Abstract
The pore-forming toxin streptolysin O (SLO) can be used to reversibly permeabilize adherent and nonadherent cells, allowing delivery of molecules with up to 100 kDa mass to the cytosol. Using FITC-labeled albumin, 105–106 molecules were estimated to be entrapped per cell. Repair of toxin lesions depended on Ca2+-calmodulin and on intact microtubules, but was not sensitive to actin disruption or to inhibition of protein synthesis. Resealed cells were viable for days and retained the capacity to endocytose and to proliferate. The active domains of large clostridial toxins were introduced into three different cell lines. The domains were derived from Clostridium difficile B-toxin and Clostridium sordelli lethal toxin, which glycosylate small G-proteins, and from Clostridium botulinum C2 toxin, which ADP-ribosylates actin. After delivery with SLO, all three toxins disrupted the actin cytoskeleton to cause rounding up of the cells. Glucosylation assays demonstrated that G-proteins Rho and Ras were retained in the permeabilized cells and were modified by the respective toxins. Inactivation of these G-proteins resulted in reduced stimulus-dependent granule secretion, whereas ADP-ribosylation of actin by the C. botulinum C2-toxin resulted in enhanced secretion in cells. The presented method for introducing proteins into living cells should find multifaceted application in cell biology.
Keywords: protein delivery, pore-forming toxin
Methods for delivering proteins to the cytosol under retention of cell viability are scarce. Microinjection is possible but technically demanding and restricted to small cell numbers. Electroinjection with the voltage-discharge technique appears promising, but a limitation is that it cannot be used on adherent cells (1). Other methods for introducing macromolecules into cells have been reviewed recently; all require considerable methodological expertise, and no single method is widely used (2). The “Trojan” peptide penetration system is potentially useful, but is restricted to delivery of oligopeptides (3). The viral hemagglutinin mediated fusion technique works well but is not generally easy to perform (4). Here, we describe a very simple method to bring exogenous proteins into living cells.
Streptolysin O (SLO), prototype of the cholesterol-binding family of bacterial exotoxins, forms very large pores in the plasma membrane of mammalian cells (5). The three-dimensional structure of perfringolysin, a member of this family, has been solved (6), and the molecular events leading to pore formation by SLO are understood in some detail (7–9). After binding to membranes, toxin monomers diffuse laterally in the bilayer and oligomerize to form homotypic aggregates that represent very large transmembrane pores whose diameters can reach 35 nm. With currently used protocols, SLO attack is generally lethal, so the possibilities of conducting cell biological studies after permeabilization have been restricted. Nevertheless, the toxin is being widely used to investigate cellular processes on a short time scale (10).
Previous work by Giles et al. (11, 12) has indicated that, under certain conditions, cells can repair SLO lesions. Membrane permeabilization appeared to be reversible because anti-sense oligonucleotides could be introduced and their activity demonstrated. Prompted by these reports, we ventured to establish a protocol for delivering proteins. Although our experiments were underway, Fawcett et al. (13) reported that fluorescein-labeled dextran and albumin could be visualized in rat myocytes after their permeabilization with low doses of SLO. Microscopic examination indicated localization of the markers in the cytosol, but, in the absence of functional assays, it was not possible to conclude that the method could be used to deliver biologically active molecules to their intracellular targets (13). Here, we describe a simple, generally applicable protocol for reversible permeabilization of adherent and nonadherent cells. To demonstrate that cellular processes remain intact, we delivered active domains of large bacterial exotoxins into three different targets and showed that the toxins exerted their function in the resealed cells.
Materials and Methods
Materials.
Recombinant SLO, CDB546 from Clostridium difficile toxin B, CS546 from Clostridium sordelli lethal toxin, and C2I from Clostridium botulinum C2 toxin were prepared as described (14–16). Trinitrophenyl (TNP)-ovalbumin and IgE were kindly donated by A. Hoffmann (Paul Ehrlich Institute, Langen, Germany). Chemicals were purchased from Sigma. Goat F(ab′)2-FITC was from Coulter; and goat IgG-FITC was from Becton Dickinson.
Cell Culture.
THP-1 cells were grown in RPMI medium (Life Technologies, Eggenstein, Germany) supplemented with 10% FCS, 2 mM l-glutamine, 1 mM sodium pyruvate, 50 μM mercaptoethanol, 100 units/ml penicillin, and 100 μg/ml streptomycin. Entry of propidium iodide, FITC-antibodies, FITC-albumin, or FITC-dextran into cells was assessed by flow cytometry.
COS-7 cells were grown in DMEM with glutamax-I, with pyridoxine (Life Technologies) supplemented with 10% FCS, 100 units/ml penicillin, and 100 μg/ml streptomycin.
Rat basophile leukemia cells transfected with the human muscarinic receptor (RBL 34) (2H3hm1; a gift from G. Schultz, Berlin, and P. Jones, Burlington, MA) were grown in Eagle's minimum essential medium with Earle's salts supplemented with 15% (vol/vol) heat-inactivated FCS, 4 mM glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. Cells were detached from culture plates with 125 mM NaCl, 1.5 mM EDTA, 5.6 mM glucose, and 10 mM Hepes, pH 7.2. Cells were preloaded with anti-TNP IgE (0.3 μg/ml) 12–24 h before antigen stimulation experiments.
Protocol for Reversible Permeabilization with SLO.
Reversible permeabilization of cells with SLO requires selection of the correct toxin dose. The permeabilization step is conducted in the absence of Ca2+, and resealing is induced by addition of Ca2+ after 10–15 min. Resealing is temperature-independent and occurs effectively at 4°C. The agent to be delivered to the cytosol is included at the permeabilization step. The required toxin concentration varies depending on cell target and must be determined by titration. The goal is to identify the concentration that effects permeabilization of 60–80% of the total cell population within 10–15 min.
Procedure.
Cells were suspended in HBSS without Ca2+ containing 30 mM Hepes, pH 7.2, for 15 min. SLO was added to final concentrations as given. After 15 min at 37°C, cells were stained with Trypan blue or propidium iodide, and the percentage of positively staining cells was approximated by microscopy or by flow cytometry. SLO concentrations that caused positive staining of 50–80% of the cells were selected. Resealing was induced by adding Ca2+ to the incubation mix. In a typical experiment, cells in a 24-well plate were permeabilized with SLO in 500 μl of buffer. To reseal, 1.5 ml of ice-cold medium containing 1–2 mM Ca2+ was added. Reducing agents such as mercaptoethanol were avoided. Over 50% of the permeabilized cells would reseal within 60 min in a successful experiment, as evidenced by the reduction in numbers of cells staining with Trypan blue or propidium iodide. Chemiluminescence measurements of cellular ATP were conducted as described (17) in parallel to ascertain that recuperation had occurred.
Assays.
Cell proliferation was assayed by using the In Situ Cell Proliferation Kit (FLUOS) from Roche Diagnostics. Endocytosis was assessed by flow cytometric determination of FITC-dextran uptake. Hexosaminidase release and glucosylation assays were performed as described (18, 19).
Results
Part I: Methodology.
Reversible permeabilization requires use of low SLO doses.
It is possible to reversibly permeabilize adherent and nonadherent cells. In every case, selecting the correct toxin dose is pivotal to success. The required toxin concentration will vary depending on cell target and density and must be determined by titration. The goal is to identify the toxin concentration that causes permeabilization of 60–80% of the total cell population within 10–15 min at 37°C. For nonadherent cells, this can conveniently be determined by flow cytometry. An experiment using THP cells is shown in Fig. 1A. Cells were suspended in Ca2+-free buffer and treated with 100 or 20 ng/ml SLO for 10 min. Staining with propidium iodide revealed that approximately 95% and 80% became permeabilized, respectively. Membrane repair was initiated by adding a low concentration of Ca2+ to the buffer. It was not necessary to add serum or serum components. Surprisingly, resealing occurred even at 4°C. When propidium iodide staining was performed after 2 h, it was found that, following treatment with 100 ng/ml SLO, a small population of the cells became resealed, as evidenced from the presence of approximately 7% propidium iodide-negative cells. Membrane repair was efficient in cells that had been treated with 20 ng/ml SLO. Then, approximately 63% of the cells were no longer stained with propidium iodide after the recovery period of 2 h.
Figure 1.
(A) Demonstration of membrane resealing by flow cytometric analysis of propidium iodide influx into cells. THP cells were incubated with SLO at the given concentrations. Addition of propidium iodide after 10 min resulted in red fluorescence of permeabilized cells. After repair (t120), the majority of cells treated with 20 ng/ml SLO became impermeable to the dye. (B) Proliferation assay. Flow cytometric analysis of 5-brom-2′-desoxyuridine incorporation performed after 2 h and 26 h following resealing revealed no difference to the controls. (C) Endocytosis assay. No alterations in endocytic uptake of FITC-dextran were observed by flow cytometry between toxin-treated and control cells.
Permeabilized cells remain viable.
Microscopically, we discerned no alterations even during a period of 5 days following permeabilization of cells. When proliferation assays were conducted to test for viability, no differences were detected compared with the controls (Fig. 1B). In the experiment of Fig. 1C, endocytic activity was tested by using FITC-dextran. Again, no differences to the controls were observed.
Kinetics of lesion repair.
THP cells were permeabilized with 20 ng/ml SLO for 15 min and repair was initiated. The number of propidium iodide-positive cells was approximately 75% at time 0, and then steadily decreased to approximately 25% after 60 min. In a typical case, therefore, approximately 25% of the original cell population would remain nonpermeabilized, approximately 25% would be dead, and approximately 50% would be resealed after 1 h.
Quantification of cellular ATP-levels complemented the propidium iodide uptake assays. In the experiment of Fig. 2, adherent COS cells were permeabilized with SLO at the depicted concentrations for 10 min, after which resealing was initiated. Total cellular ATP was quantified at the given times. It was found that when SLO was applied at doses effecting approximately 80% ATP depletion, cells were able to recuperate and gradually replenish their ATP stores, which reached 50–90% of the original levels after 4 h.
Figure 2.
Recovery of cellular ATP levels following SLO-treatment. Adherent COS-cells were permeabilized with SLO at the depicted concentrations for 10 min, after which resealing was initiated. Total cellular ATP was quantified at the given times. The experiment was reproduced two times with similar results.
Size limit of deliverable macromolecules.
To estimate the size of the transient pores, cells were permeabilized with 20 ng/ml SLO in the presence of FITC-dextran of varying mass, or in the presence of FITC-IgG or FITC-F(ab′)2. Flow cytometry was performed after 2 h. It was found that the approximate Mr cutoff was ≈100 kDa. Thus, FITC-dextran 70, FITC F(ab′)2 and also FITC-albumin entered the cells, whereas FITC-IgG and FITC-dextran with a Mr of ≥260,000 did not.
Fig. 3 depicts the results obtained by fluorescence microscopy in analogous experiments conducted with FITC-dextrans of various sizes. The dextrans were present during permeabilization, and propidium iodide was added after resealing. The panels on the right show the phase-contrast photographs, and the corresponding fluorescence microscopic pictures are depicted on the left. In the absence of SLO, no permeabilization occurred and cells exhibited neither green nor red fluorescence. When SLO was applied at 80 ng/ml, four populations of cells were identifiable at the end of the experiment: (i) primarily nonpermeabilized, with no fluorescence; (ii) permeabilized and resealed, with green fluorescence; (iii) permeabilized and nonresealed, with red fluorescence; and (iv) permeabilized and incompletely resealed, with yellow (dual) fluorescence. It was found that the molecular weight cut-off for dextrans deliverable to resealed cells was also in the range of 150 kDa.
Figure 3.
Entrapment of FITC-dextrans of varying size. THP cells were permeabilized with 20 ng/ml SLO in the presence of FITC-dextrans of the given masses, and propidium iodide was added after the resealing period. Panels on the right show the phase contrast photographs, and the corresponding fluorescence microscopic pictures are depicted on the left. The molecular weight cutoff for dextrans that could be delivered was in the range of 150 kDa.
Estimation of deliverable protein molecules.
THP1 cells were permeabilized in the presence of 50 μg/ml FITC-albumin. After resealing, cells were washed and solubilized with 1% SDS in 1 M NaOH. By using fluorimetry, we obtained rough estimates of 105–106 entrapped albumin molecules per cell.
Analysis of factors governing lesion repair.
Cells were exposed to 20 ng/ml SLO for 15 min, and the dependence of resealing on candidate factors was determined by propidium iodide-staining. Resealing did not occur in the absence of extracellular Ca2+ and failed in the presence of the calmodulin inhibitor calmidazolium. Resealing was also impaired by nocodazol, the disruptor of microtubuli. In contrast, membrane repair occurred in the presence of cytochalasin D and cycloheximide. These findings were congruent with those that have been reported for the resealing of physical membrane lesions (20, 21).
Part II: Functional Studies.
That delivered proteins could exert their function was demonstrated with three cell lines and three bacterial toxins. SLO-concentrations eliciting 50–70% permeabilization as estimated by Trypan-blue staining were used.
Delivery of the enzymatic domain of C.
sordelli results in glycosylation of G-proteins of the Rho and Ras family.
C. sordelli lethal toxin is a 270-kDa protein that specifically glycosylates and inactivates G-proteins of the Rho and Ras family (22). The N-terminal 546 amino acids harbor the functional domain (CS546) whose activity can be demonstrated in cell lysates (23). With the exception of microinjection, methods do not exist to deliver the isolated domain to the cytosol of living cells.
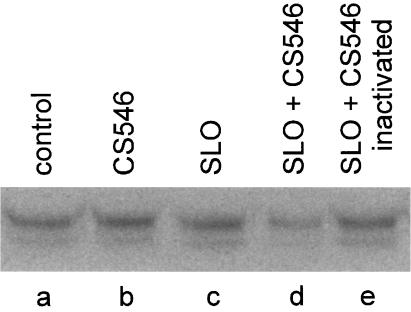
Cells were treated with 100 ng/ml SLO in the presence or absence of active or heat-inactivated CS546. Controls were left unpermeabilized in buffer, with or without CS546. After resealing, the cells were washed and incubated for 2 h. Cell lysates were then prepared, and small G-proteins were postglycosylated with 14C-labeled UDP-glucose in the presence of CS546. Fig. 4 depicts the results obtained after SDS/PAGE and autoradiography. The control (no SLO, no CS546) displaying the postglycoslyated small G-protein band is shown in lane a. Addition of CS546 to nonpermeabilized cells was essentially without effect (lane b). Treatment with SLO alone similarly caused no alteration of the glycosylation pattern. This control indicated that cells could tolerate SLO permeabilization and that the cellular components required for performance of the assay were not lost into the extracellular compartment. When cells were permeabilized in the presence of CS546, a marked reduction in the intensity of the labeled Ras band was observed (lane d), reflecting blockade of the glycosylation site with unlabeled cellular glucose. Heat-inactivated CS546 was without effect (lane e). These results demonstrated that the isolated functional domain of C. sordelli lethal toxin had indeed exerted its action in the resealed cells.
Figure 4.
Glucosylation of RBL 2H3 lysates. RBL 2H3 cells were permeabilized with SLO (100 ng/ml) alone or in the presence or absence of active or heat-inactivated CS546 (3 μg/ml) for 10 min. Controls were left unpermeabilized in buffer, with or without active CD546 (3 μg/ml). After resealing, the cells were washed and incubated for 2 h. Cells were then scraped off in the presence of lysis buffer and sonicated five times for 5 s on ice. Thereafter, the lysates (1 mg/ml) were incubated with 1 μg/ml CS546 and 20 μM UDP[14C] glucose for 30 min at 37°C. Labeled proteins were analyzed by SDS/PAGE. PhosphorImager data from SDS/PAGE are shown.
Delivery of the functional domains of large clostridial toxins provokes toxin-specific morphological changes.
Glycosylation of small G-proteins by C. sordelli and C. difficile B toxin leads to their inactivation, which is followed by disruption of the actin cytoskeleton and rounding of adherent cells (24). The next experiments were performed to discern whether delivery of the isolated functional domain of either C. sordelli or C. difficile toxin B would cause the typical morphological changes that are provoked by the holotoxins. The affirmative findings are depicted in Fig. 5. Cells treated with SLO alone exhibited normal morphology after the recovery period of 2 h (Fig. 5A), and no actin alterations could be discerned with phalloidin staining (not shown). Cells permeabilized with SLO in the presence of CS546 are shown in Fig. 5B. Approximately 70% displayed the alterations characteristic of the action of this toxin. The cell bodies rounded up, but elongated protrusions remained adherent to the cover slips. Fig. 5C shows cells in which the functional domain of C. difficile toxin B was delivered. Here, rounding up occurred without the “sticky” protrusions, again in accord with what has been described for the holotoxin (24). The morphological effects were absent when heat-inactivated toxins were used (not shown). They were also absent when the toxin fragments were applied to cells in the absence of SLO for 8 h. Analogous experiments conducted with C2-I showed that disruption of the actin cytoskeleton by ADP-ribosylation of monomeric actin similarly caused characteristic rounding up of the cells (not shown).
Figure 5.
Morphological changes of RBL 2H3 cells upon treatment with SLO, CDB546, or CS546. Cells were incubated with SLO (100 ng/ml) alone for 10 min (A), or in combination with either 3 μg/ml CDB546 (B) or 3 μg/ml CS546 (C). Pictures were taken after 90 min resealing.
Clostridial toxins differentially influence receptor-triggered granule secretion.
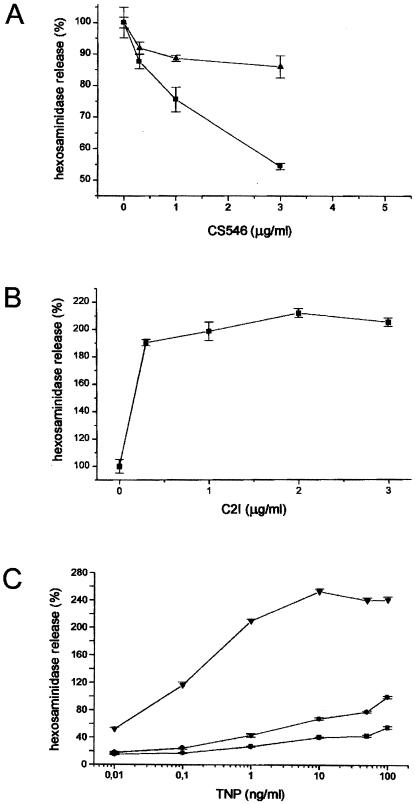
Receptor-triggered granule secretion depends on the function of small G-proteins, whose inactivation of these G-proteins by clostridial toxins has been shown to impede secretion (25). Similar inhibitory effects were found to be induced by delivery of the functional toxin domains to the cells. RBL 2H3 cells were exposed to 100 or 50 ng/ml SLO in the presence of CS546 in the depicted concentrations. Permeabilization was approximated to be 60% and 20% at the higher and lower toxin dose, respectively. Controls were either permeabilized in the absence of CS546, or in the presence of heat-inactivated toxin. After the 2-h resealing period, the cells were stimulated with TNP, and hexosaminidase release was quantified. Controls were set at 100%. As shown in Fig. 6A, active CS546 dose-dependently reduced secretion of hexosaminidase. Similar results were obtained with CDB546.
Figure 6.
(A) Decreased hexosaminidase release in SLO permeabilized RBL 2H3 cells after treatment with CS546 or CDB546. RBL cells were exposed to 100 ng/ml (■) or 50 ng/ml (▴) SLO in the presence of CD546 (A) or CDB546 (B) in the depicted concentrations. After 2 h resealing, cells were stimulated with TNP (50 ng/ml) and hexosaminidase release was measured. Hexosaminidase release of cells treated with SLO only was set at 100%. (B) Increased hexosaminidase release in SLO permeabilized RBL 2H3 cells after treatment with C2I. RBL cells were exposed to 100 ng/ml SLO in the presence of C2I in the depicted concentrations. After 2 h resealing, cells were stimulated with TNP (50 ng/ml). Secretion in control cells was set as 100%. (C) Hexosaminidase release in SLO permeabilized RBL 2H3 cells after treatment with C2I or CS546. RBL cells were exposed to 100 ng/ml SLO alone (●) or in the presence of C2I (2 μg/ml, ▴) or CS546 (5 μg/ml, ■). After 2 h resealing, cells were stimulated with TNP in the depicted concentrations and hexosaminidase release was measured.
Evidence has recently been obtained that actin disruption ensuing from ADP ribosylation of monomeric actin with C. botulinum C2 toxin in the absence of small G-protein inactivation enhances receptor-triggered secretion (25). We reproduced this finding by using the active C2I subunit. As shown in Fig. 6B, delivery of this toxin by means of SLO permeabilization resulted in doubling of hexosaminidase release.
Dose-response analyses concluded the series. Cells were loaded with either C2I or CS546, and secretion was subsequently measured in response to varying ligand concentrations. The results are shown in Fig. 6C. Maximal secretion in controls stimulated with 50 ng/ml TNP was set at 100%. The stimulant dose-dependently provoked secretion, and dose-response curves were shifted to higher or lower levels after delivery of C2I or CS546, respectively.
Discussion
Few other procedures for introducing proteins into living cells have been described. A literature search has confirmed that no currently available method is being widely used, and we therefore anticipate that the presented methodology will render good service to investigators in many fields.
Some technical aspects require mention. Quality of SLO preparations is important, because contaminations with proteases or DNases may create deleterious artefacts. The correct SLO concentration must always be initially determined by titrations. This is done by estimating the percent of permeabilized cells after 10–15 min of exposure to the toxin. The true protein concentration should be given rather than using arbitrary hemolytic units (HU), which vary considerably depending on definition. In our laboratory, a 1 mg/ml solution of SLO has an activity of approximately 50,000 HU/ml. This is equivalent to the statement that a 20-ng/ml solution will lyse over 90% of an erythrocyte suspension containing 2.5 × 108 cells in the presence of 1% albumin. It is our experience that 20–500 ng/ml SLO is required to reversibly permeabilize most cell types. We use the C530A substitution mutant that is oxygen-stable, so a reducing agent need not be included in the permeabilization buffer. Another modification of earlier protocols is the avoidance of FCS, because the only requirement for initiating resealing is the addition of Ca2+. Lesion repair then occurs promptly. When 70–80% of the cells were initially permeabilized, we regularly found that two-thirds recovered. Thus, the method will usually permit proteins to be delivered to approximately 50% of the total cell population under retention of viability. If required, it may be possible to isolate the resealed cells, e.g., by cointroducing a fluorescent dye and subsequently sorting the cells.
The fact that cell recovery functions only at initial permeabilization rates of 60–80% allow tentative calculations to be made. Permeabilization of 63% of the cells reflects the presence of one functional lesion per cell, i.e., 100 functional lesions are then distributed randomly in 100 cells. Thereby, 37% remain nonpermeabilized, and 63% will carry one or more lesions, the upper limit at this toxin dose being essentially three. Hence, it can be concluded that recovery is successful only if cells carry very few (probably one or two) lesions.
The mechanisms underlying lesion repair have not been delineated. We previously found that cells treated with low SLO doses shed a fraction of toxin into the supernatant. However, the amount of remaining, cell-bound toxin was always considerable, and exceeded 50% at any dose (26). Hence, shedding alone cannot explain recovery. Experiments with inhibitors revealed a behavior pattern mimicking that reported for repair of physical lesions (20, 21). Admittedly, the fact that repair occurred at 4°C remains entirely inexplicable.
Experiments using FITC-albumin led to estimates that 105–106 protein molecules could be entrapped in a resealed cell. That cells remained viable was ascertained with the use of assays for endocytosis and proliferation. Delivery of active domains from large clostridial toxins was used to demonstrate the utility of the permeabilization system, because excellent readout systems could be used to confirm their cytosolic delivery. The N-terminal fragments of C. difficile toxin B and C. sordelli lethal toxin comprise amino acid residues 1–546 harboring the catalytic domains, but devoid of cell binding and translocation activities (23). It was shown that the catalytic domains indeed glucosylated small GTPases in the cytosol. The typical toxin phenotype ensued, characterized by destruction of the actin cytoskeleton and rounding up of the cells. The actin ADP-ribosylating enzyme component C2I of C2 toxin could also be delivered, showing that protein uptake was unspecific. As observed for the respective holotoxins, the active toxin fragments caused the same effects on receptor-regulated secretion in RBL cells. The secretion experiments went an important step further in demonstrating that the cells had truly recuperated, because dead cells would not respond to the secretion stimulus. The very fact that introduction of an isolated, functional toxin domain into cells produced effects identical to those discovered for the holotoxins is noteworthy. Because of their exceptionally large size, efforts to produce recombinant clostridial holotoxins have uniformly failed. Although generation of mutant functional domains is feasible, experimentation has been hampered because of the impossibility of delivering the isolated domains to cells. Our protocol now solves the specific dilemma in this field.
Acknowledgments
We thank Wieslawa Bobkiewicz for excellent technical assistance, Michael Hombach for performing ATP measurements, and Klaus Adler for photographical work. This investigation was supported by the Deutsche Forschungsgemeinschaft (SFB 490, Mainz, and SFB 388, Freiburg). The manuscript contains part of the M.D. thesis of S.C.B.
Abbreviations
- SLO
streptolysin O
- TNP
trinitrophenyl
- RBL
rat basophile leukemia
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Wilson A K, Horwitz J, de Lanerolle P. Am J Physiol. 1991;260:C355–C363. doi: 10.1152/ajpcell.1991.260.2.C355. [DOI] [PubMed] [Google Scholar]
- 2.Lauer J L, Fields G B. Methods Enzymol. 1997;289:564–571. doi: 10.1016/s0076-6879(97)89064-7. [DOI] [PubMed] [Google Scholar]
- 3.Derossi D, Chassaing G, Prochiantz A. Trends Cell Biol. 1998;8:84–87. [PubMed] [Google Scholar]
- 4.Doxsey S J, Sambrook J, Helenius A, White J. J Cell Biol. 1985;101:19–27. doi: 10.1083/jcb.101.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhakdi S, Bayley H, Valeva A, Walev I, Walker B, Kehoe M, Palmer M. Arch Microbiol. 1996;165:73–79. doi: 10.1007/s002030050300. [DOI] [PubMed] [Google Scholar]
- 6.Rossjohn J, Feil SC, McKinstry W J, Tweten R K, Parker M W. Cell. 1997;89:685–692. doi: 10.1016/s0092-8674(00)80251-2. [DOI] [PubMed] [Google Scholar]
- 7.Palmer M, Vulicevic I, Saweljew P, Valeva A, Kehoe M, Bhakdi S. Biochemistry. 1998;37:2378–2383. doi: 10.1021/bi9720890. [DOI] [PubMed] [Google Scholar]
- 8.Palmer M, Harris R, Freytag C, Kehoe M, Tranum-Jensen J, Bhakdi S. EMBO J. 1998;17:1598–1605. doi: 10.1093/emboj/17.6.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shatursky O, Heuck A P, Shepard L A, Rossjohn J, Parker M W, Johnson A E, Tweten R K. Cell. 1999;99:293–299. doi: 10.1016/s0092-8674(00)81660-8. [DOI] [PubMed] [Google Scholar]
- 10.Bhakdi S, Weller U, Walev I, Martin E, Jonas D, Palmer M. Med Microbiol Immunol. 1993;182:167–175. doi: 10.1007/BF00219946. [DOI] [PubMed] [Google Scholar]
- 11.Giles RV, Spiller D G, Grzybowski J, Clark R E, Nicklin P, Tidd D M. Nucleic Acids Res. 1998;26:12567–1575. doi: 10.1093/nar/26.7.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giles R V, Grzybowski J, Spiller D G, Tidd D M. Nucleosides Nucleotides. 1997;16:1155–1163. doi: 10.1080/07328319908044855. [DOI] [PubMed] [Google Scholar]
- 13.Fawcett J M, Harrison S M, Orchard C H. Exp Physiol. 1998;83:293–303. doi: 10.1113/expphysiol.1998.sp004114. [DOI] [PubMed] [Google Scholar]
- 14.Weller U, Muller L, Messner M, Palmer M, Valeva A, Tranum Jensen J, Agrawal A P, Biermann C, Dobereiner A, Kehoe M A, Bhakdi S. Eur J Biochem. 1996;236:34–39. doi: 10.1111/j.1432-1033.1996.00034.x. [DOI] [PubMed] [Google Scholar]
- 15.Busch C, Hofmann F, Selzer J, Munro S, Jeckel D, Aktories K. J Biol Chem. 1998;31:19566–19572. doi: 10.1074/jbc.273.31.19566. [DOI] [PubMed] [Google Scholar]
- 16.Barth H, Hofmann F, Olenik C, Just I, Aktories K. Infect Immun. 1991;66:1364–1369. doi: 10.1128/iai.66.4.1364-1369.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhakdi S, Muhly M, Korom S, Hugo F. Infect Immun. 1989;57:3512–3519. doi: 10.1128/iai.57.11.3512-3519.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozawa K, Szallasi Z, Kazanietz M G, Blumberg P M, Mischak H, Mushinski J F, Beaven M A. J Biol Chem. 1993;268:1749–1756. [PubMed] [Google Scholar]
- 19.Just I, Selzer J, Hofmann F, Green G A, Aktories K. J Biol Chem. 1996;271:10149–10153. doi: 10.1074/jbc.271.17.10149. [DOI] [PubMed] [Google Scholar]
- 20.Bi G Q, Alderton J M, Steinhardt R A. J Cell Biol. 1995;131:1747–1758. doi: 10.1083/jcb.131.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNeil P L, Steinhardt R A. J Cell Biol. 1997;137:1–4. doi: 10.1083/jcb.137.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Just I, Hofmann F, Aktories K. In: Bacterial Protein Toxins: Handbook of Experimental Pharmacology. Aktories K, Just I, editors. Vol. 145. Berlin: Springer; 2000. pp. 307–331. [Google Scholar]
- 23.Hofmann F, Busch C, Aktories K. Infect Immun. 1998;66:1976–1981. doi: 10.1128/iai.66.3.1076-1081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ottlinger M E, Lin S. Exp Cell Res. 1988;174:215–229. doi: 10.1016/0014-4827(88)90156-5. [DOI] [PubMed] [Google Scholar]
- 25.Prepens U, Just I, von Eichel-Streiber C, Aktories K. J Biol Chem. 1996;271:7324–7329. doi: 10.1074/jbc.271.13.7324. [DOI] [PubMed] [Google Scholar]
- 26.Walev I, Palmer M, Valeva A, Weller U, Bhakdi S. Infect Immun. 1995;63:1188–1194. doi: 10.1128/iai.63.4.1188-1194.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]