Abstract
Purpose
Allergic rhinitis is clinically defined as a disorder of the nose induced by IgE mediated inflammation after allergen exposure of the nasal mucosa. Many reports have stated that Panax ginseng and fermented red ginseng have anti-inflammatory effects, especially against Th2-type inflammation. This study was conducted to evaluate the therapeutic effects of fermented red ginseng in allergic rhinitis.
Methods
In this 4-week, double-blind, placebo-controlled study, 59 patients with persistent perennial allergic rhinitis were randomly divided into two groups: those receiving fermented red ginseng tablets (experimental group) and those receiving placebo (control group). The primary efficacy variable was the total nasal symptom score (TNSS; rhinorrhea, sneezing, itchy nose, and nasal congestion). Secondary efficacy variables were the Rhinitis Quality of Life (RQoL) score and skin reactivity to inhalant allergens, as determined by the skin prick test.
Results
There was no significant difference in the TNSS score and TNSS duration score between the experimental and placebo groups in weeks 1, 2, 3, or 4. For nasal congestion, fermented red ginseng was significantly effective (P<0.005), while placebo caused no change. The activity and emotion of RQoL improved markedly secondary to treatment with fermented red ginseng (P<0.05), while placebo caused no change. Additionally, fermented red ginseng reduced skin reactivity to sensitized perennial allergens (P<0.05). Fermented red ginseng was well tolerated.
Conclusions
Fermented red ginseng improved nasal congestion symptoms and RQoL in patients with perennial allergic rhinitis.
Keywords: Allergic rhinitis, alternative medicine, fermented ginseng, ginsenoside
INTRODUCTION
Allergic rhinitis results from specific IgE-mediated allergic reactions in the nasal mucosa, characterized by a runny nose, nasal itching, sneezing, and nasal congestion. Nasal biopsy reveals accumulations of mast cells, eosinophils, and basophils in the submucosal layer and accumulations of eosinophils in the lamina propria.1 Treatment of allergic rhinitis has generally focused on suppressing these inflammatory reactions, and the main medications used are antihistamines, nasal steroids, and leukotriene receptor antagonists. Apart from these treatments, immunotherapy can be performed in some patients.2 In cases of perennial allergic rhinitis, the symptoms are often prolonged for extended periods of time and thus require long-term medication. However, antihistamines can be ineffective against nasal congestion, the most common symptom, and long-term use can lead to weight gain in some patients. Thus, patients who do not want to receive such long-term medication often seek complementary and alternative therapies the effects of which have not yet been verified.
Ginseng has long been used as an herbal medication throughout East Asia, including Korea, and is a member of the scientific family Araliaceae, genus Panax. Of the species of ginseng, the four most commonly used for medicinal purposes are Panax ginseng, P. japonicum, P. notoginseng, and P. quinquefolium; the term "ginseng" generally refers to P. ginseng.3 Commercialized ginseng is broadly categorized into three types depending on its age and manufacturing process: fresh ginseng that is less than 4 years old and has not been dried, white ginseng that is 4-6 years old and has been peeled or either sun-dried or oven-dried, and red ginseng that is older than 6 years and steamed before being dried. The specific drying process of red ginseng leads to a nonenzymatic color change that gives it its characteristic hue. These various methods of preparing ginseng alter the formation and concentration of the saponin within the ginseng.4,5 Fermented red ginseng has been treated with micro-organisms and enzymes that enhance the saponin content of the red ginseng for maximum efficacy; this is based on the fact that ginseng's efficacy in different subjects varies based on the number and activity of each individual's intestinal flora. Several studies have reported ginseng's effect in increasing immunity and exerting an anti-inflammatory effect.6,7 Hyun et al.8 reported that red ginseng and fermented red ginseng had a much greater effect in suppressing allergic inflammation than did white ginseng.
Thus, the purpose of this study was to analyze whether fermented red ginseng results in clinically significant symptomatic improvement in patients with allergic rhinitis.
MATERIALS AND METHODS
Patients
This study included patients aged 18-55 years who had been continuously experiencing allergic rhinitis symptoms for at least 2 years, whose skin prick test result was positive for more than one perennial allergen (house dust mites, fungi, cockroaches, or animal hair), and who had experienced at least two of the four major symptoms: itching, sneezing, runny nose, and congestion, with a minimum symptom score of 2 points. The following patients were excluded from the study: (1) those who were taking medication for allergic diseases, (2) those who had taken antihistamines or H2-blockers within 2 weeks of the study, (3) those who had asthma, hypertension, or diabetes mellitus, (4) those who were pregnant or who might become pregnant within 3 months of the study, and (5) those who showed abnormal results in blood tests prior to the study.
Materials
Our study utilized soft capsules (250 mg/capsule) that were made from fermented red ginseng (FRG). FRG powder was provided by Bifido Inc. (Hongcheon, Korea). The dried FRG contained 236.7 mg crude saponin, 2.3 mg Rb2, 0.1 mg Rb3, 0.6 mg Rc, 9.5 mg Rd, 0.5 mg Re, 0.6 mg Rg1, 8.2 mg Rg2, 27.7 mg Rg3, 12.1 mg Rh1, 3.1 mg Rh2, and 61.0 mg compound K per gram. The placebo capsules were the same size and shape and were filled with starch.
Study design
Our randomized, double-blind, placebo-controlled study was approved by the Institutional Review Board of Seoul National University Hospital and was conducted between September 28 and November 23, 2009 (H-008-042-290). Patients who met our inclusion criteria and did not fall into any exclusion criteria were randomly placed into the experimental group, who received fermented red ginseng capsules, or the control group, who received placebo capsules. All patients were instructed to take three capsules two times daily for 4 weeks. A Rhinitis Quality of Life (RQoL) survey was performed directly before and after the 4-week treatment period, and the total nasal symptom score (TNSS) was also recorded daily throughout the treatment period.
Skin prick test
Each patient underwent a skin prick test for 14 allergens (Bencard, Bretford, UK), including house dust mites (Dermatophagoides pteronyssinus and D. farinae), fungi (Alternaria spp., Cladosporium spp., and Aspergillus spp.), cockroaches, and animal hair (dog and cat fur) as perennial allergens, and trees (tree mixture and hazel), grass (grass mixture and orchard grass), and weeds (mugwort and ragweed) as seasonal allergens. We used a saline solution as a negative control and histamine as a positive control (1 mg/mL). We measured the skin reaction 15 minutes after application of the allergens; an allergen wheal that had a size ratio greater than 1 when compared with a histamine wheal was considered to be a positive reaction.
Efficacy measurements
Changes in the TNSS of each patient were considered the primary efficacy variable, and changes in the RQoL and skin prick test were considered secondary efficacy variables. The TNSS evaluates symptoms of itching, sneezing, runny nose, and congestion on a four-point scale: 0=no symptoms, 1=mild symptom( s) (present but bearable), 2=moderate symptom(s) (present and uncomfortable), and 3=severe symptom(s) (unbearable). The symptoms were further scored based on their duration per day: 0=none, 1=less than 30 minutes, 2=between 30 minutes and 2 hours, and 3=longer than 2 hours.9,10 The TNSS values were averaged on a weekly basis during the study and compared with the TNSS values from before the study (week 0) to determine whether there were differences between them. The mean TNSS score and mean change in TNSS were described as averages of the four TNSS items and changes. The RQoL evaluated quality of life on a five-point scale in 28 items that could broadly be divided into seven domains: practical problems, sleep, nasal symptoms, non-nose/non-eye symptoms, activity state, emotional state, and eye symptoms.11 RQoL scores were taken and compared before (week 0) and after the study and were evaluated for statistical significance. The sizes of the wheals and flares in the skin prick tests from before and after the study were taken and compared.
Safety evaluation
Before and after the study, we conducted numerous blood tests to check for adverse reactions, and all patients were requested to immediately contact us if they noticed any kind of adverse reaction.
Statistical analyses
The sample size was calculated on the basis of a previous study12 that compared the efficacy of montelukast versus montelukast-levocetirizine for the treatment of persistent allergic rhinitis. Assuming a mean baseline TNSS of 7.95 and standard deviation of 0.68, it was estimated that a sample size of 23 patients in each active group would provide 80% power to show a statistically significant difference between the two treatments at a 0.05 significance level. The sample size of each group was then determined to be 33 patients, allowing a 30% dropout rate.
All statistical analyses were performed using the SPSS software (ver. 16.0, SPSS Inc., Chicago, IL, USA), and values are presented as means±standard errors. Variables were evaluated using Fischer's exact test and the Mann-Whitney U-test. The before and after comparisons of the TNSS, RQoL, and skin test results were analyzed with the paired t-test. A P value of <0.05 was considered to indicate statistical significance.
RESULTS
Patient characteristics
In total, 66 patients were enrolled in this randomized, double-blind, placebo-controlled clinical trial, and seven did not complete the study because they withdrew their consent. Thus, in total, 59 patients completed the study; 30 were randomly placed in the experimental group, and 29 were placed in the control group.
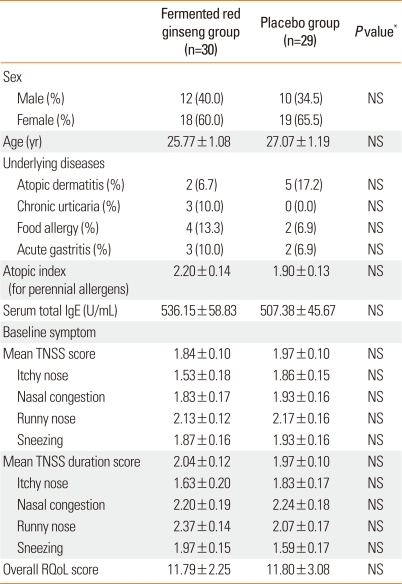
The age of the patients ranged from 19 to 48 years (mean, 26.6 years). There were 21 male patients, and they made up 35.6% of the participants. The mean number of positive reactions to all allergens was 2.64±0.20, and that to perennial allergens was 2.05±0.10. The mean numbers of positive reactions to all and perennial allergens were not significantly different between the two groups. There were no significant differences between the two groups in age, gender, underlying diseases, or serum total IgE at the time of the first visit. The mean TNSS score, mean TNSS duration score, and RQoL were not significantly different between the two groups (Table 1).
Table 1.
Demographic characteristics of the study population
*Analyzed by Fischer's exact test and the Mann-Whitney U-test.
NS, not significant; TNSS, total nasal symptom score; RQoL, Rhinitis Quality of Life.
Efficacy assessment of primary variables
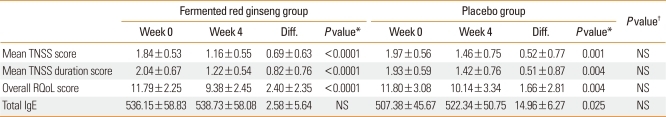
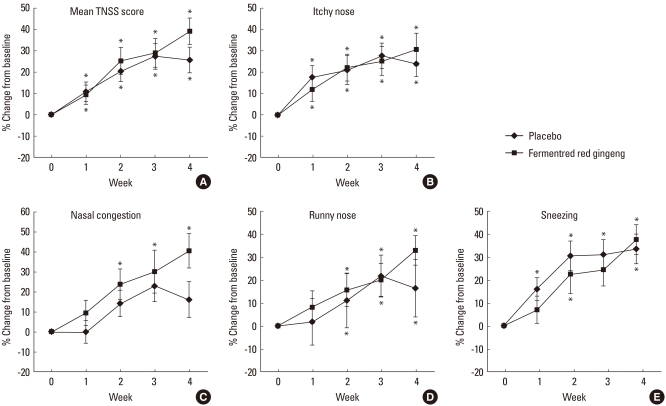
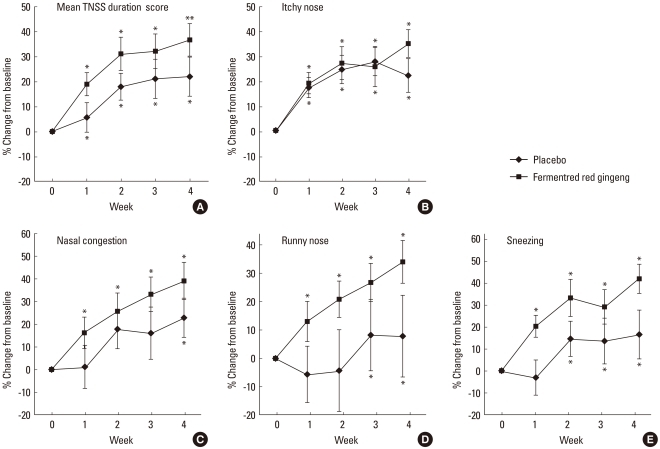
There was no significant difference in TNSS score or TNSS duration score between the experimental and placebo groups in weeks 0 and 4 (Table 2). However, both groups showed an improvement in TNSS over time. When the symptoms were separately assessed as itching, runny nose, sneezing, and nasal congestion, there was similar improvement over time for the first three symptoms. However, for nasal congestion, the experimental group showed a significantly greater improvement in the TNSS in weeks 2, 3, and 4, but the control group did not (Fig. 1). For the four major symptoms, there was a similar reduction in symptom duration over the course of the study. However, for nasal congestion, the symptom duration was significantly reduced in week 4 in the control group, whereas it was significantly reduced from week 1 in the experimental group (Fig. 2). Based on this result, it was determined that fermented red ginseng had an early effect on nasal congestion. There were similar trends in runny nose and sneezing symptoms as well, with the control group showing improvements in weeks 2 and 3 and the experimental group showed improvement from week 1.
Table 2.
Mean TNSS score, mean TNSS duration score, overall RQoL score, and total IgE (U/mL) from baseline to week 4
*Analyzed by the paired t-test. †Analyzed by the Mann-Whitney U-test.
TNSS, total nasal symptom score; RQoL, Rhinitis Quality of Life; Diff., difference between weeks 0 and 4; NS, not significant.
Fig. 1.
Weekly improvement from baseline to week 1, 2, 3, and 4 in the total nasal symptom score (TNSS). (A) Mean TNSS score, (B) itchy nose, (C) nasal congestion, (D) runny nose, and (F) sneezing score.
*P<0.05, vs. baseline, analyzed by the paired t-test.
Fig. 2.
Weekly improvement from baseline to week 1, 2, 3, and 4 in the total nasal symptom score (TNSS) symptom duration score. (A) Mean TNSS duration score, (B) itchy nose, (C) nasal congestion, (D) runny nose, and (F) sneezing score.
*P<0.05, vs. baseline, analyzed by the paired t-test.
Efficacy assessment of secondary variables
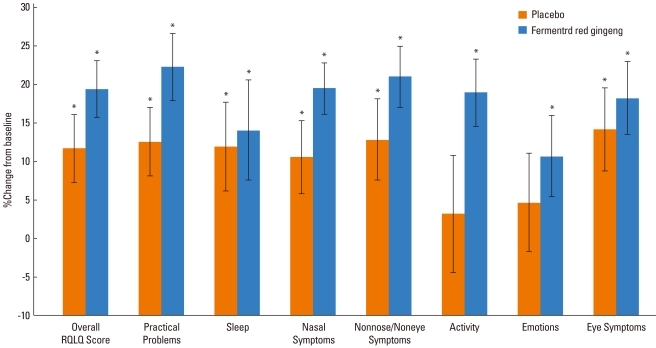
When comparing RQoL scores between before and after the 4-week medication, both the experimental and control groups showed an improvement over the course of the study. However, the control group did not show a significant improvement in activity or emotional state, whereas the experimental group showed significant improvements (Fig. 3).
Fig. 3.
Change in mean value from baseline to week 4 in overall Rhinitis Quality of Life (RQoL) score and individual RQoL domain scores.
*P<0.05, vs. baseline, analyzed by the paired t-test.
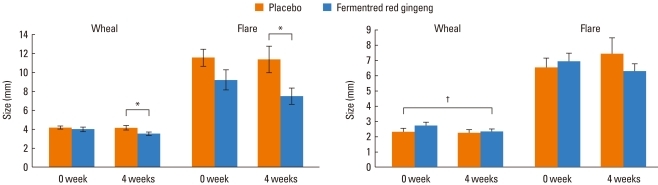
In the skin prick test performed before the 4-week medication, there was no significant difference in skin reactions to histamine between the two groups. However, in the skin prick test performed after the 4-week medication, the experimental group showed smaller wheal and flare sizes to histamine than the control group. The experimental group also showed a significant reduction in mean wheal sizes for the eight perennial allergens tested, whereas the control group did not (Fig. 4).
Fig. 4.
(A) Skin reactivity size to histamine. (B) Mean size to perennial inhalant allergen.
*P<0.05, analyzed by Student's t-test. †P<0.05, analyzed by the paired t-test.
Serum total IgE levels
When total IgE levels from before and after the 4-week medication were compared, the control group showed a significant increase, whereas the experimental group did not (Table 2).
Safety assessment
Of the 59 patients, three developed mild hepatic dysfunction as an adverse reaction. Of those three patients, one in the experimental group showed an increase in alanine aminotransferase (ALT) (27 vs. 42 IU/L), but further examination 2 weeks later showed that ALT had returned to normal. In the other two patients, who were both in the control group, one developed an increase in total bilirubin (1.0 vs. 1.5 mg/mL), and one showed an increase in ALT (36 vs. 43 IU/L). There was no other report of adverse reactions, and there was no significant difference between the two groups in reported adverse reactions.
DISCUSSION
Allergic rhinitis, an allergic inflammatory disease of the nasal airway, causes a variety of chronic symptoms that continuously fluctuate in severity over time, causing discomfort and a decrease in quality of life. Thus, early symptomatic treatment through inflammation control is important. Because of the chronic nature of allergic inflammation, some patients are reluctant to take long-term medication and so turn to unverified alternative medications. Alternative medicine is used by approximately 80% of the world's population. The United States spends roughly $34 billion annually on alternative medicine.13 A previous study documented that Germany alone spends roughly €90 million on alternative treatments for allergic diseases, including bronchial asthma.14 However, because the majority of these alternative treatments have not been medically evaluated, they often lead to symptom aggravation, adverse effects, and the inability to complete regular medical treatments; the medical costs associated with these problems are not trivial. Many of these alternative treatments are in need of experimental and clinical evaluation.
Some of the "health foods" that are currently being studied for their effects on allergic rhinitis include probiotics,15 spirulina,16 and extracts from green tea, guajava leaves, and rose petals.17 In fact, many current medications for the treatment of allergic rhinitis are derived from the plants and substances used in alternative medicine. For example, ephedrine came from the ephedra plant, anticholinergics were developed from henbane leaves, theophylline was taken from tea tree leaves, and cromolyn came from Ammi visnaga.18
It is believed that when a substance isolated from fermented red ginseng is effective in treating allergic diseases, it can be reproduced for medication. Various studies have indicated that ginseng has anti-inflammatory and anti-allergenic effects. Ginseng induces vasodilation and reduces platelet aggregation, thus improving cardiovascular function, has antioxidant and hypoglycemic properties, and stimulates the immune system through activation of the pituitary-adrenal axis.19 Ginseng can alter blood adrenocorticotropic hormone and corticosterone levels,20,21 and ginseng's saponin components, Rb1 and Rg1, are ginsenosides that act as ligands for glucocorticoid receptors, thus inducing glucocorticoid activity.22 Ligor et al.23 indicated that ginsenoside reverses the dexamethasone-induced down-regulation of glucocorticoid receptors and that it has pharmacological activities similar to those of glucocorticoids. When ginseng root extracts are administered to mice, the production of anti-allergic cytokines, such as IFN-γ and IL-10, is increased; additionally, RANTES (Regulated on Activation, Normal T-cell Expressed and Secreted) synthesis and secretions of IL-4, IL-5, and IL-12, which provoke allergic inflammation, are suppressed.24 Bae et al.6 reported that administration of the ginsenoside Rb1 metabolite compound K to mice had the effect of suppressing passive cutaneous anaphylaxis caused by IgE-antigen complexes. The reduction of IL-4 and TNF-α manifestation and suppression of NF-κB and CCL7 (C-C motif) ligand 7 activities have been proposed as its mechanism.6 Additionally, Babayigit et al.7 demonstrated that when ginseng extract is administered in a murine asthma model, chronic airway remodeling is prevented compared with the placebo group; further, because the degree of airway remodeling was similar to that in the dexamethasone group, ginseng was considered to be as effective as steroids for asthma treatment. From these results, it is conceivable that ginseng increases the Th1 response and suppresses the Th2 response, thus reducing or preventing allergic inflammation.
It is known that during ginseng processing, ginseng, red ginseng, and fermented red ginseng contain different compositions of ginsenosides, which lead to their differences in anti-inflammatory properties.16 Red ginseng and fermented red ginseng are more effective than white ginseng at suppressing the transcription of NF-κB and subsequent synthesis of proinflammatory cytokines, such as TNF-α and IFN-γ, as well as synthesizing nitric oxide induced by lipopolysaccharides.16 Jeong et al.25 reported that in a murine asthma model, red ginseng decreased airway resistance as well as the total cell count and number of eosinophils in the bronchoalveolar lavage fluid.
In this study, nasal congestion was significantly improved in the experimental group. Nasal congestion is related to both an acute allergic inflammation of mast cells and chronic inflammation. The therapeutic effect of antihistamines, which are generally effective on mast cells, on nasal congestion has been unclear. Furthermore, the use of nasal decongestants may worsen symptoms through a rebound phenomenon when the use of the medication is stopped. Nasal topical steroids, while effective in reducing nasal congestion through their anti-inflammatory effects, are associated with many adverse reactions when used for long periods of time, such as nasal mucosal atrophy, nasal pain, epistaxis, and nasal septal perforation; thus, they require special precautions in their application. Therefore, it was expected that consistent ingestion of fermented red ginseng may allow patients with chronic allergic rhinitis to reduce nasal congestion without steroid therapy. Allergic rhinitis does not merely cause nasal discomfort due to persistent nasal symptoms, but also leads to losses in concentration and headaches, which can affect sleep, social interaction, school work, and even workplace productivity, leading to socioeconomic losses.26 While the control group showed an improvement in TNSS after the 4-week medication, their emotional and activity states were unaffected, whereas the experimental group showed not only a reduction in nasal symptoms but also significant improvement in emotional and activity states after the 4-week medication. From this result, it was found that fermented red ginseng can not only serve to reduce symptoms in patients with allergic rhinitis, but also improve their quality of life.
In the skin prick test, the experimental group showed a reduction in the size of wheals and flares to histamine and showed a smaller wheal size to perennial allergens than did the control group. This led us to conclude that fermented red ginseng has an effect on allergic inflammatory reactions to both histamine and specific allergens. Our results suggest a similar mechanism to the passive cutaneous anaphylaxis reaction reported in a previous animal study.6 IgE plays an important role in the initiation of allergic inflammatory reactions. In our study, the control group showed an increase in total IgE, whereas the experimental group did not. Based on this result, it is thought that fermented red ginseng has a potential anti-IgE effect.
In this study, there was no remarkable adverse effect, aggravation of symptoms, or significant abnormality in the results of routine blood tests. Thus, we are led to conclude that fermented red ginseng can be used to treat allergic rhinitis without any remarkable adverse effects.
Because this was a relatively small-scale, short-term study, further large-scale, long-term studies are needed to fully evaluate the safety and efficacy of fermented red ginseng. Additionally, comparisons with other nonmedical allergic rhinitis treatments may be necessary. Another limitation of our study is that we relied on patient-reported symptom scores and quality of life surveys, and were unable to use measurements of nasal resistance or nasal secretion smear tests for objective analyses.
In conclusion, the results of this study suggest that fermented red ginseng can be safely used as a health food to treat allergic rhinitis, can improve both nasal symptoms and overall quality of life, and has potential for further development.
ACKNOWLEDGMENTS
This work was supported by grants from the Ministry for Health, Welfare and Family Affairs (A080664), and the Gangwon Regional Innovation Agency, Ministry of Knowledge Economy, Republic of Korea.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Howarth PH, Salagean M, Dokic D. Allergic rhinitis: not purely a histamine-related disease. Allergy. 2000;55(Suppl 64):7–16. doi: 10.1034/j.1398-9995.2000.00802.x. [DOI] [PubMed] [Google Scholar]
- 2.Cruz AA, Popov T, Pawankar R, Annesi-Maesano I, Fokkens W, Kemp J, Ohta K, Price D, Bousquet J. Common characteristics of upper and lower airways in rhinitis and asthma: ARIA update, in collaboration with GA(2)LEN. Allergy. 2007;62(Suppl 84):1–41. doi: 10.1111/j.1398-9995.2007.01551.x. [DOI] [PubMed] [Google Scholar]
- 3.Yun TK. Brief introduction of Panax ginseng C.A. Meyer. J Korean Med Sci. 2001;16(Suppl):S3–S5. doi: 10.3346/jkms.2001.16.S.S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nocerino E, Amato M, Izzo AA. The aphrodisiac and adaptogenic properties of ginseng. Fitoterapia. 2000;71(Suppl 1):S1–S5. doi: 10.1016/s0367-326x(00)00170-2. [DOI] [PubMed] [Google Scholar]
- 5.Kim ST, Jang JH, Kwon JH, Moon KD. Change in the chemical components of red and white ginseng after puffing. Korean J Food Preserv. 2009;16:355–361. [Google Scholar]
- 6.Bae EA, Trinh HT, Yoon HK, Kim DH. Compound K, a metabolite of ginsenoside Rb1, inhibits passive cutaneous anaphylaxis reaction in mice. J Ginseng Res. 2009;33:93–98. [Google Scholar]
- 7.Babayigit A, Olmez D, Karaman O, Bagriyanik HA, Yilmaz O, Kivcak B, Erbil G, Uzuner N. Ginseng ameliorates chronic histopathologic changes in a murine model of asthma. Allergy Asthma Proc. 2008;29:493–498. doi: 10.2500/aap.2008.29.3137. [DOI] [PubMed] [Google Scholar]
- 8.Hyun MS, Hur JM, Shin YS, Song BJ, Mun YJ, Woo WH. Comparison study of white ginseng, red ginseng, and fermented red ginseng on the protective effect of LPS-induced inflammation in RAW 264.7 cells. J Appl Biol Chem. 2009;52:21–27. [Google Scholar]
- 9.Dahl R, Kapp A, Colombo G, de Monchy JG, Rak S, Emminger W, Riis B, Grønager PM, Durham SR. Sublingual grass allergen tablet immunotherapy provides sustained clinical benefit with progressive immunologic changes over 2 years. J Allergy Clin Immunol. 2008;121:512–518.e2. doi: 10.1016/j.jaci.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 10.Fokkens WJ, Jogi R, Reinartz S, Sidorenko I, Sitkauskiene B, van Oene C, Faris MA, Ellsworth A, Caldwell MF. Once daily fluticasone furoate nasal spray is effective in seasonal allergic rhinitis caused by grass pollen. Allergy. 2007;62:1078–1084. doi: 10.1111/j.1398-9995.2007.01522.x. [DOI] [PubMed] [Google Scholar]
- 11.Park KH, Cho JS, Lee KH, Shin SY, Moon JH, Cha CI. Rhinoconjunctivitis quality of life questionnaire (RQLQ) as an evaluator of perennial allergic rhinitis patients-the first report. Korean J Otolaryngol-Head Neck Surg. 2002;45:254–262. [Google Scholar]
- 12.Ciebiada M, Gorska-Ciebiada M, DuBuske LM, Gorski P. Montelukast with desloratadine or levocetirizine for the treatment of persistent allergic rhinitis. Ann Allergy Asthma Immunol. 2006;97:664–671. doi: 10.1016/S1081-1206(10)61098-8. [DOI] [PubMed] [Google Scholar]
- 13.MacLennan AH, Wilson DH, Taylor AW. The escalating cost and prevalence of alternative medicine. Prev Med. 2002;35:166–173. doi: 10.1006/pmed.2002.1057. [DOI] [PubMed] [Google Scholar]
- 14.Schafer T. Epidemiology of complementary alternative medicine for asthma and allergy in Europe and Germany. Ann Allergy Asthma Immunol. 2004;93:S5–S10. doi: 10.1016/s1081-1206(10)61481-0. [DOI] [PubMed] [Google Scholar]
- 15.Vliagoftis H, Kouranos VD, Betsi GI, Falagas ME. Probiotics for the treatment of allergic rhinitis and asthma: systematic review of randomized controlled trials. Ann Allergy Asthma Immunol. 2008;101:570–579. doi: 10.1016/S1081-1206(10)60219-0. [DOI] [PubMed] [Google Scholar]
- 16.Cingi C, Conk-Dalay M, Cakli H, Bal C. The effects of spirulina on allergic rhinitis. Eur Arch Otorhinolaryngol. 2008;265:1219–1223. doi: 10.1007/s00405-008-0642-8. [DOI] [PubMed] [Google Scholar]
- 17.Kim SY, Kang HR, Kim JH, Son KM, Jeong JM, Park SH, Hwang YI, Jang SH, Kim DG, Jung KS. The effects of extracts from green tea, guajava leaves and rose petals on allergic rhinitis: a randomized double blind control study. Korean J Asthma Allergy Clin Immunol. 2009;29:89–95. [Google Scholar]
- 18.Lee HB. Alternative and complement therapies for asthma. Pediatr Allergy Respir Dis. 2002;12:247–252. [Google Scholar]
- 19.Kiefer D, Pantuso T. Panax ginseng. Am Fam Physician. 2003;68:1539–1542. [PubMed] [Google Scholar]
- 20.Fulder SJ. Ginseng and the hypothalamic-pituitary control of stress. Am J Chin Med. 1981;9:112–118. doi: 10.1142/s0192415x81000159. [DOI] [PubMed] [Google Scholar]
- 21.Hiai S, Yokoyama H, Oura H, Kawashima Y. Evaluation of corticosterone secretion-inducing activities of ginsenosides and their prosapogenins and sapogenins. Chem Pharm Bull (Tokyo) 1983;31:168–174. doi: 10.1248/cpb.31.168. [DOI] [PubMed] [Google Scholar]
- 22.Lee YJ, Chung E, Lee KY, Lee YH, Huh B, Lee SK. Ginsenoside-Rg1, one of the major active molecules from Panax ginseng, is a functional ligand of glucocorticoid receptor. Mol Cell Endocrinol. 1997;133:135–140. doi: 10.1016/s0303-7207(97)00160-3. [DOI] [PubMed] [Google Scholar]
- 23.Ligor T, Ludwiczuk A, Wolski T, Buszewski B. Isolation and determination of ginsenosides in American ginseng leaves and root extracts by LC-MS. Anal Bioanal Chem. 2005;383:1098–1105. doi: 10.1007/s00216-005-0120-8. [DOI] [PubMed] [Google Scholar]
- 24.Liou CJ, Huang WC, Tseng J. Short-term oral administration of ginseng extract induces type-1 cytokine production. Immunopharmacol Immunotoxicol. 2006;28:227–240. doi: 10.1080/08923970600816681. [DOI] [PubMed] [Google Scholar]
- 25.Jeong YJ, Paeng JW, Choi DC, Lee BJ. Effects of Korean red ginseng extracts on airway hyperresponsiveness and inflammation in a murine asthma model. Korean J Asthma Allergy Clin Immunol. 2010;30:43–49. [Google Scholar]
- 26.van Oene CM, van Reij EJ, Sprangers MA, Fokkens WJ. Quality-assessment of disease-specific quality of life questionnaires for rhinitis and rhinosinusitis: a systematic review. Allergy. 2007;62:1359–1371. doi: 10.1111/j.1398-9995.2007.01482.x. [DOI] [PubMed] [Google Scholar]