Abstract
Because genetic characteristics vary among subjects, the therapeutic effects of a certain drug differ among patients with the same disease. For this reason, special interest has focused on tailored treatments. Although it is well known that sex is genetically determined, little attention has been paid to sex differences in the clinical features and treatment of asthma. Females are more likely to suffer allergic asthma, to have difficulty controlling asthma symptoms, and to show adverse effects to drugs. As asthma symptoms show cyclic changes depending on female hormone levels in many women of child-bearing age, the use of contraceptives may specifically help to treat female patients with asthma such as those with perimenstrual asthma and severe asthma. Generally, testosterone seems to suppress asthma, and dehydroepiandrosterone (DHEA), a less virilizing androgen, may be effective for treating asthma. Evidence exists for a therapeutic and steroid-sparing effect of DHEA. However, further studies on the optimal dose and route of DHEA for each sex are needed. Monitoring of the serum DHEA-S level is necessary for patients with asthma on inhaled steroid treatment, and at minimum, replacement therapy for patients with a low level of DHEA may be helpful for treating their asthma.
Keywords: Asthma, dehydroepiandrosterone, sex, perimenstrual, treatment
INTRODUCTION
Asthma is defined by characteristic features such as airway hyper-responsiveness (AHR), reversible airflow obstruction, and paroxysmal cough, dyspnea, and wheezing, which are associated with airway inflammation.1 Therefore, it is not a disease entity, but a syndrome caused by a variety of etiologic agents. Because the clinical features of asthma and responses to anti-asthma drugs are similar regardless of cause, most patients are treated with the same drugs under the same diagnostic name of asthma. However, etiology-based specific treatments have long been given, such as avoidance of offending allergens and allergen-specific immunotherapy when specific allergens have been identified. Moreover, with advances in genetics, the association of HLA-DPB*0301 or cysteinyl leukotriene receptor 1 promotor-634C>T and the necessity for leukotriene receptor antagonists has been identified in patients with aspirin-intolerant asthma.2 Thus, treatments tailored for individuals have gained the attention of clinicians. Although it is premature to apply such tailored gene-analysis treatments in general practice, differential treatment according to sex can be easily introduced because genetically determined sex can be identified without the need for a gene analysis. In 2003, Osman3 mentioned sex-specific treatments in patients with asthma. However, few systematic reviews of studies on this issue have been reported so far.
SEX DIFFERENCES IN ASTHMA PREVALENCE
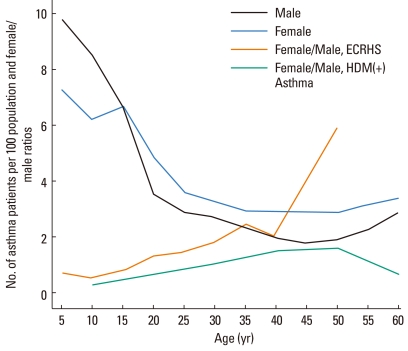
The prevalence of asthma is higher in males than in females before adolescence, but this trend is reversed after adolescence.3-5 In 1977, Wormald6 reported that the incidence of asthma with a positive response to skin prick tests using house dust mite allergens was three times higher in males before the age of 10 years, but it changed to 1.5 times and 1.6 times higher in females in their third and fourth decades (child bearing years), respectively, and was higher again in males in their fifth decade (Figure). A cross-sectional analysis of the data of the European Community Respiratory Health Survey by de Marco et al.7, conducted in 2000 with more than 18,000 subjects in 16 countries, found that the incidence of asthma was 0.56 times lower in females at the age of 5-10 years but increased in females after adolescence, and it was 5.91 times higher in females after their fourth decade; furthermore, this trend was observed in all 16 countries. Osman et al.8 also reported in 2007 an analysis of more than 260,000 asthmatic patients retrieved from the General Practice Research Database which showed that the prevalence of asthma per 100 population was 9.8 for males and 7.3 for females at the age of 5 years, 6.7 and 6.7 at 15 years, 2.9 and 3.6 at 25 years, 2.3 and 3.0 at 35 years, 1.8 and 2.9 at 45 years, and 2.3 and 3.2 at 55 years, respectively, suggesting that the sex ratio reverses after adolescence. They also reported a similar trend in patients with allergic rhinitis. Similarly, Poulos et al.9 reported in 2007 that the hospital admission rates for urticaria, angioedema, and anaphylaxis were higher in boys than in girls among children aged less than 15 years, whereas those rates were higher in females than in males among those aged 15 years and older.
Figure.
Sex differences in asthma prevalence. The ratios of female to male patients with asthma and a positive response to skin prick tests using house dust mites (HDM) (redrawn from Wormald6) and in patients who were reported to have asthma in the European Community Respiratory Health Survey (ECRHS) (redrawn from de Marco et al.7), and the number of asthma patients per 100 population for each sex in the General Practice Research Database (redrawn from Osman et al.8).
This reversal in sex ratio after puberty is not caused by increased loss of established asthma in boys, but by late incidence of asthma among girls.4,10 The sex difference in the prevalence of asthma is reflected in the sex difference in the hospitalization rate and asthma severity.4 The reasons for the higher prevalence of asthma in boys may be that their airways develop relatively slowly compared with lung volume (dysanapsis)3,4 and are easily sensitized to indoor allergens such as house dust mites and cat dander,11 whereas girls and young women tend to be treated after their asthma becomes aggravated (Yentl syndrome).3,4 Actually, the sex difference begins during gestation; the lung matures more slowly and surfactant is produced later in the male than in the female fetus.12 Consequently, male neonates tend to have lower specific airway conductance than do female neonates.
In adults, because the airway caliber and lung function of males are greater than those of females, a smaller airway caliber in females may contribute to the reversal of the sex ratio for asthma prevalence after puberty.7 Because airway resistance is inversely proportionate to the fourth power of airway diameter, airway resistance easily increases when airway caliber is small, and CO2 retention in blood may occur in females due to the small airway caliber even when the degree of airflow obstruction is not as severe although frank ventilatory failure generally occurs when forced expiratory volume in 1 second (FEV1) is ≤25% during an asthma attack.13 Some genetic polymorphisms are associated with asthma in females and not in males, such as cytotoxic T lymphocyte-associated 4 receptor (+49 A/G), cyclooxygenase-2 (-165 G/C), defensin β-1, toll-like receptor 4, and estrogen receptor α.12 In addition to these genetic, anatomical, and physiological differences, apparent immunological differences must exist between the sexes because not only allergic asthma,6 but also other allergic diseases,8,9 occur more frequently in females after puberty, as mentioned above. Moreover, females are more sensitive to environmental factors such as smoking, cooking gas, ozone, and pets, and body mass index (BMI) is closely related to asthma and atopy in women.4 Sex hormones may play an important role in this sex difference.
SEX HORMONES AND ASTHMA
The immunological system interacts with the endocrine system. In general, female sex hormones aggravate asthma and other allergic diseases, whereas male sex hormones suppress such diseases.3 Females seem to be born with a Th2 bias, as humans naturally tend to shift to Th2 to prevent mothers from rejecting their fetuses during pregnancy, and immunity becomes mature in a Th2-suppressing manner following exposure to infections after birth (the so-called hygiene hypothesis).
Women who reached menarche before the age of 12 years have a 2.08-fold higher risk of asthma after puberty compared with those who reached menarche after the age 12 years.14 During the menstrual cycle, skin-test responses to allergens,15,16 adenosine-AHR,17 and exhaled nitric oxide level16 vary according to the levels of sex hormones, and these responses are abolished by the use of oral contraceptives.16,17 Progesterone18 and estrogen,19,20 the representative female sex hormones, increase the secretion of interleukin (IL)-4 and the total IgE level, respectively. However, progesterone inhibits histamine release from mast cells,21 and estrogen induces FoxP3+ regulatory T (Treg) cells22,23; thus, these hormones do not simply work in one direction. Nevertheless, generally speaking, when the luteal phase, with its increased blood levels of progesterone and estrogen, is suppressed by the use of oral contraceptives, associated cyclic changes in AHR are inhibited.17 The risk of asthma decreases by 7% per year during oral contraceptive pill use in young women24 and increases 2.29 times with hormone replacement therapy in postmenopausal women.25
Male mice have higher numbers of CD4+/CD25+ T cells, so it is difficult to establish an asthma model using male mice; ovalbumin sensitization and challenge in male mice increases the levels of ovalbumin-specific IgE, IL-4, and IL-13 and the numbers of CD4+ T cells, B cells, and eosinophils less remarkably than in female mice.26 During a transient medical castration in men, not only serum testosterone, but also CD4+/CD25+ T cell numbers and CD8+ T cell interferon (IFN)-γ expression decreases, and this is prevented by testosterone replacement.27 Androgen inhibits leukotriene synthesis by controlling the activity of extracellular signal-regulated kinases.28 Dehydroepiandrosterone (DHEA), a weak androgen, which is produced in the adrenal gland, has similar activity, so there is a significant relationship between the serum level of DHEA sulfate ester (DHEA-S), a precursor of DHEA, and the number of IFN-γ-secreting cells.29 Because the serum DHEA-S level is relatively low in patients with asthma30 or atopic dermatitis,31 and because it decreases in a dose-dependent manner with the use of inhaled corticosteroids,32 screening tests for serum DHEA-S level may be useful for examining adrenal function and for determining DHEA replacement therapy in patients with asthma who are receiving inhaled steroid treatment. Testosterone replacement therapy in male patients with rheumatoid arthritis and low testosterone levels increases CD8+ T cells and decreases IgM rheumatoid factor and medication requirements.3
SEX, OBESITY, AND ASTHMA
Although women are usually under stress to be lean, and their greatest concern is eating habits, obesity develops more frequently in women. When obesity is defined as a BMI ≥30 kg/m2, the prevalence of obesity is 24.1% in men and 24.9% in women in the United Kingdom, and 28.6% in men and 34.2% in women in the United States.33 Although women must store excess fat for reproduction and lactation, the exact mechanism underlying the need for excess body weight in women is not well understood; however, lower energy expenditure due to shorter stature and less fat-free body mass in women must play an important role.34 Estrogen rather decreases appetite and body weight. This hormone decreases abdominal and visceral fat, which is associated with metabolic syndrome, but it distributes fat to the gluteo-femoral area, and this fat is used through lipolysis by lipoprotein lipase activity during lactation.
Recently, the prevalence of asthma and obesity have simultaneously increased, so it is presumed that obesity may be one of the causes of asthma.35 Camargo et al.36 reported in 1999 that the risk for developing asthma was 2.7 times higher in obese women and 2.5 times higher when body weight had increased by 25 kg at the 4-year follow-up after the age of 18 years. The mechanisms by which obesity affects asthma include 1) mechanical factors such as lower lung volume and compliance and smaller diameter of the peripheral airways, 2) comorbidities such as gastroesophageal reflux disease (GERD), 3) systemic inflammation caused by fat-cell-secreting adipokines including IL-6, tumor necrosis factor (TNF)-α and eotaxin, 4) a decrease in adiponectin, an obesity hormone with an anti-inflammatory effect, 5) an increase in AHR by leptin, which has a similar structure as IL-6, and 6) increased oxidative stress.37,38 Unlike patients with Th2-mediated asthma, obese asthmatics show unremarkable airway inflammation, suggesting a distinct asthma phenotype.38 Thus, these patients may have relative resistance to conventional corticosteroid therapy,38,39 and special attention should be paid to frequently comorbid GERD.38 However, Yoo et al.40 have reported that the prevalence of atopy is higher in obese Korean male adolescents. Jeon et al.41 have documented that the FEV1/forced vital capacity (FVC) ratio is significantly lower in obese women or during the menstrual period, but unfortunately, they did not observe whether obese patients with asthma showed a decreased FEV1/FVC ratio during the menstrual period.
Insulin resistance is associated with obesity; diacylglycerol accumulates in the skeletal muscle and liver and triggers protein kinase C activation, with subsequent impairments in insulin signalling.42 Insulin has an anti-inflammatory effect by inhibiting nuclear factor κB and inducing FoxP3+Treg cells, so insulin may affect athma.23 Despite the observation that sex hormone replacement therapy increases the risk of developing postmenopausal asthma in lean women without insulin resistance, it may improve asthma in obese postmenopausal women with insulin resistance by decreasing body weight and insulin resistance.
FEMALE-SPECIFIC ASTHMA
Perimenstrual (catamenial) asthma
As mentioned above, asthma and allergy markers vary during the menstrual cycle according to sex hormone levels,15-17 and perimenstrual asthma occurs in 30-40% of female asthmatics during the child-bearing years, regardless of allergy.23 Recently, in a study monitoring daily respiratory symptoms of fertile asthmatic females during two consecutive menstrual cycles, Pereira Vega et al.43 reported that 59.6% of patients showed premenstrual exacerbation of symptoms in at least one of the two cycles, but only 22.3% in both cycles. Autoantibodies against sex hormones are also able to induce perimenstrual asthma and miscarriage.44 Since perimenstrual asthma was first described by Frank et al.45 in 1931, numerous studies have reported on this type of asthma. During the perimenstrual period, not only adenosine-AHR,17 but also methacholine-AHR46 is aggravated, and the use of oral contraceptives can prevent such aggravation17,46 and improve clinical symptoms.23
Asthma-related menstrual irregularity
As asthmatics show changes in sex hormones such as a decrease in serum DHEA-S level regardless of drug therapy, they can also have associated menstrual irregularities. In a study at seven institutions with approximately 8,000 women of childbearing age, Svanes et al.47 found that the prevalence of menstrual irregularity was 1.54 times higher in patients with asthma and 1.29 times higher in those with allergic rhinitis regardless of the use of antiasthmatic medications, and this difference was similar at all seven institutions. They suggested that insulin resistance may correlate with both asthma and menstrual irregularity.
Pregnancy
Pregnancy affects asthma and vice versa, including mechanical changes in lung function. The purpose of asthma treatment during pregnancy is delivery of a healthy baby, which is an important goal in addition to those for general asthma. Since the considerable number of congenital malformation cases due to thalidomide in the 1960s, careful administration of drugs during pregnancy has become common sense. Details about this issue can be found elsewhere.48
SEX DIFFERENCES IN THE EFFECTIVENESS OF AND ADVERSE REACTIONS TO ASTHMA TREATMENT
Treatment effectiveness
Because a sex difference exists in the prevalence of asthma and allergic diseases and sex hormones affect allergic diseases, it is postulated that responses to anti-asthmatic drugs differ between the sexes; however, few investigations have examined this issue. Montelukast, but not fluticasone, increases FEV1 in females more than in males among patients aged 6-17 years with asthma.49 Johnston et al.50 reported that montelukast prevents asthma's worsening in younger boys aged 2-5 years (odds ratio [OR], 0.03; P<0.001) or 6-9 years (OR, 0.27; P=0.028) and in older girls aged 10-14 years (OR, 0.17; P=0.001). Such sex differences in treatment effectiveness may be mainly attributable to dysanaptic lung growth in boys3,4,51 and female sex hormone after menarche, which are associated with more active asthma.51 BCG and DHEA are more effective in female mice than in male mice.52 Male mice have a high IFN-γ/IL-5 ratio and low allergen-specific IgE level, but they develop asthma despite this and may require treatment to help overcome these conditions. Furthermore, male children have higher theophylline clearance53 and lower drug adherence during asthma exacerbation,54 but controlling their asthma is relatively easier.55
Adverse reactions
Adverse drug reactions occur more frequently in females than in males. For instance, the rate of a positive skin test to penicillin is 3.2 times higher in females than in males.56 Additionally, as females have a lower drug clearance rate, adverse reactions due to theophylline53 and hypersomnia resulting from first-generation antihistamines and cetirizine occur more frequently in females than males.57 Asthmatic women using fluticasone dry powder inhalers complain of hoarseness and dysphonia more frequently than men do.58 Estrogen inhibits osteoclast proliferation and bone turnover, so deficiency in this hormone in postmenopausal women makes them prone to developing steroid-induced osteoporosis.59 Testosterone prevents the development of osteoporosis by directly acting on osteoblasts or after being converted to estrogen by aromatase.
SEX-SPECIFIC ASTHMA TREATMENT
Females
Female-specific asthma including perimenstrual asthma may require appropriate therapy such as oral contraceptives. Considering that asthma and other allergic diseases as well as adverse reactions to drugs occur more frequently in females, it is conceivable that DHEA, an androgen with a less virilizing effect, could be used as a treatment option for female-specific asthma. DHEA reduced the steroid requirement without significant adverse reactions, other than mild acne, in patients with systemic lupus erythematosus.60 A phase IIa clinical trial of an inhalation formulation of synthetic DHEA sulfate EPI-12323 (Naturasone) found a significant inhibition of allergen-induced late airway reactions,61 suggesting its potential use in clinical practice.
Although DHEA is the crucial precursor for converting both testosterone and estrogen, DHEA administration increases circulating androgens in women.62 T lymphocytes and endothelial cells have high-affinity binding sites for DHEA. A previous study demonstrated that DHEA inhibits AHR/airway eosinophilia and that its effects are related to a decrease in IL-5 concentration in mice.63 DHEA inhibits cell proliferation but maintains Th1 bias64 and may also work through IL-10 production.65 Furthermore, the antagonistic action of DHEA against glucocorticoid-induced Th2 deviation can probably occur through peroxisome proliferator-activated receptor-α.66 DHEA inhibits airway smooth muscle proliferation and airway remodeling by inhibiting DNA binding of activator protein (AP)-1, and it may produce a steroid-sparing effect by suppressing AP-1-induced glucocorticoid resistance.67 Moreover, DHEA inhibits production of TNF-α68 and neutrophilic inflammation,69 through which it exerts a therapeutic effect on severe asthma associated with such factors. However, the long-term systemic use of DHEA may induce chronic heart failure by deleting ubiquinone through the inhibition of glucose 6-phosphate dehydrogenase and hydroxymethylglutaryl coenzyme A reductase activities.70 Therefore, an inhaled preparation of DHEA is desirable for patients to avoid any unexpected serious adverse reactions.
Males
Serum DHEA-S level significantly correlates with IFN-γ level,29 and men have a high serum DHEA-S level. Thus, allergic diseases do not frequently develop in men. However, the therapeutic effectiveness of DHEA may be low because an increase in the IFN-γ/IL-5 ratio by DHEA is inversely related to serum DHEA-S level.64 In that case, men would require treatment modalities that induce Th1 cells more strongly or act through different mechanisms. However, clinicians should remember that if the dosage of DHEA is excessively high, its therapeutic effect tends to decrease.63 Because the dose of inhaled steroid correlates with the degree of decrease in serum DHEA-S level,32 DHEA replacement therapy would be ideal for asthma treatment in men with a decreased DHEA-S level detected through regular monitoring.
CONCLUSIONS
Females are more prone to suffer allergic asthma, to having difficulty controlling asthma symptoms, and to showing adverse responses to drugs. As asthma symptoms show cyclic changes depending on female hormone levels in many women of child-bearing age, the use of contraceptives in women may specifically help treat such perimenstrual asthma and severe asthma as well. Generally, testosterone seems to suppress asthma, and DHEA, a less virilizing androgen, may be effective for treating asthma. Evidence exists for the therapeutic and steroid-sparing effect of DHEA. However, further studies on the optimal dose and route of DHEA for each sex are needed. Monitoring of the serum DHEA-S level is necessary for patients with asthma who are receiving inhaled steroid treatment, and at least replacement therapy for patients with a low level of DHEA may be helpful for treating their asthma.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Global strategy for asthma management and prevention [Internet] Global Initiative for Asthma. [updated 2009]. Available from: http://www.ginasthma.org.
- 2.Kim SH, Ye YM, Hur GY, Lee SK, Sampson AP, Lee HY, Park HS. CysLTR1 promoter polymorphism and requirement for leukotriene receptor antagonist in aspirin-intolerant asthma patients. Pharmacogenomics. 2007;8:1143–1150. doi: 10.2217/14622416.8.9.1143. [DOI] [PubMed] [Google Scholar]
- 3.Osman M. Therapeutic implications of sex differences in asthma and atopy. Arch Dis Child. 2003;88:587–590. doi: 10.1136/adc.88.7.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almqvist C, Worm M, Leynaert B working group of GA2LEN WP 2.5 'Gender'. Impact of gender on asthma in childhood and adolescence: a GA2LEN review. Allergy. 2008;63:47–57. doi: 10.1111/j.1398-9995.2007.01524.x. [DOI] [PubMed] [Google Scholar]
- 5.Chen W, Mempel M, Schober W, Behrendt H, Ring J. Gender difference, sex hormones, and immediate type hypersensitivity reactions. Allergy. 2008;63:1418–1427. doi: 10.1111/j.1398-9995.2008.01880.x. [DOI] [PubMed] [Google Scholar]
- 6.Wormald PJ. Age-sex incidence in symptomatic allergies: an excess of females in the child-bearing years. J Hyg (Lond) 1977;79:39–42. doi: 10.1017/s0022172400052827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Marco R, Locatelli F, Sunyer J, Burney P. Differences in incidence of reported asthma related to age in men and women. A retrospective analysis of the data of the European Respiratory Health Survey. Am J Respir Crit Care Med. 2000;162:68–74. doi: 10.1164/ajrccm.162.1.9907008. [DOI] [PubMed] [Google Scholar]
- 8.Osman M, Hansell AL, Simpson CR, Hollowell J, Helms PJ. Gender-specific presentations for asthma, allergic rhinitis and eczema in primary care. Prim Care Respir J. 2007;16:28–35. doi: 10.3132/pcrj.2007.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poulos LM, Waters AM, Correll PK, Loblay RH, Marks GB. Trends in hospitalizations for anaphylaxis, angioedema, and urticaria in Australia, 1993-1994 to 2004-2005. J Allergy Clin Immunol. 2007;120:878–884. doi: 10.1016/j.jaci.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 10.Nicolai T, Pereszlenyiova-Bliznakova L, Illi S, Reinhardt D, von Mutius E. Longitudinal follow-up of the changing gender ratio in asthma from childhood to adulthood: role of delayed manifestation in girls. Pediatr Allergy Immunol. 2003;14:280–283. doi: 10.1034/j.1399-3038.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 11.Sears MR, Burrows B, Flannery EM, Herbison GP, Holdaway MD. Atopy in childhood. I. Gender and allergen related risks for development of hay fever and asthma. Clin Exp Allergy. 1993;23:941–948. doi: 10.1111/j.1365-2222.1993.tb00279.x. [DOI] [PubMed] [Google Scholar]
- 12.Postma DS. Gender differences in asthma development and progression. Gend Med. 2007;4(Suppl B):S133–S146. doi: 10.1016/s1550-8579(07)80054-4. [DOI] [PubMed] [Google Scholar]
- 13.Nowak RM, Tomlanovich MC, Sarkar DD, Kvale PA, Anderson JA. Arterial blood gases and pulmonary function testing in acute bronchial asthma. Predicting patient outcomes. JAMA. 1983;249:2043–2046. [PubMed] [Google Scholar]
- 14.Salam MT, Wenten M, Gilliland FD. Endogenous and exogenous sex steroid hormones and asthma and wheeze in young women. J Allergy Clin Immunol. 2006;117:1001–1007. doi: 10.1016/j.jaci.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Kalogeromitros D, Katsarou A, Armenaka M, Rigopoulos D, Zapanti M, Stratigos I. Influence of the menstrual cycle on skin-prick test reactions to histamine, morphine and allergen. Clin Exp Allergy. 1995;25:461–466. doi: 10.1111/j.1365-2222.1995.tb01078.x. [DOI] [PubMed] [Google Scholar]
- 16.Mandhane PJ, Hanna SE, Inman MD, Duncan JM, Greene JM, Wang HY, Sears MR. Changes in exhaled nitric oxide related to estrogen and progesterone during the menstrual cycle. Chest. 2009;136:1301–1307. doi: 10.1378/chest.09-0604. [DOI] [PubMed] [Google Scholar]
- 17.Tan KS, McFarlane LC, Lipworth BJ. Modulation of airway reactivity and peak flow variability in asthmatics receiving the oral contraceptive pill. Am J Respir Crit Care Med. 1997;155:1273–1277. doi: 10.1164/ajrccm.155.4.9105066. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell VL, Gershwin LJ. Progesterone and environmental tobacco smoke act synergistically to exacerbate the development of allergic asthma in a mouse model. Clin Exp Allergy. 2007;37:276–286. doi: 10.1111/j.1365-2222.2007.02658.x. [DOI] [PubMed] [Google Scholar]
- 19.Hamano N, Terada N, Maesako K, Hohki G, Ito T, Yamashita T, Konno A. Effect of female hormones on the production of IL-4 and IL-13 from peripheral blood mononuclear cells. Acta Otolaryngol Suppl. 1998;537:27–31. [PubMed] [Google Scholar]
- 20.Holt PG, Britten D, Sedgwick JD. Suppression of IgE responses by antigen inhalation: studies on the role of genetic and environmental factors. Immunology. 1987;60:97–102. [PMC free article] [PubMed] [Google Scholar]
- 21.Vasiadi M, Kempuraj D, Boucher W, Kalogeromitros D, Theoharides TC. Progesterone inhibits mast cell secretion. Int J Immunopathol Pharmacol. 2006;19:787–794. doi: 10.1177/039463200601900408. [DOI] [PubMed] [Google Scholar]
- 22.Tai P, Wang J, Jin H, Song X, Yan J, Kang Y, Zhao L, An X, Du X, Chen X, Wang S, Xia G, Wang B. Induction of regulatory T cells by physiological level estrogen. J Cell Physiol. 2008;214:456–464. doi: 10.1002/jcp.21221. [DOI] [PubMed] [Google Scholar]
- 23.van den Berge M, Heijink HI, van Oosterhout AJ, Postma DS. The role of female sex hormones in the development and severity of allergic and non-allergic asthma. Clin Exp Allergy. 2009;39:1477–1481. doi: 10.1111/j.1365-2222.2009.03354.x. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins MA, Dharmage SC, Flander LB, Douglass JA, Ugoni AM, Carlin JB, Sawyer SM, Giles GG, Hopper JL. Parity and decreased use of oral contraceptives as predictors of asthma in young women. Clin Exp Allergy. 2006;36:609–613. doi: 10.1111/j.1365-2222.2006.02475.x. [DOI] [PubMed] [Google Scholar]
- 25.Barr RG, Wentowski CC, Grodstein F, Somers SC, Stampfer MJ, Schwartz J, Speizer FE, Camargo CA., Jr Prospective study of postmenopausal hormone use and newly diagnosed asthma and chronic obstructive pulmonary disease. Arch Intern Med. 2004;164:379–386. doi: 10.1001/archinte.164.4.379. [DOI] [PubMed] [Google Scholar]
- 26.Melgert BN, Postma DS, Kuipers I, Geerlings M, Luinge MA, van der Strate BW, Kerstjens HA, Timens W, Hylkema MN. Female mice are more susceptible to the development of allergic airway inflammation than male mice. Clin Exp Allergy. 2005;35:1496–1503. doi: 10.1111/j.1365-2222.2005.02362.x. [DOI] [PubMed] [Google Scholar]
- 27.Page ST, Plymate SR, Bremner WJ, Matsumoto AM, Hess DL, Lin DW, Amory JK, Nelson PS, Wu JD. Effect of medical castration on CD4+ CD25+ T cells, CD8+ T cell IFN-gamma expression, and NK cells: a physiological role for testosterone and/or its metabolites. Am J Physiol Endocrinol Metab. 2006;290:E856–E863. doi: 10.1152/ajpendo.00484.2005. [DOI] [PubMed] [Google Scholar]
- 28.Pergola C, Dodt G, Rossi A, Neunhoeffer E, Lawrenz B, Northoff H, Samuelsson B, Rådmark O, Sautebin L, Werz O. ERK-mediated regulation of leukotriene biosynthesis by androgens: a molecular basis for gender differences in inflammation and asthma. Proc Natl Acad Sci U S A. 2008;105:19881–19886. doi: 10.1073/pnas.0809120105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verthelyi D, Klinman DM. Sex hormone levels correlate with the activity of cytokine-secreting cells in vivo. Immunology. 2000;100:384–390. doi: 10.1046/j.1365-2567.2000.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunn PJ, Mahood CB, Speed JF, Jury DR. Dehydroepiandrosterone sulphate concentrations in asthmatic patients: pilot study. N Z Med J. 1984;97:805–808. [PubMed] [Google Scholar]
- 31.Tabata N, Tagami H, Terui T. Dehydroepiandrosterone may be one of the regulators of cytokine production in atopic dermatitis. Arch Dermatol Res. 1997;289:410–414. doi: 10.1007/s004030050213. [DOI] [PubMed] [Google Scholar]
- 32.Kannisto S, Korppi M, Remes K, Voutilainen R. Serum dehydroepiandrosterone sulfate concentration as an indicator of adrenocortical suppression in asthmatic children treated with inhaled steroids. J Clin Endocrinol Metab. 2001;86:4908–4912. doi: 10.1210/jcem.86.10.7975. [DOI] [PubMed] [Google Scholar]
- 33.Global obesity prevalence in adults [Internet] International Obesity Task Force. Available from: http://www.iotf.org.
- 34.Lovejoy JC, Sainsbury A Stock Conference 2008 Working Group. Sex differences in obesity and the regulation of energy homeostasis. Obes Rev. 2009;10:154–167. doi: 10.1111/j.1467-789X.2008.00529.x. [DOI] [PubMed] [Google Scholar]
- 35.Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol. 2005;115:897–909. doi: 10.1016/j.jaci.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 36.Camargo CA, Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med. 1999;159:2582–2588. doi: 10.1001/archinte.159.21.2582. [DOI] [PubMed] [Google Scholar]
- 37.Delgado J, Barranco P, Quirce S. Obesity and asthma. J Investig Allergol Clin Immunol. 2008;18:420–425. [PubMed] [Google Scholar]
- 38.Lugogo NL, Kraft M, Dixon AE. Does obesity produce a distinct asthma phenotype? J Appl Physiol. 2010;108:729–734. doi: 10.1152/japplphysiol.00845.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sutherland ER, Goleva E, Strand M, Beuther DA, Leung DY. Body mass and glucocorticoid response in asthma. Am J Respir Crit Care Med. 2008;178:682–687. doi: 10.1164/rccm.200801-076OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoo S, Kim HB, Lee SY, Kim BS, Kim JH, Yu JH, Kim BJ, Hong SJ. Association between obesity and the prevalence of allergic diseases, atopy, and bronchial hyperresponsiveness in Korean adolescents. Int Arch Allergy Immunol. 2010;154:42–48. doi: 10.1159/000319207. [DOI] [PubMed] [Google Scholar]
- 41.Jeon YH, Yang HJ, Pyun BY. Lung function in Korean adolescent girls: in association with obesity and the menstrual cycle. J Korean Med Sci. 2009;24:20–25. doi: 10.3346/jkms.2009.24.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375:2267–2277. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pereira Vega A, Sánchez Ramos JL, Maldonado Pérez JA, Alvarez Gutierrez FJ, Ignacio García JM, Vázquez Oliva R, Romero Palacios P, Bravo Nieto JM, Sánchez Rodríguez I, Gil Muñoz F. Variability in the prevalence of premenstrual asthma. Eur Respir J. 2010;35:980–986. doi: 10.1183/09031936.00045109. [DOI] [PubMed] [Google Scholar]
- 44.Jensen-Jarolim E, Untersmayr E. Gender-medicine aspects in allergology. Allergy. 2008;63:610–615. doi: 10.1111/j.1398-9995.2008.01645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frank RT. The hormonal cause of premenstrual tension. Arch Neurol Psychiatry. 1931;26:1053–1057. [Google Scholar]
- 46.Dratva J, Schindler C, Curjuric I, Stolz D, Macsali F, Gomez FR, Zemp E SAPALDIA Team. Perimenstrual increase in bronchial hyperreactivity in premenopausal women: results from the population-based SAPALDIA 2 cohort. J Allergy Clin Immunol. 2010;125:823–829. doi: 10.1016/j.jaci.2009.12.938. [DOI] [PubMed] [Google Scholar]
- 47.Svanes C, Real FG, Gislason T, Jansson C, Jögi R, Norrman E, Nyström L, Torén K, Omenaas E. Association of asthma and hay fever with irregular menstruation. Thorax. 2005;60:445–450. doi: 10.1136/thx.2004.032615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luskin AT, Lipkowitz MA. The diagnosis and management of asthma during pregnancy. Immunol Allergy Clin North Am. 2000;20:745–761. [Google Scholar]
- 49.Szefler SJ, Phillips BR, Martinez FD, Chinchilli VM, Lemanske RF, Strunk RC, Zeiger RS, Larsen G, Spahn JD, Bacharier LB, Bloomberg GR, Guilbert TW, Heldt G, Morgan WJ, Moss MH, Sorkness CA, Taussig LM. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005;115:233–242. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 50.Johnston NW, Mandhane PJ, Dai J, Duncan JM, Greene JM, Lambert K, Sears MR. Attenuation of the September epidemic of asthma exacerbations in children: a randomized, controlled trial of montelukast added to usual therapy. Pediatrics. 2007;120:e702–e712. doi: 10.1542/peds.2006-3317. [DOI] [PubMed] [Google Scholar]
- 51.de Benedictis FM, Baraldi E, Boner A. Gender differences in the effectiveness of asthma treatment. Pediatrics. 2008;121:1289. doi: 10.1542/peds.2008-0373. [DOI] [PubMed] [Google Scholar]
- 52.Cui Y, Choi IS, Koh YA, Lin XH, Cho YB, Won YH. Effects of combined BCG and DHEA treatment in preventing the development of asthma. Immunol Invest. 2008;37:191–202. doi: 10.1080/08820130801967833. [DOI] [PubMed] [Google Scholar]
- 53.Igarashi T, Iwakawa S. Effect of gender on theophylline clearance in the asthmatic acute phase in Japanese pediatric patients. Biol Pharm Bull. 2009;32:304–307. doi: 10.1248/bpb.32.304. [DOI] [PubMed] [Google Scholar]
- 54.Sundberg R, Torén K, Franklin KA, Gislason T, Omenaas E, Svanes C, Janson C. Asthma in men and women: treatment adherence, anxiety, and quality of sleep. Respir Med. 2010;104:337–344. doi: 10.1016/j.rmed.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 55.Hermosa JL, Sánchez CB, Rubio MC, Mínguez MM, Walther JL. Factors associated with the control of severe asthma. J Asthma. 2010;47:124–130. doi: 10.3109/02770900903518835. [DOI] [PubMed] [Google Scholar]
- 56.Park MA, Matesic D, Markus PJ, Li JT. Female sex as a risk factor for penicillin allergy. Ann Allergy Asthma Immunol. 2007;99:54–58. doi: 10.1016/S1081-1206(10)60621-7. [DOI] [PubMed] [Google Scholar]
- 57.Nicolas JM, Espie P, Molimard M. Gender and interindividual variability in pharmacokinetics. Drug Metab Rev. 2009;41:408–421. doi: 10.1080/10837450902891485. [DOI] [PubMed] [Google Scholar]
- 58.Ishizuka T, Hisada T, Aoki H, Yanagitani N, Kaira K, Utsugi M, Shimizu Y, Sunaga N, Dobashi K, Mori M. Gender and age risks for hoarseness and dysphonia with use of a dry powder fluticasone propionate inhaler in asthma. Allergy Asthma Proc. 2007;28:550–556. doi: 10.2500/aap2007.28.3049. [DOI] [PubMed] [Google Scholar]
- 59.Frenkel B, Hong A, Baniwal SK, Coetzee GA, Ohlsson C, Khalid O, Gabet Y. Regulation of adult bone turnover by sex steroids. J Cell Physiol. 2010;224:305–310. doi: 10.1002/jcp.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petri MA, Lahita RG, Van Vollenhoven RF, Merrill JT, Schiff M, Ginzler EM, Strand V, Kunz A, Gorelick KJ, Schwartz KE GL601 Study Group. Effects of prasterone on corticosteroid requirements of women with systemic lupus erythematosus: a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2002;46:1820–1829. doi: 10.1002/art.10364. [DOI] [PubMed] [Google Scholar]
- 61.Shah S. European Respiratory Society--14th Annual Congress. Drug highlights. 4-8 September 2004, Glasgow, UK. IDrugs. 2004;7:914–916. [PubMed] [Google Scholar]
- 62.Arlt W. Dehydroepiandrosterone replacement therapy. Curr Opin Endocrinol Diabetes. 2006;13:291–305. [Google Scholar]
- 63.Lin XH, Choi IS, Koh YA, Cui Y. Effects of combined bacille Calmette-Guerin and dehydroepiandrosterone treatment on established asthma in mice. Exp Lung Res. 2009;35:250–261. doi: 10.1080/01902140802626656. [DOI] [PubMed] [Google Scholar]
- 64.Choi IS, Cui Y, Koh YA, Lee HC, Cho YB, Won YH. Effects of dehydroepiandrosterone on Th2 cytokine production in peripheral blood mononuclear cells from asthmatics. Korean J Intern Med. 2008;23:176–181. doi: 10.3904/kjim.2008.23.4.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng GF, Tseng J. Regulation of murine interleukin-10 production by dehydroepiandrosterone. J Interferon Cytokine Res. 2000;20:471–478. doi: 10.1089/10799900050023889. [DOI] [PubMed] [Google Scholar]
- 66.Hernandez-Pando R, De La Luz Streber M, Orozco H, Arriaga K, Pavon L, Al-Nakhli SA, Rook GA. The effects of androstenediol and dehydroepiandrosterone on the course and cytokine profile of tuberculosis in BALB/c mice. Immunology. 1998;95:234–241. doi: 10.1046/j.1365-2567.1998.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dashtaki R, Whorton AR, Murphy TM, Chitano P, Reed W, Kennedy TP. Dehydroepiandrosterone and analogs inhibit DNA binding of AP-1 and airway smooth muscle proliferation. J Pharmacol Exp Ther. 1998;285:876–883. [PubMed] [Google Scholar]
- 68.Kipper-Galperin M, Galilly R, Danenberg HD, Brenner T. Dehydroepiandrosterone selectively inhibits production of tumor necrosis factor α and interleukin-6 in astrocytes. Int J Dev Neurosci. 1999;17:765–775. doi: 10.1016/s0736-5748(99)00067-2. [DOI] [PubMed] [Google Scholar]
- 69.Corporate overview [Internet] Epigenesis Pharmaceuticals. Available from: http://www.epigene.com/about/overview.html.
- 70.Nyce JW. Liquid inhalation formulations of dehydroepiandrosterone derivatives [Internet] Available from: http://www.faqs.org/patents/app/20090053143.