Abstract
Arginase (ARG), the enzyme that catalyzes the conversion of arginine to ornithine and urea, is the first and committed step in polyamine biosynthesis in Leishmania. The creation of a conditionally lethal Δarg null mutant in Leishmania mexicana has established that ARG is an essential enzyme for the promastigote form of the parasite and that the enzyme provides an important defense mechanism for parasite survival in the eukaryotic host. Furthermore, human ARGI (HsARGI) has also been implicated as a key factor in parasite proliferation. Thus, inhibitors of ARG offer a rational paradigm for drug design. To initiate a search for inhibitors of the L. mexicana ARG (LmARG), recombinant LmARG and HsARGI enzymes were purified from Escherichia coli. Both LmARG and HsARGI were specific for L-arginine and exhibited no activity with either D-arginine or agmatine as possible substrates. LmARG exhibited a Km of 25 ± 4 mM for L-arginine, a pH optimum ~9.0, and was dependent upon the presence of a divalent cation, preferentially manganese. A Km of 13.5 ± 2 mM for L-arginine was calculated for the HsARGI. A collection of 37 compounds was evaluated against both enzymes. Twelve of these compounds were identified as being either strong inhibitors of both LmARG and HsARGI or differential inhibitors between the two enzymes. Of the 12 compounds, six were selected for further analysis and the type and extent of inhibition determined.
Keywords: Leishmania, Arginase, Polyamines, Inhibitors, Amino acids
1. Introduction
Leishmania are protozoan parasites that cause a spectrum of disfiguring to deadly diseases in humans. The genus is digenetic with the extracellular, flagellated promastigote existing in the phlebotomine sandfly host, while the intracellular, non-motile amastigote resides within the phagolysosome of macrophages and other reticuloendothelial cells in the mammalian host. A key effector in mammalian macrophages that inhibits the extent of infection of a variety of obligatory intracellular pathogens, including Leishmania, is nitric oxide, which is synthesized from L-arginine by the inducible nitrogen oxide synthase (iNOS) (Mauel et al., 1991; Wanasen and Soong, 2008). iNOS shares its amino acid substrate with arginase (L-arginine ureohydrolase; EC 3.5.3.1, ARG), a metalloenzyme that catalyzes the hydrolysis of L-arginine to L-ornithine and urea. Ornithine is an intermediate in the urea cycle in mammalian cells and can also serve as a precursor for the synthesis of proline, glutamate and polyamines. Whether macrophages kill or tolerate Leishmania depends on the delicate balance between the two competing iNOS and ARG activities that are reciprocally regulated by cytokines secreted by Th1 and Th2 CD4+ T helper cells, respectively (Iniesta et al., 2001; Wanasen and Soong, 2008).
Human cells express two ARG enzymes; human ARG I (HsARGI) is a cytosolic enzyme that primarily functions in hepatocytes as a component of the urea cycle, while human arginase II (HsARGII) is broadly distributed among tissues and primarily found in the mitochondrial matrix. Interestingly, murine bone marrow and peritoneal macrophages express robust levels of HsARGI mRNA and protein after up-regulation by Th2 cytokines, although quiescent macrophages express negligible levels of HsARGI (Louis et al., 1999; Munder et al., 1999). Unstimulated macrophages also constitutively express HsARGII at levels that are unresponsive to Th2 cytokines (Louis et al., 1999; Munder et al., 1999). Both HsARGI and HsARGII have been extensively characterized at the biochemical level, and high resolution crystal structures of the two enzymes have been determined (Cox et al., 2001; Cama et al., 2003a, c; Shin et al., 2004; Di Costanzo et al., 2005). In contrast, Leishmania only express a single ARG enzyme. The availability of genetic Δarg knockouts of both Leishmania mexicana and Leishmania major have proven that the sole function of the leishmanial ARG, a glycosomal enzyme, is to serve as precursor for the biosynthesis of polyamines (Roberts et al., 2004; Reguera et al., 2009), ubiquitous aliphatic cations found in virtually every eukaryotic cell that play vital roles in such physiological processes as growth, differentiation and macromolecular biosynthesis (Pegg and McCann, 1982; Pegg, 2009).
Because robust activity of host ARG removes substrate available for nitric oxide synthesis via iNOS, ARG is widely viewed as a viable therapeutic target. Furthermore, it is well-documented in the murine infectivity model of L. major that an increased expression of host ARGI in susceptible Balb/c mice is associated with exacerbation of parasitemia in activated macrophages (Iniesta et al., 2001, 2002, 2005; Taylor-Robinson, 2001; Kropf et al., 2003, 2005). Employing inhibitors of ARG, several groups have independently demonstrated that ARG activity is important for the intracellular survival and growth of L. major in murine macrophages and mice (Iniesta et al., 2001, 2002; Kropf et al., 2005). Nω-hydroxy-L-arginine (NOHA) dramatically reduces parasite loads in infected macrophages, a result that can be reversed by supplementation with ornithine (Iniesta et al., 2001). In addition, Nω-hydroxy-nor-L-arginine (nor-NOHA) has been shown to diminish ARG activity, lesion size, and tissue parasite burden in infected mice (Iniesta et al., 2005; Kropf et al., 2005). While nor-NOHA does not reduce parasite ARG activity in intact parasites (Kropf et al., 2005), NOHA inhibits proliferation of L. major promastigotes by targeting ARG (Reguera et al., 2009).
ARG was found to be an indispensable enzyme for promastigote proliferation, since L. mexicana and L. major Δarg parasites rely on ornithine or polyamine supplementation for survival (Roberts et al., 2004; Reguera et al., 2009). The abilities of L. mexicana and L. major Δ arg null mutants to retain their capacity to infect Balb/c mice implies both that amastigotes of these cutaneous species can salvage sufficient host ornithine or polyamines to at least partially meet their own polyamine requirements and that the parasite ARG by itself is not essential for maintenance of intracellular infection. However, the considerably reduced infectivity phenotypes of the L. mexicana and L. major Δarg mutants in mice also suggest that the parasite ARG is necessary for optimal infectivity.
Intriguingly, the reduced infectivity of the L. mexicana Δarg parasites appears to correlate with an increased production of nitric oxide by the infected macrophages (Gaur et al., 2007). Similarly, immunohistochemistry of tissues from mice infected with L. mexicana Δarg revealed higher levels of nitrosylated tyrosine residues compared with tissues from mice infected with wild type parasites (Gaur et al., 2007). The reduced infectivity phenotype of the L. major Δarg parasites, in contrast, does not appear to correlate with increased nitric oxide production (Muleme et al., 2009).
Due to the relevance of both the host and parasite ARG activities in the maintenance of leishmanial virulence, we performed an initial pharmacological profile of the L. mexicana ARG (LmARG), with a particular focus on its comparative pharmacological features with HsARGI. We purified LmARG in large and replenishable quantities, determined its kinetic parameters and response to various divalent cations and pH changes, and compared its pharmacological profile with that of the purified HsARGI with respect to a battery of 37 potential inhibitors (http://www.brenda-enzymes.org/). Potent inhibitors of either LmARG or HsARGI were analyzed further for their mechanism of inhibition. This pharmacological profile provides a basis for differential or dual inhibition of the human and LmARG activities.
2. Materials and methods
2.1. Chemicals and reagents
Metal salts, isopropyl β-D-1-thiogalactopyranoside (IPTG), ARG substrates and commercially available ARG inhibitors were purchased from Sigma-Aldrich® (St. Louis, MO, USA) and Fisher Scientific (Pittsburgh, PA, USA). Ni2+-nitrilotriacetate (Ni-NTA) agarose beads were procured from Qiagen (Valencia, CA, USA). Complete Mini EDTA-free protease inhibitor and bovine pancreatic RNAase were bought from Roche Diagnostics Corp. (Indianapolis, IN, USA). The TOPO-TA and pET200/D-TOPO® expression kits, the HiFi Platinum Supermix for PCR, oligonucleotide primers, and the Qubit™ fluorometer and protein quantification kits were all obtained from Invitrogen (Carlsbad, CA, USA). The QuantiChrom™ Arginase Assay Kit was acquired from BioAssay Systems (Hayward, CA, USA) and Biosafe™ Coomassie from Bio-Rad Laboratories Life Science Research (Hercules, CA, USA). The two known inhibitors of the human ARGs, 2(S)-amino-6-boronohexanoic acid (ABH), and S-(2-boronoethyl)-L-cysteine (BEC), as well as a hexahistidine-(His6-)tagged version of the HsARGI cDNA ligated into the pET11d vector were graciously supplied by Dr. David Christianson, University of Pennsylvania (Philadelphia, PA, USA). For the purposes of this article, the HsARGI cDNA expression plasmid is referred to as pETHsARGI.
2.2. Expression and purification of LmARG
The cloning of LmARG (GenBank™ accession number AF038409) has been reported (Roberts et al., 2004). The LmARG open reading frame (ORF) was amplified from a cosmid DNA template using PCR technology. Oligonucleotide sense and antisense primers were 5'-CACCATGGAGCACGTGCAGCAG-3’ and 5'-CTACAGCTTGGAGCTCGTATGCGGAGT-3', respectively. The amplified LmARG ORF was inserted into the pET200/D-TOPO® Escherichia coli expression vector, which automatically attaches a (His6)-tag to the NH2-terminus of the expressed protein and transformed into One Shot® TOP10 E. coli according to the manufacturer's directions. The chimeric plasmid was designated pETLmARG. Appropriate segments within pETLmARG were sequenced in both directions in order to ensure sequence fidelity and correct insertion of the LmARG ORF within the expression plasmid. pETLmARG was then purified from the One Shot® TOP10 E. coli and transformed into BL21 Star ™ (DE3) One Shot® E. coli for over-expression. LmARG was expressed using 1 mM IPTG according to the manufacturer's brochure except that the E. coli were grown at 30 °C and the growth media included 10% glycerol. Four liters of transformed E. coli that had been treated with IPTG were lysed in 200 ml of 50 mM sodium phosphate buffer, pH 8.0 containing 0.5 M NaCl, a dissolved protease inhibitor cocktail tablet (Roche Diagnostics, Indianopolis, IN, USA), and 5 µg ml−1 units of DNAase (Sigma-Aldrich Corp., St. Louis, MO, USA) by means of two passages through a French press at 12,000 psi. The bacterial lysate was then clarified by centrifugation at 10,000 g for 20 min at 4 °C. Lysate was loaded onto a 3.0 ml Ni-NTA column and washed with 5 vol. of E. coli lysis buffer. Purified LmARG protein was eluted by the addition of 250 mM imidazole to the lysis buffer. Individual 0.5 ml elution fractions were collected, and eluted protein in each fraction was quantified on a Qubit® fluorometer using the Quant-iT™ Protein Assay Kit. The purity of the final eluted product was confirmed by fractionation on a 10% SDS-PAGE and subsequent staining with Biosafe™ Coomassie.
His6-tagged HsARGI was purified using the identical purification protocol as described above for LmARG except the expression plasmid contained the pET11d backbone and the enzyme was purified from 500 ml of transformed E. coli.
2.3. LmARG and HsARGI activity measurements
All LmARG and HsARGI assays were performed on freshly purified protein at 37 °C in 100 mM N-cyclohexyl-2-aminoethanesulfonic acid (CHES) buffer, pH 9.5, unless otherwise noted. Immediately prior to the assay, HsARGI and LmARG were activated in 25 mM maleic acid and 2 mM MnCl2 (unless otherwise noted) at 37 °C for 15 min. ARG assays were carried out using three adjacent rows of 96-well microtiter plates (Becton-Dickinson Biosciences, Le Pont de Claix, France) in 200 µl vol.: the first row was employed for dilution of activated LmARG or HsARGI protein into the CHES buffer, the second row contained L-arginine in buffer to initiate the enzyme reaction, and the subsequent rows were pre-loaded with 20 µl glacial acetic acid to quench the ARG reaction. The reactions were initiated by the addition of an appropriate dilution of activated LmARG or HsARGI protein that gave linear kinetics to the corresponding well containing the L-arginine. The ARG reactions were terminated by removal of 20 µl aliquots from the reaction mixture and their addition to the glacial acetic acid. The urea product of the ARG reactions was quantified by removal of a 5 µl aliquot of the quenched reaction and measuring the urea colorimetrically using the Quantichrom™ urea assay kit. Appropriate amounts of standards, e.g., urea (0 – 10 mM) and arginine (0 – 200 mM), diluted in CHES buffer, were also quantified using the Quantichrom™ kit. Two types of measurements were obtained: i) the kinetics of ARG activity with respect to time were acquired over five to six time points that were generally spaced 30 s apart, and production of urea versus time calculated by linear regression; and ii) endpoint assays were performed under linear conditions in which a single aliquot was taken at a single time point and total urea product measured.
2.4. Determination of ARG kinetic parameters
Km determinations were performed at L-arginine concentrations ranging from 7.8 – 50 mM for LmARG and 2.5 – 200 mM for HsARGI. Protein concentrations in the assay were calculated with the Qubit™ fluorometer in order to calculate the kcat value.
2.5. pH optima
pH optima for LmARG were determined over a pH range of 7.5 – 11.0 in 0.5 pH unit increments in appropriate adjusted buffers containing 150 mM 3-(N-morpholino)propanesulfonic acid, 150 mM CHES, and 150 mM N-cyclohexyl-3-aminopropanesulfonic acid. The concentration of L-arginine in these assays was 250 mM. Velocity assays were used to determine the reaction rate for each pH value, and ARG activity calculated as a percentage of the average peak velocity of three independent measurements.
2.6. Ion dependence
To determine the ion dependence of LmARG, residual ions from the purified enzyme preparation were first chelated with 2 mM EDTA, followed by dialysis at 4 °C against 1 L of 25 mM maleic acid buffer, pH 7.0, to remove the EDTA. Divalent cations (BaCl2, CaCl2, CdCl2, CoCl2, CuSO4, FeSO4, HgCl2, MgSO4, MnCl2, NiCl2, Pb(NO3)2, SnCl2, or ZnCl2) at 2 mM concentrations were then added back to the dialyzed enzyme preparation, and the mixture heat-activated at 37 °C for 15 min. Control enzyme samples without any divalent cation or to which 2 mM EDTA was added were included in each experiment. The LmARG reaction in these experiments was performed in the presence of 250 mM L-arginine and was terminated after 5 min, a time point during which ureohydrolysis was linear.
2.7. Inhibitor profiles
A panel of 37 compounds bearing some structural relationship to L-arginine was tested for the ability to inhibit LmARG and HsARGI activities. These compounds are listed in Table 1. Each of these compounds was evaluated for its ability to inhibit LmARG and HsARGI at a concentration of 40 mM, and the L-arginine substrate concentration was fixed at 40 mM to establish the inhibitory effects of these compounds against the L. mexicana and human enzymes. Residual rates of ARG activity were calculated as a percentage of the uninhibited velocity for both enzymes. Selected inhibitors were subjected to additional kinetic analysis by either performing velocity assays under a variety of inhibitor and substrate concentrations or by calculating the Ki from the panel data.
Table 1.
Compounds tested for inhibition of Leishmania mexicana arginase (LmARG) and human ARGI (HsARGI).
| Adenosine | Guanidinoacetic acid |
| Agmatine | L-Homoargenine |
| 2(S)-amino-6-boronohaxanoic acid (ABH) | DL-Homocysteine |
| γ-Amino-butyric acid (GABA) | L-Leucine |
| 8-Aminoguanine | L-Lysine |
| D-Arginine | L-Methionine |
| Blasticidin S | NG-Methyl-L-arginine |
| Boric acid | Mitoguazone (MGBG) |
| S-(2-boronoethyl)-L-cysteine (BEC) | α-Methyl-ornithine |
| 8-Bromoguanine | Monoglutathionyl-spermidine disulphide |
| Cadaverine | Nω-Hydroxy-nor-L-arginine (nor-NOHA) |
| Creatine | Nω-Hydroxy-L-arginine (NOHA) |
| L-Cysteine | Ornithine |
| Difluoromethylornithine | L-Proline |
| Ethylenediamine | Putrescine |
| L-Glutamate | Spermidine |
| L-Glutamine | Trypanothione |
| Glutathione | Urea |
| Guanidine thiocyanate |
Two of the compounds, D-arginine and agmatine, were also tested as possible substrates for both LmARG and HsARGI. These reactions were carried out with either 50 mM D-arginine or 50 mM agmatine in the absence of L-arginine and allowed to progress for 30 min. A separate tube containing 50 mM L-arginine was employed as a positive control in this substrate specificity analysis. Urea produced from D-arginine or agmatine was then quantified as above for L-arginine.
2.8. Data analysis
To determine initial velocities, data were analyzed by linear regression using Microsoft Excel. If the data were significantly non-linear (r2 < 0.85), then the data point was eliminated. Non-linear regression, curve-fitting and transformation of data to create Dixon and Haynes-Woolf plots were performed in Prism4 for Macintosh (version 4.0b, GraphPad Software, San Diego California USA, www.graphpad.com). Prism4 was also employed for error analysis of the data. For calculation of Ki values, data presented in Fig. 5 were used with the following equations (Segel, 1975). Competitive inhibition:
| (1) |
Uncompetitive inhibition:
| (2) |
where [I] is the concentration of inhibitor, [S] is the concentration of substrate, and a is the relative velocity measured in the reaction.
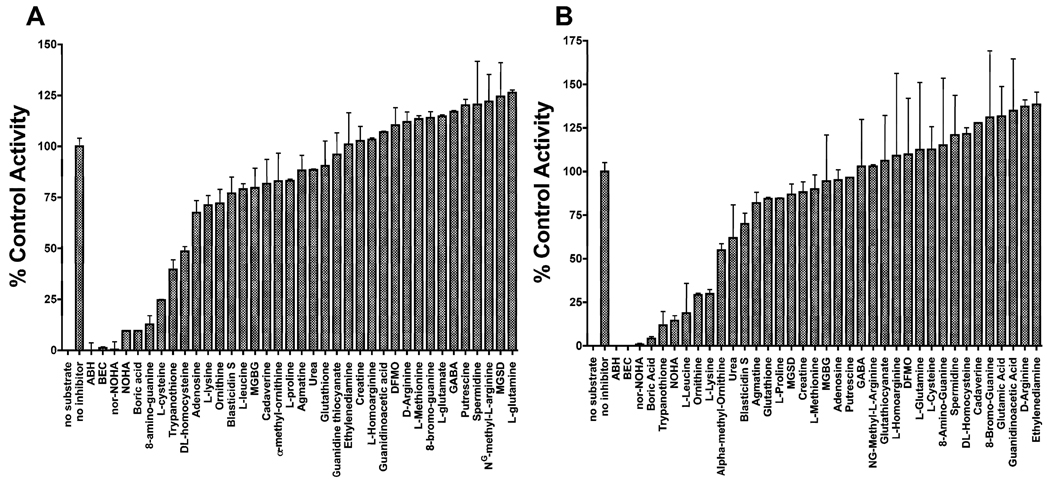
Fig. 5.
Inhibition profiles for Leishmania mexicana arginase (LmARG) (A) and human ARGI (HsARGI) (B). The inhibitor effects of a variety of different chemicals on the LmARG (A) and HsARGI (B) catalyzed hydrolysis of L-arginine were measured in the presence of 40 mM of each chemical. Data are expressed as a percentage of no-inhibitor control. Presented are the mean ± S.D. of two separate experiments. ABH, 2(S)-amino-6-boronohexanoic acid; BEC, S-(2-boronoethyl)-L-cysteine; Nor-NOHA, Nω-Hydroxy-nor-L-arginine; NOHA, Nω-hydroxy-L-arginine; MGBG, methylglyoxal bis-guanylhydrazone; DFMO, α-difluoromethylornithine; GABA, γ-aminobutyric acid; and MGSD, monoglutathionyl-spermidine disulphide.
2.9. Parasite proliferation assays
Leishmania mexicana (MNYC/BZ/62/M379) promastigotes were cultivated in DME-L medium, a completely defined Dulbecco’s modified Eagle-based medium that was specifically designed for growing Leishmania promastigotes (Iovannisci and Ullman, 1983). To determine toxicity of select compounds against L. mexicana, 5 × 104 promastigotes were seeded in 100 µl vol. with serial two-fold dilutions of inhibitor. After 5 days, alamarBlue™ (Biosource International, Camarillo, CA, USA) was added and reduction of alamarBlue™ to resorufin quantified after 16 h at 570 and 600 nm on a Multiskan Ascent plate reader (Thermo Labsystems, Vantaa, Finland). Results of experiments are expressed as a percentage of alamarBlue™ reduction in inhibitor-treated cultures compared with untreated control cultures and are the mean values and S.D.s of triplicate experiments.
3. Results
3.1. Expression and purification of LmARG
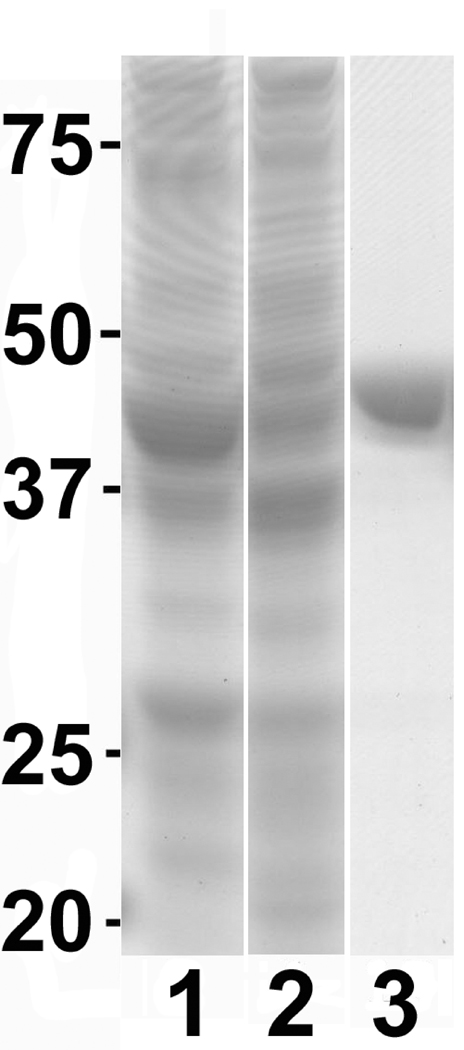
LmARG expression in E. coli was induced with IPTG from the pETLmARG expression construct and purified to effective homogeneity over a Ni-NTA column (Fig. 1). Fractionation of the purified protein on a denaturing gel revealed a single polypeptide that migrated slightly more slowly than its predicted ~39 kDa molecular mass (Fig. 1). The overall yield of the pure recombinant LmARG was ~10 mg per liter. HsARGI was also purified to homogeneity, as previously reported (Di Costanzo et al., 2007), from an extract of the pETHsARGI E. coli transformant with similar yields (data not shown).
Fig. 1.
Leishmania mexicana arginase (LmARG) expression and purification. Recombinant LmARG was over-expressed and purified from Escherichia coli as described in Section 2.2. Shown are the crude lysates from E. coli transformed with LmARG following a 4 h induction with isopropyl β-D-1-thiogalactopyranoside (IPTG) (lane 1), without induction (lane 2), and the first elution fraction from a Ni2+-nitrilotriacetate (Ni-NTA) agarose column (lane 3), as fractionated by SDS-PAGE. The migration and size in kDa of molecular mass markers is indicated to the left of the figure.
3.2. Kinetic analysis of LmARG
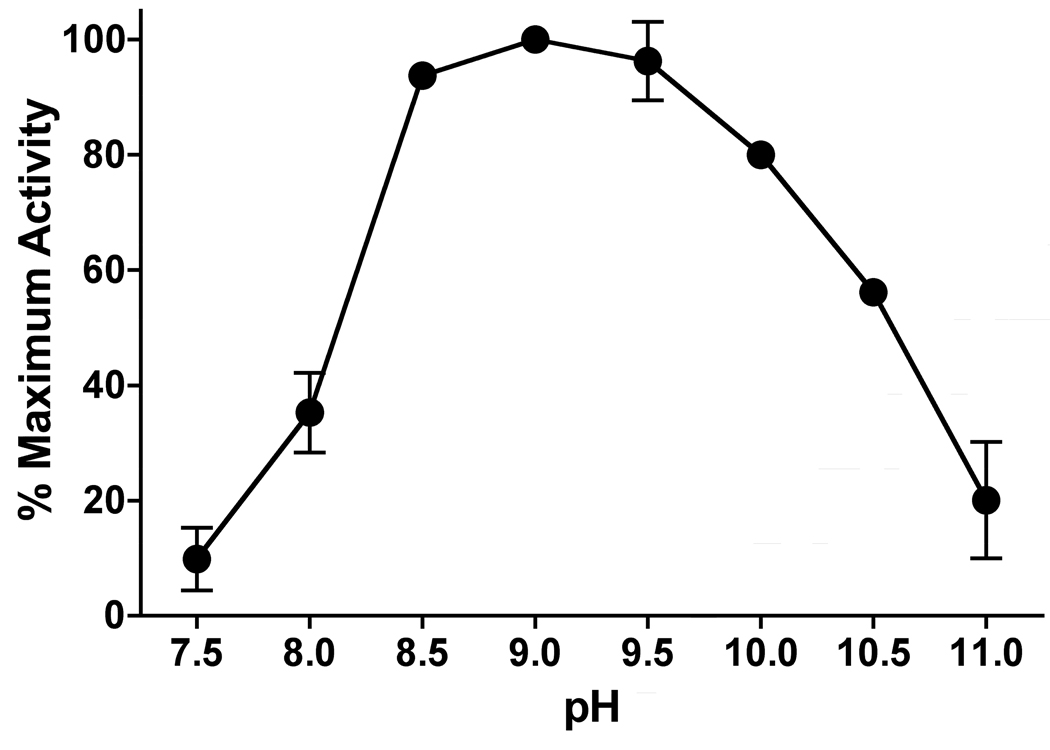
In order to evaluate the pharmacophore profile of LmARG, an assessment of ideal assay conditions and kinetic parameters was an essential prerequisite. LmARG displayed a broad pH optimum peak centered at pH 9.0 (Fig. 2). Activity dropped off steeply below pH 8.5, and more gradually at pH values greater than 9.5. At these pH values, the α-carboxylate and guanidino groups of the L-arginine substrate are fully charged, while the α-NH2 moiety is partially protonated. The basic pH optimum for LmARG is similar to that previously determined for HsARGI (Beruter et al., 1978). Based on these pH optimum data, all subsequent ARG activity measurements were performed at pH 9.0.
Fig. 2.
Effect of pH on Leishmania mexicana arginase (LmARG) activity. LmARG-catalyzed urea production from L-arginine was measured over a pH range of 7.5 – 10.5. Data shown are the mean ± S.E.M. of three independent experiments.
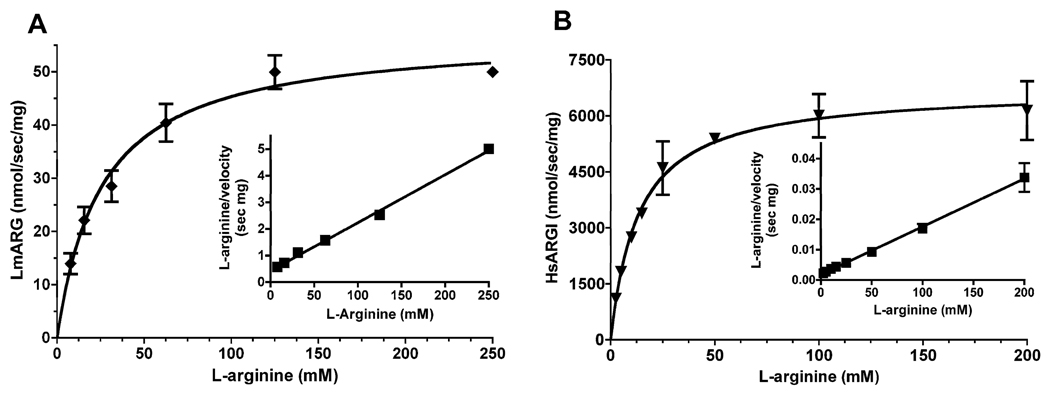
Steady state kinetic analysis showed that LmARG displayed classic Michaelis-Menten kinetics as a function of L-arginine concentration. Non-linear regression revealed a Km of 25 ± 4 mM for L-arginine (Fig. 3A), a value virtually identical to that (21.5 ± 0.90 mM) previously obtained for the Leishmania amazonensis enzyme (da Silva et al., 2008). The kcat for LmARG was calculated to be ~1.7 s−1. The HsARGI also exhibited Michaelis-Menten kinetics with a Km of 13 ± 2 mM for L-arginine (Fig. 3B). LmARG did not recognize either D-arginine or L-agmatine (decarboxylated L-arginine) as substrate (data not shown).
Fig. 3.
Michaelis-Menten kinetics of Leishmania mexicana arginase (LmARG) and human ARGI (HsARGI). The velocities of the LmARG (A) and HsARGI (B) were determined as a function of L-arginine concentration of substrate. The data were plotted by non-linear regression with r2 values of 0.9188 and 0.8913 for LmARG and HsARGI, respectively. Hanes-Woolf plots of the Michaelis-Menten are presented in the insets of each panel and verify the results obtained by non-linear regression. The line in the Haynes-Woolf plot is the standard linear regression line with an r2 value of 0.9857 for LmARG and 0.9399 for HsARGI, respectively. The data are the mean ± S.D. of three independent measurements for each enzyme.
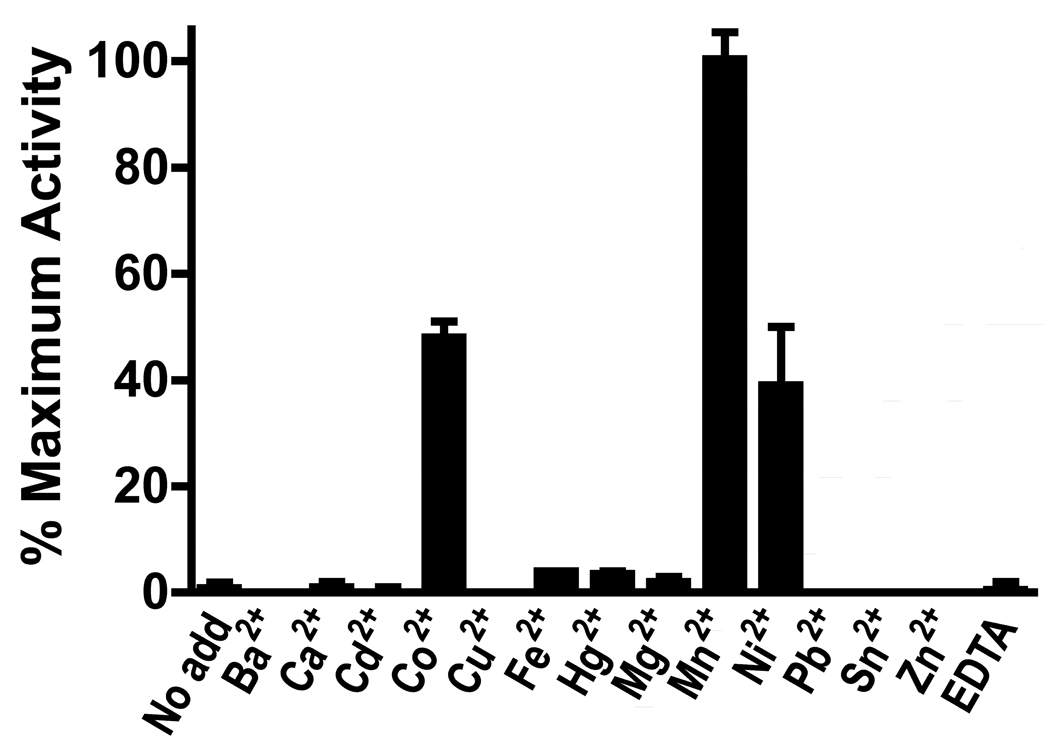
Manganese is the customary divalent cation required by members of the ureohydrolase family (Cama et al., 2003b). LmARG was inactive in the absence of added cation or in the presence of EDTA (Fig. 4). The enzyme exhibited the most robust activity in the presence of manganese, but cobalt and nickel were also able to support LmARG activity at ~48% and ~39%, respectively, of the maximal levels obtained with manganese (Fig. 4). Other metal ions did not support ARG activity. Because the LmARG amino acid sequence (Roberts et al., 2004) contains a potential zing finger motif (residues 289–320), the effect of zinc on manganese activation of LmARG was examined. Co-activation with manganese and zinc reduced activity to zero (data not shown).
Fig. 4.
Effects of divalent cation on Leishmania mexicana arginase (LmARG) activity. LmARG activity was measured in the absence or presence of a variety of different cations at 2 mM and/or 2 mM EDTA. Data are shown as a percentage of LmARG activity in the presence of Mn2+ and are the mean ± S.D. of two separate experiments.
3.3. Inhibitor profiles of LmARG and HsARGI
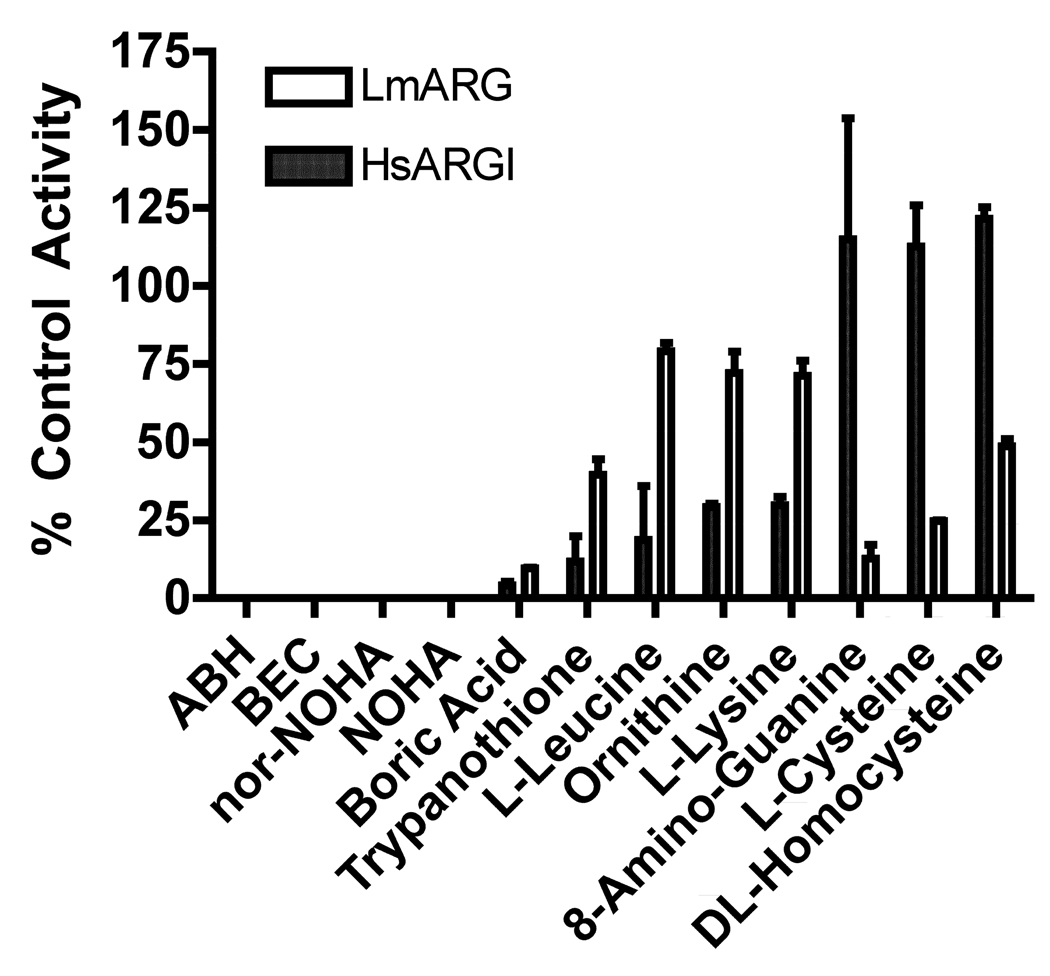
A collection of 37 compounds that are either known inhibitors of HsARGI activity or structural analogs of L-arginine (http://www.brenda-enzymes.org/) was evaluated for the ability to inhibit both LmARG and HsARGI activities. Each compound was screened at a 40 mM concentration in the presence of 40 mM L-arginine substrate. The majority of these chemicals did not significantly (<25%) inhibit either LmARG or HsARGI under these conditions (Fig. 5). Nine compounds, ABH, BEC, nor-NOHA, NOHA, boric acid, 8-amino-guanine, L-cysteine, trypanothione, and DL-homocysteine inhibited the LmARG enzyme by >50% under these conditions, while nine chemicals, ABH, BEC, nor-NOHA, NOHA, boric acid, trypanothione, L-leucine, L-ornithine, and L-lysine inhibited the human counterpart by >50% (Figs. 5 and 6). However, four compounds, ABH, BEC, nor-NOHA, and NOHA, completely blocked both HsARGI and LmARG activities under the assay conditions (Fig. 5). Furthermore, selective inhibition was observed among several of the inhibitors. Five compounds, boric acid, trypanothione, L-leucine, L-ornithine and L-lysine, and 8-aminoguanine, preferentially inhibited the HsARGI activity, whereas three compounds, 8-aminoguanine, L-cysteine, DL-homocysteine, demonstrated greater potency toward LmARG (Fig. 5).
Fig. 6.
Comparison of selected arginase (ARG) inhibitors. The inhibitory effects of the most potent inhibitors of ARG in Fig. 1 were compared for Leishmania mexicana ARG (LmARG) and human ARGI (HsARGI). The data presented are the means ± S.D. of two independent experiments. ABH, 2(S)-amino-6-boronohexanoic acid; BEC, S-(2-boronoethyl)-L-cysteine; Nor-NOHA, Nω-Hydroxy-nor-L-arginine; NOHA, Nω-hydroxy-L-arginine.
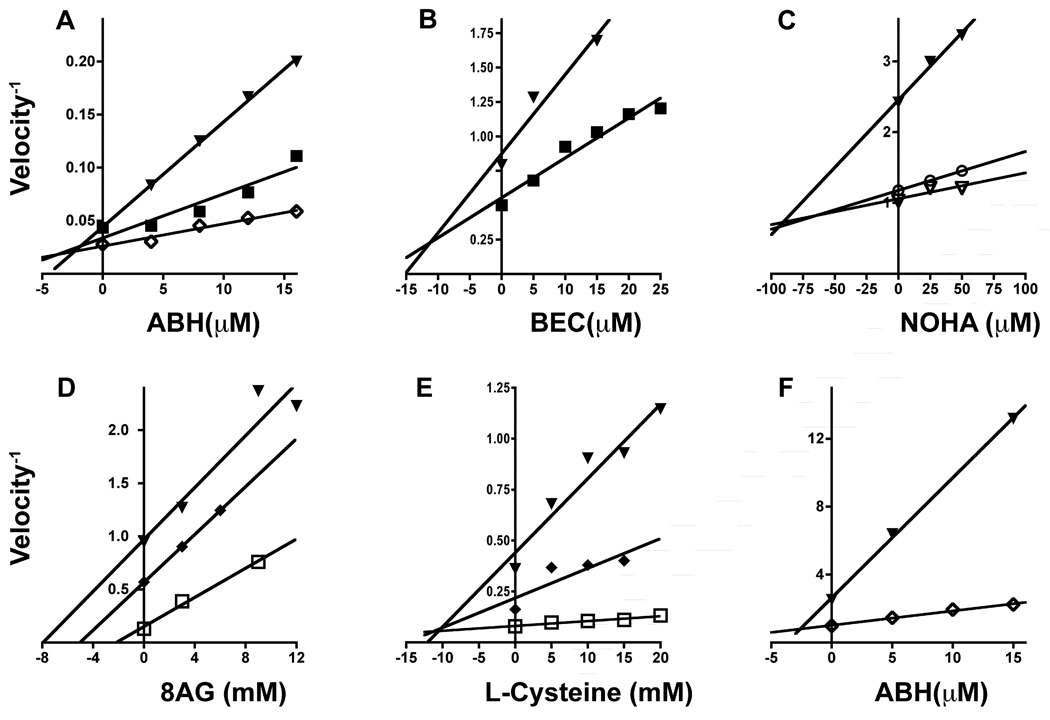
Six of these inhibitors were selected for further investigation by Dixon plot analysis to determine the mode of inhibition and inhibition constants (Ki) of the LmARG-inhibitor complexes (Fig. 7 and Table 2). The Dixon plots revealed that three of the four most potent inhibitors, ABH, BEC and NOHA, all inhibited LmARG in a competitive fashion with Ki values ranging from 1.3 – 85 µM (Fig. 7A–C). Nor-NOHA was not evaluated further due to its structural similarity to NOHA. None of the four compounds, however, was specific for LmARG (Table 2). The type and extent of inhibition of the four most potent inhibitors against HsARGI have already been established (Di Costanzo et al., 2010). 8-aminoguanine, trypanothione and L-cysteine were much less potent inhibitors with Ki values in the mM range for LmARG and HsAGI (Fig. 7 and Table 2). 8-aminoguanine and trypanothione inhibited LmARG in an uncompetitive fashion, while L-cysteine was a competitive inhibitor (Fig. 7D–E). The inhibition of AHB against the HsARGI was tested experimentally by Dixon plot analysis in order to verify the accuracy of our assay method (Fig. 7F).
Fig. 7.
Dixon plots for select inhibitors. 2(S)-amino-6-boronohexanoic acid (ABH) (A), S-(2-boronoethyl)-L-cysteine (BEC) (B), Nω-hydroxy-L-arginine (NOHA) (C), 8-aminoguanine (D) and Lcysteine (E) inhibition of Leishmania mexicana arginase (LmARG) is shown. ABH (F) inhibition of human ARGI (HsARGI) is also exhibited. Data points were obtained by regression of velocity assay data with r2 > 0.85, and linear regression performed on the reciprocals of velocity versus concentration of inhibitor in the reaction for indicated concentrations of L-arginine present as substrate. The units of velocity−1 are given in sec mg protein nmol−1 for all six panels. The selected concentrations of L-arginine are: 10 mM (▼), 20 mM (◆), 25 mM (■), 50 mM (▽), 100 mM (◇), and 150 mM (□).
Table 2.
Summary of type and extent of inhibition for selected compounds against Leishmania mexicana arginase (LmARG) and human ARGI (HsARGI). The extent and type of inhibition for LmARG and HsARGI were determined by Dixon plot analysis or (for those marked with 'a') by calculation from panel data assuming the type of inhibition shown in the final column and using equations (1) and (2) (Section 2.8).
| Inhibitor | Ki vs. LmARG | Ki vs. HsARGI | Type of Inhibition |
|---|---|---|---|
| ABH | 1.3 µM | 3.5 µM | Competitive |
| BEC | ~10 µM | 14 µMa | Competitive |
| nor-NOHA | ~50 µM* | ~10 µMa | Competitive |
| NOHA | 85 µM | ~70 µMa | Competitive |
| 8-Aminoguanine | < 2 mM | N/A | Uncompetitive |
| L-Cysteine | 11 mM | > 30 mMa | Competitive |
| Trypanothione | < 5 mM | < 5 mMa | Uncompetitive |
ABH, 2(S)-amino-6-boronohaxanoic acid; BEC, S-(2-boronoethyl)-L-cysteine; nor-NOHA, Nω-Hydroxy-nor-L-arginine; NOHA, Nω-Hydroxy-L-arginine.
3.4. Effect of LmARG inhibitors on cultured promastigotes
To determine the toxicity of the LmARG inhibitors toward L. mexicana promastigotes, parasites were incubated with various concentrations of the compounds that inhibited LmARG activity by >50% and growth was assessed after 5 days. The only compounds that exhibited any toxicity toward the parasites were NOHA and nor-NOHA with effective concentrations of compound that inhibited growth by 50% (EC50 values) of 6 mM and 3 mM, respectively (data not shown).
4. Discussion
A variety of studies have implicated ARG activity in ameliorating Leishmania infections in mammalian macrophages via the provision of carbon skeletons for polyamine biosynthesis and/or through the depletion of substrate for nitric oxide synthesis (Iniesta et al., 2001, 2002, 2005; Taylor-Robinson, 2001; Kropf et al., 2003, 2005). Thus, inhibitors of ARG offer a rational therapeutic paradigm for the treatment of leishmaniasis. This study was undertaken to assess the effects of known inhibitors of the HsARGI (Iniesta et al., 2001, 2002; Di Costanzo et al., 2005, 2010; Kropf et al., 2005), as well as amino acids, polyamines and a variety of other compounds, on LmARG activity. The recombinant LmARG exhibited similar kinetics, e.g., Km value and pH and ion dependence, to the previously characterized ARG enzyme from L. amazonensis (da Silva et al., 2008), as well as to the human ARGI (Ash et al., 2000; Ash, 2004). At equimolar concentrations of inhibitor and substrate, four compounds, ABH, BEC, nor-NOHA and NOHA, obliterated both LmARG and HsArgI activity, with Ki values in the µM range. ABH and BEC are synthetic boronic acid analogs that form tetrahedral intermediates within the active site of human ARG enzymes (Di Costanzo et al., 2005), whereas NOHA, a hydroxy-arginine analog, is an intermediate of nitric oxide synthesis and nor-NOHA an analog of NOHA. All four are well-characterized inhibitors of mammalian ARG enzymes (Di Costanzo et al., 2005, 2010) and inhibited both LmARG and HsARGI in a competitive manner. Several other compounds tested (Fig. 6) appeared to be selective for either the leishmanial or human enzymes. Dixon plot analysis revealed that 8-aminoguanine and L-cysteine, both of which affect LmARG to a much more significant extent than HsARGI, inhibited LmARG in an uncompetitive and competitive mode, respectively.
Although NOHA and nor-NOHA are both potent inhibitors of the recombinant LmARG enzyme, their effects on ARG activity in intact parasites is disparate. While nor-NOHA does not inhibit the ability of intact L. major promastigotes to convert arginine to ornithine (Kropf et al., 2005), another study showed that NOHA has an EC50 of 40 µM against L. major promastigotes, while L. major ARG over-producers were resistant to the drug (Reguera et al., 2009). Our own studies indicate that only millimolar concentrations of NOHA or nor-NOHA even slightly impaired growth of L. mexicana promastigotes, although this modest inhibition could be overcome by putrescine supplementation (data not shown). It remains to be determined whether the lack of efficacy of NOHA and nor-NOHA against intact L. mexicana is due to limited uptake into the parasite or delivery to the glycosome, the organelle in which ARG resides (Roberts et al., 2004).
HsARGI has emerged as a key factor for parasite virulence (Iniesta et al., 2001, 2002, 2005; Taylor-Robinson, 2001; Kropf et al., 2003, 2005; Wanasen and Soong, 2008). Numerous studies have found an augmented activity of HsARGI associated with increased parasitemia (Iniesta et al., 2001, 2002, 2005; Taylor-Robinson, 2001; Kropf et al., 2003, 2005). Furthermore, pharmacological inhibition of host ARG with either NOHA or nor-NOHA causes significant reduction of parasite numbers in macrophages or tissue parasite burden and lesion size in infected mice, respectively (Iniesta et al., 2001, 2002; Kropf et al., 2005). Because the selective inhibition host ARG by nor-NOHA is not sufficient to clear parasitemia in mice, it is reasonable to presume that the parasite ARG also plays a significant role in infectivity. However, it cannot be ruled out in these studies with nor-NOHA that the host ARGI is not completely inhibited or that polyamines are being produced by HsARGII or via a newly described alternative pathway using arginine decarboxylase and agmatinase (Sastre et al., 1998).
ARG-deficient strains of L. mexicana (Roberts et al., 2004; Gaur et al., 2007) and L. major (Muleme et al., 2009; Reguera et al., 2009) that were created via double targeted gene replacement are still capable of eliciting cutaneous disease in Balb/c mice, although the virulence phenotypes were clearly attenuated. Because the Δarg Leishmania are auxotrophic for polyamines, the diminished virulence phenotype of these knockouts implies that the cutaneous macrophages in which these parasites reside have residual supplies of either polyamines or polyamine precursors to sustain amastigote growth. The diminished virulence of the Δarg L. mexicana null mutant is associated with an increased amount of nitric oxide production, a phenomenon that has been observed in vitro and in vivo (Gaur et al., 2007). This increased capacity of the host to produce nitric oxide may be due to higher levels of available arginine caused by decreased arginine scavenge from the Δarg L. mexicana parasites. The conjecture that the parasite may counteract the accumulation of intracellular arginine by reducing arginine import reasonable, since it has been demonstrated that expression and activity of the L. donovani LdAAP3 arginine transporter is regulated by intracellular levels of arginine (Darlyuk et al., 2009). Thus, it remains unclear whether the reduced infectivity of the Δarg L. mexicana is due to to polyamine scavenge being less efficient than endogenous polyamine biosynthesis and/or due to the ability of the host to synthesize more nitric oxide. Regardless, it is evident that while parasite ARG activity is not essential for parasite survival, it is important for disease pathogenesis.
Taken together, previously published reports intimate that both host and parasite ARG activities contribute to the pathogenesis of Leishmania parasites. It is apparent that inhibition of leishmanial ARG activity by itself, at least in cutaneous strains, is insufficient to eliminate infection in vivo, and has also been shown that inhibition of the host ARG alone appears to be inadequate as a therapeutic strategy (Iniesta et al., 2001; Kropf et al., 2005). The hypothesis that inhibition of host parasite ARG alone is insufficient to prevent Leishmania infections can presumably be tested in recently developed mice strains in which the ARGI gene has been specifically eliminated in murine macrophages by gene targeting strategies (El Kasmi et al., 2008). Mice harboring the conditional gene deletion at the HsARGI locus were considerably less susceptible to experimental infections of both Mycobacterium tuberculosis and Toxoplasma gondii (El Kasmi et al., 2008).
Whether dual inhibition of both enzymes is a superior therapeutic strategy merits further investigation. To this end, we have provided evidence that LmARG and HsARGI are similar enough in their kinetic behavior to be targeted by an inhibitor. Furthermore, it should be noted that the ARG activity assay established in this work can easily be scaled for high throughput in screening assays for discovery of novel inhibitors of the LmARG and/or HsARGI enzymes.
Acknowledgements
This work was supported in part by grant R01 AI041622 from the National Institute of Allergy and Infectious Disease, USA. We thank Dr. David Christianson and Ms. Heather Gennadios [University of Pennsylvania, USA] for supplying ABH, BEC, and the HsARGI vector.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ash DE. Structure and function of arginases. J Nutr. 2004;134:2760S–2764S. doi: 10.1093/jn/134.10.2760S. [DOI] [PubMed] [Google Scholar]
- Ash DE, Cox JD, Christianson DW. Arginase: a binuclear manganese metalloenzyme. Met Ions Biol Syst. 2000;37:407–428. [PubMed] [Google Scholar]
- Beruter J, Colombo JP, Bachmann C. Purification and properties of arginase from human liver and erythrocytes. Biochem J. 1978;175:449–454. doi: 10.1042/bj1750449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cama E, Colleluori DM, Emig FA, Shin H, Kim SW, Kim NN, Traish AM, Ash DE, Christianson DW. Human arginase II: crystal structure and physiological role in male and female sexual arousal. Biochemistry. 2003a;42:8445–8451. doi: 10.1021/bi034340j. [DOI] [PubMed] [Google Scholar]
- Cama E, Emig FA, Ash DE, Christianson DW. Structural and functional importance of first-shell metal ligands in the binuclear manganese cluster of arginase I. Biochemistry. 2003b;42:7748–7758. doi: 10.1021/bi030074y. [DOI] [PubMed] [Google Scholar]
- Cama E, Shin H, Christianson DW. Design of amino acid sulfonamides as transition-state analogue inhibitors of arginase. J Am Chem Soc. 2003c;125:13052–13057. doi: 10.1021/ja036365b. [DOI] [PubMed] [Google Scholar]
- Cox JD, Cama E, Colleluori DM, Pethe S, Boucher JL, Mansuy D, Ash DE, Christianson DW. Mechanistic and metabolic inferences from the binding of substrate analogues and products to arginase. Biochemistry. 2001;40:2689–2701. doi: 10.1021/bi002318+. [DOI] [PubMed] [Google Scholar]
- da Silva ER, da Silva MF, Fischer H, Mortara RA, Mayer MG, Framesqui K, Silber AM, Floeter-Winter LM. Biochemical and biophysical properties of a highly active recombinant arginase from Leishmania (Leishmania) amazonensis and subcellular localization of native enzyme. Mol Biochem Parasitol. 2008;159:104–111. doi: 10.1016/j.molbiopara.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Darlyuk I, Goldman A, Roberts SC, Ullman B, Rentsch D, Zilberstein D. Arginine homeostasis and transport in the human pathogen Leishmania donovani. J Biol Chem. 2009;284:19800–19807. doi: 10.1074/jbc.M901066200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Costanzo L, Ilies M, Thorn KJ, Christianson DW. Inhibition of human arginase I by substrate and product analogues. Arch Biochem Biophys. 2010;496:101–108. doi: 10.1016/j.abb.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Costanzo L, Moulin M, Haertlein M, Meilleur F, Christianson DW. Expression, purification, assay, and crystal structure of perdeuterated human arginase I. Arch Biochem Biophys. 2007;465:82–89. doi: 10.1016/j.abb.2007.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Costanzo L, Sabio G, Mora A, Rodriguez PC, Ochoa AC, Centeno F, Christianson DW. Crystal structure of human arginase I at 1.29-A resolution and exploration of inhibition in the immune response. Proc Natl Acad Sci U S A. 2005;102:13058–13063. doi: 10.1073/pnas.0504027102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kasmi KC, Qualls JE, Pesce JT, Smith AM, Thompson RW, Henao-Tamayo M, Basaraba RJ, Konig T, Schleicher U, Koo MS, Kaplan G, Fitzgerald KA, Tuomanen EI, Orme IM, Kanneganti TD, Bogdan C, Wynn TA, Murray PJ. Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat Immunol. 2008;9:1399–1406. doi: 10.1038/ni.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur U, Roberts SC, Dalvi RP, Corraliza I, Ullman B, Wilson ME. An effect of parasite-encoded arginase on the outcome of murine cutaneous leishmaniasis. J Immunol. 2007;179:8446–8453. doi: 10.4049/jimmunol.179.12.8446. [DOI] [PubMed] [Google Scholar]
- Iniesta V, Carcelen J, Molano I, Peixoto PM, Redondo E, Parra P, Mangas M, Monroy I, Campo ML, Nieto CG, Corraliza I. Arginase I induction during Leishmania major infection mediates the development of disease. Infect Immun. 2005;73:6085–6090. doi: 10.1128/IAI.73.9.6085-6090.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniesta V, Gomez-Nieto LC, Corraliza I. The inhibition of arginase by N(omega)-hydroxyl-arginine controls the growth of Leishmania inside macrophages. J Exp Med. 2001;193:777–784. doi: 10.1084/jem.193.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniesta V, Gomez-Nieto LC, Molano I, Mohedano A, Carcelen J, Miron C, Alonso C, Corraliza I. Arginase I induction in macrophages, triggered by Th2-type cytokines, supports the growth of intracellular Leishmania parasites. Parasite Immunol. 2002;24:113–118. doi: 10.1046/j.1365-3024.2002.00444.x. [DOI] [PubMed] [Google Scholar]
- Iovannisci DM, Ullman B. High efficiency plating method for Leishmania promastigotes in semidefined or completely-defined medium. J Parasitol. 1983;69:633–636. [PubMed] [Google Scholar]
- Kropf P, Fuentes JM, Fahnrich E, Arpa L, Herath S, Weber V, Soler G, Celada A, Modolell M, Muller I. Arginase and polyamine synthesis are key factors in the regulation of experimental leishmaniasis in vivo. FASEB J. 2005;19:1000–1002. doi: 10.1096/fj.04-3416fje. [DOI] [PubMed] [Google Scholar]
- Kropf P, Herath S, Weber V, Modolell M, Muller I. Factors influencing Leishmania major infection in IL-4-deficient BALB/c mice. Parasite Immunol. 2003;25:439–447. doi: 10.1111/j.1365-3024.2003.00655.x. [DOI] [PubMed] [Google Scholar]
- Louis CA, Mody V, Henry WL, Jr, Reichner JS, Albina JE. Regulation of arginase isoforms I and II by IL-4 in cultured murine peritoneal macrophages. Am J Physiol. 1999;276:R237–R242. doi: 10.1152/ajpregu.1999.276.1.R237. [DOI] [PubMed] [Google Scholar]
- Mauel J, Ransijn A, Buchmuller-Rouiller Y. Killing of Leishmania parasites in activated murine macrophages is based on an L-arginine-dependent process that produces nitrogen derivatives. J Leukoc Biol. 1991;49:73–82. doi: 10.1002/jlb.49.1.73. [DOI] [PubMed] [Google Scholar]
- Muleme HM, Reguera RM, Berard A, Azinwi R, Jia P, Okwor IB, Beverley S, Uzonna JE. Infection with arginase-deficient Leishmania major reveals a parasite number-dependent and cytokine-independent regulation of host cellular arginase activity and disease pathogenesis. J Immunol. 2009;183:8068–8076. doi: 10.4049/jimmunol.0803979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munder M, Eichmann K, Moran JM, Centeno F, Soler G, Modolell M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol. 1999;163:3771–3777. [PubMed] [Google Scholar]
- Pegg AE. Mammalian polyamine metabolism and function. IUBMB Life. 2009;61:880–894. doi: 10.1002/iub.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg AE, McCann PP. Polyamine metabolism and function. Am J Physiol. 1982;243:C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Reguera RM, Balana-Fouce R, Showalter M, Hickerson S, Beverley SM. Leishmania major lacking arginase (ARG) are auxotrophic for polyamines but retain infectivity to susceptible BALB/c mice. Mol Biochem Parasitol. 2009;165:48–56. doi: 10.1016/j.molbiopara.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SC, Tancer MJ, Polinsky MR, Gibson KM, Heby O, Ullman B. Arginase plays a pivotal role in polyamine precursor metabolism in Leishmania. Characterization of gene deletion mutants. J Biol Chem. 2004;279:23668–23678. doi: 10.1074/jbc.M402042200. [DOI] [PubMed] [Google Scholar]
- Sastre M, Galea E, Feinstein D, Reis DJ, Regunathan S. Metabolism of agmatine in macrophages: modulation by lipopolysaccharide and inhibitory cytokines. Biochem J. 1998;330(Pt 3):1405–1409. doi: 10.1042/bj3301405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segel I. Enzyme kinetics; behaviour and analysis of rapid equilibrium and steady state enzyme systems. New York: Wiley, John and Sons; 1975. [Google Scholar]
- Shin H, Cama E, Christianson DW. Design of amino acid aldehydes as transition-state analogue inhibitors of arginase. J Am Chem Soc. 2004;126:10278–10284. doi: 10.1021/ja047788w. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson A. Th1/Th2-regulated arginase availability modulates Leishmania infection. Trends Parasitol. 2001;17:262. doi: 10.1016/s1471-4922(01)02003-7. [DOI] [PubMed] [Google Scholar]
- Wanasen N, Soong L. L-arginine metabolism and its impact on host immunity against Leishmania infection. Immunol Res. 2008;41:15–25. doi: 10.1007/s12026-007-8012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]