Abstract
This study investigated how aging compromises the control of saccades and eye-hand coordination when accuracy constraints and termination requirements of hand movement are altered. Seventeen older adults and seventeen young controls performed two-segment aiming movements. The first segment had two target sizes to alter accuracy constraints. Two-segment eye movements were always made to first and second targets, whereas hand movements were varied across three hand-movement types with different termination requirements: 1) stop both at the first and second targets, 2) stop at the first target and discontinue, and 3) move through the first target and discontinue. Compared to the young adults, the older adults produced hypometric primary saccades and delayed gaze fixation to the first target. The older adults also modified eye movements less depending on the hand termination and accuracy requirements. After pointing completion to the first target, the older adults maintained their gaze fixation to that target for a longer duration than young adults. However, this prolonged gaze fixation was minimized when a hand termination was not required. Conversely, the prolongation of gaze fixation was magnified when the hand termination was required at the first target while the eye movement was continuing to the next target. Thus, older adults have difficulties in concurrent control of inhibiting hand movement and initiating eye movement at a target within a sequence. Taken together, it is suggested that aging reduces the ability to modify eye movements to meet various behavioral constraints imposed on manual aiming tasks.
Keywords: Gaze anchoring, Kinematics, Eye-hand coordination, Limb control, Oculomotor control, Aging
1 INTRODUCTION
Slowness of movements is a prominent feature of aiming movements in older adults, and it is emphasized when the complexities of tasks are increased (Ketcham et al., 2002; Salthouse, 1985). Older adults are also known to have difficulty coordinating multiple body segments during complex motor tasks. For example, they exhibited deficits in the performance of a multi-joint movement compared to a single-joint movement (Seidler et al., 2002; Ketcham et al., 2004). Age-related changes in a coordination pattern were observed between the arms during bimanual movements (Serrien et al., 2000; Stelmach et al., 1988) as well as among the individual fingers during a multiple-finger force production (Shim et al., 2004; Shinohara et al., 2004).
Despite well documented declines of limb motor function in older adults, evidence of declines in oculomotor function due to aging is mixed (see a brief review in Rand & Stelmach, 2010b). Furthermore, very little is known about how aging affects eye-hand coordination. This is because most of the previous studies focused on investigating age-related deficits of one effector. To our knowledge, only two previous studies examined aging on eye-hand coordination by measuring eye and hand movements together (Rand & Stelmach, 2010b; Warabi et al., 1986). It was shown that when eye movement was accompanied by a hand pointing movement, older adults delayed the initiation of saccades (Warabi et al., 1986) and decreased the velocity of primary saccades (Rand & Stelmach, 2010b). Older adults also anchored their gazes to a pointing target for a longer duration than young adults. These findings suggest that the control of eye movements is compromised in older adults when performing manual aiming movements.
Since increased complexity of motor tasks is known to aggravate the difficulties for older adults in controlling manual movements (Ketcham et al., 2002; Salthouse, 1985), it is pertinent to examine systematically how aging alters eye movements depending on various task complexities and constraints imposed on manual movements. Such an investigation of eye-hand coordination will provide valuable insights into age-related declines in coordinating multiple effectors to perform complex motor tasks. The present study explores these issues by applying a recently developed two-segment aiming task (Rand & Stelmach, 2010a) to older adults. In this task, eye movements were always made to the first and second targets, while the complexities of hand movement were changed by altering both accuracy and termination requirements.
In the current study the accuracy requirement was changed using two target sizes at the first target location. Recent studies have shown that a target size manipulation does not alter the duration of a primary saccade when the eyes move alone to a target (Rand et al., 2010a; Wu et al., 2010). Harris & Wolpert (2006) speculated that it is so because a target in the visual scene needs to be placed within the fovea. Thus, in saccades the fovea is equivalent to target width, thereby being indifferent to a target size manipulation. In contrast, a target size manipulation significantly alters the duration of hand movements (Fitts, 1954). Taking these aspects into consideration, the eyes have a constant level of accuracy constraint across two target sizes for movement execution, while the hand has different levels depending on the target size. Therefore, when the eyes and hand are performed together, the target size manipulation enables us to examine the influence of hand accuracy control on the execution of eye movement.
The termination requirement of hand movements was also altered in three different ways over the first and second targets in this study: 1) stop at both the first and second targets, 2) stop at the first target and discontinue, and 3) move through the first target and discontinue. While hand movements were manipulated, eye movements were always made to both the first and second targets for all conditions. More specifically, eye movements were performed naturally to accompany hand movements to the first target for all conditions. Eye movements to the second target were performed naturally with hand movements for the condition 1 above, but performed separately from hand movements for the conditions 2 and 3 above. The hand-termination requirement at the first target imposes an additional control demand on the first segment compared to moving through the target. This is so because movement amplitude has to be planned and energy of the moving arm has to be dissipated to stop the arm at the target (Dounskaia et al., 2005). Furthermore, the hand-termination requirement at the second target imposes an additional control demand on the second segment compared to no hand movement. As a result, the global task complexity over the entire sequence is lowest when the hand moves through the first target and discontinues, but is highest when the hand stops both at the first and second targets. Applying these accuracy and hand-termination manipulations to older adults and young controls allows us to examine age-related deficits in the control of saccades and eye-hand coordination depending on the complexities of manual aiming movements.
One of the prominent features of the saccades during manual aiming movements is that gaze is fixated to a reach target until the hand movement is completed (gaze anchoring, Neggers & Bekkering, 2000 gaze anchoring, Neggers & Bekkering, 2001). Previous studies in young adults reported that gaze anchoring was terminated in a predictive manner prior to or very shortly after the completion of reaching (Bowman et al., 2009; Flanagan & Johansson, 2003; Johansson et al., 2001; Rand & Stelmach, 2010a). Therefore, the current study also sought to determine whether older adults terminate gaze anchoring in a predictive manner, and whether it is influenced by the complexities of eye and hand movements within a sequence. Preliminary findings have been presented in an abstract form (Rand & Stelmach, 2009).
2 METHODS
2.1 Participants
Seventeen older adults (mean±SD=74.0±3.9 years old; 8 males and 9 females) and seventeen young controls (mean±SD=23.1±3.8 years old; 9 males and 8 females) participated in the study. All participants filled out a health-history questionnaire to exclude those who had a history of diabetics, stroke, arthritis, or other neurological or movement impairments. To assess general cognitive function, the Mini-Mental State Exam (MMSE, Folstein, Folstein, & McHugh, 1975) was administered to all participants; everyone fell in the normal range. The average total score and attention score of MMSE were 29.5±1.4 (mean±SD) and 4.9±0.5 for the older adults, respectively, and 29.9±0.3 and 4.9±0.2 for the young controls, respectively. All participants were right-handed and had normal or corrected-to-normal visual acuity. Everyone was able to read text typed in Arial 12 font presented on a table at a distance of 60 cm. This study was approved by Arizona State University’s Institutional Review Board overseeing the use of human subjects in research. All participants provided written informed consent prior to participation.
2.2 Apparatus and Procedure
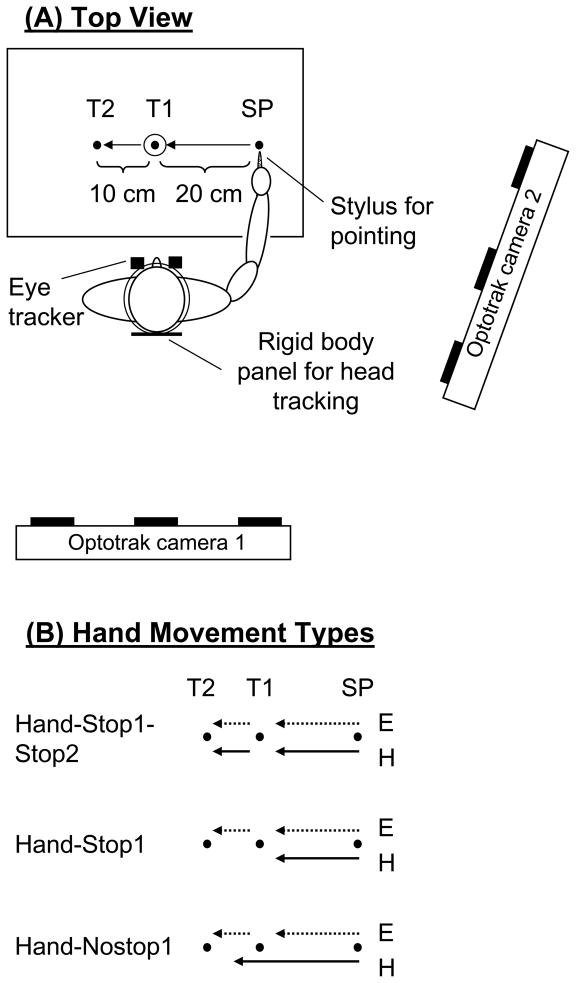
The participants were comfortably seated in front of a table (Fig. 1A). A starting position (SP, 0.5 cm in diameter), a first target (T1) and second target (T2, 0.5 cm in diameter [index of difficulty (ID, Fitts, 1954): 5.3]) were horizontally aligned and displayed in black on a white background on the table. To manipulate terminal accuracy requirement of the first segment, T1 had two sizes (0.5 cm [ID: 6.3] or 4.0 cm [ID: 3.3]). T1 and T2 were located 20 cm and 30 cm left of SP, respectively. The table was tilted at 45° angle from the horizontal. T1 was aligned along the participant’s midline. The viewing distance of the head relative to T1 was 60 cm, resulting in that visual angles from the SP to T1 and from T1 to T2 were 18.4° and 9.5°, respectively.
Figure 1.
Top view of the experimental setup (A). The Optotrak system was used for hand movement recording, and the ASL eye tracker was used for eye movement recording. Two-segment movements were made from a starting position (SP) to a first target (T1), and then to a second target (T2). Two different target sizes (0.5 cm and 4.0 cm in diameter) were used for T1 to vary an accuracy constraint. B: Experimental conditions related to hand termination requirement. For all conditions, the eyes made two-segment movements from the SP to T1, and subsequently to T2. Conversely, hand movements were varied across conditions. The small-T1 size conditions are shown in the figure.
All participants performed single- or two-segment arm movements with their dominant hand, while making two-segment eye movements. Participants held a non-ink stylus as a pointing device and made two distinct movements from the SP to T1 (1st-segment) and then to T2 (2nd-segment). The SP, T1 and T2 were displayed throughout a trial. At the beginning of each trial, the participants placed the tip of the stylus on the SP and fixated their gaze on the SP, and then the examiner said “ready.” After random delay between one-two seconds, an auditory go-signal was delivered. In response to the go-signal, the participants initiated the two-segment movements. No specific instruction was given regarding which effector (eye or hand) to be initiated first. Participants were instructed to reach to the second target as quickly and as accurately as possible. Participants were also instructed to slide the stylus tip on the table surface during movements.
To examine how termination requirements and accuracy constraints of hand movements influenced the control of eye movements, hand movements were varied across conditions (Fig. 1B). However, eye movements were always made to T1 and T2 for all conditions. There were three conditions of hand movement type: 1) terminate the hand movement at both T1 and T2 [hand-stop1-stop2], 2) terminate the hand movement at T1 then to discontinue [hand-stop1], 3) pass through T1 without stopping on it and discontinue [hand-nostop1]. For the hand-nostop1 condition, no specific hand termination location was specified after the hand passed through T1, thereby minimizing the control demand of hand termination. In terms of hand movements, the participants were encouraged not to make pointing errors at both targets. In terms of eye movements, in order to investigate the influence of hand movements on eye movements, no specific instruction was given to the participants whether to look at T1. However, due to the gaze anchoring phenomenon (i.e., gaze is fixated to a pointing target until pointing completion), eye movements were naturally made to T1 for all conditions and also to T2 for the hand-nostop1 condition. For the hand-stop1 and hand-nostop1 conditions, the participants were instructed to make pointing movement to T1 (stop or pass through), and then look at T2.
The three hand movement type conditions were combined with the two sizes of T1, yielding six experimental conditions (3 [hand movement type] × 2 [T1 sizes]). All six conditions were randomized and counterbalanced across participants to reduce practice and fatigue effects. A few familiarization trials were performed before collecting the experimental trials of each condition, so that participants were fully confident with the task requirements. A block of 10 trials was recorded for each condition. When a participant made obvious errors during tasks (such as missing the targets or initiating the movements prior to the go-signal), that trial was repeated at the end of each condition. A total of 60 trials were analyzed.
In this study the stylus was utilized as a pointing device for two reasons. One reason was that precise pointing to the small targets (0.5 cm in diameter) was not easy with the finger tip, because it was much larger than these targets. The other reason was to maximize the control demand of hand termination at a target by employing a sliding (tracing) movement on the table surface instead of lifting the hand off the table for pointing. If the hand were lifted for pointing, the pointing movements would have been automatically stopped when the finger landed on a target, which would have reduced the control demand of hand termination. For sliding movements, it was important to minimize the friction between the effector and the table surface and the inter-subject variability of that friction. Therefore, the stylus was utilized.
Participants’ hand movements were recorded at 240 Hz by using an Optotrak 3-D system (Northern Digital). Horizontal eye movements were recorded by using ASL Eye-Trac 6000 (Applied Science Laboratories). This noninvasive system, which is based on using infrared light to visualize the pupil, samples the movement of each eye as well as the head at 240 Hz. The eye tracker was calibrated for each participant before data recording, using nine saccadic targets across the aforementioned table. Two sets of optotrak cameras were mounted in two separate elevated positions in order to visualize all infrared emitting diode markers (IREDs). The IREDs were attached to the tip of the stylus, three corners of the table top and on 6 locations of a rigid-body panel (HR-06221, Northern Digital). The panel was mounted to the back of a headgear with which the ASL eye cameras were attached (Fig. 1A). The eye tracker was calibrated in relation to the Optotrak system using x, y, z coordinates of three IREDs placed on the table, so that ocular gaze coordinates (the intersection of gaze direction with the table) and the position of the stylus tip were transformed to a common coordinate system. Head position during participants’ performance was not restrained and was recorded from the six IREDs on the back panel of the headgear. Head position and the angles of the eyes in the head allowed the calculation of the ocular gaze coordinates on the table by the ASL eye tracker. Spatial resolution of the optotrak system was 0.001 cm and that of the ASL system was 0.1 degree. To synchronize the Optotrak and ASL systems, the Optotrak controlled the initiation of data recording of both devices. The participant’s gaze position during the experiment was displayed on-line on a computer screen viewed by the experimenters. This enabled them to monitor the participant’s performance during each trial.
2.3 Data Analysis
Data analysis was focused on the first segment and the transition from the first segment to the second. For the analysis of hand movement, velocity was calculated as the first derivative of horizontal position data. Derivatives were calculated using the sliding window technique, where the data points within the window (the window width was 7 points) were approximated with a quadratic polynomial. The polynomial was then used for calculating the analytic derivative at the window’s center. Thus, calculating derivatives using this method also provided data filtering. Subsequently, peak velocity was measured during the first segment. Movement onset was determined as the first data point after a velocity threshold (1.5 cm/s) was reached. Movement offset of the first segment was detected when velocity fell below 1.5 cm/s. One exception to this was the movement offset of the hand-nostop1 condition, where the offset was determined as the time in which the tip of the stylus passed through the center of T1. These landmarks were first automatically detected using computer software. Subsequently, the results of this automatic procedure were inspected, and corrected manually as needed. Acceleration time and deceleration time of the first segment were measured from the onset to the peak velocity and from the peak velocity to the offset, respectively.
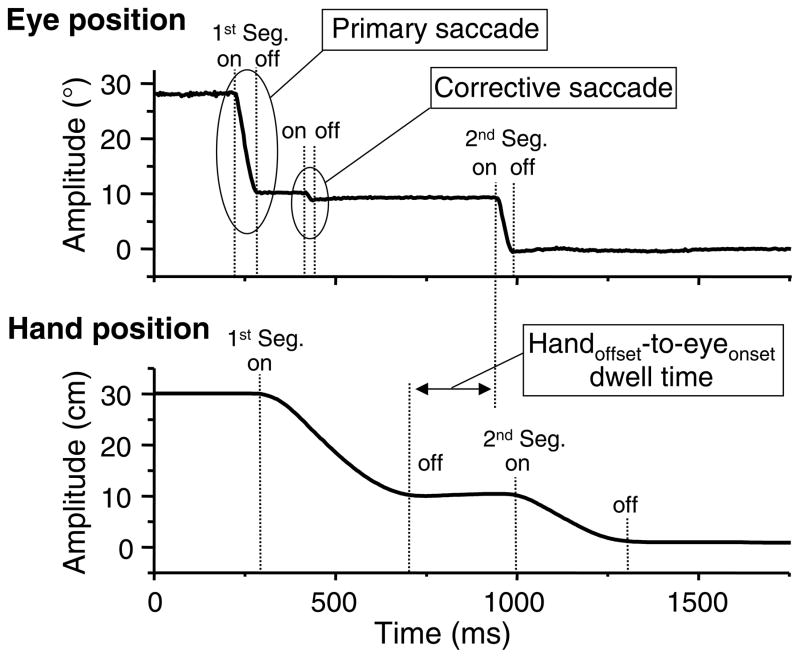
For the analysis of eye movement, velocity of saccades was calculated as the first derivative of horizontal position data from the left eye by applying a standard procedure of two-point signal differentiation. Data filtering was not performed to avoid any types of distortion of signal. Saccade onset (for each segment) and offset (for the first segment) of primary saccades as well as the onset and offset of corrective saccades (for the first segment) were determined using a threshold criterion of 18.4°/s. This value was equivalent to 20 cm/s on gaze coordinates (the intersection of gaze direction with the table) of this study. This criterion was determined empirically by examining a large sample of eye velocity profiles. Fig. 2 shows an example of a primary saccade and a corrective saccade. Peak velocity of primary saccade to T1 was determined. These landmarks were first automatically detected using computer software. Subsequently, the obtained results were inspected, and corrected manually as needed. Duration of primary saccade to T1 was measured from the onset to offset. Amplitude of primary saccade to T1 was measured as a distance between two horizontal gaze locations recorded at the saccade onset and offset. In terms of corrective saccades to T1, percentage of trials containing corrective saccades was calculated for each condition. To examine the time spent to stabilize gaze fixation to T1, time of visual capture was measured from the onset of primary saccade to the end of last corrective saccade.
Figure 2.
An example of eye and hand movements. Horizontal eye and hand positions from a trial of the hand-stop1-stop 2 condition are plotted against time. The eye and hand movements are made to both T1 (first segment movement [1st Seg.]) and T2 (second segment movement [2nd Seg.]). The eye movement shows an example of a primary saccade followed by a corrective saccade during the first segment movement. The eye and hand movements also show gaze anchoring at T1 (the gaze is held on T1 until the completion the hand movement). Handoffset-to-eyeonset dwell time is measured from the hand offset at T1 to the saccade onset to T2.
For the transition from the first to the second segment, handoffset-to-eyeonset dwell time was measured from the offset of hand movement to T1 to the saccadic onset to T2 (Fig. 2). This parameter was used to assess whether gaze was anchored to the first target until pointing to that target was completed and the time spent to prepare a gaze shift to T2 after the pointing completion to T1.
For each participant, a mean value for the ten trials was obtained for each of the experimental conditions. These mean values were used for statistical analysis. A 2 (Group) × 3 (Hand movement type: hand-stop1-stop2, hand-stop1, hand-nostop1) × 2 (T1 size: small vs. large) ANOVA with repeated measures was applied to determine the aging effect and effects of termination requirements and accuracy constraints on each variable. The probability level for statistical significance was p<0.05. When appropriate, post-hoc analysis was carried out using t-test with Bonferroni adjustments.
3 RESULTS
3.1 Hand movements for the first segment
Before examining the influence of hand movements on the control of saccades, we first report kinematic characteristics of hand movements altered depending on the hand termination and accuracy requirements.
3.1.1 Movement time, acceleration time, and deceleration time
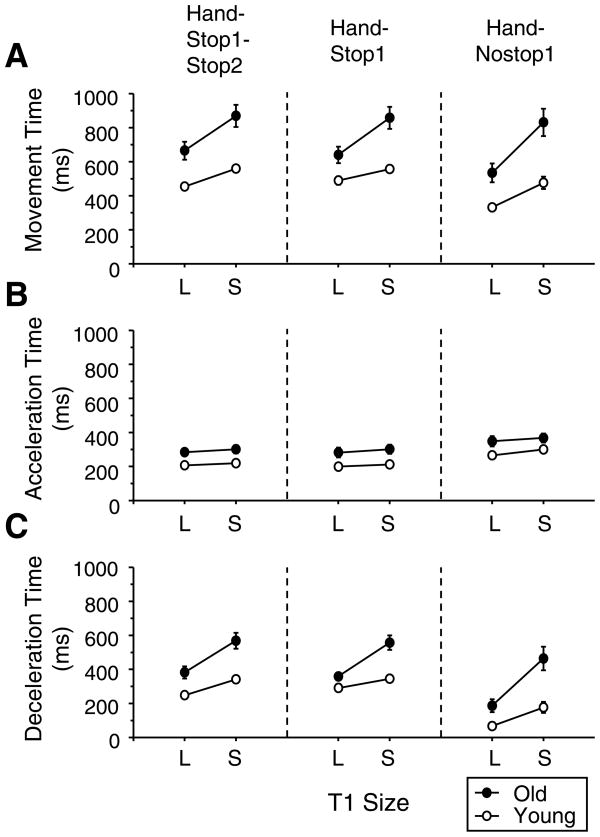
The older adults exhibited significantly longer movement times to T1 than the young adults across all conditions (Fig. 3AF(1,32)=17.5, p<0.001), being consistent with numerous previous studies (Bellgrove et al., 1998; Cooke et al., 1989; Darling et al., 1989; Ketcham et al., 2002; Walker et al., 1997). When movement time was divided into the acceleration (Fig. 3B) and deceleration time (Fig. 3C), the older adults had a significantly longer acceleration time (F(1,32)= 8.9, p<0.001) and deceleration time (F(1,32)= 19.9, p<0.001) than the young adults. Across both groups, the movement durations were altered depending on the hand movement type (movement time: F(2,64)=22.9, p<0.001; acceleration time: F(2,64)=64.9, p<0.001; deceleration time: F(2,64)=71.9, p<0.001). A post-hoc test indicated that the hand movement was of shorter duration when the hand moved through the T1 (hand-nostop1) compared to when the hand landed on the T1 for the hand-stop1-stop2 and hand-stop1 conditions (p <0.001 in all three parameters). The two latter conditions did not differ from each other. Both groups also spent significantly longer time for the small T1 compared to the large T1 (movement time: F(1,32)=130.7, p<0.001; acceleration time: F(1,32)=13.2, p<0.001; deceleration time: F(1,32)=89.2, p<0.001).
Figure 3.
Mean movement times of hand movements are presented for all hand movement type conditions from the first segment (A). The movement time is divided into acceleration time (B) and deceleration time (C). Movements were analyzed from the start position to T1 for all conditions. L and S refer to the large and small T1 conditions, respectively. The error bars represent the SE.
As expected from previous studies (Bellgrove et al., 1998; Cooke et al., 1989; Darling et al., 1989; Ketcham et al., 2002; Romero et al., 2003), the older adults showed a disproportionately prolonged deceleration phase for the small T1 from the large T1 compared to the young adults (see Fig. 3C). Accordingly, there was a significant interaction between group and T1 size for the movement time (F(1,32)=19.6, p<0.001) and the deceleration time (F(1,32)=17.3, p<0.001). An interaction between hand movement type and T1 size was also significant for the movement time (F(2,64)=4.7, p<0.001) and deceleration time (F(2,64)=6.1, p<0.001). As can be seen in Fig. 3A and C, this was due to the fact that the difference between the two target sizes became substantially greater for the hand-nostop1 condition than other hand-type conditions. No other main effect was found.
3.1.2 Peak velocity
Peak velocity of hand movements for the older adults was significantly lower (806±46 [SE] mm/s on average across participants) than the young adults (573±62 mm/s, F(1,32)=9.1, p<0.01). Peak velocity was significantly slower for the small T1 than for the large T1 (F(1,32)=63.3, p<0.001). The average peak velocity for the large T1 across two groups was 708±45 mm/s (hand-stop1-stop2), 750±47 mm/s (hand-stop1), and 860±69 mm/s (hand-nostop1). The corresponding value for the small T1 was 611±39 mm/s (hand-stop1-stop2), 643±41 mm/s (hand-stop1), and 566±40 mm/s (hand-nostop1). There was an interaction between hand movement type and T1 size (F(2,64)=13.9, p<0.001). This was because both groups disproportionately altered the peak velocity depending on the T1 size in the hand-nostop1 condition compared to other hand-type conditions. No other main effect was found.
3.2. Saccadic eye movements for the first segment
3.2.1 Duration and amplitude of primary saccades
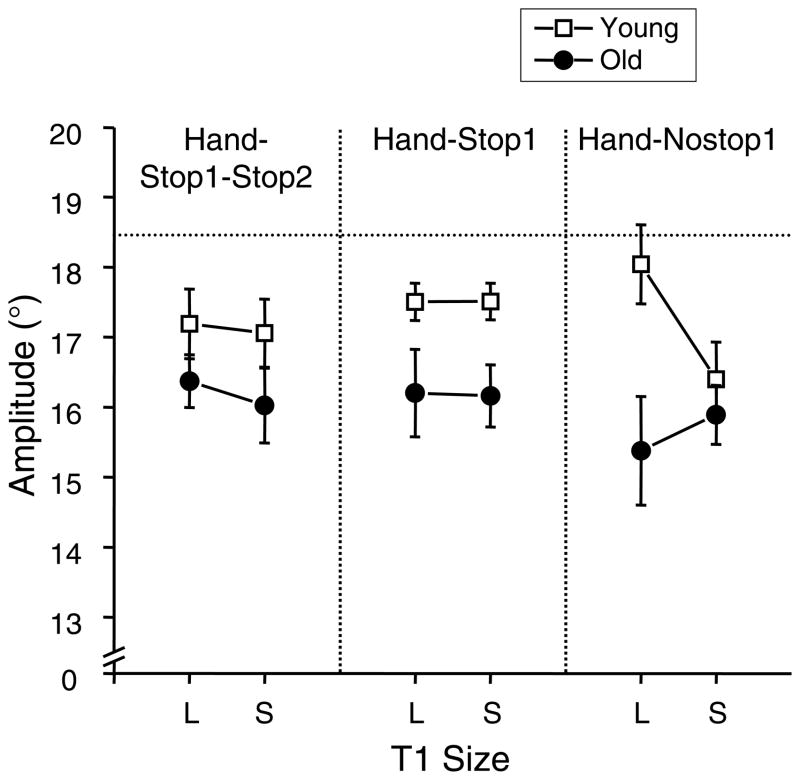
Mean duration of primary saccade to T1 across all participants and all conditions was 64±3 (SE) ms for the young adults and 65±3 ms for the older adults. The saccade duration was not affected by aging, the hand movement type manipulation or the T1 size manipulation. This finding of the T1 size manipulation is in agreement with a previous study (Wu et al., 2010). In contrast, the saccade amplitude was affected by aging and these manipulations. The older adults made significantly shorter saccades compared to the young adults (Fig. 4F(1,32)=5.2, p<0.05). Even though there was no hand-type main effect (p>0.05), an interaction effect between group, hand movement type, and T1 size was significant (F(2,64)=5.6, p<0.01). As can be seen in Fig. 4, this effect was caused by that the T1 size effect was different between two groups for the hand-nostop1 condition, while that effect was similar for other hand-type conditions. In the hand-nostop1 condition, the young adults made the largest saccadic amplitude among all hand movement type conditions for the large T1, but made the smallest amplitude for the small T1. The T1 size difference for this condition was significant for the young adults (p<0.05), but non-significant for the older adults (p>0.05).
Figure 4.
Mean amplitudes of primary saccades during the first segment are plotted for all hand movement type conditions. The filled circle symbol refers to the older adults, and the open square symbol refers to the young controls. L and S refer to the large and small T1 conditions, respectively. The horizontal dotted line refers to the target amplitude. The error bars represent the SE.
3.2.2 Frequency of corrective saccades and time of visual capture
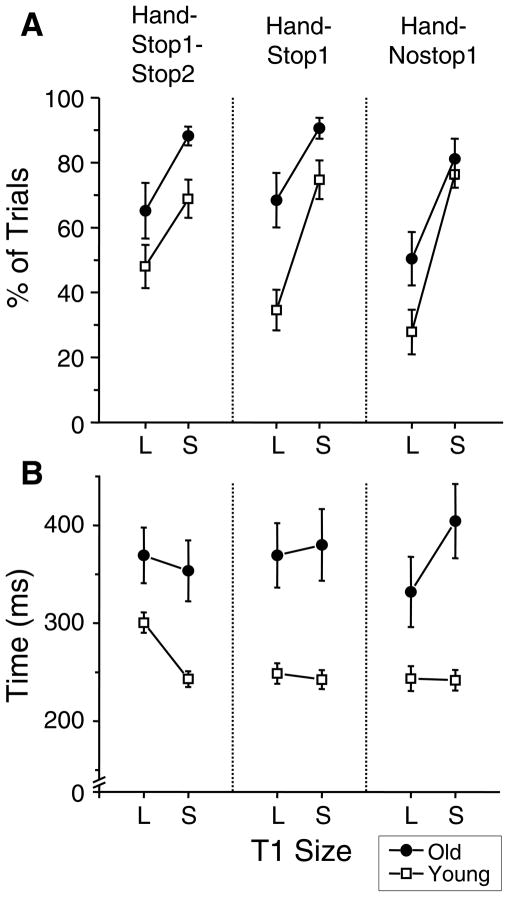
After completing the primary saccades, the participants often made some corrective saccades before gaze was stabilized at T1. The percentage of trials in which the participants made a corrective saccade is presented in Fig. 5A. The older adults made a corrective saccade more frequently across trials than the young adults (F(1,32)=7.8, p<0.01). Across groups, the corrective saccades were made more frequently for the small target than for the large target (F(1,32)=91.9, p<0.001), which is consistent with a previous study (Wu et al., 2010). The percentage of trials with the corrective saccades also changed depending on the hand movement type (F(2,64)=6.5, p<0.001). A post-hoc analysis revealed that the hand-nostop1 condition had lower percentage of trials with corrective saccades compared to the hand-stop1-stop2 condition (p<0.05) and hand-stop1 condition (p<0.05). Across groups, the difference between the two target sizes in this parameter was the greatest for the hand-nostop1 condition and the smallest for the hand-stop1-stop2 condition (see Fig. 5A). Accordingly, an interaction between the hand movement type and T1 size was significant (F(2,64)=3.2, p<0.05). Additionally, there was a trend of an interaction between group and target size. As seen in Fig. 5A, the difference between small and large T1 in this parameter tended to be greater for the young adults than that for the older adults.
Figure 5.
Mean percentages of trials containing corrective saccades (A) and mean times of target capture (B) are presented for all hand movement type conditions. The filled circle symbol refers to the older adults, and the open square symbol refers to the young controls. L and S refer to the large and small T1 conditions, respectively. The error bars represent the SE.
For trials containing corrective saccades, the time of visual capture was measured from the onset of primary saccade to the end of last corrective saccade in order to examine the time spent to stabilize the gaze fixation (Fig. 5B). The older adults spent a significantly longer time to stabilize the gaze than the young adults (F(1,32)=16.2, p<0.001). There was a significant interaction between hand movement type and T1 size (F(2,64)=4.5, p<0.05). There was also a significant interaction between group and T1 size (F(1,32)=4.5, p<0.05), because older adults mostly increased the time of visual capture for the small T1 compared to the large T1, whereas young adults decreased it (see Fig. 5B).
3.3 Gaze shift for the second segment
The time that the eyes spent from hand-offset at T1 to eye-onset toward T2 was analyzed to examine whether the initiation of saccades to T2 relative to the pointing completion to T1 was delayed for the older adults. Fig. 6 shows the average handoffset-to- eyeonset dwell times across all participants and across two T1 sizes. For most conditions, the dwell time had positive values. This indicates that the gazes were anchored at T1 during pointing movement and the gazes were continued to be anchored until shortly after the hand reached that target (Fig. 2). Such a gaze anchoring phenomenon was expected from previous studies (Neggers & Bekkering, 2000,Neggers & Bekkering, 2001).
Figure 6.
Mean handoffset-to-eyeonset dwell times across participants and across T1 size conditions are plotted for all hand movement type conditions. The dwell time was measured from the hand offset at T1 to the saccade onset to T2. White and black columns refer to the young and older adult groups, respectively. The error bars represent the SE.
The older adults had a significantly longer dwell time than the young adults (F(1,32)=11.6, p<0.01). Across groups, the initiation of the saccades to T2 was significantly delayed for the small T1 compared to the large T1 (F(1,32)=125.0, p<0.01). There was a significant main effect of hand movement type (F(2,64)=10.7, p<0.001) as well as an interaction between group and hand movement type (F(2,64)=4.6, p<0.01). As can be seen in Fig. 6, this was due to the fact that the young adults differentially shortened the dwell time compared to the older adults for the hand-stop1 condition. At the same time, the older adults substantially shortened the dwell time for the hand-nostop1 condition compared to other conditions, thereby reducing the difference between the two groups. A post-hoc test revealed that the group difference was significant when the hand terminated at the T1 (hand-stop1-stop2: p<0.05, hand-stop1: p<0.01), but not when the hand moved through the T1 (hand-nostop1: p>0.05).
It is also noteworthy that young adults produced a negative dwell time on average for the hand-stop1 condition with large T1 (−94±28 [SE] ms), indicating that the gaze was shifted to the second target prior to the completion of pointing. In this condition, the average value for the older adults was still positive (64±38 ms). Very interestingly, however, the older adults were able to shift their gazes nearly simultaneously to the pointing completion to T1 for the hand-nostop1 condition with large T1. As a result, the average value for the older adults (−4±36 ms) for this condition was similar to that for the young adults (−2±19 ms).
4 DISCUSSION
4.1 Execution of saccadic eye and hand movements during the first segment
For both young and older adults, the hand-nostop1 condition showed the largest modification of hand kinematic characteristics among all hand movement type conditions depending on the accuracy constraint (Fig. 3). When the accuracy requirement was low, both groups benefited from the low control demand of the hand for this condition (i.e., no hand termination at T1 and no hand movement to T2), which resulted in the fastest hand movements. Conversely, when accuracy constraint was high, both groups produced the slowest hand movements with a prolonged deceleration phase. Thus, instead of benefiting from the low hand control demand, the participants were disadvantaged by the unnatural dual task between two effectors at T1 (eye to stop vs. hand to move through). The pronounced movement slowing is likely due to an increased processing demand both for increasing the hand movement accuracy and preparing two different movements between the two effectors.
Reflecting these changes, the amplitude of primary saccades for the young adults was similarly modified in the hand-nostop1 condition. The young adults produced the longest saccade amplitude when the accuracy requirement was low, but the shortest saccade amplitude when it was high (Fig. 4). This suggests that the oculomotor system takes into account the complexity of hand movements when controlling eye movements. In contrast, the saccadic amplitude for the older adults was hypometric and unchanged regardless of the accuracy and hand movement type conditions (Fig. 4). Even when the control demand of the hand was lowest (hand-nostop1 condition with large T1), the older adults did not increase the saccade amplitude. Thus, the older adults executed eye movements independently from the complexity of hand movements, indicating a low integration between the two effectors. A hypometric saccade amplitude in older adults was previously reported when hand movements accompanied eye movements instead of eye movements alone (Rand & Stelmach, 2010b). The current findings further suggest that older adults have a difficulty in modifying eye movements based on task complexities imposed on the hand movements.
In this study, the amplitude of primary saccades changed depending on the groups or experimental conditions, but saccade duration did not change by these factors. Thus, the relationship between the amplitude and duration of primary saccades was changed between the groups as well as among experimental conditions. These findings appear to contradict a well-known consistent linear relationship between duration, peak velocity and amplitude of saccades (the ‘main sequence’, Bahill et al., 1975; Harris & Wolpert, 2006). However, research has shown that this relationship can change depending on different experimental paradigms (Groh and Sparks 1996; Smit et al., 1987; Zambarbieri et al., 1982) and whether or not eye movements are accompanied by hand movements (Epelboim et al., 1997). The current results are in agreement with these studies. In terms of the aging effect on this relationship, saccade duration was the same between the two groups. However, the saccade amplitude of older adults was shorter than that of the young adults (Fig. 4). Their peak velocity (387±18 °/s in average across all conditions) was also lower than that of the young adults (411±15 °/s). Thus, the older adults executed saccades more slowly and in a more hypometric manner relative to a given saccade duration compared to the young adults. This suggests that aging alters the main sequence when eye movements are executed with hand movements.
The results of corrective saccades showed that the target size manipulation significantly affected the occurrence of these saccades in both groups (Fig. 5). The same effect was previously observed when eye movements were performed alone (Rand et al., 2010a; Wu et al., 2010). On the other hand, the target size manipulation did not affect the duration and amplitude of primary saccades in both groups except for the saccade amplitude of the hand-nostop1 condition for young adults. These observations together suggest that the oculomotor system deals with various target sizes through a utilization of corrective saccades (Wu et al., 2010). Conversely, the limb motor system deals with those by altering the kinematic parameters of hand movements (Fig. 3). An important finding is that the occurrence of the corrective saccades was modified by the combinations of the hand termination and accuracy requirements (Fig. 5). Thus, the occulomotor system takes into account the complexities of hand movements when controlling corrective saccades.
In terms of aging effects on corrective saccades, the older adults produced a greater percentage of trials with corrective saccades than the young adults for all conditions (Fig. 5A). These age-related changes were in part attributed to the fact that the older adults tended to make shorter primary saccades compared to the young adults (Fig. 4), thereby increasing a need to adjust gaze location to the target. However, the older adults also differentially increased the time needed to fixate gazes on T1 compared to the young adults when the accuracy constraint of hand movement was high (Fig. 5B). Thus, there likely is an age-related deficit in stabilizing gazes. Taken together, the results of corrective saccades suggest that older adults are more limited than young adults in controlling gaze fixation in response to various accuracy constraints.
4.2 Gaze shift at the transition between two segments
Older adults anchored their gazes to T1 until pointing to T1 was completed (Fig. 6), being in agreement with previous studies from young adults (Bowman et al., 2009; Neggers & Bekkering, 2000; 2001) and older adults (Rand & Stelmach, 2010b; Warabi et al., 1986). An informative finding of this study is that older adults significantly delayed their gaze shifts compared with young adults only when the hand termination requirement at T1 was imposed (hand-stop1-stop2, hand-stop1). Thus, the hand termination is a factor that causes the delay in ending gaze anchoring for older adults. One possible reason for this is that older adults have a problem to dissipate the energy of the moving hand to make a complete stop at the target. In fast aiming movements, a triphasic (agonist-antagonist-agonist) EMG profile is typically observed, and the second and third EMG bursts are used for the complete stop at the target (Berardelli et al., 1996; Brown & Cooke, 1990; Engelhorn, 1983; Takatoku & Fujiwara, 2010). Thus, the older adults may have problems with controlling these specific muscular activities, which in turn causes a delay in terminating gaze anchoring.
Another possible reason is that older adults find it difficult to effectively assess the complete stop of hand movement at the target. The assessment of such complete stop is made by multi-modal sensory signals (such as visual, proprioceptive, tactile) (Bowman et al., 2009; Johansson et al., 2001). Thus, the older adults may have difficulty with processing these various sensory signals to determine the complete stop of hand movements. Indeed, previous research has reported an age-related proprioceptive degeneration (Ferrell et al. 1992; Hay et al. 1996; Petrella et al. 1997) and position sense deterioration (Hurley et al. 1998; Madhavan & Shields, 2005; Neeuwsen et al. 1993; Romero et al. 2003; Verschueren et al. 2002) as well as an age-related tactile sense decline (Cole et al. 1999; 2001; Desrosiers et al. 1999; Ranganathan et al. 2001). These deficits likely necessitate older adults to utilize more visual feedback to estimate the hand termination, thereby resulting in a delay in ending gaze anchoring.
Another informative finding is that differences between the two groups in terminating gaze anchoring were most pronounced in the hand-stop1 condition (Fig. 6). The result suggests that older adults have a deficit in a concurrent control of two opposing actions between the two effectors at T1 (i.e., an inhibition of hand movement vs. an initiation of eye movement) during a sequence. This contention is in accordance with the general assumption that a decline of inhibitory functions is a major component of the aging process (Andrés et al., 2008; Dustman et al., 1996; Hasher and Zacks, 1988; Kramer et al., 1994; West, 1996). For example, older adults often show deficits in anti-saccades task, where a reflexive saccade toward a target needs to be inhibited and a saccade to the opposite direction needs to be initiated (Abel & Douglas, 2007; Klein et al., 2000; Oliency et al., 1997; Peltsch et al., 2009; Sweeney et al., 2001). Our study extends these observations made in the oculomotor system by demonstrating that older adults have a difficulty in inhibiting one action and initiating another across the oculomotor and limb motor systems.
It should be noted, however, that there has been a suggestion in literature that declines in working memory capacity, rather than an inhibitory function, is the underlying cause for age-related declines in anti-saccade task performance (Eenshuistra et al., 2004). This contention is based on an assumption that active inhibition requires a fair amount of working memory capacity (Conway & Engle, 1994; Roberts et al., 1994). Furthermore, various dual-task studies showed that eye or hand movements of older adults were deteriorated when a second task was concurrently performed, which suggests a limited working memory capacity in older adults (Eenshuistra et al., 2004; Fraser et al., 2010; Hartley & Little, 1999; McPhee et al. 2004; Voelcker-Rehage et al., 2006). Thus, it is possible that when in the current study two opposing demands on the hand and eyes were imposed concurrently at T1, a limited working memory capacity in older adults may have caused the observed delay in gaze anchoring termination.
Interestingly, the older adults were able to terminate gaze anchoring at T1 in a similar manner as the young adults for the hand-nostop1 condition (Fig. 6). Thus, when no hand termination is required, older adults are as capable as young adults to terminate gaze anchoring in relation to a task completion of hand movement. Furthermore, when the T1 was large in this condition, the older adults shifted their gaze simultaneously with the pointing completion to T1. This finding is pertinent from the perspective of predictive control. Research in young adults has shown that the termination of gaze anchoring is often controlled in a predictive mode based on an estimation of pointing completion instead of an actual completion of pointing (Bowman et al., 2009; Flanagan & Johansson 2003; Johansson et al., 2001, Rand & Stelmach 2010a). The current finding suggests that older adults are capable of such predictive control when a task requirement is conducive. More specifically, older adults are able to estimate the state of the arm and its relation to the target during pointing and predict the timing of the hand arrival to that target. Based on that prediction, older adults are capable of terminating the gaze anchoring as effectively as young adults. However, older adults are less able to predict whether the hand would be precisely stopped at a target when hand termination was required. Such deficit was revealed in other hand movement type conditions of this study.
In conclusion, older adults modify the characteristics of eye movements and gaze anchoring less compared to young adults depending on the hand termination and accuracy constraints. Furthermore, the capability of older adults in predictive termination of gaze-anchoring is diminished by the hand termination requirement. Thus, aging reduces the ability of older adults to coordinate eye and hand movements to meet various manual task constraints. All of these observations are consistent with the postulate that aging compromises coordination among multiple body segments during complex goal-directed movements.
Research highlights.
Older adults make hypometric saccades and delay gaze fixation to a pointing target.
Older adults modify eye movements less depending on hand termination requirements.
Older adults enhance gaze anchoring when hand termination is required.
Aging reduces the ability to modify eye movements for various manual task demands.
Acknowledgments
This research was supported by a grant from National Institute on Aging AG31366. We thank Ms. Lydia Anderson and Ms. Morgan Fairman for their help in data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel LA, Douglas J. Effects of age on latency and error generation in internally medicated saccades. Neurobiol Aging. 2007;28:627–37. doi: 10.1016/j.neurobiolaging.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Andrés P, Guerrini C, Phillips LH, Perfect TJ. Differential effects of aging on executive and automatic inhibition. Dev Neuropsychol. 2008;33:101–23. doi: 10.1080/87565640701884212. [DOI] [PubMed] [Google Scholar]
- Bahill AT, Clark MR, Stark L. Dynamic overshoot in saccadic eye movements is caused by neurological control signal reversals. Exp Neurol. 1975;48:107–22. doi: 10.1016/0014-4886(75)90226-5. [DOI] [PubMed] [Google Scholar]
- Bellgrove MA, Phillips JG, Bradshaw JL, Gallucci RM. Response (re-)programming in aging: A kinematic analysis. J Gerontol A Biol Sci Med Sci. 1998;53A:M222–7. doi: 10.1093/gerona/53a.3.m222. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Hallett M, Rothwell JC, Agostino R, Manfredi M, Thompson PD. Singlejoint rapid arm movements in normal subjects and in patients with motor disorders. Brain. 1996;119:661–74. doi: 10.1093/brain/119.2.661. [DOI] [PubMed] [Google Scholar]
- Brown SH, Cooke JD. Movement-related phasic muscle activation. I. Relations with temporal profile of movement. J Neurophysiol. 1990;63:455–64. doi: 10.1152/jn.1990.63.3.455. [DOI] [PubMed] [Google Scholar]
- Bowman MC, Johannson RS, Flanagan JR. Eye-hand coordination in a sequential target contract task. Exp Brain Res. 2009;195:273–83. doi: 10.1007/s00221-009-1781-x. [DOI] [PubMed] [Google Scholar]
- Cole KJ, Rotella DL, Harper JG. Mechanisms for age-related changes of fingertip forces during precision gripping and lifting in adults. J Neurosci. 1999;19:3238–47. doi: 10.1523/JNEUROSCI.19-08-03238.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole KJ, Rotella DL. Old age affects fingertip forces when restraining an unpredictably loaded object. Exp Brain Res. 2001;136:535–42. doi: 10.1007/s002210000613. [DOI] [PubMed] [Google Scholar]
- Cooke JD, Browns SH, Cunningham DA. Kinematics of arm movements in elderly humans. Neurobiol Aging. 1989;10:159–65. doi: 10.1016/0197-4580(89)90025-0. [DOI] [PubMed] [Google Scholar]
- Conway ARA, Engle RW. Working memory and retrieval: A resource-dependent inhibition model. J Exp Psychol Gen. 1994;123:354–73. doi: 10.1037//0096-3445.123.4.354. [DOI] [PubMed] [Google Scholar]
- Darling WG, Cooke JD, Brown SH. Control of simple arm movements in elderly humans. Neurobiol Aging. 1989;10:149–57. doi: 10.1016/0197-4580(89)90024-9. [DOI] [PubMed] [Google Scholar]
- Desrosiers J, Hebert R, Bravo G, Rochette A. Age-related changes in upper extremity performance of elderly people: A longitudinal study. Exp Gerontol. 1999;34:393–405. doi: 10.1016/s0531-5565(99)00018-2. [DOI] [PubMed] [Google Scholar]
- Dounskaia N, Wisleder D, Johnson T. Influence of biomechanical factors on substructure of pointing movements. Exp Brain Res. 2005;164:505–16. doi: 10.1007/s00221-005-2271-4. [DOI] [PubMed] [Google Scholar]
- Dustman RE, Emmerson RY, Shearer DE. Life span changes in electrophysiologial measures of inhibition. Brain Cogn. 1996;30:109–26. doi: 10.1006/brcg.1996.0007. [DOI] [PubMed] [Google Scholar]
- Eenshuistra RM, Ridderinkhof KR, van der Molen MW. Age-related changes in antisaccade task performance: inhibitory control or working-memory engagement? Brain Cogn. 2004;56:177–88. doi: 10.1016/j.bandc.2004.02.077. [DOI] [PubMed] [Google Scholar]
- Engelhorn R. Agonist and antagonist muscle EMG activity pattern changes with skill acquisition. Res Q Exerc Sport. 1983;54:315–23. [Google Scholar]
- Epelboim J, Steinman RM, Kowler E, Pizlo Z, Erkelens CJ, Collewijn H. Gaze-shift dyndamics in two kinds of sequential looking tasks. Vision Res. 1997;37:2597–607. doi: 10.1016/s0042-6989(97)00075-8. [DOI] [PubMed] [Google Scholar]
- Ferrell WR, Crighton A, Sturrock RD. Age-dependent changes in position sense in human proximal interphalangeal joints. Neuroreport. 1992;3:259–61. doi: 10.1097/00001756-199203000-00011. [DOI] [PubMed] [Google Scholar]
- Fitts PM. The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol. 1954;47:381–91. [PubMed] [Google Scholar]
- Flanagan JR, Johansson RS. Action plans used in action observation. Nature. 2003;424:769–71. doi: 10.1038/nature01861. [DOI] [PubMed] [Google Scholar]
- Folstein M, Folstein S, McHugh P. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fraser SA, Li KZ, Penhune VB. Dual-task performance reveals increased involvement of executive control in fine motor sequencing in healthy aging. J Gerontol B Psychol Sci Soc Sci. 2010;65:526–35. doi: 10.1093/geronb/gbq036. [DOI] [PubMed] [Google Scholar]
- Groh JM, Sparks D. Saccades to somatosensory targets. I. Behavioural characteristsics. J Neurophysiol. 1996;75:412–27. doi: 10.1152/jn.1996.75.1.412. [DOI] [PubMed] [Google Scholar]
- Harris CM, Wolpert DM. The main sequence of saccades optimizes speed-accuracy trade-off. Biol Cybern. 2006;95:21–9. doi: 10.1007/s00422-006-0064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley AA, Little DM. Age-related differences and similarities in dual-task interference. J Exp Psychol Gen. 1999;128:416–49. doi: 10.1037//0096-3445.128.4.416. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: A review and a new view. In: Bower GH, editor. The psychology of learning and motivation. Vol. 22. New York: Academic Press; 1988. pp. 193–225. [Google Scholar]
- Hay L, Bard C, Fleury M, Teasdale N. Availability of visual and proprioceptive afferent messages and postural control in elderly adults. Exp Brain Res. 1996;108:129–39. doi: 10.1007/BF00242910. [DOI] [PubMed] [Google Scholar]
- Hurley MV, Rees J, Newham DJ. Quadriceps function, proprioceptive acuity and functional performance in healthy young, middle aged, and elderly subjects. Age Ageing. 1998;27:55–62. doi: 10.1093/ageing/27.1.55. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G, Bäckström A, Flanagan JR. Eye-hand coordination in object manipulation. J Neurosci. 2001;21:6917–32. doi: 10.1523/JNEUROSCI.21-17-06917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketcham CJ, Dounskaia NV, Stelmach GE. Age-related differences in the control of multijoint movements. Motor Control. 2004;8:422–36. doi: 10.1123/mcj.8.4.422. [DOI] [PubMed] [Google Scholar]
- Ketcham CJ, Seidler RD, Van Gemmert AWA, Stelmach GE. Age-related kinematic differences as influenced by task difficulty, target size, and movement amplitude. Journals Gerontol B Psychol Sci. 2002;57B:P54–64. doi: 10.1093/geronb/57.1.p54. [DOI] [PubMed] [Google Scholar]
- Klein C, Fischer B, Hartnegg K, Heiss WH, Roth M. Optomotor and neuropsychological performance in old age. Exp Brain Res. 2000;135:141–54. doi: 10.1007/s002210000506. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Humphrey DG, Larish JF, Logan GD, Strayer DL. Aging and inhibition: beyond a unitary view of inhibitory processing in attention. Psychol Aging. 1994;9:491–12. [PubMed] [Google Scholar]
- Madhavan S, Shields RK. Influence of age on dynamic position sense: evidence using a sequential movement task. Exp Brain Res. 2005;164:18–28. doi: 10.1007/s00221-004-2208-3. [DOI] [PubMed] [Google Scholar]
- McPhee LC, Scialfa CT, Dennis WM, Ho G, Caird JK. Age differences in visual search for traffic signs during a simulated conversation. Hum Factors. 2004;46:674–85. doi: 10.1518/hfes.46.4.674.56817. [DOI] [PubMed] [Google Scholar]
- Neeuwsen HJ, Sawicki TM, Stelmach GE. Improved foot position sense as a result of repetitions in older adults. J Gerontol. 1993;48:P137–41. doi: 10.1093/geronj/48.3.p137. [DOI] [PubMed] [Google Scholar]
- Neggers SFW, Bekkering H. Ocular gaze is anchored to the target of an ongoing pointing movement. J Neurophysiol. 2000;83:639–51. doi: 10.1152/jn.2000.83.2.639. [DOI] [PubMed] [Google Scholar]
- Neggers SFW, Bekkering H. Gaze anchoring to a pointing target is present during the entire pointing movement and is driven by a non-visual signal. J Neurophysiol. 2001;86:961–70. doi: 10.1152/jn.2001.86.2.961. [DOI] [PubMed] [Google Scholar]
- Oliency A, Ross RG, Young DA, Freedman R. Age diminishes performance on an antisaccade eye movement task. Neurobiol Aging. 1997;18:483–89. doi: 10.1016/s0197-4580(97)00109-7. [DOI] [PubMed] [Google Scholar]
- Peltsch A, Hemraj A, Garcia A, Munoz DP. Age-related trends in saccade characteristics among the elderly. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging. In press. [DOI] [PubMed] [Google Scholar]
- Petrella RJ, Lattanzio PJ, Nelson MG. Effect of age and activity on knee joint proprioception. Am J Phys Med Rehabil. 1997;76:235–41. doi: 10.1097/00002060-199705000-00015. [DOI] [PubMed] [Google Scholar]
- Rand MK, Stelmach GE. Eye-hand coordination during two-stroke aiming movements in older adults. Abstr Soc Neurosci. 2009:853.4. [Google Scholar]
- Rand MK, Stelmach GE. Effects of hand termination and accuracy constraint on eye-hand coordination during sequential two-segment movements. Exp Brain Res. 2010a;207:197–211. doi: 10.1007/s00221-010-2456-3. [DOI] [PubMed] [Google Scholar]
- Rand MK, Stelmach GE. Effect of aging on coordinated eye and hand movements with two-segment sequence. Motor Control. 2010b doi: 10.1123/mcj.16.4.447. In review. [DOI] [PubMed] [Google Scholar]
- Ranganathan VK, Siemionow V, Sahgal V, Yue GH. Effects of aging on hand function. J Am Geriatr Soc. 2001;49:1478–84. doi: 10.1046/j.1532-5415.2001.4911240.x. [DOI] [PubMed] [Google Scholar]
- Roberts RJ, Jr, Hager LD, Heron C. Prefrontal cognitive processes: Working memory and inhibition in the antisaccade task. J Exp Psychol Gen. 1994;123:374–93. [Google Scholar]
- Romero DH, Van Gemmert AWA, Adler CH, Bekkering H, Stelmach GE. Time delays prior to movement alter the drawing kinematics of elderly adults. Hum Mov Sci. 2003;22:207–20. doi: 10.1016/s0167-9457(02)00160-4. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. A theory of cognitive aging. In: Stelmach GE, Vroon PA, editors. Advances in Psychology. Vol. 28. North Holland, The Netherlands: Elsevier Science; 1985. [Google Scholar]
- Seidler RD, Alberts JL, Stelmach GE. Changes in multi-joint performance with age. Motor Control. 2002;6:19–31. doi: 10.1123/mcj.6.1.19. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Swinnen SP, Stelmach GE. Age-related deterioration of coordinated interlimb behavior. J Gerontol B Psychol Sci Soc Sci. 2000;55:P295–303. doi: 10.1093/geronb/55.5.p295. [DOI] [PubMed] [Google Scholar]
- Shim JK, Lay BS, Zatsiorsky VM, Latash ML. Age-related changes in finger coordination in static prehension tasks. J Appl Physiol. 2004;97:213–24. doi: 10.1152/japplphysiol.00045.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, Scholz JP, Zatsiorsky VM, Latash ML. Finger interaction during accurate multi-finger force production tasks in young and elderly persons. Exp Brain Res. 2004;156:282–92. doi: 10.1007/s00221-003-1786-9. [DOI] [PubMed] [Google Scholar]
- Smit AC, Van Gisbergen JA, Cools AR. A parametric analysis of human saccades in different experimental paradigms. Vision Res. 1987;27:1745–62. doi: 10.1016/0042-6989(87)90104-0. [DOI] [PubMed] [Google Scholar]
- Stelmach GE, Amrhein PC, Goggin NL. Age differences in bimanual coordination. J Gerontol. 1988;43:P18–23. doi: 10.1093/geronj/43.1.p18. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Rosano C, Berman RA, Luna B. Inhibitory control of attention declines more than working memory during normal aging. Neurobiol Aging. 2001;22:39–47. doi: 10.1016/s0197-4580(00)00175-5. [DOI] [PubMed] [Google Scholar]
- Takatoku N, Fujiwara M. Muscle activity patterns during quick increase of movement amplitude in rapid elbow extensions. J Electromyogr Kinesiol. 2010;20:290–7. doi: 10.1016/j.jelekin.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Verschueren SM, Brumagne S, Swinnen SP, Cordo PJ. The effect of aging on dynamic position sense at the ankle. Behav Brain Res. 2002;136:593–603. doi: 10.1016/s0166-4328(02)00224-3. [DOI] [PubMed] [Google Scholar]
- Voelcker-Rehage C, Stronge AJ, Alberts JL. Age-related differences in working memory and force control under dual-task conditions. Neuropsychol Dev Cogn. 2006;13:366–84. doi: 10.1080/138255890969339. [DOI] [PubMed] [Google Scholar]
- Walker N, Philbin DA, Fisk AD. Age-related differences in movement control: Adjusting submovement structure to optimize performance. J Gerontol B Psychol Sci. 1997;52B:P40–52. doi: 10.1093/geronb/52b.1.p40. [DOI] [PubMed] [Google Scholar]
- Warabi T, Noda H, Kato T. Effect of aging on sensorimotor functions of eye and hand movements. Exp Neurol. 1986;92:686–97. doi: 10.1016/0014-4886(86)90309-2. [DOI] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120:272–92. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Wu CC, Kwon OS, Kowler E. Fitts’s Law and speed/accuracy trade-offs during sequences of saccades: Implications for strategies of saccadic planning. Vision Res. 2010;50:2142–57. doi: 10.1016/j.visres.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambarbieri D, Schmid R, Magenes G, Prablanc C. Saccadic responses evoked by presentation of visual and auditory targets. Exp Brain Res. 1982;47:417–27. doi: 10.1007/BF00239359. [DOI] [PubMed] [Google Scholar]