Abstract
Purpose
The present study investigates the long-term effects of intravenous immunoglobulin (IVIg) therapy for the treatment of moderate to severe childhood atopic dermatitis (AD). Previous research indicates that IVIg can treat severe AD; however, the effectiveness of IVIg has not been confirmed in prospective, blinded clinical trials.
Methods
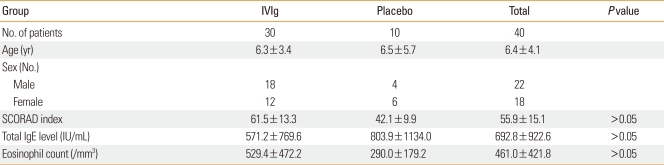
Forty eligible children with moderate to severe AD, as defined by the criteria of Hanifin and Rajka, were enrolled in a randomized, placebo-controlled study. After the completion of an initial screening visit (V0), the patients were randomly allocated into therapy (n=30) and control (n=10) groups (V1). Thirty children were each treated with three injections of 2.0 g/kg IVIg at 1-month intervals over a 12-week period. Ten children were treated with placebo. Assessments were conducted after each injection (V2, V3, and V4) and at 3 (V5) and 6 months (V6) after completed treatment.
Results
The disease severity index was significantly decreased at V5 compared with the value at V1 (P<0.05). There were no significant changes in the total IgE level or total eosinophil count in peripheral blood at the last injection (V4) compared with the value at V1. The interleukin (IL)-5/interferon (IFN)-γ ratio was assessed in T-helper 1 (Th1) and Th2 cells. The ratio significantly decreased between V1 and V5, after which it increased, such that the ratio at V6 was not significantly different from that at V1. Compared with the level at V1, the intercellular cell adhesion molecule-1 level at V4 did not differ significantly, but the level at V5 was lower.
Conclusions
This study suggests that IVIg therapy may clinically improve AD in patients after 3 months of therapy, but the improvement may decline by 6 months after therapy.
Keywords: Intravenous immunoglobulin, atopic dermatitis
INTRODUCTION
Atopic dermatitis (AD) is a persistent, chronically relapsing inflammatory skin disease in which abnormal hematopoietic cells circulate and infiltrate skin and respiratory tissue at sites perturbed by irritants, antigens, and infectious agents. AD is associated with multiple immunological abnormalities, and 80% of patients suffer from defective regulation of IgE production.1 AD can range from mild, localized lesions to generalized eczematous lesions. Although a number of patients with AD respond to topical therapy, others have severe resistant disease which is not controlled with first- or second-line topical therapies. Therefore, AD may be considered as a systemic disease for which there are no satisfactory systemic therapies, although topically applied medications, including corticosteroid and cyclosporine agents, give relief in some patients.2-4 Long-term, chronic application of topical steroids may supplant the need for systemic therapy in many cases. Newer therapies such as tacrolimus are effective, but are limited to second-line therapy after initial treatments have failed. Oral cyclosporine has also been shown to be effective; however, its use is limited by adverse effects such as renal toxicity. Approximately 20% of patients discontinue cyclosporine treatment within 2 years because of adverse effects.5,6
Intravenous immunoglobulin (IVIg) is prepared by cold ethanol fractionation of pooled plasma, followed by additional viral inactivation procedures. The final product is a pure concentrate of IgG (with small amounts of IgA and IgM) with intact function and minimal impurities.7,8 The immunomodulatory mechanism of IVIg is thought to be mediated by interactions of the Fc portion of IgG with Fc receptors and complement, interactions of the antigen-binding variable region F(ab)2, or substances other than antibodies in IVIg preparations.9 Previous research has shown that IVIg significantly modulates the production of several cytokines, including interleukin (IL)-1, IL-2, IL-3, IL-4, IL-5, IL-10, tumor necrosis factor-α, and granulocyte-macrophage colony-stimulating factor, as well as cytokine antagonists (IL-1 receptor antagonist), by monocyte-macrophages and lymphocytes.10 It appears that the biological anti-inflammatory effects of IVIg involve the participation of both the F(ab)2 and Fc regions in the formation of an IgG-antigen complex. These regions are associated with the inhibition of lymphocyte proliferative responses and the modulation of T-helper 1 (Th1) and Th2 cytokine production. This effect of IVIg is mediated by the inhibition of differentiation and maturation of dendritic cells (DCs) in vitro, with down-regulated expression of co-stimulatory molecules, thereby impairing the ability of mature DCs to produce IL-12 and enhancing their ability to produce IL-10. The consequences are the inhibition of autoreactive and alloreactive T-cell activation and proliferation.11,12
IVIg therapy has consistently demonstrated activity in patients with AD, including severe AD,13 although its effectiveness has not been confirmed in prospective, blinded clinical trials. Patients with Kawasaki disease or idiopathic thrombocytopenic purpura and concomitant AD who received IVIg showed improvement of their dermatitis.14,15 One study gave mixed results, with the best response occurring in patients with extremely high IgE levels.16 Reports on severe childhood AD suggest that IVIg can lead to improved symptoms when used as a monotherapy; nine out of 10 children improved when given 2 g/kg IVIg.17 However, most published research has evaluated only the short-term effects of IVIg.
The present study was conducted to assess the long-term effects of IVIg therapy in moderate to severe childhood AD.
MATERIALS AND METHODS
Patients
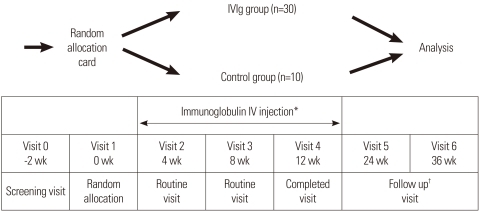
Forty eligible children with moderate to severe AD, as defined by the criteria of Hanifin and Rajka18, were enrolled in a randomized, placebo-controlled study after an initial screening visit (V0) (Fig. 1). Eligibility criteria included AD present on more than 30% of the body surface, no response to conventional therapy, and age older than 2 years. Informed consent was obtained from either the child or a parent. Allergy skin prick tests or in vitro allergy tests (Unicap: Phardia, Uppsala, Sweden) were conducted at the screening visit (V0). The patients were randomized at the next clinic visit (V1) (Fig. 1). Patients were allowed to use only steroid-free hydrophilic or emollient ointment on the skin as adjunctive therapy. Seven patients did not complete the study: five IVIg subjects suffered side effects, including headache, nausea, and abdominal pain, and two control subjects did not complete the trial for personal reasons. This study was approved by the Korean FDA (IVIg IIT Atopy-No. 48) and the Hanyang University Guri Hospital IRB committee.
Fig. 1.
Study design of intravenous immunoglobulin (IVIg) therapy, blood specimen collection for laboratory analyses, and evaluation of clinical efficacy.
*Immunoglobulin IV injection: three times (2 g/kg month IV) for 12 weeks; †Follow up: after completing injection, every 4 weeks for 6 months.
Methods
The IVIg patients received an injection of IVIg (Greencross Pharm Co, Seoul, Korea) at 2.0 g/kg body weight/month at each monthly visit (V2, V3, and V4) for 12 weeks (Fig. 1). The patients were also assessed at each clinic visit and at 3 (V5) and 6 months (V6) after the final injection. Patients with headache or nausea were permitted to take oral acetaminophen. Placebo group received general topical moisturizing lotion, 1% hydrocortisone cream and took oral antihistamines if they complaint itching for controlling itching skin as same to IVIg group. At V1, V4, V5, and V6, laboratory tests were performed for blood chemistry, total IgE, eosinophil cationic protein (ECP), IL-5, interferon (IFN)-γ, and intercellular cell adhesion molecule (ICAM)-1. Disease severity was assessed by the criteria of Hanifin and Rajka18, and included clinical severity and total body surface area (TBSA). Disease severity was assessed with the SCORAD index, which was calculated as A/5+7×B/2+C, where A is the TBSA measured as a percentage of lesional skin according to the rule of nines; B is the clinical severity consisting of six parameters: erythema, edema/papulation, excoriation, oozing/vesicle, lichenification, and dryness with pigmentation/depigmentation; and C is a subjective measurement of symptoms such as pruritus and loss of sleep. Parameters A and B were scaled from 0 to 3; parameter C, from 0 to 10. To minimize potential variation, the same physician measured the disease severity at each visit (Table 1).
Table 1.
Subjective characteristics in study
IVIg, intravenous immunoglobulin.
Determination of cytokine concentrations
The concentrations of IL-5, IFN-γ, and ICAM-1 were determined by cytometric bead array assays (Bio-Plex; Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer's protocol. All measurements were made in duplicate, with <10% variation between the two measurements.
Statistical analysis
The characteristics of the IVIg and placebo groups at V0 were compared using the Mann-Whitney U test. Disease severity was compared between the groups at baseline (V1) and at V2, V3, V4, V5, and V6. Differences between visits were analyzed by multiple comparisons as ANOVA. Analyses were performed using the SPSS statistical package, version 11.5 (SPSS Inc., Chicago, IL, USA). A value of P<0.05 was taken to indicate statistical significance.
RESULTS
Clinical effects of IVIg therapy in children with moderate to severe AD
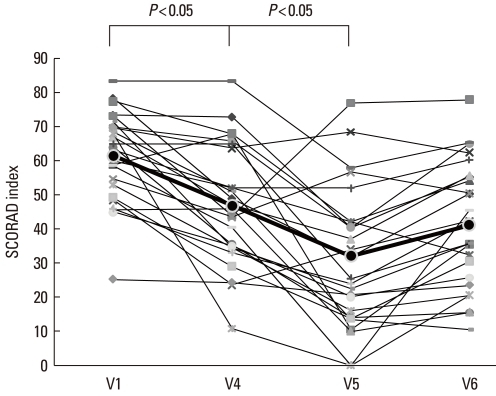
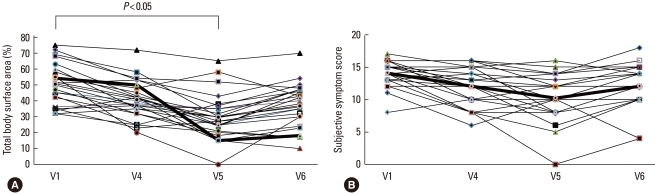
Fig. 2 presents the mean percentage improvements of clinical parameters at each visit. The SCORAD index declined until 3 months after the last IVIg injection (V5) and did not change significantly during the subsequent 3 months (V6) (V1: 61.5±13.0, V4: 46.9±17.3, V5: 32.1±19.4, and V6: 39.3±18.4; V1 vs. V5: P<0.05) (Fig. 2, Table 2). The TBSA parameter of the SCORAD index improved until V5, but declined by V6 (V1: 50.5±12.8, V4: 40.7±11.9, V5: 29.7±14.2, and V6: 36.8±13.7; V1 vs. V5: P<0.05) (Fig. 3A). The scores for subjective symptoms such as itching and loss of sleep did not change significantly during the study (V1: 14.1±2.0, V4: 12.0±2.9, V5: 10.2±3.6, and V6: 12.0±3.2) (Fig. 3B). The SCORAD index of the control group did not improve significantly until V6.
Fig. 2.
SCORAD index before (V1) and during therapy (V4), and at 3 (V5) and 6 months (V6) after the last intravenous immunoglobulin (IVIg) injection. The SCORAD index was significantly lower at the 3-month follow-up visit (V5) compared with the index at V1, but it had increased by the 6-month follow-up visit (V6).
Table 2.
Comparison of SCORAD index, serum IgE, and eosinophil count between children with IVIg treatment and the controls
*There shows significantly differences of SCORAD between IVIg and control at V1 vs. V4 (P=0.005) and V1 vs. V5 (P=0.17).
IVIg, intravenous immunoglobulin; V1, before therapy; V4, during therapy; V5, 3-month follow-up visit; V6, 6-month follow-up visit.
Fig. 3.
Total body surface area of lesions (A) and subjective symptoms score (B) before (V1) and during therapy (V4), and at 3 (V5) and 6 months (V6) after the last IVIg injection. The total body surface area of lesions was significantly lower at the 3-month follow-up visit (V5) compared with the area at V1, but it had increased by the 6-month follow-up visit (V6). The subjective symptoms score did not change significantly.
Immunological parameters in peripheral blood
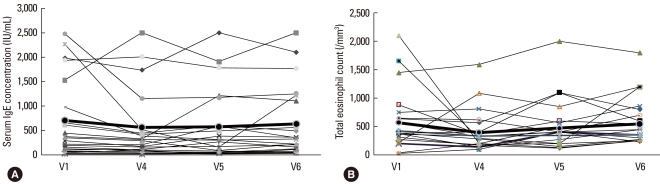
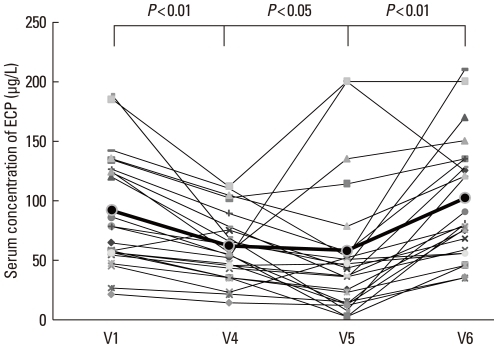
The total serum IgE concentration was initially elevated in all subjects (V0). The levels declined during IVIg treatment, were higher at the 3-month post-treatment visit (V5), and returned to initial levels by 6 months after treatment (V1: 571.2±753.4, V4: 384.9±519.5, V5: 493.9±620.3, and V6: 548.9±677.2 IU/mL) (Fig. 4A, Table 2). The total eosinophil count in peripheral blood demonstrated a similar pattern: it decreased during IVIg treatment, but returned to the initial level by 6 months post-treatment (V1: 529.4±462.6, V4: 397.6±342.8, V5: 470.8±411.7, V6: 547.6±393.3 per mm2) (Fig. 4B, Table 2). The ECP level was also decreased at 3 months post-treatment, but returned to the initial level by 6 months post-treatment (V1: 91.7±49.5, V4: 62.1±30.5, V5: 58.1.2±57.9, V6: 94.8±41.6 µg/L) (Fig. 5).
Fig. 4.
The serum IgE concentration (A) and total eosinophil count (B) before (V1) and during therapy (V4), and at 3 (V5) and 6 months (V6) after the last IVIg injection. These parameters did not change significantly.
Fig. 5.
Eosinophil cationic protein (ECP) concentration before (V1) and during therapy (V4), and at 3 (V5) and 6 months (V6) after the last IVIg injection. The ECP concentration was significantly lower (P < 0.05) at the 3-month follow-up visit (V5) compared with the concentration at V1, but it had increased significantly by the 6-month follow-up visit (V6).
Changes in IL-5, INF-γ, and ICAM-1 levels with IVIg treatment
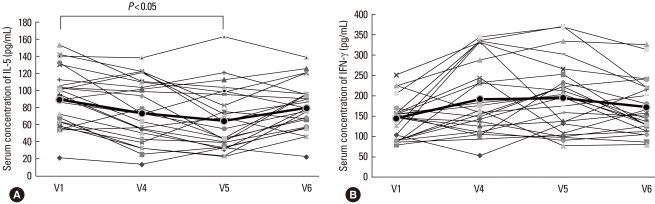
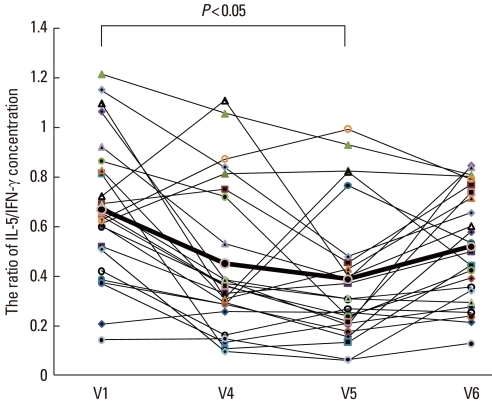
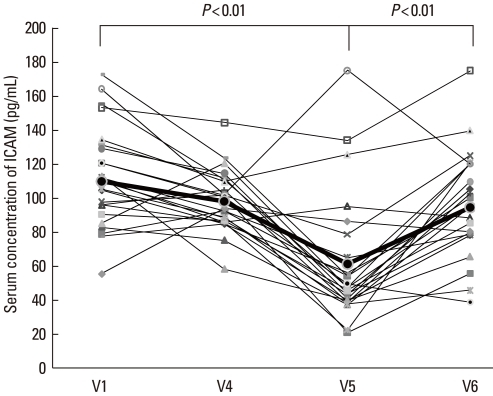
The IL-5 concentration decreased between V1 and V4, but the difference was not statistically significant. Between V1 and V5, the IL-5 level decreased significantly (P<0.05). By 6 months post-treatment, the IL-5 level had returned to the initial concentration (V1: 89.2±34.8, V4: 73.3±36.7, V5: 64.3±37.9, and V6: 80.1±30.7 pg/mL; V1 vs. V5: P<0.05) (Fig. 6A). The IFN-γ concentration was higher at V4 and V5 compared with that at V1 and decreased by V6, although the differences were not significant (V1: 143.7±51.2 pg/mL, V4: 195.0±98.6 pg/mL, V5: 194.0±85.6 pg/mL, and V6: 171.2±68.8 pg/mL) (Fig. 6B). The IL-5/IFN-γ ratio, assessed for Th1 and Th2, was significantly lower at V4 and V5 compared with that at V1 and increased by V6 (V1: 0.67±0.28, V4: 0.45±0.30, V5: 0.39±0.27, and V6: 0.51±0.23; V1 vs. V4, and V1 vs. V5: P<0.05) (Fig. 7). The concentration of ICAM-1 was lower at V4 than at V1, but not significantly lower. The ICAM-1 concentration decreased significantly between V1 and V5 (P<0.05), but was higher at V6, approaching the level at V1 (V1: 112.2±28.2, V4: 99.4±16.1, V5: 61.3±36.9, and V6: 94.9±31.8 pg/mL) (Fig. 8).
Fig. 6.
The IL-5 (A) and IFN-γ (B) concentrations before (V1) and during therapy (V4), and at 3 (V5) and 6 months (V6) after the last intravenous immunoglobulin (IVIg) injection. The IL-5 level was significantly lower (P<0.05) at the 3-month follow-up visit (V5) compared with the level at V1, but it had increased by the 6-month follow-up visit (V6). The IFN-γ level did not change significantly.
Fig. 7.
The IL-5/IFN-γ ratio before (V1) and during therapy (V4), and at 3 (V5) and 6 months (V6) after the last intravenous immunoglobulin (IVIg) injection. The IL-5/IFN-γ ratio was significantly lower at the 3-month follow-up visit (V5) compared with the ratio at V1, but it had increased by the 6-month follow-up visit (V6).
Fig. 8.
Intracellular cell adhesion molecule (ICAM)-1 concentration before (V1) and during therapy (V4), and at 3 (V5) and 6 months (V6) after the last IVIg injection. The ICAM-1 concentration was significantly lower at the 3-month follow-up visit (V5) compared with the concentration at V1, but it had increased by the 6-month follow-up visit (V6).
Safety of IVIg treatment
Of the 30 children initially treated with IVIg, five discontinued therapy because of side effects: four due to severe headache and nausea after the first (V2) and second (V3) IVIg injections, and the fifth due to low-grade fever, headache, and vomiting after the first IVIg injection. These side effects were transient and self-limiting, and arose during the first few hours after injection.
DISCUSSION
The results of this long-term study demonstrate that the clinical severity of AD showed improvement for 3 months (V5) after the last IVIg injection, but the improvement had declined by 6 months post-treatment (V6). Total serum IgE and eosinophil count decreased during therapy and for 3 months post-treatment (V5), but had returned to initial levels after another 3 months (V6). Similarly, immunological parameters, including the levels of ECP, IL-5, and ICAM-1, in peripheral blood decreased until 3 months post-treatment (V5), but had increased by the 6-month follow-up visit (V6). The level of IFN-γ increased until 3 months after treatment, but decreased over the subsequent 3 months.
Cumulative toxicity and the lack of efficacy may limit systemic glucocorticoid use, and patients severely affected by AD often require immunosuppressive treatment, including IFN-γ, cyclosporine, and IVIg. Patients with Kawasaki disease or idiopathic thrombocytopenic purpura with concomitant AD who received IVIg showed improvement of the dermatitis.14 In that study, four patients with AD had marked improvement of dermatitis symptoms after one dose of IVIg therapy (400 mg/kg infused for 5 days), with significant improvement on days 4-7 after treatment. Two patients with idiopathic thrombocytopenic purpura entered remission for AD and did not require further treatment. However, an open label study of IVIg in patients with severe AD or hyper-IgE syndrome showed no clinical improvement,16 and a randomized prospective study of IVIg in adults with severe AD failed to show a significant clinical effect.17 Therefore, the use of IVIg in the treatment of AD remains controversial. To date, only a few individuals (10 children and 30 adults) have been treated with IVIg, and those studies were relatively short, with durations and response times of less than 3 months.
The current study included 30 eligible children who were treated with high-dose IVIg for 12 weeks and 10 children who were treated with placebo. The subjects were administered monthly injections of IVIg (2.0 g/kg body weight/month) over a 12-week period. Assessment was conducted after each treatment and at 3 and 6 months after treatment; thus, the final assessment was at 9 months after the start of the study. The improvements in AD, assessed based on clinical severity and immunological parameters such as ECP, lasted for 3 months after the last IVIg treatment. However, at the long-term follow-up at 6 months post-treatment, both the clinical and immunological improvements showed a trend back to the initial status.
Preparations of IVIg consist of intact IgE molecules with a distribution of IG subclasses corresponding to that in normal serum. Subclass distribution varies among preparations, and some products have lower physiological levels of IgG3 and/or IgG4. IVIg contains small amounts of other proteins and products, such as albumin, IgA, IgE, IgM, sugars, and salts. The monomer and dimer contents also vary among preparations and include up to 3% non-active polymers.19 These proteins and other molecules may affect the tolerability and half-life of IVIg infusions. Generally, the half-life of injected IVIg is approximately 2-3 weeks, but this varies depending on the immune status of the patient. The present study demonstrated that IVIg therapy (2.0 g/kg/month for 3 months) showed efficacy at 3 months, but not 6 months, after the final injection.
Previous studies have reported elevated serum levels of soluble ICAM-1 and endothelial leukocyte adhesion molecule (ELAM)-1 in patients with severe atopic eczema. Moreover, E-selectin and VCAM-1 are critical adhesion molecules for the trafficking of memory T cells and eosinophils into skin lesions of AD.20-22 Serum levels of the intracellular adhesion molecules ICAM-1 and ECP in previous studies correlated with improvements in AD; therefore, we measured ICAM-1 and ECP as markers for efficacy of IVIg in the present study. IVIg reduced the levels of ICAM-1 and ECP until 3 months after the final IVIg treatment. The decreased levels of ICAM-1 and ECP suggest that IVIg therapy may decrease the influx of inflammatory cells such as eosinophils in skin lesions.
It has been proposed that Th2 cells play a key pathogenic role in AD through regulatory T-cell modulation. There is further evidence that monocytes from AD patients secrete increased amounts of the Th2 cytokines IL-4, IL-5, IL-10, and IL-13. A number of studies have indicated that the immunological balance shifts toward a Th2 response in AD,23,24 while several other reports have suggested that Th1, rather than Th2, cells are important for AD pathogenesis. One study found high levels of IFN-γ mRNA and protein expression in 80% of the eczematous skin of AD patients.25 Further research has suggested that acute inflammation is mediated by Th2 cell cytokines, whereas chronic lesions had an increased number of cells expressing IL-12 mRNA compared with unaffected skin.26 However, these cells were limited to the skin lesion and were not found in the peripheral blood. In the present study, the IL-5 level was significantly decreased at 3 months post-treatment treatment, but the IFN-γ level did not change significantly during or after treatment. These findings suggest that IVIg therapy may not influence T-cells, but that Th1 cells become more dominant. The apparent switch from Th2 to Th1 dominance may explain the clinical improvement at 3 months after IVIg therapy and its absence 3 months later, although this requires further investigation.
There were a few minor side effects associated with IVIg infusion in the present study, but most were self-limiting and occurred only during the first few hours after infusion. The side effects may be reduced by slowing the rate of infusion or administering NSAIDs or acetaminophen prior to the next IVIg infusion. Side effects such as headache and nausea might have resulted from the high dose of IVIg or might have been a consequence of a low level of IgG aggregation, immune complex formation, or complement activation in the IVIg preparationt.27,28
It is important to consider the balance between pharmacological efficacy and drug cost with respect to time commitment and financial implications of this therapy. The present study suggests that patients may need to receive IVIg therapy every 6 months, and this cost should be compared with the estimated improvement in the quality of life. Therefore, IVIg therapy should not be introduced until after the physician and patient have considered both the clinical efficacy and the cost of the therapy.
ACKNOWLEDGMENTS
This study was able to be completed by a grant of Greencross Pharm Co.
References
- 1.Hanifin JM, Chan SC. Monocyte phosphodiesterase abnormalities and dysregulation of lymphocyte function in atopic dermatitis. J Invest Dermatol. 1995;105:84S–88S. doi: 10.1111/1523-1747.ep12316116. [DOI] [PubMed] [Google Scholar]
- 2.Sonenthal KR, Grammer LC, Patterson R. Do some patients with atopic dermatitis require long-term oral steroid therapy? J Allergy Clin Immunol. 1993;91:971–973. doi: 10.1016/0091-6749(93)90208-w. [DOI] [PubMed] [Google Scholar]
- 3.Sowden JM, Berth-Jones J, Ross JS, Motley RJ, Marks R, Finlay AY, Salek MS, Graham-Brown RA, Allen BR, Camp RD. Double-blind, controlled, crossover study of cyclosporin in adults with severe refractory atopic dermatitis. Lancet. 1991;338:137–140. doi: 10.1016/0140-6736(91)90134-b. [DOI] [PubMed] [Google Scholar]
- 4.Salek MS, Finlay AY, Luscombe DK, Allen BR, Berth-Jones J, Camp RD, Graham-Brown RA, Khan GK, Marks R, Motley RJ. Cyclosporin greatly improves the quality of life of adults with severe atopic dermatitis. A randomized, double-blind, placebo-controlled trial. Br J Dermatol. 1993;129:422–430. doi: 10.1111/j.1365-2133.1993.tb03170.x. [DOI] [PubMed] [Google Scholar]
- 5.Grossman RM, Chevret S, Abi-Rached J, Blanchet F, Dubertret L. Long-term safety of cyclosporine in the treatment of psoriasis. Arch Dermatol. 1996;132:623–629. [PubMed] [Google Scholar]
- 6.Kirby B, Owen CM, Blewitt RW, Yates VM. Cutaneous T-cell lymphoma developing in a patient on cyclosporin therapy. J Am Acad Dermatol. 2002;47:S165–S167. doi: 10.1067/mjd.2002.106357. [DOI] [PubMed] [Google Scholar]
- 7.Sewell WA, Jolles S. Immunomodulatory action of intravenous immunoglobulin. Immunology. 2002;107:387–393. doi: 10.1046/j.1365-2567.2002.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spahn JD, Leung DY, Chan MT, Szefler SJ, Gelfand EW. Mechanisms of glucocorticoid reduction in asthmatic subjects treated with intravenous immunoglobulin. J Allergy Clin Immunol. 1999;103:421–426. doi: 10.1016/s0091-6749(99)70466-5. [DOI] [PubMed] [Google Scholar]
- 9.Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–486. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 10.Andersson J, Skansén-Saphir U, Sparrelid E, Andersson U. Intravenous immune globulin affects cytokine production in T lymphocytes and monocytes/macrophages. Clin Exp Immunol. 1996;104(Suppl 1):10–20. [PubMed] [Google Scholar]
- 11.Bayry J, Lacroix-Desmazes S, Delignat S, Mouthon L, Weill B, Kazatchkine MD, Kaveri SV. Intravenous immunoglobulin abrogates dendritic cell differentiation induced by interferon-alpha present in serum from patients with systemic lupus erythematosus. Arthritis Rheum. 2003;48:3497–3502. doi: 10.1002/art.11346. [DOI] [PubMed] [Google Scholar]
- 12.Bayry J, Lacroix-Desmazes S, Carbonneil C, Misra N, Donkova V, Pashov A, Chevailler A, Mouthon L, Weill B, Bruneval P, Kazatchkine MD, Kaveri SV. Inhibition of maturation and function of dendritic cells by intravenous immunoglobulin. Blood. 2003;101:758–765. doi: 10.1182/blood-2002-05-1447. [DOI] [PubMed] [Google Scholar]
- 13.Jolles S, Sewell C, Webster D, Ryan A, Heelan B, Waite A, Rustin M. Adjunctive high-dose intravenous immunoglobulin treatment for resistant atopic dermatitis: efficacy and effects on intracellular cytokine levels and CD4 counts. Acta Derm Venereol. 2003;83:433–437. doi: 10.1080/00015550310020549. [DOI] [PubMed] [Google Scholar]
- 14.Jolles S, Hughes J, Rustin M. The treatment of atopic dermatitis with adjunctive high-dose intravenous immunoglobulin: a report of three patients and review of the literature. Br J Dermatol. 2000;142:551–554. doi: 10.1046/j.1365-2133.2000.03377.x. [DOI] [PubMed] [Google Scholar]
- 15.Kimata H. High dose gammaglobulin treatment for atopic dermatitis. Arch Dis Child. 1994;70:335–336. doi: 10.1136/adc.70.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wakim M, Alazard M, Yajima A, Speights D, Saxon A, Stiehm ER. High dose intravenous immunoglobulin in atopic dermatitis and hyper-IgE syndrome. Ann Allergy Asthma Immunol. 1998;81:153–158. doi: 10.1016/S1081-1206(10)62802-5. [DOI] [PubMed] [Google Scholar]
- 17.Paul C, Lahfa M, Bachelez H, Chevret S, Dubertret L. A randomized controlled evaluator-blinded trial of intravenous immunoglobulin in adults with severe atopic dermatitis. Br J Dermatol. 2002;147:518–522. doi: 10.1046/j.1365-2133.2002.04833.x. [DOI] [PubMed] [Google Scholar]
- 18.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl. 1980;114:146–148. [Google Scholar]
- 19.Prins C, Gelfand EW, French LE. Intravenous immunoglobulin: properties, mode of action and practical use in dermatology. Acta Derm Venereol. 2007;87:206–218. doi: 10.2340/00015555-0249. [DOI] [PubMed] [Google Scholar]
- 20.Kowalzick L, Kleinheinz A, Neuber K, Weichenthal M, Köhler I, Ring J. Elevated serum levels of soluble adhesion molecules ICAM-1 and ELAM-1 in patients with severe atopic eczema and influence of UVA1 treatment. Dermatology. 1995;190:14–18. doi: 10.1159/000246627. [DOI] [PubMed] [Google Scholar]
- 21.Wakita H, Sakamoto T, Tokura Y, Takigawa M. E-selectin and vascular cell adhesion molecule-1 as critical adhesion molecules for infiltration of T lymphocytes and eosinophils in atopic dermatitis. J Cutan Pathol. 1994;21:33–39. doi: 10.1111/j.1600-0560.1994.tb00688.x. [DOI] [PubMed] [Google Scholar]
- 22.Halmerbauer G, Frischer T, Koller DY. Monitoring of disease activity by measurement of inflammatory markers in atopic dermatitis in childhood. Allergy. 1997;52:765–769. doi: 10.1111/j.1398-9995.1997.tb01236.x. [DOI] [PubMed] [Google Scholar]
- 23.Grewe M, Bruijnzeel-Koomen CA, Schöpf E, Thepen T, Langeveld-Wildschut AG, Ruzicka T, Krutmann J. A role for Th1 and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunol Today. 1998;19:359–361. doi: 10.1016/s0167-5699(98)01285-7. [DOI] [PubMed] [Google Scholar]
- 24.Chan S, Henderson WR, Jr, Li SH, Hanifin JM. Prostaglandin E2 control of T cell cytokine production is functionally related to the reduced lymphocyte proliferation in atopic dermatitis. J Allergy Clin Immunol. 1996;97:85–94. doi: 10.1016/s0091-6749(96)70286-5. [DOI] [PubMed] [Google Scholar]
- 25.Thepen T, Langeveld-Wildschut EG, Bihari IC, van Wichen DF, van Reijsen FC, Mudde GC, Bruijnzeel-Koomen CA. Biphasic response against aeroallergen in atopic dermatitis showing a switch from an initial TH2 response to a TH1 response in situ: an immunocytochemical study. J Allergy Clin Immunol. 1996;97:828–837. doi: 10.1016/s0091-6749(96)80161-8. [DOI] [PubMed] [Google Scholar]
- 26.Hamid Q, Naseer T, Minshall EM, Song YL, Boguniewicz M, Leung DY. In vivo expression of IL-12 and IL-13 in atopic dermatitis. J Allergy Clin Immunol. 1996;98:225–231. doi: 10.1016/s0091-6749(96)70246-4. [DOI] [PubMed] [Google Scholar]
- 27.Gelfand EW. Differences between IGIV products: impact on clinical outcome. Int Immunopharmacol. 2006;6:592–599. doi: 10.1016/j.intimp.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Nydegger UE, Sturzenegger M. Adverse effects of intravenous immunoglobulin therapy. Drug Saf. 1999;21:171–185. doi: 10.2165/00002018-199921030-00003. [DOI] [PubMed] [Google Scholar]