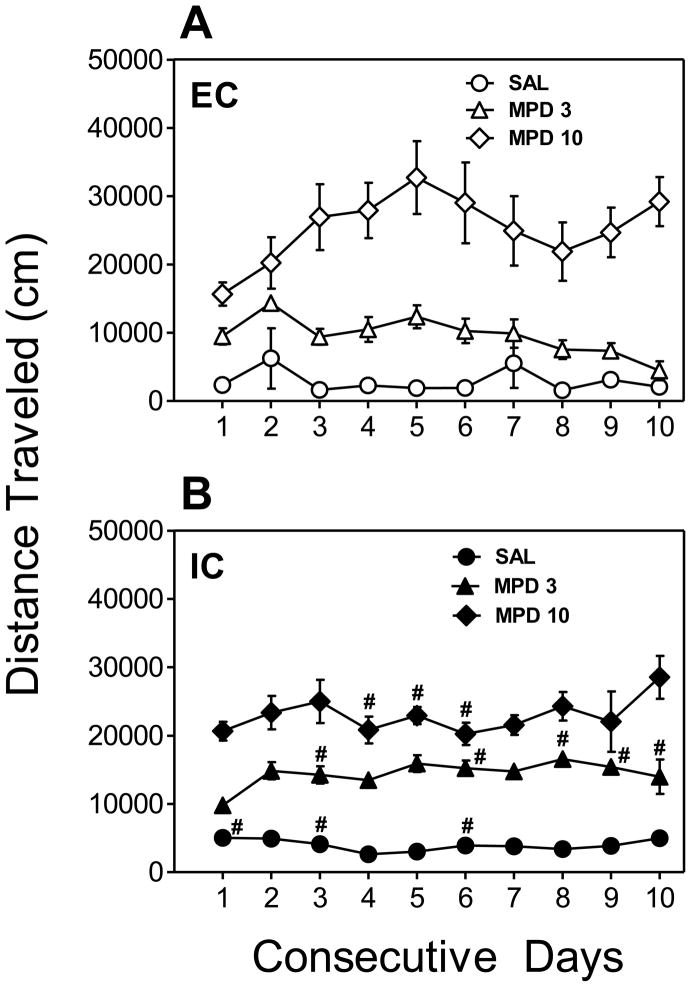
The effect of repeated administration of MPD (3 and 10 mg/kg) on total activity during Days 1–10 in EC and IC rats is illustrated in Fig. 1 (also see summary, Table 1). A three-factor ANOVA revealed significant main effects of Treatment (F (2, 29) = 81.86, p < 0.001) and Day (F (9,261) = 2.18, p < 0.05). Although the main effect of Rearing Condition did not attain significance (F (1,29) = 0.82, p > 0.05), a significant Rearing Condition x Treatment × Day interaction (F (18,261) = 1.76, p < 0.05) was found. For EC rats (Fig. 1A), a two-factor ANOVA revealed main effects of Treatment (F (2, 15) = 30.40, p < 0.001) and Day (F (9,135) = 2.37, p < 0.05), as well as a Treatment × Day interaction (F (18,135) = 2.65, p < 0.001). EC rats treated with 3 mg/kg of MPD displayed greater locomotor activity on Day 2 than on Day 1 (p < 0.05), whereas EC rats treated with 10 mg/kg of MPD displayed greater locomotor activity on Days 4, 5 and 10 than on Day 1 (p < 0.05) (Fig. 1A). There was no significant main effect of Day in EC rats treated with saline. For IC rats (Fig. 1B), a two-factor ANOVA revealed a significant main effect of Treatment (F (2, 14) = 142.73, p < 0.001), but no other main effects or interactions attained significance. IC rats treated with 3 mg/kg of MPD displayed greater locomotor activity on Days 2–9 than on Day 1 (p < 0.05) (Fig. 1B). There was no significant main effect of Day in IC rats treated with saline or 10 mg/kg of MPD.
Fig. 1.
Effect of MPD on total locomotor activity across Days 1–10 in EC (A) and IC (B) rats. Rats were treated with MPD (3 or 10 mg/kg, s.c.) or saline (SAL) on Days 1–10. Data points indicate the means ± S.E.M. total distance traveled (cm) during each 60-min daily session. Activity in EC rats treated with 3 mg/kg MPD on Day 2 was significantly greater than on Day 1, whereas activity in EC rats treated with 10 mg/kg MPD on Days 4–5 and 10 was significantly greater than on Day 1 (A). Activity in IC rats treated with MPD (3 mg/kg) on Days 2–9 was significantly greater than on Day 1. # p < 0.05, difference from EC rats of the same treatment group at the corresponding time interval. N=6 rats for EC and IC groups, with the exception of the IC SAL group (N=5).
Table 1.
Locomotor activity and [3H]WIN 35,428 binding among enriched (EC), impoverished (IC) and social (SC) condition rats treated repeatedly with saline or methylphenidate (MPD; 3 or 10 mg/kg). For locomotor activity, the first 2 columns show the mean distances traveled (cm) on Days 1 and 10, respectively, while the third column shows the percent change (% Δ) represented as a decrease (↓) or increase (↑) in activity on Day 1 compared to Day 10. For [3H]WIN 35,428 binding, the 2 columns show the mean Bmax values (pmol/mg protein) in prefrontal cortex (PFC) and striatum. Locomotor activity data are illustrated in Figures 1 - 3, 6 and 7 and [3H]WIN 35,428 binding data are illustrated in Figures 1-3, 6 and 7.
| Group | Saline |
MPD 3 mg/kg |
MPD 10 mg/kg |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Locomotor Activity | Bmax | Locomotor Activity | Bmax | Locomotor Activity | Bmax | ||||||||||
| Day 1 | Day 10 | % Δ | PFC | Striatum | Day 1 | Day 10 | % Δ | PFC | Striatum | Day 1 | Day 10 | % Δ | PFC | Striatum | |
| EC | 2340 | 2060 | ↓11.97 | 0.78 | 5.66 | 9482 | 4438 | ↓53.20 | 0.72 | 4.87 | 15665 | 29196 | ↑86.38 | 0.5 | 4.27 |
| IC | 5031 | 4985 | ↓0.91 | 0.82 | 5.58 | 9775 | 13980 | ↑59.28 | 0.78 | 4.99 | 20653 | 28531 | ↑38.14 | 0.65 | 4.59 |
| SC | 9529 | 7606 | ↓20.18 | 0.61 | 6.92 | 15020 | 23675 | ↑57.62 | 0.52 | 6.67 | 22406 | 31954 | ↑42.61 | 0.64 | 6.67 |
Based on the significant three-way interaction, additional two-factor ANOVAs (Rearing Condition × Day) were conducted (see Fig. 1). In saline-treated EC and IC rats, ANOVA revealed a significant main effect of Rearing Condition (F (1,9) = 7.28, p < 0.05); post hoc tests showed that IC rats were significantly more active than EC rats on Days 1, 3 and 6 (p < 0.05). In EC and IC rats treated with 3 mg/kg of MPD, ANOVA revealed a significant main effect of Rearing Condition (F (1,10) = 20.43, p < 0.01), as well as a Rearing Condition × Day interaction (F (9,90) = 3.74, p < 0.01); post hoc tests showed that IC rats were significantly more active than EC rats on Days 3, 6 and 8–10 (p < 0.05). In EC and IC rats treated with 10 mg/kg of MPD, although there was no main effect of Rearing Condition (F (1,10) = 1.30, p > 0.05), a significant Rearing Condition × Day interaction (F (9,90) = 2.70, p < 0.05) was found; post hoc tests showed that IC rats were less active than EC rats on Days 4–6 (p < 0.05).
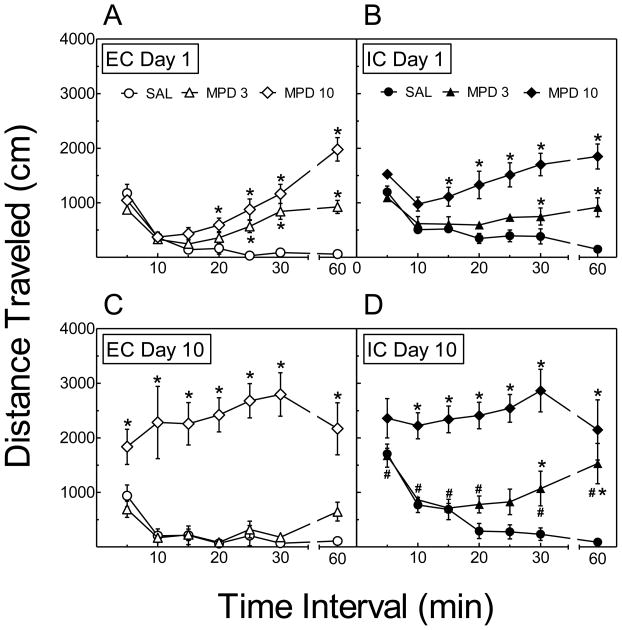
The time course effect of MPD in EC and IC rats on Days 1 and 10 is illustrated in Fig. 2. A four-factor ANOVA revealed significant main effects of Rearing Condition (F (1, 29) = 8.59, p < 0.01), Treatment (F (2, 29) = 110.46, p < 0.001), Day (F (1, 29) =12.10, p < 0.01) and Time (F (11,319) = 7.84, p < 0.001). In addition, there was a significant Rearing Condition × Treatment × Day interaction (F (2, 29) = 4.67, p < 0.05). On Day 1, the activity of EC rats treated with 3 mg/kg of MPD was significantly greater during the 25–60-min intervals, and activity of EC rats treated with 10 mg/kg of MPD was significantly greater during the 20–60-min intervals, compared to saline-treated EC rats (Fig. 2A). On Day 1, the activity of IC rats treated with 3 mg/kg of MPD was significantly greater during the 30-, 50- and 60-min intervals, and activity of IC rats treated with 10 mg/kg of MPD was significantly greater during the 15–60-min intervals, compared to saline-treated IC rats (Fig. 2B). On Day 1, while no significant difference in activity between EC and IC rats treated with 3 mg/kg of MPD was observed (F (1,10) = 0.48, p > 0.05), the activity of saline-treated IC rats was higher than that of EC rats (F (1, 9) = 10.40, p < 0.05), and the activity of IC rats treated with 10 mg/kg of MPD was higher than that of EC rats (F (1,10) = 5.19, p < 0.05). On Day 10, EC and IC rats treated with 10 mg/kg of MPD were significantly (p < 0.05) more active than the saline controls during each time interval except the 5-min interval in the IC group (Fig. 2C and D, respectively); however, the levels of activity produced by 10 mg/kg of MPD were not different between EC and IC rats. In contrast, only IC rats treated with 3 mg/kg of MPD displayed greater activity (p < 0.05) than saline-treated controls on Day 10; post hoc tests showed that 3 mg/kg of MPD produced significantly greater activity in IC rats relative to saline controls beginning at the 30-min time interval (Fig. 2D).
Fig. 2.
Time course of the effect of MPD on locomotor activity during Day 1 in EC (A) and IC (B) rats and Day 10 in EC (C) and IC (D) rats. Rats were administered MPD (3 or 10 mg/kg, s.c.) or saline (SAL) on Days 1–10. Data points indicate means ± SEM distance traveled (cm) during 5–30-min and 60-min time intervals. * p < 0.05, difference in activity compared to SAL-treated rats at the corresponding time interval. # p < 0.05, difference from EC rats within same treatment group at the corresponding time interval. N=6 rats for EC and IC groups, with the exception of the IC saline group (N=5).
On the saline challenge session that followed the 14-day drug-free period (Day 25), there was a significant main effect of Rearing Condition (F (1,29) = 14.10, p < 0.01) and Treatment (F (1,29) = 5.94, p < 0.01). IC rats showed overall higher activity than EC rats and prior MPD treatment produced a dose-dependent increase in activity indicative of conditioned hyperactivity (results not shown). Similarly, on the MPD (10 mg/kg) challenge session (Day 26), significant main effects of Rearing Condition (F (1,29) = 6.24, p < 0.05) and Treatment (F (1,29) = 11.89, p < 0.05) were found. IC rats showed overall higher activity than EC rats and prior MPD treatment produced a dose-dependent increase in activity indicative of conditioned hyperactivity (results not shown). As expected, overall activity collapsed across conditions was higher on the MPD challenge session than on the saline challenge session.
3.1.2. Experiment 2
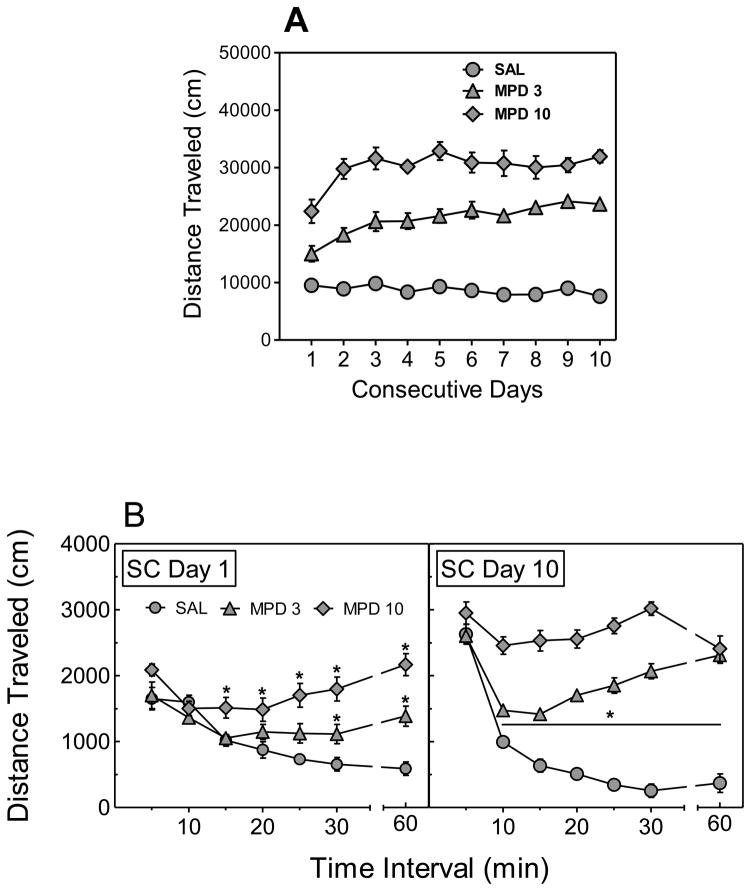
In a separate experiment using SC rats, a three-factor ANOVA revealed significant main effects of Treatment (F (2,21) = 181.30, p < 0.001) and Day (F (9,189) = 6.95, p < 0.001), as well as a significant Treatment × Day interaction (F (18,189) = 3.25, p < 0.001). Post hoc tests showed that activity on Days 2–10 was greater than activity on Day 1 in SC rats treated with 3 mg/kg of MPD, whereas locomotor activity on Days 3, 5–7 and 10 was greater than that on Day 1 in SC rats treated with 10 mg/kg of MPD (Fig. 3A). On Day 1, SC rats treated with 3 mg/kg of MPD were more active than saline-treated SC rats during the 30–60-min time intervals, whereas SC rats treated with 10 mg/kg of MPD were more active than saline-treated SC rats during the 15–60-min time intervals (Fig. 3B). On Day 10, SC rats treated with either 3 or 10 mg/kg of MPD were more active than saline-treated SC rats during the 10–60-min time intervals (Fig. 3B).
Fig. 3.
Effect of MPD on total locomotor activity across Days 1–10 (A), and time course of the effect of MPD on locomotor activity during Days 1 and 10 (B), in SC rats. Rats were administered MPD (3 or 10 mg/kg, s.c.) or saline (SAL) on Days 1–10. Data points indicate means ± SEM distance traveled (cm) during 5–30-min and 60-min time intervals. * p < 0.05, difference in activity in compared to SAL-treated rats at the corresponding time interval (* above a line indicates a significant difference from SAL for each time interval in both MPD dose groups). N=8 rats per group.
On the saline challenge session that followed the 14-day drug-free period (Day 25), there was a significant main effect of Treatment (F (2,21) = 3.74, p < 0.05); prior MPD treatment produced a dose-dependent increase in activity indicative of conditioned hyperactivity (results not shown). Similarly, on the MPD (10 mg/kg) challenge session (Day 26), there was a significant main effect of Treatment (F (2,21) = 4.26, p < 0.05); prior MPD treatment produced a dose-dependent increase in activity indicative of conditioned hyperactivity (results not shown). As expected, overall activity was higher on the MPD challenge session than on the saline challenge session in SC rats.
3.2. Effect of rearing condition on PKC-mediated DAT function
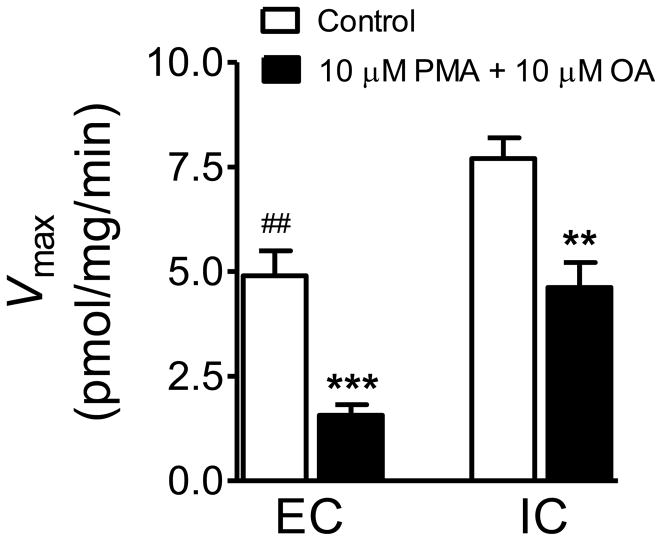
To determine the effect of rearing condition on basal levels of DAT phosphorylation, which is related to its function, kinetic analysis of [3H]DA uptake was performed in the presence or absence of the PKC activator PMA and the protein phosphatase inhibitor OA. In the absence of PMA and OA, environmental enrichment significantly decreased (37 ± 4%) the Vmax of [3H]DA uptake in PFC compared to IC rats (t (10) = 3.92, p < 0.01) with no change in Km values (EC: 21 ± 1.3 nM; IC: 22 ± 1.9 nM, respectively) (Fig. 4). In the presence of 10 μM PMA and 10 μM OA, the Vmax was lower in EC than in IC (t (10) = 4.44, p < 0.001) rats. In addition, the Vmax was further decreased by 68 ± 6% in EC rats (t (5) = 9.14, p < 0.001), and by 40 ± 4% in IC rats (t (5) = 4.92, p < 0.01) in the presence of PMA + OA relative to controls. No differences were observed between EC and IC rats in Km values (EC: 20.2 ± 1.5 nM; IC: 21.4 ± 1.8 nM, respectively).
Fig. 4.
Effect of PMA and OA on kinetic analysis of [3H]DA uptake in PFC of EC and IC rats. Synaptosomes were preincubated in the absence (control) or the presence of 10 μM PMA plus 10 μM OA at 34°C for 15 min followed by the addition of one of eight concentrations of [3H]DA (0.1 nM − 1 μM) for 10 min. Vmax values are expressed as means ± SEM pmol/mg protein. ** p < 0.01, *** p < 0.001, difference in 10 μM PMA + 10 μM OA from the respective controls. ## p < 0.01, difference from IC rats. N=6 rats per group.
3.3. Effect of acute MPD on kinetic parameters of [3H]DA uptake in PFC and striatum in EC and IC rats
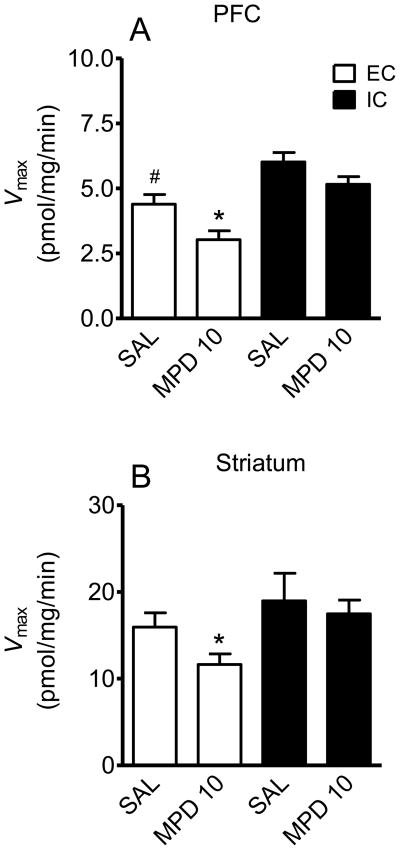
In saline-treated EC rats, the Vmax of [3H]DA uptake in PFC rats was decreased compared to IC rats (t (10) = 3.17, p < 0.01). Acute MPD (10 mg/kg) resulted in a 32 ± 3% decrease in Vmax in PFC compared to saline controls in EC rats (t (10) = 2.73, p < 0.05, Fig. 5A), with no change in Km (MPD: 19.4 ± 1.5 nM; saline: 20.0 ± 2.4 nM). Similarly, the Vmax in striatum was decreased by 26 ± 1% in EC rats compared to the saline control (t (10) = 2.71, p < 0.05, Fig. 5B), with no change in Km (MPD: 28 ± 2.5 nM; saline: 27.8 ± 2.3 nM). In contrast, MPD (10 mg/kg) did not alter the kinetic parameters of [3H]DA uptake in PFC or striatum in IC rats. Further, acute MPD (3 mg/kg) did not significantly alter the kinetic parameters of [3H]DA uptake in PFC or striatum from either EC or IC groups (results not shown).
Fig. 5.
Effect of acute MPD administration on Vmax of [3H]DA uptake in PFC and striatum in EC and IC rats. Rats were given a single injection of MPD (3 or 10 mg/kg, s.c.) or saline (SAL) and were sacrificed 60 min later. PFC and striatum were obtained from the same rat. Vmax values in PFC (A) and striatum (B) are expressed as means ± S.E.M. pmol/mg protein. *p < 0.05, different from SAL control group. # p < 0.05, difference between EC and IC SAL control group. N=6 rats per group.
3.4. [3H]WIN 35,428 binding in PFC and striatum from EC and IC rats with repeated administration of MPD
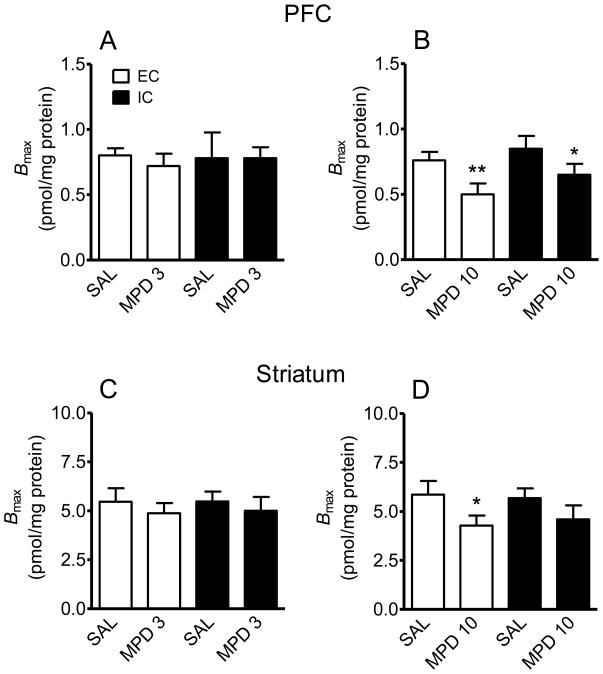
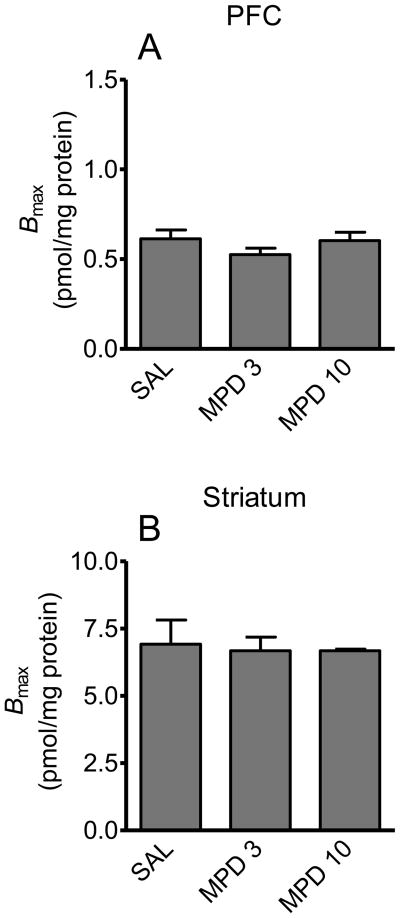
To determine if repeated administration of MPD altered radioligand binding sites on DAT, saturation analysis of [3H]WIN 35,428 binding was performed using brain regions from EC and IC rats previously injected with MPD (3 or 10 mg/kg) or saline. Fig. 6 shows Bmax values in PFC and striatum of EC and IC rats used in the behavioral experiment. The 3 mg/kg dose of MPD did not alter Bmax values in either PFC or striatum from EC and IC rats (Fig. 6A and C) or Kd (PFC: MPD, 11.5 ± 2.1 nM and saline, 12.2 ± 1.9 nM; striatum: MPD, 8.2 ± 1.9 nM and saline, 9.0 ± 1.5 nM). However, 10 mg/kg of MPD decreased Bmax in PFC by 34 ± 4% (t (10) = 3.51, p < 0.01) and decreased Bmax in striatum by 27 ± 3% (t (10) = 1.75, p < 0.05) in EC rats ( Fig. 6B and D). In IC rats, 10 mg/kg of MPD decreased Bmax by 24 ± 3% in PFC (t (10) = 2.55, p < 0.05), but no significant effect was found in striatum (t (10) = 1.81, p > 0.05, Fig. 6D). Fig. 7 shows Bmax values in PFC and striatum of SC rats. No significant differences between MPD-treated SC rats (3 or 10 mg/kg) and their saline controls for Bmax values in PFC (Fig. 7A) or striatum (Fig. 7B) were observed.
Fig. 6.
Effect of repeated MPD administration MPD on the Bmax of [3H]WIN 35,428 binding in PFC and striatum in EC and IC rats. After completion of the behavioral studies, EC and IC rats previously injected with MPD (3 or 10 mg/kg) or saline (SAL) once daily for 10 days remained in the home cage for a 14-day drug-free period. All rats were subsequently challenged with an injection of SAL and MPD (10 mg/kg), and brains were removed 2 hours after MPD injection. Bmax values in PFC (A and B) and striatum (C and D) are expressed as means ± SEM pmol/mg protein. *p < 0.05, ** p < 0.01, different from the respective SAL control. N=6 rats for EC and IC groups, with the exception of the IC SAL group (N=5).
Fig. 7.
Effect of repeated MPD administration on the Bmax of [3H]WIN 35,428 binding in PFC and striatum in SC rats. After completion of the behavioral studies, SC rats treated previously with MPD (3 or 10 mg/kg) or saline (SAL) once daily for 10 days remained in the home cage for a 14-day drug-free period. All rats were subsequently challenged with an injection of SAL and MPD (10 mg/kg), and brains were removed 2 hours after MPD injection. Bmax values in PFC (A) and striatum (B) are expressed as means ± SEM N=8 rats per group.
4. Discussion
The present findings demonstrate that environmental enrichment alters the behavioral and neurochemical effects of acute and repeated MPD administration. In Experiment 1, acute MPD (3 and 10 mg/kg) produced similar overall hyperactivity in EC and IC rats. However, across repeated administration of the low MPD dose (3 mg/kg), the hyperactivity became more pronounced in IC rats relative to EC rats. In contrast, the pattern of activity across repeated administration of the high MPD dose (10 mg/kg) was similar in EC and IC rats. Further, Experiment 2 showed that SC rats displayed enhanced hyperactivity across repeated MPD (3 mg/kg) that was comparable to IC rats, suggesting that the blunted response to repeated 3 mg/kg of MPD in EC rats was likely due to the presence of novel objects rather than social cohorts.
Regarding the neurochemical results, basal DAT function in PFC was decreased in EC rats compared to IC rats, a finding that replicates previous results [46]. More important, the current results extend this previous work by showing that EC rats also display greater PKC activation in PFC than IC rats. These enrichment-induced functional changes in PFC were not accompanied by changes in basal levels of DAT binding. Acute MPD (10 mg/kg) also decreased DAT function in PFC of EC rats, but not IC rats. With repeated administration, the high dose of MPD (10 mg/kg) also decreased DAT binding in striatum of EC rats, as well as decreasing DAT binding in PFC of EC and IC rats. Importantly, given that the changes in DAT function and expression were observed after treatment with the high dose of MPD (10 mg/kg), it does not appear that the attenuated locomotor response to 3 mg/kg of MPD in EC rats is attributable to a specific DAT alteration in PFC or striatum.
The present results are in agreement with previous work showing that differences in activity between EC and IC rats at baseline persist across experimental sessions [1, 2, 57–59]. The enrichment-induced difference in activity was apparent even during the challenge saline and MPD test days that occurred 2 weeks following termination of the repeated daily treatment. It is interesting that SC rats had the highest level of baseline activity and the greatest sensitivity to MPD-induced hyperactivity of any group (Table 1). Specifically, SC rats showed hyperactivity across repeated 3 and 10 mg/kg doses of MPD that was more robust than that observed in IC (3 and 10 mg/kg) and EC (10 mg/kg) rats. Despite the greater MPD-induced hyperactivity in SC rats, there was no change in Bmax of [3H]WIN 35,428 binding in PFC or striatum in SC rats, indicating that the environment-dependent changes in MPD-induced hyperactivity do not simply reflect a change in number of DAT binding sites. However, direct comparisons between EC and IC rats (Experiment 1) and SC rats (Experiment 2) are tempered by the caveat that these behavioral experiments were not conducted contemporaneously in the same laboratory, i.e., Experiments 1 and 2 were conducted at different institutes. While the same methodology was used in each setting, even standardized procedures can result in marked differences in results when behavioral data are collected by different experimenters [60]. In any event, the blunted hyperactivity to repeated MPD in EC rats is in accord with our previous studies showing that environmental enrichment during development produces sustained decreases in both baseline activity levels and sensitization to repeated amphetamine or nicotine [3, 8].
The present results contrast with previous work showing that environmental enrichment increases sensitization to the selective DAT inhibitor GBR12935 [2]. Importantly, repeated MPD (3 or 10 mg/kg) in the current report, while producing conditioned hyperactivity on the saline challenge day (Day 25), did not alter activity in EC, IC or SC rats on the MPD (10 mg/kg) challenge day (Day 26), indicating that long-lasting MPD sensitization was not obtained. Although there are no reports examining long-term sensitization to MPD in EC or IC rats in the literature, the current results in SC rats are consistent with other reports in standard-housed rats that MPD sensitization may not survive a long period of abstinence [61] and is dependent on time of day, dose and method of behavioral recording [42]. The differential effects of rearing environment between GBR12935- and MPD-induced sensitization in EC and IC groups may reflect differences in the neural adaptations occurring with repeated administration of GBR12935 versus MPD. Since GBR12935 is a selective DAT inhibitor [47, 62, 63], whereas MPD inhibits both DAT and NET [64–66], the discrepant findings obtained with MPD in the current report may be due to, at least in part, to the involvement of both transporters [64–66]. In accord with this notion, low doses of MPD are known to preferentially increase both DA and NE efflux in PFC compared to limbic structures [43].
The present study and our previous work [2, 46] demonstrate that EC rats exhibit a decrease in Vmax of [3H]DA uptake under basal conditions in PFC, but not in striatum or nucleus accumbens, compared to IC rats. The decrease in DAT function in PFC of EC rats is accompanied by a parallel decrease in cell surface DAT expression [46], suggesting a dynamic modulation of DAT function and cell surface DAT by rearing environments. This interpretation is also supported by the lack of change in DAT density measured by [3H]GBR12935 and [3H]WIN 35,428 binding in PFC of EC and IC rats [2]. In addition to the current results showing decreased DAT function in PFC of EC rats, environmental enrichment also reduces DA D1 receptor function in rat PFC [73], indicating that environmental factors can alter multiple aspects of DA function in this brain region. Given the important role of PFC in impulsivity and stimulant self-administration [16], the current results may also have relevance to enrichment-mediated differences in sensitivity to the effects of stimulants on certain aspects of impulsivity and drug self-administration. Determining the role of PFC DAT function in reduction of impulsive choice [11] and drug self-administration in EC rats is an interesting topic for future investigations.
Consistent with our previous findings [2], a significant reduction (36%) in Vmax was found for EC relative to IC rats in the control group (in the absence of PKC activator and the protein phosphatase inhibitor). The Vmax in PFC was decreased further by 68% in EC and 40% in IC in the presence of a PKC activator and inhibitor. There is abundant evidence that PKC activation causes decreased DAT transport capacity and DAT internalization. For example, incubation with β-PMA dramatically increases levels of 32PO4-labeled immunoprecipitated DAT in both synaptosomes [54] and cells heterologously expressing DAT [67]. Pretreatment with PKC activators acutely and rapidly reduces [3H]DA uptake without affecting the Km value and reducing the Bmax of [3H]mazindol binding to intact Xenopus oocytes [68]. Further, the induction of PKC with β-PMA results in intracellular redistribution of the DAT from the plasma membrane to recycling endosomal compartments in hDAT-expressing PC12 cells [69]. In contrast, other studies also reported that phosphorylation of DAT after PKC activation is not responsible for the internalization of DAT [70], and appears to not have a major effect on DA uptake [71]. Thus, these findings suggest that dynamic regulation of DAT involves multiple processes including transporter phosphorylation and membrane trafficking [51, 52, 72]. The current results showing an enrichment-induced difference in DAT function in the presence of the combination of PMA and OA suggest that DAT in PFC of EC rats is more sensitive to PKC-mediated down-regulation than that in IC rats.
While Bmax values in PFC or striatum of EC and IC rats were not changed after repeated administration of 3 mg/kg of MPD, enrichment-induced behavioral differences were obtained with this dose. In contrast, environmental enrichment decreased Bmax after 10 mg/kg of MPD, which produced similar levels of activity in EC and IC rats. Notably, the Bmax data were obtained from rats given 10 days of MPD, followed with 14 days later by a saline and MPD challenge. Therefore, the Bmax data do not reflect the molecular change of DAT proteins immediately after the 10 days of repeated MPD. It is possible that repeated MPD (3 mg/kg) altered DAT expression, but that this change was reversed during the drug free period. Regardless, repeated 10 mg/kg of MPD decreased Bmax in PFC and striatum of EC rats and in PFC of IC rats compared to saline controls. Again, the dose-dependent dissociation between the behavioral and neurochemical results obtained suggests that the enrichment-induced behavioral differences may occur via a DAT-independent mechanism. Further, similar to prior self-administration results [6, 74], the differential sensitivity to MPD-induced hyperactivity was noted only with the low dose of MPD, indicating that exposure to high doses of this drug are capable of eliminating differences between EC and IC rats. Another potential mechanism underlying the differential response to MPD in EC and IC rats could be an alteration in PFC glutamate afferents that provide reciprocal modulation of DA activity in ventral tegmental area and nucleus accumbens, as these regions are involved in the acute hyperactivity and behavioral sensitization produced by administration of stimulant drugs [75–77]. In fact, EC rats show greater glutamate levels in nucleus accumbens following administration of a high dose of amphetamine (2.0 mg/kg), but EC and IC do not differ in response to a low dose (0.5 mg/kg) of amphetamine [78]. Therefore, an altered balance of DA and glutamate in PFC, ventral tegmental area and nucleus accumbens may contribute to the current findings.
The MPD-induced decrease in Vmax and Bmax associated with environmental enrichment is specific to PFC. A single injection of MPD (10 mg/kg) significantly decreased Vmax in the PFC (32%) and striatum (27%) in EC but not IC rats, whereas a lower dose (3 mg/kg) of MPD did not alter DAT function in these brain regions in either EC or IC rats, indicating that basal DAT function in these regions was higher in IC rats compared to EC rats. On the other hand, repeated administration of 10 mg/kg, but not 3 mg/kg, significantly decreased Bmax of [3H]WIN 35,428 binding in PFC (33%) and striatum (27%) of EC rats, while the 10 mg/kg dose also decreased the Bmax in PFC (24%) of IC rats. Interestingly, the percentage of reduction in Vmax in PFC and striatum of EC rats following acute administration of 10 mg/kg was similar to the magnitude of the decrease in Bmax in the same regions of EC rats treated previously with 10 mg/kg. Thus, prior administration of the high dose of MPD led to decreases in DAT function and density, and may depend on a basal difference in DAT activity in EC and IC rats. In addition, neither MPD dose altered the Bmax of [3H]WIN 35,428 binding in PFC nor striatum of SC rats, although SC rats developed sensitization to repeated administration of both MPD doses. Collectively, alterations in sensitivity to MPD-induced hyperactivity following repeated injections appear to be related more strongly to differences in DAT function than to differences in WIN binding.
In conclusion, the current results suggest that environmental enrichment diminishes DAT function in PFC by enhancing basal DAT phosphorylation. These findings may have important implications for preclinical studies of the role of environment in individual differences in drug abuse, and for the ADHD-like elevations in hyperactivity and impulsivity in IC rats. Notably, exposure to an enriched environment was recently shown to improve the cognitive function of spontaneously hypertensive rats, the most common rodent model of ADHD [79]. Future studies of the impact of environmental enrichment on protein function in PFC and other regions should allow for a more complete understanding of the neurobiology of individual differences in impulsivity and drug abuse vulnerability.
Highlights.
Environmental enrichment regulates PKC-mediated down-regulation of DAT
Methylphenidate dose-dependently decreases DAT function and expression
Rearing environments differentially alter locomotor effects of methylphenidate.
Acknowledgments
This work was supported by grants from the National Institute on Drug Abuse: DA024275 (J.Z.), DA026721 (J.Z.), DA012964 (M.T.B.), DA013137 (R.M.B.) and DA023853 (T.E.W.) as well as a University of Kentucky Research Support grant (J.Z.). We acknowledge Dr. Maarten E. A. Reith (New York University, New York, NY) for commenting on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bowling SL, Rowlett JK, Bardo MT. The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology. 1993;32:885–93. doi: 10.1016/0028-3908(93)90144-r. [DOI] [PubMed] [Google Scholar]
- 2.Zhu J, Green T, Bardo MT, Dwoskin LP. Environmental enrichment enhances sensitization to GBR 12935-induced activity and decreases dopamine transporter function in the medial prefrontal cortex. Behav Brain Res. 2004;148:107–17. doi: 10.1016/s0166-4328(03)00190-6. [DOI] [PubMed] [Google Scholar]
- 3.Green TA, Cain ME, Thompson M, Bardo MT. Environmental enrichment decreases nicotine-induced hyperactivity in rats. Psychopharmacology (Berl) 2003;170:235–41. doi: 10.1007/s00213-003-1538-3. [DOI] [PubMed] [Google Scholar]
- 4.Bowling SL, Bardo MT. Locomotor and rewarding effects of amphetamine in enriched, social, and isolate reared rats. Pharmacol Biochem Behav. 1994;48:459–64. doi: 10.1016/0091-3057(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 5.Bardo MT, Valone JM, Bevins RA. Locomotion and conditioned place preference produced by acute intravenous amphetamine: role of dopamine receptors and individual differences in amphetamine self-administration. Psychopharmacology (Berl) 1999;143:39–46. doi: 10.1007/s002130050917. [DOI] [PubMed] [Google Scholar]
- 6.Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl) 2001;155:278–84. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- 7.Zhu J, Bardo MT, Green TA, Wedlund PJ, Dwoskin LP. Nicotine increases dopamine clearance in medial prefrontal cortex in rats raised in an enriched environment. J Neurochem. 2007 doi: 10.1111/j.1471-4159.2007.04951.x. [DOI] [PubMed] [Google Scholar]
- 8.Bardo MT, Bowling SL, Rowlett JK, Manderscheid P, Buxton ST, Dwoskin LP. Environmental enrichment attenuates locomotor sensitization, but not in vitro dopamine release, induced by amphetamine. Pharmacol Biochem Behav. 1995;51:397–405. doi: 10.1016/0091-3057(94)00413-d. [DOI] [PubMed] [Google Scholar]
- 9.Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006;12:559–66. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–63. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 11.Perry JL, Stairs DJ, Bardo MT. Impulsive choice and environmental enrichment: effects of d-amphetamine and methylphenidate. Behav Brain Res. 2008;193:48–54. doi: 10.1016/j.bbr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- 13.Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, et al. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–8. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Wood DA, Siegel AK, Rebec GV. Environmental enrichment reduces impulsivity during appetitive conditioning. Physiol Behav. 2006;88:132–7. doi: 10.1016/j.physbeh.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 15.Hellemans KG, Nobrega JN, Olmstead MC. Early environmental experience alters baseline and ethanol-induced cognitive impulsivity: relationship to forebrain 5-HT1A receptor binding. Behav Brain Res. 2005;159:207–20. doi: 10.1016/j.bbr.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Perry JL, Joseph JE, Jiang Y, Zimmerman RS, Kelly TH, Darna M, et al. Prefrontal cortex and drug abuse vulnerability: Translation to prevention and treatment interventions. Brain Res Rev. 2010 doi: 10.1016/j.brainresrev.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tannock R, Schachar RJ, Carr RP, Chajczyk D, Logan GD. Effects of methylphenidate on inhibitory control in hyperactive children. J Abnorm Child Psychol. 1989;17:473–91. doi: 10.1007/BF00916508. [DOI] [PubMed] [Google Scholar]
- 18.Tannock R, Schachar R, Logan G. Methylphenidate and cognitive flexibility: dissociated dose effects in hyperactive children. J Abnorm Child Psychol. 1995;23:235–66. doi: 10.1007/BF01447091. [DOI] [PubMed] [Google Scholar]
- 19.Potter AS, Newhouse PA. Effects of acute nicotine administration on behavioral inhibition in adolescents with attention-deficit/hyperactivity disorder. Psychopharmacology (Berl) 2004;176:182–94. doi: 10.1007/s00213-004-1874-y. [DOI] [PubMed] [Google Scholar]
- 20.Groman SM, James AS, Jentsch JD. Poor response inhibition: at the nexus between substance abuse and attention deficit/hyperactivity disorder. Neurosci Biobehav Rev. 2009;33:690–8. doi: 10.1016/j.neubiorev.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsden CA. Attention deficit hyperactivity disorder (ADHD): improved understanding and novel drug treatment. Neuropharmacology. 2009;57:577–8. doi: 10.1016/j.neuropharm.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Swanson JM, Volkow ND. Psychopharmacology: concepts and opinions about the use of stimulant medications. J Child Psychol Psychiatry. 2009;50:180–93. doi: 10.1111/j.1469-7610.2008.02062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volkow ND, Swanson JM. Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry. 2003;160:1909–18. doi: 10.1176/appi.ajp.160.11.1909. [DOI] [PubMed] [Google Scholar]
- 24.Swanson JM, Volkow ND. Serum and brain concentrations of methylphenidate: implications for use and abuse. Neurosci Biobehav Rev. 2003;27:615–21. doi: 10.1016/j.neubiorev.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Heron C, Costentin J, Bonnet JJ. Evidence that pure uptake inhibitors including cocaine interact slowly with the dopamine neuronal carrier. Eur J Pharmacol. 1994;264:391–8. doi: 10.1016/0014-2999(94)00502-8. [DOI] [PubMed] [Google Scholar]
- 26.Volkow ND, Wang GJ, Fowler JS, Fischman M, Foltin R, Abumrad NN, et al. Methylphenidate and cocaine have a similar in vivo potency to block dopamine transporters in the human brain. Life Sci. 1999;65:PL7–12. doi: 10.1016/s0024-3205(99)00225-8. [DOI] [PubMed] [Google Scholar]
- 27.Solanto MV. Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res. 1998;94:127–52. doi: 10.1016/s0166-4328(97)00175-7. [DOI] [PubMed] [Google Scholar]
- 28.John CE, Jones SR. Voltammetric characterization of the effect of monoamine uptake inhibitors and releasers on dopamine and serotonin uptake in mouse caudate-putamen and substantia nigra slices. Neuropharmacology. 2007;52:1596–605. doi: 10.1016/j.neuropharm.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Shaughnessy CT, Lythgoe DJ, Butcher SP, Kendall L, Wood B, Steward MC. Effects of hypoxia on fetal rat brain metabolism studied in utero by 31P-NMR spectroscopy. Brain Res. 1991;551:334–7. doi: 10.1016/0006-8993(91)90953-s. [DOI] [PubMed] [Google Scholar]
- 30.Heal DJ, Cheetham SC, Smith SL. The neuropharmacology of ADHD drugs in vivo: insights on efficacy and safety. Neuropharmacology. 2009;57:608–18. doi: 10.1016/j.neuropharm.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 31.Wayment HK, Deutsch H, Schweri MM, Schenk JO. Effects of methylphenidate analogues on phenethylamine substrates for the striatal dopamine transporter: potential as amphetamine antagonists? J Neurochem. 1999;72:1266–74. doi: 10.1046/j.1471-4159.1999.0721266.x. [DOI] [PubMed] [Google Scholar]
- 32.Sandoval V, Riddle EL, Hanson GR, Fleckenstein AE. Methylphenidate redistributes vesicular monoamine transporter-2: role of dopamine receptors. J Neurosci. 2002;22:8705–10. doi: 10.1523/JNEUROSCI.22-19-08705.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volz TJ, Farnsworth SJ, King JL, Riddle EL, Hanson GR, Fleckenstein AE. Methylphenidate Administration Alters Vesicular Monoamine Transporter-2 Function in Cytoplasmic and Membrane-Associated Vesicles. J Pharmacol Exp Ther. 2007;323:738–45. doi: 10.1124/jpet.107.126888. [DOI] [PubMed] [Google Scholar]
- 34.Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3:617–28. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- 35.Rubia K, Halari R, Cubillo A, Mohammad AM, Scott S, Brammer M. Disorder-specific inferior prefrontal hypofunction in boys with pure attention-deficit/hyperactivity disorder compared to boys with pure conduct disorder during cognitive flexibility. Hum Brain Mapp. 2010 doi: 10.1002/hbm.20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schecklmann M, Romanos M, Bretscher F, Plichta MM, Warnke A, Fallgatter AJ. Prefrontal oxygenation during working memory in ADHD. J Psychiatr Res. 2010;44:621–8. doi: 10.1016/j.jpsychires.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 37.Stahl SM. The prefrontal cortex is out of tune in attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2009;70:950–1. doi: 10.4088/jcp.09bs05416. [DOI] [PubMed] [Google Scholar]
- 38.Yang PB, Swann AC, Dafny N. Sensory-evoked potentials recordings from the ventral tegmental area, nucleus accumbens, prefrontal cortex, and caudate nucleus and locomotor activity are modulated in dose-response characteristics by methylphenidate. Brain Res. 2006;1073–1074:164–74. doi: 10.1016/j.brainres.2005.12.055. [DOI] [PubMed] [Google Scholar]
- 39.Amini B, Yang PB, Swann AC, Dafny N. Differential locomotor responses in male rats from three strains to acute methylphenidate. Int J Neurosci. 2004;114:1063–84. doi: 10.1080/00207450490475526. [DOI] [PubMed] [Google Scholar]
- 40.Yang PB, Swann AC, Dafny N. Chronic pretreatment with methylphenidate induces cross-sensitization with amphetamine. Life Sci. 2003;73:2899–911. doi: 10.1016/s0024-3205(03)00673-8. [DOI] [PubMed] [Google Scholar]
- 41.Lee MJ, Swann AC, Dafny N. Methylphenidate sensitization is prevented by prefrontal cortex lesion. Brain Res Bull. 2008;76:131–40. doi: 10.1016/j.brainresbull.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Wooters TE, Dwoskin LP, Bardo MT. Age and sex differences in the locomotor effect of repeated methylphenidate in rats classified as high or low novelty responders. Psychopharmacology (Berl) 2006;188:18–27. doi: 10.1007/s00213-006-0445-9. [DOI] [PubMed] [Google Scholar]
- 43.Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, et al. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–20. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 44.Sesack SR, Hawrylak VA, Melchitzky DS, Lewis DA. Dopamine innervation of a subclass of local circuit neurons in monkey prefrontal cortex: ultrastructural analysis of tyrosine hydroxylase and parvalbumin immunoreactive structures. Cereb Cortex. 1998;8:614–22. doi: 10.1093/cercor/8.7.614. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto BK, Novotney S. Regulation of extracellular dopamine by the norepinephrine transporter. J Neurochem. 1998;71:274–80. doi: 10.1046/j.1471-4159.1998.71010274.x. [DOI] [PubMed] [Google Scholar]
- 46.Zhu J, Apparsundaram S, Bardo MT, Dwoskin LP. Environmental enrichment decreases cell surface expression of the dopamine transporter in rat medial prefrontal cortex. J Neurochem. 2005;93:1434–43. doi: 10.1111/j.1471-4159.2005.03130.x. [DOI] [PubMed] [Google Scholar]
- 47.Zhu J, Reith ME. Role of the dopamine transporter in the action of psychostimulants, nicotine, and other drugs of abuse. CNS Neurol Disord Drug Targets. 2008;7:393–409. doi: 10.2174/187152708786927877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daniels GM, Amara SG. Regulated trafficking of the human dopamine transporter. Clathrin-mediated internalization and lysosomal degradation in response to phorbol esters. J Biol Chem. 1999;274:35794–801. doi: 10.1074/jbc.274.50.35794. [DOI] [PubMed] [Google Scholar]
- 49.Melikian HE. Neurotransmitter transporter trafficking: endocytosis, recycling, and regulation. Pharmacology & Therapeutics. 2004;104:17–27. doi: 10.1016/j.pharmthera.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 50.Zahniser NR, Sorkin A. Rapid regulation of the dopamine transporter: role in stimulant addiction? Neuropharmacology. 2004;47:80–91. doi: 10.1016/j.neuropharm.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 51.Foster JD, Adkins SD, Lever JR, Vaughan RA. Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J Neurochem. 2008;105:1683–99. doi: 10.1111/j.1471-4159.2008.05262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boudanova E, Navaroli DM, Stevens Z, Melikian HE. Dopamine transporter endocytic determinants: carboxy terminal residues critical for basal and PKC-stimulated internalization. Mol Cell Neurosci. 2008;39:211–7. doi: 10.1016/j.mcn.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu J, Apparsundaram S, Dwoskin LP. Nicotinic receptor activation increases [3H]dopamine uptake and cell surface expression of dopamine transporters in rat prefrontal cortex. J Pharmacol Exp Ther. 2009;328:931–9. doi: 10.1124/jpet.108.147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vaughan RA, Huff RA, Uhl GR, Kuhar MJ. Protein kinase C-mediated phosphorylation and functional regulation of dopamine transporters in striatal synaptosomes. J Biol Chem. 1997;272:15541–6. doi: 10.1074/jbc.272.24.15541. [DOI] [PubMed] [Google Scholar]
- 55.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 56.Zhu J, Bardo MT, Bruntz RC, Stairs DJ, Dwoskin LP. Individual differences in response to novelty predict prefrontal cortex dopamine transporter function and cell surface expression. Eur J Neurosci. 2007;26:717–28. doi: 10.1111/j.1460-9568.2007.05690.x. [DOI] [PubMed] [Google Scholar]
- 57.Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998;18:1979–86. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith JK, Neill JC, Costall B. Post-weaning housing conditions influence the behavioural effects of cocaine and d-amphetamine. Psychopharmacology (Berl) 1997;131:23–33. doi: 10.1007/s002130050261. [DOI] [PubMed] [Google Scholar]
- 59.Coolon RA, Cain ME. Effects of mecamylamine on nicotine-induced conditioned hyperactivity and sensitization in differentially reared rats. Pharmacol Biochem Behav. 2009;93:59–66. doi: 10.1016/j.pbb.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 60.Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–2. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- 61.McDougall SA, Collins RL, Karper PE, Watson JB, Crawford CA. Effects of repeated methylphenidate treatment in the young rat: sensitization of both locomotor activity and stereotyped sniffing. Exp Clin Psychopharmacol. 1999;7:208–18. doi: 10.1037//1064-1297.7.3.208. [DOI] [PubMed] [Google Scholar]
- 62.Andersen PH, Jansen JA, Nielsen EB. [3H]GBR 12935 binding in vivo in mouse brain: labelling of a piperazine acceptor site. Eur J Pharmacol. 1987;144:1–6. doi: 10.1016/0014-2999(87)90002-1. [DOI] [PubMed] [Google Scholar]
- 63.Gaytan O, Yang P, Swann A, Dafny N. Diurnal differences in sensitization to methylphenidate. Brain Res. 2000;864:24–39. doi: 10.1016/s0006-8993(00)02117-x. [DOI] [PubMed] [Google Scholar]
- 64.Easton N, Steward C, Marshall F, Fone K, Marsden C. Effects of amphetamine isomers, methylphenidate and atomoxetine on synaptosomal and synaptic vesicle accumulation and release of dopamine and noradrenaline in vitro in the rat brain. Neuropharmacology. 2007;52:405–14. doi: 10.1016/j.neuropharm.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 65.Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997;340:249–58. doi: 10.1016/s0014-2999(97)01393-9. [DOI] [PubMed] [Google Scholar]
- 66.Volkow ND, Wang GJ, Fowler JS, Ding YS. Imaging the effects of methylphenidate on brain dopamine: new model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1410–5. doi: 10.1016/j.biopsych.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 67.Huff RA, Vaughan RA, Kuhar MJ, Uhl GR. Phorbol esters increase dopamine transporter phosphorylation and decrease transport Vmax. J Neurochem. 1997;68:225–32. doi: 10.1046/j.1471-4159.1997.68010225.x. [DOI] [PubMed] [Google Scholar]
- 68.Zhu SJ, Kavanaugh MP, Sonders MS, Amara SG, Zahniser NR. Activation of protein kinase C inhibits uptake, currents and binding associated with the human dopamine transporter expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1997;282:1358–65. [PubMed] [Google Scholar]
- 69.Melikian HE, Buckley KM. Membrane trafficking regulates the activity of the human dopamine transporter. J Neurosci. 1999;19:7699–710. doi: 10.1523/JNEUROSCI.19-18-07699.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Granas C, Ferrer J, Loland CJ, Javitch JA, Gether U. N-terminal truncation of the dopamine transporter abolishes phorbol ester- and substance P receptor-stimulated phosphorylation without impairing transporter internalization. J Biol Chem. 2003;278:4990–5000. doi: 10.1074/jbc.M205058200. [DOI] [PubMed] [Google Scholar]
- 71.Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, et al. N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol. 2004;2:E78. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Holton KL, Loder MK, Melikian HE. Nonclassical, distinct endocytic signals dictate constitutive and PKC-regulated neurotransmitter transporter internalization. Nat Neurosci. 2005;8:881–8. doi: 10.1038/nn1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Del Arco A, Segovia G, Canales JJ, Garrido P, de Blas M, Garcia-Verdugo JM, et al. Environmental enrichment reduces the function of D1 dopamine receptors in the prefrontal cortex of the rat. J Neural Transm. 2007;114:43–8. doi: 10.1007/s00702-006-0565-8. [DOI] [PubMed] [Google Scholar]
- 74.Green TA, Gehrke BJ, Bardo MT. Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacology (Berl) 2002;162:373–8. doi: 10.1007/s00213-002-1134-y. [DOI] [PubMed] [Google Scholar]
- 75.Swanson CJ, Kalivas PW. Regulation of locomotor activity by metabotropic glutamate receptors in the nucleus accumbens and ventral tegmental area. J Pharmacol Exp Ther. 2000;292:406–14. [PubMed] [Google Scholar]
- 76.Sesack SR, Bunney BS. Pharmacological characterization of the receptor mediating electrophysiological responses to dopamine in the rat medial prefrontal cortex: a microiontophoretic study. J Pharmacol Exp Ther. 1989;248:1323–33. [PubMed] [Google Scholar]
- 77.Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–42. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- 78.Rahman S, Bardo MT. Environmental enrichment increases amphetamine-induced glutamate neurotransmission in the nucleus accumbens: a neurochemical study. Brain Res. 2008;1197:40–6. doi: 10.1016/j.brainres.2007.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pamplona FA, Pandolfo P, Savoldi R, Prediger RD, Takahashi RN. Environmental enrichment improves cognitive deficits in Spontaneously Hypertensive Rats (SHR): relevance for Attention Deficit/Hyperactivity Disorder (ADHD) Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1153–60. doi: 10.1016/j.pnpbp.2009.06.012. [DOI] [PubMed] [Google Scholar]