Abstract
Disruption of visual input to one eye during early development leads to marked functional impairments of vision, commonly referred to as amblyopia. A major consequence of amblyopia is the inability to encode binocular disparity information leading to impaired depth perception or stereo acuity. If amblyopia is treated early in life (before 4 years of age), then recovery of normal stereoscopic function is possible. Treatment is rarely undertaken later in life (adulthood) because declining levels of neural plasticity are thought to limit the effectiveness of standard treatments. Here, the authors show that a learning-based therapy, designed to exploit experience-dependent plastic mechanisms, can be used to recover stereoscopic visual function in adults with amblyopia. These cases challenge the long-held dogma that the critical period for visual development and the window for treating amblyopia are one and the same.
Background
Amblyopia is a common monocular developmental visual disorder (affecting 2–4% of the UK population1–3) and accounts for the majority (~90%) of children's eye appointments in the UK.4 The effectiveness of standard treatments (patching or occlusion of the unaffected eye) is thought to be tightly coupled to the critical periods of visual development – ending around 9 years of age.2 Despite the fact that many adults with this condition do not have normal 3D vision, treatment is rarely undertaken in adulthood. However, new evidence suggests that the adult visual brain retains more experience-dependent neural plasticity than previously thought. This capacity for change in adulthood can be expressed in many different forms – from molecular transformations through to completely re-organised cortical maps.5 If this residual brain plasticity can be exploited, it then raises the possibility of being able to treat the condition much later in life. Consistent with this proposal, several research groups have used perceptual learning – a behavioural manifestation of experience-dependent neural plasticity – to successfully reverse the monocular visual deficit in adulthood.6–9 Here, we develop this approach to show complete recovery of stereo acuity in adults presenting with anisometropic amblyopia.
We present two case studies, both of which show it is possible to completely resolve long-standing stereo acuity deficits well into adulthood. These results demonstrate that the adult visual system retains a significant capacity for change and directly challenges the century-old notion that treatment for stereo loss is only effective if initiated before the end of the critical period(s) of visual development.
These cases address an area of clinical vision science that has generated much recent public interest. A prominent neuroscientist (Professor Susan Barry) published a book (Fixing my Gaze: A Scientist's Journey into Seeing in Three Dimensions) describing of her own personal case study, and how she recovered stereo function in adulthood.10 Here, we provide an independent assessment of stereo recovery in adults. More importantly, we examine patients with very different clinical presentations (anisometropia, amblyopia) from Professor Barry.
Case presentation
-
▶
Two adults with anisometropic amblyopia (clinical details provided in table 1).
-
▶
Both subjects underwent monocular perceptual learning (described in ‘Treatment’) to reduce the difference in visual acuity between the two eyes.
-
▶
After monocular learning, both subjects demonstrated gross stereopsis on a standard clinical test (TNO stereo test).
-
▶
Both subjects underwent stereoscopic learning (described in ‘Treatment’) to improve stereo acuity.
-
▶
Stereo acuity was re-measured in both subjects 7 months after the cessation of learning to establish the permanence of treatment.
Table 1.
Clinical details of amblyopic subjects
| Observer | Age (years) | Gender | Eye | Refractive error | Cover test | Type of amblyopia | Treatment history |
|---|---|---|---|---|---|---|---|
| RC | 29 | M | RE | +0.25DS | NMD | Anisometropic | Occlusion (PT from age 5; duration unknown) |
| LE | +2.50/−1.00×60 | ||||||
| CM | 24 | F | RE | +0.25DS | NMD | Anisometropic | Occlusion (PT from age 3–5) |
| LE | +0.25/−2.50×55 |
| Visual acuity (logMAR) | ||||||
|---|---|---|---|---|---|---|
| Before monocular learning | End of monocular learning | End of stereo learning | ||||
| RE | LE | RE | LE | RE | LE | |
| RC | 0.00 | 0.24 | 0.02 | 0.14 | 0.00 | 0.10 |
| CM | −0.08 | 0.28 | −0.08 | 0.14 | −0.06 | 0.16 |
LE, left eye; NMD, no movement detected; PT, part time, RE, right eye.
Investigations
-
▶
Measures of monocular visual acuity before and after learning assessed using standard clinical procedures (logMAR acuity).
-
▶
Computer-based measures of stereo acuity using random dot stereograms in combination with standard psychophysical procedures.
Treatment
Refractive adaptation
Before perceptual learning, subjects wore their best optical correction (spectacles) for a period of at least 8 weeks. In some cases of childhood amblyopia, refractive adaption alone can produce improvements in monocular visual acuity.11 12
Treatment of monocular visual deficit
Both subjects trained on a monocular spatial acuity task for nine sessions (1 session/day; 10–15 min/session). One subject learnt on a task where the orientation of a target (Landolt C that reduced in size) had to be identified. The other subject learnt on a task where they had to identify whether a target was present or not (sinusoidal grating that increased in spatial frequency). Each task employed standard psychophysical procedures and scoring criteria to establish the smallest spatial detail that could be resolved.
Treatment of binocular visual deficit
Both subjects trained on a stereo acuity task for nine sessions (1 session/day; 10 min/session). Stereo acuity was measured using a mirror stereoscope in conjunction with stereogram pairs, where random dot images were viewed independently by each eye. A disparity-defined target (Landolt C) was created by shifting a region of one image relative to the other – larger shifts producing greater disparities. The task was to identify the orientation of the letter. Each target was presented five times and the disparity reduced until the subject made four errors at any single disparity level. This process was repeated three times during each session.
Outcome and follow-up
Refractive adaptation
Neither subject showed any significant change in their monocular visual acuity after refractive adaptation. This finding is not entirely surprising because both subjects have been wearing a full optical correction for close work for a number of years.
Treatment of monocular visual deficit
Changes in visual performance across learning sessions, normalised to the estimate obtained in the first session, are plotted in figure 1. Both subjects showed improvements on the monocular task they trained on (RC: 20%; CM: 40%). More importantly, they both showed significant changes in letter-based visual acuity on a Bailey–Lovie chart at the end of learning. Full details of the magnitude of monocular improvement are provided in table 1.
Figure 1.
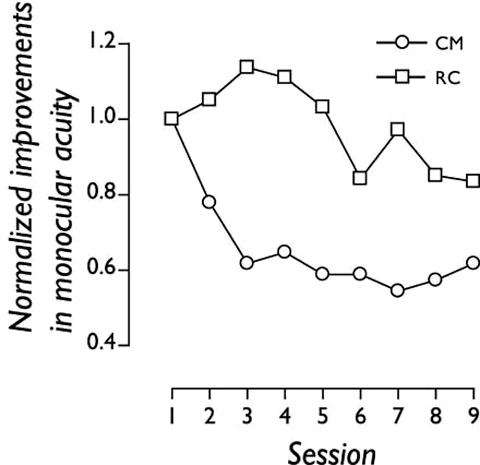
Nine daily sessions of perceptual learning improved two adult amblyopic subjects’ monocular spatial acuity. Changes in visual performance across learning sessions are normalised to the estimate obtained in the first session.
Treatment of binocular visual deficit
The data presented in figure 2 show that both adult amblyopic subjects improved their stereo acuity to normal levels over nine sessions (black symbols). As a control, we used dioptric blur (+1.00DS lenses) to degrade stereo acuity back to around 200 s of arc in two visually normal observers (grey symbols). In contrast to the amblyopic subjects, the observers with blur-limited stereo acuity showed little or no improvements over the same time course, ruling out simple procedural explanations (learning how to perform the task) for the visual improvements of amblyopic subjects. More rapid learning was associated with better performance at the start of training, consistent with other work. Subjects were not able to perform the stereo task either with one eye covered or with a +1.50DS (or greater dioptric power) lens placed in front of one eye, confirming the absence of any monocular cues in the stereograms.
Figure 2.
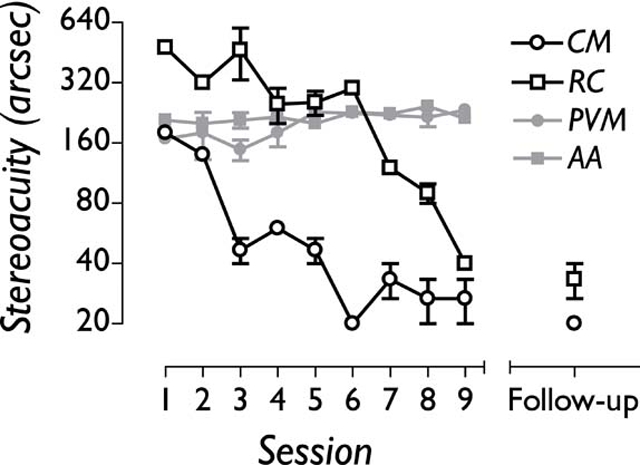
Nine daily sessions of perceptual learning improved two adult amblyopic subjects’ stereo acuity to normal levels (open symbols), but had little effect on the stereo acuity of two visually normal subjects who had their stereo acuity degraded using monocular dioptric blur (grey symbols). Both amblyopic subjects had retained the improvements in stereo acuity 7 months after the cessation of training (follow-up).
Follow-up
The stereo acuity measures were repeated 7 months after the cessation of training. Both amblyopic subjects showed no significant slippage in stereo performance (see figure 2).
Discussion
The results of the monocular learning phase are consistent with a number of other case reports and clinical studies, demonstrating that perceptual learning can significantly improve monocular visual acuity in adult amblyopia.6–9 The key point of this case study is not how the improvements in monocular visual acuity are generated, as this could arise from either refractive adaptation, perceptual learning or simply covering the unaffected eye for short periods during testing (occlusion). What we show for the first time is that learning-based therapies can improve stereo acuity (3D vision) to near-normal levels beyond the improvements delivered by monocular learning. The data presented in table 1 show that for both amblyopic subjects, improvements in stereo acuity are established independently of any further changes in visual acuity.
As a control, two visually normal adults underwent the same training procedure but had their stereo acuity degraded using monocular dioptric blur (+1.00 DS). The subjects with blur-limited stereo acuity thresholds showed little or no improvements over the same time course, ruling out a procedural explanation for the learnt improvements in the amblyopic subjects (ie, simply learning how to perform the task).
Professor Susan Barry's dramatic recovery of stereo function was first brought to public attention in the New Yorker magazine in 2006 by Oliver Sacks.13 The studies we present here differ in a number of critical ways. Barry did not have amblyopia and her surgical treatment for strabismus was undertaken relatively early in life. Our subjects, on the other hand, presented with amblyopia, did not have surgery and had occlusion therapy later in childhood.
Learning points.
-
▶
Plastic neural mechanisms can be harnessed to develop new treatment tools for ameliorating human amblyopia in adulthood.
-
▶
A learning-based therapy, designed to exploit experience-dependent plasticity, successfully reversed the monocular visual loss resulting from amblyopia.
-
▶
Monocular improvements in visual performance promoted the independent recovery of stereoscopic visual function in adults with amblyopia.
-
▶
It is possible to recover normal levels of stereo function in amblyopic subjects with mature visual systems, challenging the long-held dogma that the critical period for visual development and the window for treating amblyopia are one and the same.
Acknowledgments
Wellcome Trust; European Commission FP7 Programme; College of Optometrists.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Crawford ML, Smith EL, 3rd, Harwerth RS, et al. Stereoblind monkeys have few binocular neurons. Invest Ophthalmol Vis Sci 1984;25:779–81 [PubMed] [Google Scholar]
- 2.Vaegen TD, Taylor D. Critical period for deprivation amblyopia in children. Trans Ophthalmol Soc UK 1979;99:432–9 [PubMed] [Google Scholar]
- 3.von Noorden GK. Binocular Vision and Ocular Motility: Theory and Management of Strabismus. Fifth edition St Louis: Mosby; 1996 [Google Scholar]
- 4.Stewart CE, Fielder AR, Stephens DA, et al. Design of the Monitored Occlusion Treatment of Amblyopia Study (MOTAS). Br J Ophthalmol 2002;86:915–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci 1998;21:149–86 [DOI] [PubMed] [Google Scholar]
- 6.Polat U, Ma-Naim T, Belkin M, et al. Improving vision in adult amblyopia by perceptual learning. Proc Natl Acad Sci USA 2004;101:6692–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levi DM. Perceptual learning in adults with amblyopia: a reevaluation of critical periods in human vision. Dev Psychobiol 2005;46:222–32 [DOI] [PubMed] [Google Scholar]
- 8.Huang CB, Zhou Y, Lu ZL. Broad bandwidth of perceptual learning in the visual system of adults with anisometropic amblyopia. Proc Natl Acad Sci USA 2008;105:4068–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li RW, Klein SA, Levi DM. Prolonged perceptual learning of positional acuity in adult amblyopia: perceptual template retuning dynamics. J Neurosci 2008;28:14223–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barry SR. Fixing My Gaze: A Scientist's Journey into Seeing in Three Dimensions. New York: Basic Books; 2009 [Google Scholar]
- 11.Moseley MJ, Neufeld M, McCarry B, et al. Remediation of refractive amblyopia by optical correction alone. Ophthalmic Physiol Opt 2002;22:296–9 [DOI] [PubMed] [Google Scholar]
- 12.Moseley MJ, Fielder AR, Stewart CE. The optical treatment of amblyopia. Optom Vis Sci 2009;86:629–33 [DOI] [PubMed] [Google Scholar]
- 13.Sacks O. A neurologist's notebook, “Stereo Sue”. New Yorker 2006;19:64 [Google Scholar]


