Abstract
An infant rat’s chance of survival is increased when it remains close to the nest. Early olfactory learning supports such adaptive behavior. Previous experiments indicated that non-associative odor exposure immediately after birth promoted later attachment to a similarly scented artificial nipple. The goal of the current experiments was to extend these findings on olfactory learning in the hours after birth by: exposing pups to more than one odor exposure (Exp. 1), dissecting the role of timing versus order of odor exposure (Exp. 2), testing the odor specificity of these effects (Exp. 3 and 4), and evaluating associative odor conditioning soon after birth (Exp. 5). Without explicit prior odor experience, pups only hours old do not respond much to a novel odor. Prior non-associative odor experience increases later motor activity to that same odor and to novel odors. Furthermore, these findings may be specific to certain amodal dimensions of the (in our case) lemon odor exposure. Single odor non-associative and associative conditioning was equally effective immediately after birth and during the third postnatal hour. Nevertheless, pups given two mere odor exposures responded to the first one more than the second at test, regardless of whether the exposures began immediately or two hours after birth. Possible mechanisms for these findings concerning early olfactory learning are discussed.
Keywords: Early Experience, Rodent, Learning, Neonatal, Smell/Olfaction
For many species, procuring food, warmth, and protection requires a close physical proximity between the infant and caregiver sometimes mediated by a psychological closeness known as attachment. Attachment theories began to take shape in the 1950’s with landmark studies by John Bowlby and Harry Harlow in human and non-human primates respectively (Bowlby, 1965; Harlow & Harlow, 1965). Both Bowlby and Harlow recognized a sensitive period early in development, after which establishment of attachment is difficult or less effective. Decades prior to this work, Konrad Lorenz had discovered imprinting in young goslings (Lorenz, 1937). Imprinting is an adaptive, stereotypic, and relatively immutable response wherein young fowl, in Lorenz’s case, follow (attach to) moving objects seen within a critical period soon after birth.
In addition to traditional imprinting usually restricted to fowl, researchers have studied mammalian imprinting in several different species (rats, Hudson, 1993; deer, Muller-Schwarze & Muller-Schwarze, 1971; spiny mice, Porter & Etscorn, 1974; guinea pigs, Sluckin, 1968). Instead of a moving object, mammals typically imprint onto an olfactory stimulus, usually maternally derived odors. A sensitive period has been suggested for this rapid and robust early olfactory preference learning in the rat as well as in human infants (Moriceau, Wilson, Levine, & Sullivan, 2006; Romantshik, Porter, Tillmann, & Varendi, 2007).
Early olfactory learning begins in the prenatal environment. Three-day-old human infants preferentially orient (via head turning) toward their own mother’s amniotic fluid as opposed to another mother’s (Schaal, Marlier, & Soussignan, 1998). Odor learning continues into the postnatal environment and can play a role in the feeding behaviors of infants. A crying infant will stop fussing and begin to elicit mouthing at the smell of their mother’s odor (Sullivan & Toubas, 1998). Early olfactory learning thus has implications for soothing premature infants and for promoting nipple attachment and bonding (Schaal, Hummel, & Soussignan, 2004; Winberg & Porter, 1998). Classical olfactory conditioning may explain how these olfactory preferences are formed. For instance, one-day-old human infants successfully orient towards a previously novel odor stimulus 24 hours after it had been paired with tactile stimulation (Sullivan et al., 1991). In the previous example, the mother’s odors were likely associated with feeding and thus promoted mouthing.
Much of the related research on the neurobiology of early learning and memory has focused on animals such as the rat for obvious ethical considerations. Like the human infant, many tasks vital to the newborn rat (i.e., suckling, huddling, and home orientation) are learned through classical olfactory conditioning wherein the odor of the dam, conspecifics or home is associated with rewards such as nutrition, warmth, and protection (Alberts, 2007; Cheslock, Varlinskaya, Petrov, & Spear, 2000; Rosenblatt, 1983). The first nipple attachment, however, is a unique situation. Prenatal and postnatal chemosensory experiences with amniotic fluid create a powerful cue for the neonate, which helps the pup initiate orienting movements towards the dam and promotes the first suckling experience (Hofer, Shair & Singh, 1976; Teicher & Blass, 1977).
What about these pre- and postnatal experiences with amniotic fluid makes them capable of initiating orienting behaviors? Perhaps mere exposure imbues amniotic fluid with a sense of familiarity thereby evoking comfort. It is also possible that reinforcing stimuli, such as the warmth of the uterine environment where the amniotic fluid is experienced prenatally combined with licking stimulation taking place while amniotic fluid is experienced postnatally, could support orienting to the amniotic fluid odor through classical conditioning. Alternatively, catecholamines might support odor learning in the absence of an external appetitive stimulus such as stroking or milk, which typically activate the noradrenergic system (Sullivan & Wilson, 1994). Catecholamines are one of many neurotransmitters and neuromodulators surging during the perinatal period (Herlenius & Lagercrantz, 2001). Functionally, neurochemicals within this surge are responsible for a number of events, from promoting independent respiration (Ronca & Alberts, 1995) to mediating postnatal learning (Leon, 1998). Of the catecholamines, norepinephrine (NE) in particular facilitates early olfactory conditioning (Sullivan & Wilson, 1994). For example, a noradrenergic agonist paired with an odor is sufficient to induce a preference for that odor in infant rats (Bordner & Spear, 2006; Sullivan, McGaugh, & Leon, 1991). Similarly, head turn preference scores towards an odor experienced soon after birth were positively related to levels of NE measured in umbilical cord blood of human infants (Varendi, Porter, & Winberg, 2002). Perhaps the perinatal catecholamine surge per se supports early odor learning in the absence of traditional unconditioned stimuli. The time period just after birth, when NE levels are at their highest, may provide a unique window into the neurobiology of early olfactory learning (Ronca, Abel, Ronan, Renner, & Alberts, 2006).
A study by Cheslock, Sanders and Spear (2004) found that although simple retention of a learned association was similar for both ages, pups 3-4 hours old (P0) were resistant to retroactive interference of their first experimentally acquired association whereas pups 24 hours old (P1) were not. Retroactive interference is said to have occurred when newly learned information interferes with the recall of information learned in the past. Retroactive interference was inferred by Cheslock et al. (2004) when a learned association (e.g., odor—saccharin) was forgotten due to a subsequently learned association (odor—quinine). Better recall of the first salient event experienced in the postnatal context (cognitive primacy) and physiological consequences of the birthing process were hypotheses discussed by the authors to explain this age-related effect.
A subsequent study tested the effects of mere odor exposure upon later reexposure to that odor in a nursing context. Postnatal age (in hours) at odor exposure was manipulated (Miller & Spear, 2008). Rat pups were exposed to an odor immediately, one hour, or two hours after cesarean delivery to study the effects this might have on later attachment to a surrogate nipple scented with that same odor and behavioral activation in its presence. Exposure to lemon odor within the first three hours after cesarean section resulted in increased behavioral activation and decreased latency to attach to a surrogate nipple upon reexposure at test, four hours postpartum. Romantshik et al. (2007), however, found that with human infants only odors experienced immediately after birth were learned.
One purpose of the current experiments was to extend the findings of Miller & Spear (2008) by further characterizing the features of olfactory learning in the hours just after birth. Experiment 1 tested the effects of multiple odor exposures on later odor-evoked motor activity. Experiment 2 assessed the relative impact of time versus order of odor exposure. Experiment 3 and 4 evaluated the odor specificity of this early olfactory learning. Finally, Experiment 5 measured associative odor conditioning soon after birth as a function of age (in hours) at conditioning.
General Method
Subjects
Subjects (see Table 1 for sample size information) were pups cesarean sectioned from Sprague-Dawley (Taconic, Germantown, NY) females bred in large wire hanging cages to a male. When a sperm plug was found (Embryonic day zero, E0) females were removed from the hanging cages and placed in opaque breeder tubs (45 cm long × 23 cm wide × 20 cm high) partially filled with shavings with one to two other pregnant rat(s) until E20 when they were separated into identical individual breeding tubs. Ambient temperatures in the colony room were maintained at 22°C on a 14-10 hour light/dark cycle (lights on at 0700) with ad libitum access to food (Breeders Purina Rat Chow, Lowell, MA) and water. Rats used in the following experiments were maintained and treated in accordance with the guidelines for animal care and use established by the National Institutes of Health (1986) and the Binghamton University Institutional Care and Use Committee.
Table 1.
Sample size information for Exp 1-5
| Experiment | 1 | 2 | 3 | 4 | 5 |
| N | 144 | 96 | 110 | 137 | 96 |
| # of groups | 18 | 12 | 12 | 18 | 12 |
| n per group | 8 | 7-9 | 7-11 | 7-8 | 8 |
Note. Each litter of subjects contributed no more than one pup per sex in each condition.
Cesarean delivery
Near expected term (E21), pups were delivered by cesarean section. Isoflurane anesthetized (Baxter, Deerfield, IL; VetEquip, Pleasanton, CA) the dam during cesarean delivery. A midline incision was made through the abdominal wall to expose the uterine horns. A small incision into each amniotic sac allowed externalization of the pups. The umbilical cords were ligated with sewing thread and cut. Finally, extra embryonic membranes were removed by gentle rolling of the neonate on a sanitary paper towel. Each pup was placed into a plastic container (12 cm long × 12 cm wide × 6 cm high) lined with moist paper towels. This container held pups before and after odor exposures and testing in a 35°C ± 1°C incubator. Once the cesarean section was completed, the dam was sacrificed via rapid cervical dislocation. The litters’ time of birth was noted when the median pup (e.g., fourth out of seven pups) was delivered.
Heating chamber and odor presentation
Since conditioning procedures persisted for an hour, pups were exposed to stimuli within a temperature maintained heating chamber (Microplate Incubator, Boekel Scientific, Feasterville, PA). These heating chambers were maintained at 35°C ± 1°C. Within the heating chamber, pups were placed into a hexagonal shaped shallow cup (8.5 mm wide at the top, 5.5 mm wide at the bottom, 2 mm deep) lined with synthetic fur (for a schematic depiction see Miller & Spear, 2008). All conditions had two pups in each cup, one male and one female. Odors were presented by placing a cotton swab above and in the center of the conditioning cup. This cotton swab remained there for the duration of the conditioning procedures.
Test box
Testing occurred in a transparent glove box (63 cm long × 50 cm wide × 25 cm high). The pup was placed on a mirror atop a heating pad maintained at 35.5°C ± 0.5°C. The mirror allows the 35.5°C ± 0.5°C temperature to radiate up and around the pup. For this reason the ambient temperature in the box was kept at a lower temperature (28.0°C ± 1.0°C). These temperatures have been used in many similar paradigms (Cheslock et al., 2004; Cheslock, Varlinskaya, Petrov, & Spear, 2000; Miller & Spear, 2008; Petrov, Varlinksaya, & Spear, 2001). Heating pad temperature was maintained using a temperature controller (Model 40-90-8B, Frederick Haer, Inc., Brunswick, ME). Ambient temperature was obtained with the use of commercial heating pads. In order to facilitate and standardize the odor exposure procedure the individual subject was strapped and fastened into a ‘vest’ made from ultra-thin, elastic rubber (Petrov, Varlinskaya, & Spear, 2001). The vest was designed to hold the pup in a semi-supine posture, which simulates the natural position of neonatal rats suckling at the maternal nipple (Eilam & Smotherman, 1998). This gentle restraint also prevents a righting reflex but does not otherwise produce apparent discomfort or behavioral incompetence.
Test
Duration of behavioral activation was measured by a blind experimenter (via video playback) in a continuous fashion for the entire four-minute test (inter-rater reliability: Pearson’s correlation coefficient > 0.93). Pups were placed into a vest and then into the testing box for a two-minute baseline period. Next, exposure to the test odor was presented on a cotton swab lightly waved 1 cm in front of the pups’ snout for two more minutes for a total of a four-minute motor activity test. A two-minute odor presentation period seemed more than sufficient to reveal effects of early odor exposure on later behavioral activation considering past research has accomplished this in the first minute of odor presentation alone (Miller & Spear, 2008). Furthermore, although the aforementioned study also measured nipple attachment behavior in the presence of the preexposed odor, the present study only used the motor activation test. This test has led to more consistent and robust effects of previous odor exposure than the nipple attachment test.
The duration of head and bursting movements were both scored in the present study. Head movements (e.g., probing, scanning) have been thought of as a measure of orienting behavior imperative for locating the nipple, whereas bursting movements are typically seen once an unconditioned stimulus (e.g., milk, stroking) is encountered. Despite the conventional distinction between head movements (a measure of orientation) and full body movements or bursts (a general index of arousal) these measures were positively correlated in the present study. Furthermore, results did not differ as a function of this distinction so the duration of head and burst movements was combined into a single motor activity score.
Data analysis
The average motor activity duration score from the first two minutes of the test (baseline) was subtracted from each of the odor exposure minutes for baseline-adjusted data. For example: motor activity during Minute 3 – [(motor activity during Minute 1 + motor activity during Minute 2)/2]. The same was done for Minute 4. In the following experiments, minute was a within subjects variable (Minute 1 and Minute 2 of odor exposure) and duration of motor activity in seconds was the dependent measure. Planned comparisons were conducted with Fisher’s least significant difference test. Results of the ANOVA’s were considered significant if p < 0.05.
Experiment 1
Previous experiments investigated early odor learning in the rat as a function of time since birth (Miller & Spear, 2008). This study found that motor activity at four hours postpartum in the presence of a previously experienced odor was independent of when the odor exposure took place during the pup’s first three postnatal hours. The present experiment sought to replicate and extend these findings with additional control conditions and variation in number of odor exposures as well as number of odorants exposed. Exposure to two different odors allowed for assessment of susceptibility to olfactory interference. Previous experiments have found special resistance to retroactive interference in newborns, beyond that seen in 1-day old pups (Cheslock et al., 2004). We predicted accordingly that newborn pups would more readily respond to the target odor when it was experienced before a different odor than if experienced after a different odor. Pups exposed to the same odor twice allowed us to assess the impact of familiarity on responding at test. If familiarity was the primary mechanism underlying later odor-evoked motor activity, we hypothesized that more experience with the odor would lead to greater levels of familiarity and therefore odor-evoked motor activity.
Method
Experimental design
A 2 (Sex) × 3 (Odor 1) × 3 (Odor 2) factorial design was used. Male and female pups were assigned to two orthogonal odor exposure conditions: immediately after birth for an hour (Odor 1: lemon odor, ethanol odor, or no odor), and also at two hours postpartum, again for an hour (Odor 2: lemon odor, ethanol odor, or no odor). Thus, pups experienced one odor twice, two different odors, one odor only, or no odors at all.
A schematic of the design for Experiment 1 can be seen in Figure 1a. In the following, two-letter abbreviations are used to describe a condition. The first letter signifies the odor given during the first postnatal hour (Odor 1) whereas the second letter signifies the odor given during the third postnatal hour (Odor 2). For example, the LE group experienced lemon odor immediately after birth for an hour and ethanol odor in the third hour of life, also for an hour.
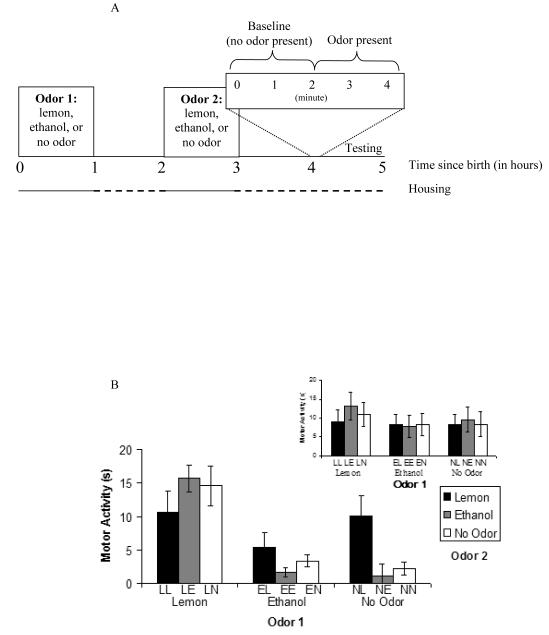
Figure 1.
Odor exposure in the neonate rat as a function of time since birth. a, Schematic of odor exposure and testing for Experiment 1. Newborn pups are exposed to an odor (or not) twice: Odor 1 (lemon, ethanol or no odor) and Odor 2 (lemon, ethanol or no odor) occur in heating chambers (solid line) during the first and third hour postpartum respectively. During the second and fourth postpartum hour and while waiting to be tested the pup is housed in an incubator (dashed line). The four-minute motor activity test occurs in a novel context. b, Mean ± SE duration of motor activity scores (in seconds) measured during testing in the presence of lemon odor (inset illustrates testing in the presence of ethanol odor) after exposure to lemon odor, ethanol odor or no odor immediately after birth (Odor 1) and two hours later (Odor 2). The abbreviations located under each bar represent the group (Odor 1 and Odor 2). For example, LE represents the group receiving lemon for Odor 1 and ethanol for Odor 2.
Procedures
For each litter, six pups were delivered via cesarean section, three males and three females. All pups were placed immediately into one of three heating chambers. One group of pups was exposed to 0.1 ml of lemon odor (Lorann oils, Inc. Lansing, MI), the second group was exposed to 0.1 ml of 190-proof-ethanol (Pharmco, Brookfield, CT) and the third group received no odor exposure (cotton swab only). After an hour of exposure in the heating chambers the pups were numbered in accordance with their experimental condition and placed into an incubator based on odor exposure to avoid cross contamination of odors possibly clinging to the skin of the pup.
At two hours postpartum, pups were placed back into the heating chambers. Again, one third of the pups were exposed to lemon odor, one third to ethanol odor, and the remaining third to no odor. Thus, some pups received odor exposure only once, some pups never experienced odor exposure, other pups were exposed to two different odors, and some were exposed to the same odor twice. After the second odor exposure, pups were once again placed into separate incubators based on their most recent odor exposure. Pups were tested at four hours postnatally with lemon odor in a four-minute motor activity test (see general method section Test for details). In the following repeated measures ANOVA, Sex, Odor 1, and Odor 2 were the between subjects variables. The two test minutes containing odor presentation and adjusted for baseline activity were the repeated dependent measures.
Lemon odor is an artificial odor with a history of use in many studies that have tested neonates (Cheslock et al., 2000; Pedersen & Blass, 1982). The logic for choosing alcohol odor was with the prospect of obtaining baseline data for alcohol odor preference in future studies, due to the authors’ interest in how early ethanol exposure affects later responsiveness to the drug (Spear & Molina, 2005). Results using ethanol odor at test revealed no significant effects (see inset of Figure 1b). For this reason, only data from lemon-tested subjects were presented. Inadequate detection of ethanol odor during odor exposure may have contributed to the present null effects. Ethanol has a much higher vapor pressure than lemon oil (59.02 mm/Hg and 0.950 mm/Hg at 25 °C respectively) indicating a greater level of evaporation throughout conditioning, especially since odor exposure occurred in a heated chamber. Obviously the result of this would be a less salient odor throughout the exposure period. For this reason, alcohol odor was not used in the remaining experiments.
Results
Prior to adjusting the data according to individual baseline differences, a check was made to ensure there were no differences in baseline activity between groups. No differences were found (ps > .1). Baseline-adjusted data revealed a main effect of Odor 1, F (2, 126) = 22.20, p < .01, an interaction of Sex and Odor 1, F (2, 126) = 3.62, p < .05, and an interaction of Odor 1 and Odor 2, F (4, 126) = 3.49, p < .01. There were no main effects or interactions involving the repeated measure, minute (p > .05). Thus, all reported data are collapsed across the two minutes.
The main effect of Odor 1 indicated that when lemon odor was experienced immediately after birth there was greater motor activation to lemon odor at test than when ethanol odor or no odor was administered at this time. The interaction of Sex and Odor 1 suggested that males in particular exhibited more activation to lemon odor at test after lemon odor exposure immediately after birth. The reliability of this sex effect awaits future replications. In general, pups responded to a test odor with motor activity when that same odor was experienced immediately after birth.
The interaction of a pup’s first and third hour odor experience can be seen in Figure 1b. Questions regarding the effect of time of exposure, the effect of interference by a different odor, and the effect of double odor exposure on memory for a simple odor exposure were clarified by planned comparisons using Fisher’s analyses (Table 2). Due to the large number of comparisons made (14) a Bonferroni corrected p value criteria of 0.004 (.05/14) was used to determine significance.
Table 2.
Fisher’s planned comparison p values for analyzed group comparisons
| Group | LL | LE | LN | EL | EE | EN | NL | NE | NN |
|---|---|---|---|---|---|---|---|---|---|
| LL | |||||||||
| LE | NA | ||||||||
| LN | > 0.1 | > 0.1 | |||||||
| EL | NA | <0.005* | NA | ||||||
| EE | NA | NA | NA | NA | |||||
| EN | NA | NA | NA | NA | NA | ||||
| NL | > 0.1 | NA | > 0.1 | > 0.1 | NA | NA | |||
| NE | NA | NA | NA | NA | NA | NA | NA | ||
| NN | 0 | <0.005* | <0.005* | > 0.1 | >0.1 | >0.1 | 0 | >0.1 |
Note. Due to the large number of comparisons, Bonferonni corrections were made. Alpha levels less than 0.004 were considered significant and were denoted by an asterisk.
NA = not analyzed
The effect of exposure time was analyzed by comparing groups with only one exposure to lemon (the test odor). Comparing pups exposed to lemon odor during the first (LN) or third (NL) postnatal hour revealed an effect of time of exposure on later behavioral activation in the presence of that same odor at test. Pups exposed to lemon odor immediately after birth (LN) activated to lemon odor at test more than naïve pups (NN). Exposure to lemon odor after a delay, as in the case of NL pups, however, did not increase responding at test using the corrected alpha value (p = .007). A more conservative comparison directly compared early exposure groups (LN) to late exposure groups (NL). Exposure to lemon odor during the first postnatal hour (LN) and exposure during the third postnatal hour (NL) yielded similar increases in behavioral activation to lemon odor at the test. Thus, there is some evidence that immediate odor exposure is more effective at eliciting motor activity to that same odor at test than odors experienced hours later, however this evidence is not strong.
The effect of interference on memory for odor exposure was explored by comparing those pups tested after exposure to two different odors. Pups exposed to lemon odor during the first postnatal hour followed by ethanol odor during the third hour (LE) responded more to lemon odor at test than naïve pups (NN). Pups exposed to the reverse (EL), however, failed to differ from naïve pups. Direct comparison of these two groups (i.e., LE and EL) further confirmed that motor activity to lemon at test was higher when the target odor was experienced first. The fact that groups exposed to both odors were no different than groups exposed to lemon odor at the equivalent time followed or preceded by no odor exposure (e.g., LE = LN and EL = NL) suggests minimal susceptibility to nonassociative interference in this age. It is also possible that ethanol odor is less intense smelling than lemon and perhaps even evaporated to some degree in the heating chamber over the hour of odor exposure. Nevertheless, it is interesting to note that in single odor exposure conditions (LN and NL) timing of odor exposure did not affect later odor-elicited behavioral activation as impressively as when pups were exposed to two different odors (LE and EL).
The effect of double exposure to the same odor on activity towards that odor was assessed by comparing pups exposed to lemon odor twice (LL) to pups experiencing lemon odor once (LN or NL) and to nonexposed pups (NN). Exposure to lemon odor twice did not lead to activation values higher than that of pups exposed only once. Double exposure to lemon odor, however, did result in levels of activation at test moderately higher than that of naïve pups (NN) (p = .0045). Finally, pups without lemon exposure (EN, NE, EE) did not differ from no odor controls (NN) in their response to lemon odor at test.
In summary, when only one odor was experienced, time of odor exposure did not seem to play a large role; both early and later exposed pups responded more to lemon odor at test than naïve controls. This finding nicely replicates previous results (Miller & Spear, 2008). When two odors were experienced, however, motor activity to the lemon odor at test was highest when the lemon odor exposure occurred prior to the alternative odor. This agrees with predictions based on neonatal work conducted using associative conditioning (Cheslock et al., 2004). Finally, exposure to the same odor twice did not increase responding over that of single exposure groups. This suggests that responding to the target odor at test is not driven solely by degree of familiarity.
Experiment 2
In Experiment 1, responding to lemon odor at test was greatest when this odor was experienced immediately after birth. An equally valid statement could be made in another way: responding to lemon odor at test was greatest when lemon odor had been experienced before exposure to a different odor. Previous studies that have noted differences in learning in the three-hour old pup compared to one-day olds did not determine whether these differences were due to high levels of certain neurochemicals detected soon after birth or to the unique primacy of information presented to a pup only hours old (Cheslock et al., 2004). Experiment 2 attempted to disentangle these possibilities by teasing apart time of odor exposure from order of odor exposure. Vanilla odor was used as an interfering odor in the present experiment, instead of the ethanol odor used in Exp. 1. The reason for this change was due to the concern that ethanol odor may have been a less salient odor than lemon odor. In a study of adults’ rankings of 3 odorants including these same volumes of ethanol and lemon odor, 90% ranked the ethanol as the least intense odor; ethanol was never ranked most intense (Miller, 2006). There was a possibility, due to ethanol’s volatile nature, that the odor may have evaporated and become weaker over the hour-long exposure in a heating chamber.
Method
Experimental design
A 2 (Sex) × 3 (Odor exposure) × 2 (Exposure time) design was used. Male and female pups were exposed to lemon odor and vanilla odor in a given order or received no odor exposure (lemon-vanilla [LV], vanilla-lemon [VL], or no odor-no odor [NN]). These odor exposures occurred either immediately after birth (Exposure time: “early”), as in Exp. 1, or 90 minutes after birth (Exposure time: “late”). Figure 2a illustrates how this design aids in the dissociation between time of odor exposure and order of odor exposure. Odor 2 in the “early” group and Odor 1 in the “late” group occur at the same postnatal time. The interstimulus interval was changed from an hour (as in Exp. 1) to 30 minutes to allow for earlier testing in the “late” exposure time group. Unpublished data from our laboratory suggests that maternal deprivation begins to alter pup nipple attachment behavior after six hours postpartum. It is possible that motor activity is also affected although this possibility has never been studied. Since retention interval and test time could not both be held constant across Exposure time groups, a decision was made to hold retention interval constant between “early” and “late” exposure groups. This decision was made because any possible effects of test time could be studied by comparing baseline values of motor activity across “early” and “late” groups as well as by detecting any significant main effects of this variable.
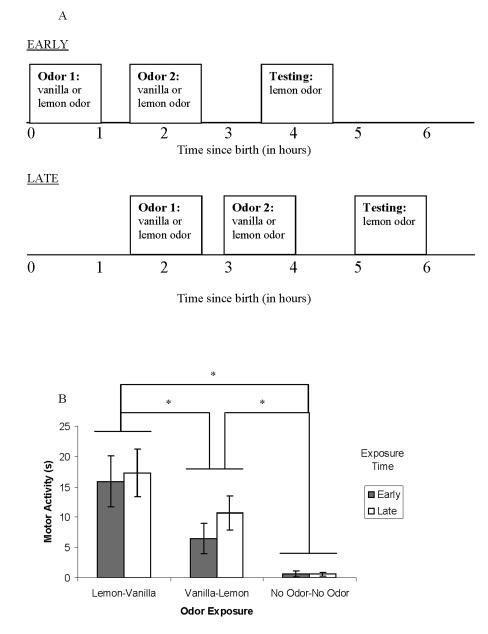
Figure 2.
Dissociating the effects of timing and order of odor exposure in the neonate rat. a, Schematic of odor exposure and testing for Experiment 2. Newborn pups were exposed to vanilla odor followed by lemon odor, lemon odor followed by vanilla odor, or received no odor exposure. This resulted in three odor exposure groups: vanilla-lemon, lemon-vanilla, and no odor-no odor (not pictured). These odor exposures occurred early (beginning immediately after birth) or late (beginning 90 minutes after birth). Testing with lemon odor followed an hour after the last odor exposure. b, Mean ± SE duration of motor activity scores (in seconds) measured during testing in the presence of lemon odor after one of three odor exposures (lemon-vanilla, vanilla-lemon, and no odor-no odor), which occurred either early or late. Although early and late groups never differ within an Odor Exposure condition, each Odor Exposure condition differs from one another. * indicates p < .05
Procedures
For each litter, six pups were delivered via cesarean section, three males and three females. Pups in the “early” exposure time group were placed into a heating chamber immediately after birth. Pups in the “late” exposure time group were first placed into a heating chamber 90 minutes after birth. Pups were exposed to either 0.1 ml of lemon odor (Lorann oils, Inc. Lansing, MI), 0.1 ml of vanilla odor (Hope Rose, USA) or received no odor exposure (cotton swab only). After an hour of exposure in the heating chambers the pups were numbered in accordance with their experimental condition and placed into an incubator based on odor exposure to avoid cross contamination of odors possibly clinging to the skin of the pup. Half an hour after their first odor exposure, pups were placed back into the heating chambers. Pups previously exposed to lemon were now exposed to vanilla odor and visa versa. Pups given no odor exposure continued to receive no odor exposure 30 minutes later. This resulted in three odor exposure groups: lemon-vanilla (LV), vanilla-lemon (VL), and no odor-no odor (NN). After the second odor exposure, pups were once again placed into separate incubators based on their most recent odor exposure. Pups were tested an hour after their last odor exposure with lemon odor in a four-minute motor activity test. A repeated measures ANOVA was used to analyze the effect of Sex, Odor Exposure and Exposure Time on motor activity during the behavioral activation test.
Results
Analyses conducted on baseline activity revealed that pups tested early were slightly (mean difference: 0.5 sec) more active than pups tested late, F (1, 84) = 6.48, p = .01. Furthermore, pups exposed to VL were slightly (mean difference: 0.75 sec) more active than LV or NN pups during baseline, F (2, 84) = 5.97, p = .004. Using baseline-adjusted scores, there were no main effects of Sex, Exposure Time, or the repeated measure, Minute. There was, however, a main effect of Odor Exposure, F (2, 84) = 18.94, p < .001. Planned comparisons revealed that motor activity in response to lemon odor was highest when exposure to lemon odor had occurred first and lowest when no exposure to lemon odor had occurred at all (see Figure 2b). All groups were significantly different from one another (all p’s < .01). There were no interactions among any of the variables under study.
The present experiment replicates the finding that when two different odors are experienced, responding to lemon odor at test is greater when lemon is experienced immediately after birth rather than after a delay. The current experiment was critical however, in tempering the conclusion that time of odor exposure was the primary characteristic involved in high levels of responding to lemon odor at test. Regardless of whether lemon odor was first experienced immediately after birth or 90 minutes later, high levels of motor activity to this odor during testing depended on the fact that it preceded rather than followed a different odor exposure.
Experiment 3
Experiment 1 and 2 found increased motor activity to a previously experienced odor. Additionally, when presented with two different odor exposures, the neonatal rat responded more to the first odor exposure. What is driving these increases in motor activity? Perhaps the motor activity is a sign of recognition, facilitated perception or a change in affect towards that odor. One strategy to aid in choosing among these various hypotheses is to test whether the increase in responding is odor specific. If responding to the test odor is not specific to the previously experienced odor, then perhaps early odor exposure alters arousal or sensory perception in general, or response to the odor is based on its amodal attributes of only its membership in the general category, “novel odorant” (e.g., Spear & Hyatt, 1993; Kraebel & Spear, 2000; Spear & McKinzie, 1995). On the other hand, increased motor activity at test would suggest recognition of the target odor’s unique properties only if these results were odor specific. Experiment 3 tests odor specificity of the finding that odors experienced first evoke greater responding at test than those experienced after prior odor exposure.
Method
Experimental design
A 2 (Sex) × 3 (Odor exposure) × 2 (Test odor) design was used. As in Experiment 2, male and female pups were exposed to lemon odor and vanilla odor in a given order or received no odor exposure (Odor exposure: lemon-vanilla [LV], vanilla-lemon [VL], no odor-no odor [NN]). The interstimulus interval was one hour as in Exp. 1. The first odor exposure began immediately after birth and lasted an hour. The second odor exposure began two hours after birth also for an hour. Testing occurred four hours postpartum with either lemon or vanilla odor (Test odor). Figure 1a illustrates this experimental design.
Procedures
For each litter, six pups were delivered via cesarean section, three males and three females. One pup of each sex was placed into one of three heating chambers (lemon, vanilla, or no odor) immediately after birth. One hour later pups were removed from the heating chambers and placed into 3 separate incubators based on their previous odor exposure to avoid odor contamination. One hour later, pups were removed from the incubators and placed into a heating chamber. As in Exp. 2, these two odor exposures resulted in three odor exposure groups: lemon-vanilla (LV), vanilla-lemon (VL), and no odor-no odor (NN). After an hour in the heating chambers pups were once again removed and placed into separate incubators until testing commenced one hour later. As in the previous two experiments, testing lasted four minutes and was conducted with a two-minute baseline period followed by presentation of an odor (lemon or vanilla odor) for two more minutes. A repeated measures ANOVA was used to test how Sex, Odor Exposure and Test Odor affected motor activity during Minutes 3 and 4 of the behavioral activation test.
Results
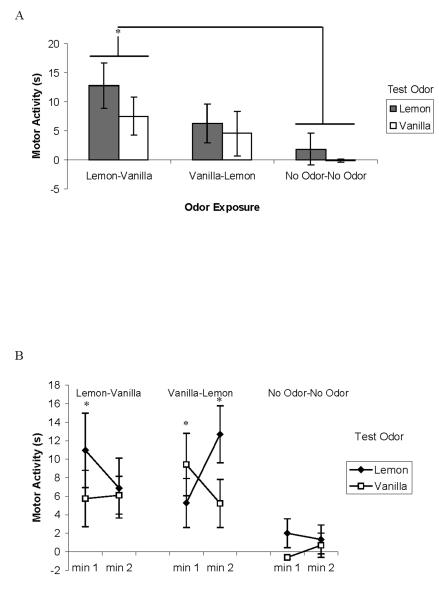
Analysis on baseline activity revealed no differences based on any of the independent variables (ps > .1). Using baseline-adjusted scores, there was a main effect of Odor Exposure, F (2, 98) = 8.12, p = .001. Pups receiving lemon and vanilla, regardless of the order (LV or VL), responded more to either test odor than pups with no prior odor experience. There was also a significant four-way interaction (Minute × Sex × Odor Exposure × Test Odor), F (2, 98) = 3.31, p = .04. To dissect this interaction further, ANOVAs were conducted separately for each sex. For males (see Figure 3a), there was a significant main effect of Odor Exposure, F (2, 49) = 4.39, p = .02. Male pups that experienced LV were more active at test than pups without prior odor exposure. Pups with VL exposures did not differ from either LV or NN groups. For females (see Figure 3b), there was the same main effect of Odor Exposure as for males, F (2, 49) = 5.12, p = .01, but also a three-way interaction (Minute × Odor Exposure × Test Odor), F (2, 49) = 4.93, p = .01. Responding to lemon odor at test was greater in LV pups than no odor controls during the first minute and VL pups during the second minute of odor presentation. Responding to vanilla odor at test was greater than no-odor controls only for VL pups during the first minute of odor presentation.
Figure 3.
Two-odor olfactory learning as a function of test odor in the neonate rat. A schematic for Experiment 3 can be found in Figure 1A. a, Males: Mean ± SE duration of motor activity scores (in seconds) measured during testing in the presence of lemon or vanilla odor after one of three odor exposures (lemon-vanilla, vanilla-lemon, and no odor-no odor). b, Females: Mean ± SE duration of motor activity scores (in seconds) measured during testing in the presence of lemon or vanilla odor after one of three odor exposures (lemon-vanilla, vanilla-lemon, and no odor-no odor). Baseline-adjusted scores are presented for each odor presentation minute during testing. * indicates a group mean that is significantly (p < .05) higher than the no-odor control group.
In summary, after exposure to lemon odor followed an hour later by vanilla odor, high levels of responding to lemon were observed for both males and females, as seen in Exp. 2. Nevertheless, odor specificity was not clear. Male pups showed no evidence of odor specificity but females demonstrated some odor-specific responding. Although both LV- and VL-exposed females responded to lemon odor at test in levels exceeding no odor controls, the pattern of responding differed between the first and second minute of the test. As predicted, responding to vanilla at test was only exhibited in the VL exposed group. Like LV pups’ responding to lemon odor, however, VL pups responded differentially only during the first minute of vanilla odor presentation.
Experiment 4
Results of the test for odor specificity in Experiment 3 were unexpectedly complicated. Males showed no evidence of odor-specific responding. Females showed some evidence of odor specificity but this depended on testing minute. The lack of odor specificity seen in Experiment 3 was not too surprising given that odor-exposed animals had experience with both test odors. In other words, no pups exposed to odor in Experiment 3 were tested on a completely novel odorant. Additionally, odor exposure in Experiment 3 utilized the two-odor exposure design. To simplify our tests of odor specificity, Experiment 4 exposed newborn pups to a single odor and tested on either a familiar or novel odor. To test whether prior odor exposure affects motor activity patterns in general, the present experiment also included a group that had no odor presented at test.
Method
Experimental design
A 2 (Sex) × 3 (Odor exposure) × 3 (Test odor) design was used. Male and female pups were exposed to lemon, cineole (Sigma-Aldrich, St. Louis, MO), or no odor (Odor exposure) immediately after birth for an hour. Three hours later (at four hours postpartum) pups were tested on lemon, cineole or no odor (Test odor). Figure 4a illustrates this design. The reason this experiment used cineole instead of the vanilla odor used in Experiment 3 was because the present experiment also served to collect parametric data for another series of experiments using this particular odorant.
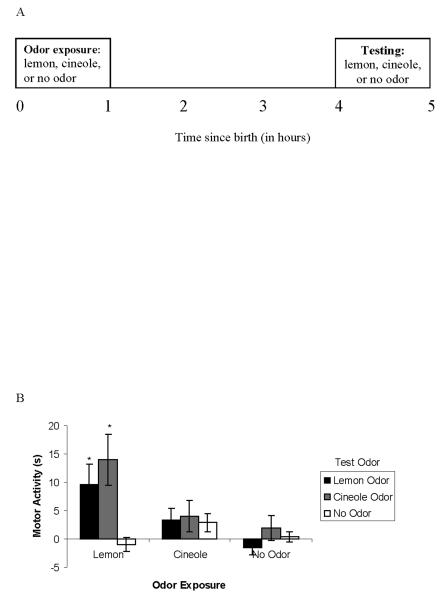
Figure 4.
Single odor olfactory learning as a function of test odor in the neonate rat. a, Schematic of odor exposure and testing for Experiment 4. Newborn pups were exposed to an odor (lemon, cineole, or no odor) immediately after birth for an hour. A four-minute motor activity test (using lemon, cineole, or no odor) took place during the pups’ fifth postnatal hour. b, Mean ± SE duration of motor activity scores (in seconds) measured during testing in the presence of lemon, cineole, or no odor. * indicates a group mean that is significantly (p < .05) higher than no-odor control groups: no odor exposure within each test odor group.
Procedures
For each litter, six pups were delivered via cesarean section, three males and three females. One pup of each sex was placed into one of three heating chambers (lemon, cineole, or no odor) immediately after birth. One hour later pups were removed from the heating chambers and placed into three separate incubators based on their previous odor exposure in order to avoid odor contamination. Testing occurred four hours postnatally and was conducted with one of three test odor conditions (lemon, cineole or no odor). A repeated measures ANOVA was used to test the effects of Sex, Odor Exposure and Test Odor on motor activity during Minutes 3 and 4 of the behavioral activation test.
Results
Analysis on baseline activity revealed no differences based on Sex, Odor Exposure or Test Odor. Using baseline-adjusted scores, there was a main effect of Odor Exposure, F (2, 119) = 7.18, p = .001, and of Test Odor, F (2, 119) = 4.62, p = .01. Pups that were exposed to lemon odor exhibited higher levels of activity at test than pups exposed to cineole or no odor, which did not differ from each other. In contrast, pups tested with cineole odor exhibited more behavioral activation at test than pups tested with lemon or no odor, which did not differ from one another.
In addition to these main effects, an interaction between Odor Exposure and Test Odor was also found, F (4, 119) = 3.29, p = .01. The interaction found between Odor Exposure and Test Odor is depicted in Figure 4b. There were no sex effects and results did not differ as a function of minute during test. Thus, data presented in Figure 4b are collapsed across Sex and Minute of test. As in previous experiments, pups given lemon odor exposure responded more to lemon odor at test than pups with no prior odor exposure that were tested on lemon odor. It is notable that pups given exposure to lemon odor also responded significantly more than no odor controls to cineole odor at test. Although responding to lemon and cineole odor at test was also equivalent after cineole odor exposure, levels of responding were rather low. Pups with no odor exposure responded similarly to pups with cineole exposure. Furthermore, there were no differences between pups with cineole odor exposure regardless of whether they were tested on cineole, lemon or no odor.
In summary, lemon odor exposure immediately after birth resulted in increased motor activity at test to lemon odor as well as to a novel odor, cineole. Exposure to lemon odor immediately after birth appears to promote motor activity induced by either lemon or cineole odor three hours later.
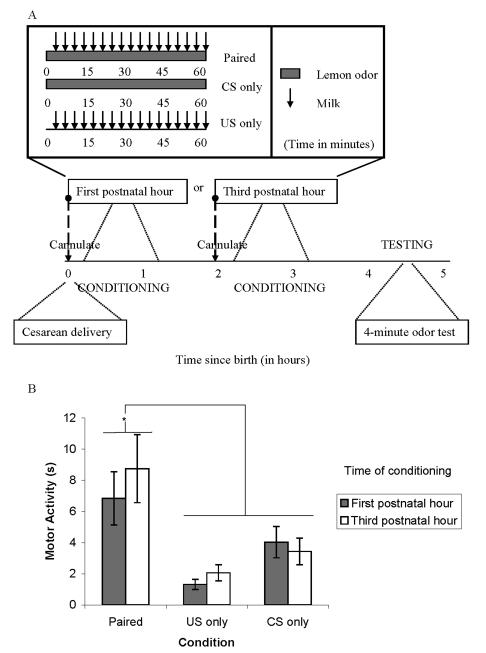
Experiment 5
How does introducing associative conditioning—an explicit source of reinforcement (unconditioned stimulus, US) paired with the odor (conditioned stimulus, CS)—alter the pattern of behavioral effects seen after mere odor (non-associative) exposure? Previous informal experiments in our laboratory have failed to observe associative conditioning earlier than 3-4 hours postnatal. These previous efforts focused on conditioned attachment, at 3-4 hours postnatal, to an artificial nipple scented with an odor that had been paired with an intraoral infusion of milk. This previous measure of associative conditioning did not include the measure of general activity applied to assess nonassociative conditioning in Experiments 1-4. To assess associative conditioning at an earlier postnatal age than previously established, Experiment 5 applied the same general activity measure as in Experiments 1-4. Experiment 5 revealed that adding an explicit reinforcer (US) enhances motor activity to the odor (CS) compared to motor activity resulting from odor exposure without an explicit reinforcer, as in Experiments 1-4. Nevertheless, the similarity in responding (increased motor activity) for an odor after mere exposure and after paired appetitive conditioning may imply that in both cases activity is more likely a positive response to the odor than an aversive reaction. A more detailed characterization of aversive responses, such as facial wiping, could add support to this possibility or might weaken it.
Method
Experimental design
A 2 (sex) × 2 (age, in hours) × 3 (condition) design was used. Male and female pups were placed in one of three conditions (paired, unconditioned stimulus [US] only, conditioned stimulus [CS] only) at a given age (first or third postnatal hour). The CS was lemon odor and the US was milk. An unpaired condition could not be included due to time constraints created by CS-US pairings given so soon after birth, i.e., since the CS and US were each experienced for an entire hour, an explicitly unpaired group specific to each age was not possible. A schematic of this design can be seen in Figure 5a.
Figure 5.
Associative conditioning in the neonate rat as a function of time since birth. a, Schematic of odor exposure and testing for Experiment 2. Newborn pups were exposed to lemon odor (CS), milk (US) or both stimuli paired for 60 minutes beginning 15 minutes or 2 hours and 15 minutes after delivery (there was a 15 minute recovery period after cannulation). A four-minute motor activity test took place during the pups’ fifth postnatal hour. b, Mean ± SE duration of motor activity scores (in seconds) measured during testing in the presence of lemon odor after conditioning (paired, CS only, US only) during the first or third postnatal hour. CS = conditioned stimulus, US = unconditioned stimulus. * indicates p < .05
Procedures
Just after cesarean section, pups in the younger age group were cannulated. For this procedure, a wire was connected to polyethylene size 10 (PE-10, Clay Adams, MD) tubing with one flanged end and inserted through the cheek. This procedure took less than 30 seconds per pup. In four-day-old pups this procedure does not significantly increase serum corticosterone levels beyond that of untreated controls (Spear, Specht, Kirstein, & Kuhn, 1989). After 15 minutes of recovery (from cannulation) in an incubator, the training procedures began. Two pups (one male and one female) were placed into a heating chamber together. Each PE-10 cannula was attached to PE-50 tubing (for paired and US only pups), which in turn was connected to a rotary microsyringe capable of precisely delivering room temperature milk infusions via a toggle switch. Length of the conditioning session was one hour.
Animals to be conditioned in close proximity to birth were given the CS (lemon odor) for an hour beginning 15 minutes after cesarean delivery (paired condition; see Figure 5a). The US (milk infusions: 1.5 μL each) was administered every 90 seconds from minute 1 of conditioning to minute 60. The US only animals received identical milk infusions in the absence of lemon odor, while CS only animals were exposed to an hour of lemon odor without milk infusions. Thus, the CS only group resembled groups from Exp 1 and 4 given an hour of lemon odor exposure immediately after birth. After conditioning, pups were placed into an incubator until testing. Pups to be conditioned during the third hour postpartum waited in the incubator until two hours postpartum. Cannulation procedures were done at this time and recovery took place for 15 minutes in the incubator. Conditioning for these pups was identical to that of pups conditioned 15 minutes after birth. Testing with lemon odor took place at four hours postpartum and lasted four minutes. In the following analyses, Sex, Age and Condition were independent variables. Motor activity during Minutes 3 and 4 of the behavioral activation test as well as body weight gain and conditioning cup weight gain (indicating spillage of milk from the pups mouth) taken after conditioning were dependent measures.
Results
To assess intake of the milk, body weight gain and weight gain of the conditioning cup were measured. There was a significant effect of age, F (1, 84) = 47.48, p < .001, and condition, F (2, 84) = 79.98, p < .001, on body weight gain such that pups conditioned later, as well as paired and US only pups, gained more weight than pups conditioned immediately after birth and pups receiving no milk (CS only), respectively. The fact that pups conditioned earlier did not gain as much weight as the later conditioned pups was not due to less milk intake but rather because younger pups seemed to experience minor weight loss due perhaps to a humidity deficit in the heating chamber. This statement is supported by (a) the absence of a Condition × Age interaction on body weight gain (p > .1), (b) the absence of a main effect of age on cup weight gain (p > .1) and (c) a significant effect of age on weight loss (body weight after – body weight before conditioning) in the CS only pups, which had no milk exposure F (1, 28) = 17.94, p < .001, (weight loss of pups conditioned immediately after birth [mean ± standard deviation]: −0.06 g ± 0.02; conditioned 2 hrs later: −0.03 ± .02). There was also a significant main effect of condition on cup weight gain, F (2, 81) = 5.84, p < .01, indicating that conditioning cups containing paired pups included more unconsumed milk than those containing control pups in the US or CS only conditions. That pups in the paired condition had more milk spill out of their mouths implies that the lemon odor was interfering with ingestion of the milk. This may be due to the intensity of this chemosensory stimulus, which when infused into the mouth causes facial wiping reliably in fetal rats (Brumley & Robinson, 2004).
In measures of motor activity, there were no main effects or interactions with age or sex. There was, however, a significant main effect of condition (Figure 5b), F (2, 84) = 10.23, p < .001. During testing, the paired group spent significantly more time active in the presence of the lemon odor than either the US only or CS only controls, which did not differ from each other. This suggests that associative conditioning occurred and was equivalent during the first and third postnatal hours. It is notable that the level of responding in the CS only group conditioned immediately after birth (mean ± standard deviation: 4.03 ± 5.61) is less than that of the LN group (14.59 ± 14.44) from Experiment 1. Recall that this CS only group was cannulated after delivery and that odor exposure did not begin until a 15-minute recovery period had elapsed. Additionally, in Experiment 1 the LN group was handled twice, whereas the CS only group in the present experiment was only handled once. These factors may have contributed to the discrepancy between two seemingly similar experimental groups, but this has yet to be tested.
In general, paired pups classically conditioned (odor and milk pairing) during their first or third postnatal hour behaviorally activated to the odor CS more than CS only and US only control groups. Like data in Exp. 1 with single odor exposures (groups LN and NL), the present experiment found no differences in responding at test based on when the odor exposure (CS only group) or conditioning (paired group) took place. It seems that associative conditioning, like nonassociative conditioning, can be effective during the first hour after birth.
General Discussion
Early olfactory learning is crucial to survival in the newborn rat. Still, olfactory learning in the hours immediately after birth has received relatively little attention. The present series of experiments was designed to characterize such olfactory learning and to test whether associative conditioning was feasible immediately after birth. Odor exposure(s) occurred within the first hours of life. Pups were exposed to either one or two odors and tested later for motor activity elicited by a test odor. Time of odor exposure (first or third postnatal hour) did not play a large role in the level of motor responding in pups receiving only a single odor exposure. When two different odors were experienced, however, the first odor was responded to more at test (Exp. 1, 2). This was specifically a function of the order of odor exposure and not the time of odor exposure (Exp. 2). The recurrent finding that previous odor exposure increases later responsiveness to that odor was not odor-specific for pups exposed to either a single odor exposure (Exp. 4) or to two different odors (Exp. 3). Finally, associative olfactory learning occurred immediately after birth. A pup conditioned to an odor and appetitive gustatory stimulus had greater responding to that same odor at test than CS-only and US-only controls regardless of whether learning occurred immediately after birth or two hours later (Exp. 5).
Experiment 1 found that, regardless of later odor exposure, males were more responsive than females to lemon odor after they experienced this odor immediately after birth. A similar experiment also found males more responsive than females to a previously experienced odor. This was seen, however, during an odor exposure occurring two hours after birth; no sex differences were found immediately after birth (Miller & Spear, 2008). In the aforementioned study as well as the current series, sex differences were otherwise largely absent.
Experiments utilizing two different odor exposures were aimed at studying non-associative interference in the neonatal rat. In Experiment 1, interference referred to the disruption of lemon odor responsiveness at test due to exposure to a different odor either before or after lemon odor exposure (proactive and retroactive interference respectively). Adults typically show better recall for the last event learned after a short retention interval and the first event after a long retention interval (Bouton & Peck, 1992; Spear, 1971). In Exp. 1, which used a short retention interval, pups did not differ in their lemon-evoked motor activity at test based on the presence or absence of a different odor exposure. There were concerns, however, that the interfering odor in Exp. 1, ethanol odor, was less salient than the lemon odor. Although Exp. 2-4 used a different interfering odor to address these concerns, single odor exposed controls were unfortunately not used in these experiments and thus we were unable to internally replicate the lack of interference seen in Exp. 1. Nevertheless, better responding to lemon odor was seen when lemon odor exposure preceded rather than followed a different odor exposure (Exp. 1-3). This seems congruent with a study by Cheslock et al. (2004), which also found proactive interference in neonatal rats. Infant rats were taught two conflicting associations and tested after a short retention interval. Newborn pups three to four hours old (P0) recalled the first association, whereas one-day-old pups (P1) recalled the second.
Cheslock et al. (2004) discussed the possible contributions of NE and cognitive primacy to their findings but could not dissociate them. This is because the P0 pups, which had fewer experiences prior to conditioning (and therefore more primacy) than the P1 pups, also had presumably higher NE levels (Ronca et al., 2006). In Experiment 1, pups exposed to two different odors had greater responsiveness to the odor experienced first, which was also the odor experienced immediately after birth. Experiment 2 was designed to disentangle time of odor exposure and order of odor exposure. Experiment 2 revealed that order of odor exposure but not timing was critical in driving odor-evoked motor responding at test. This finding seems to diminish the importance of hour by hour differences in NE levels on olfactory learning during the first hours after birth. In a similar study conducted with human infants, only those exposed to an odor soon after birth (within about 30 minutes) but not two hours later became familiar with the odor (Romantshik, Porter, Tillmann, & Varendi, 2007). In this experiment, however, testing occurred several days after odor exposure as opposed to several hours as was the case in the present study.
It is possible that NE levels in rats differ only slightly among the first hours after birth compared to the more dramatic differences between newborns and one-day-olds. Unpublished data comparing P0 and P1 pups in their ability to learn about an odor after an hour of exposure suggests that there may be something unique about olfactory learning in the first hours of life compared to just 24 hours later (Miller & Spear, 2009). A study with human infants documented a correlation between plasma NE levels at birth and odor preferences for an odor, which was experienced for 30 minutes shortly after birth (Varendi, Porter, & Winberg, 2002). Future experiments are currently being designed to compare blood and brain levels of NE between P0 and P1 pups and to pharmacologically manipulate levels of this neurochemical in order to explicitly test the effects of NE on olfactory learning soon after birth.
The present experiments lend some support to the importance of experiential or cognitive primacy in directing odor-evoked motor activity after previous odor exposure. The lack of odor specificity found after early olfactory exposures (Exp 3 and 4) underscored the need to scrutinize possible mechanisms underlying these effects. Not only was there a lack of odor specificity, it seems that there was also a lack of generality. In other words, early odor exposure increased later responding to odors but under the limited conditions tested herein this was seen only for lemon odor exposure. It is not our belief that lemon is as ecologically relevant for neonatal rats as some other odorants such as amniotic fluid. Rather, it is possible that, for example, lemon maintained a relatively constant intensity throughout the exposure period thereby making exposure odor identical to the test odor. As mentioned previously, ethanol odor may have evaporated in the heating chambers making a less concentrated odorant during exposure compared to testing. Since odor quality changes with concentration any differences in the concentration of exposure odor and test odor would potentially lead to less responding at test (Wilson & Stevenson, 2006). Unfortunately, our procedures did not permit us to measure the precise concentration of odorants tested, so the validity of this possibility for ethanol, as well as the other odorants used, is not known. Future experiments could explicitly vary intensity to study what effect this variable has on early olfactory learning and the specificity of this effect.
The notion that early olfactory learning guides attachment to caregivers gave way to the hypothesis that responding to lemon odor at test may reflect odor recognition and thus, familiarity with that particular odor. This hypothesis however, was not clearly supported in the present series of experiments. If familiarity played a critical role, one would expect responding to lemon at test to be stronger with two than with one prior lemon odor exposure, but this was not the case. Although double exposure to lemon odor led to higher levels of lemon-evoked motor activity than pups without any odor exposure, levels achieved with double exposure did not exceed those found after a single exposure. If an increased level of activation to previously exposed odors is based on a mere exposure effect mediated by familiarity, these results are surprising unless the function relating familiarity to number of exposures were negatively accelerated to an extreme at this age. It is also possible that motor activity is not sensitive enough to differentiate between one and two trials of familiarity. Past studies employing and studying familiarization have historically tended to use more extensive exposure to stimuli (Alberts & May, 1984; Wilson, Sullivan, & Leon, 1985). One would expect that more exposures to an odor would result in greater familiarity. The fact that simple familiarity with odor quality does not appear to determine responding at test seems to be in accord with the lack of odor specificity found with these same procedures. Familiarity implies that the subject has experience with a particular odor quality. Thus we would not expect the subject to respond to novel odors if familiarity were the main mechanism driving odor-evoked activity at test. Yet the attribute of the odor actually encoded by the neonate, and so its “familiarity” might not be primarily its olfactory quality, but instead an amodal dimension such as its intensity, rise-time or duration (e.g., Kraebel & Spear, 2000; Spear & Molina, 1987).
On the other hand, that two exposures to lemon odor did not result in greater levels of activity at test than single lemon odor exposures may be a consequence of habituation. Groups that had the highest rates of activation to lemon odor at test in Exp. 1 were given only a single exposure to lemon odor followed by either a dishabituation stimulus (i.e., ethanol in LE) or more time to recover from habituation (no odor during third postnatal hour as in the LN group). Although no data exist on retention of habituation in hour-old-rats, retention of habituation in the infant and fetal rat is very short compared to older rats (Parsons, Fagan, & Spear, 1973; Smotherman & Robinson, 1992). Therefore, while it is unlikely that habituation in the current experiment lingered after an hour, this remains to be tested. Sensitization, or an increase in responding resulting from repeated exposures to a stimulus, may underlie the general finding of the current experiment that previous exposure to an odor increases later behavioral activation in the presence of that odor. This account is not likely, however, since one would expect two previous lemon odor exposures to induce more motor activity in a lemon odor test than just one exposure, and this was not the case. Differentiation of “nonassociative conditioning” and sensitization in the present context is nevertheless problematic.
If not familiarity or sensitization, what mechanism underlies the increased motor activity seen upon reexposure to a previously experienced odor? Although it was initially presumed that increased activity at test indicated recognition of odor quality, without odor specificity new interpretations must be discussed. It is possible that early odor exposure non-specifically alters later odor perception. Human subjects rated a previously smelled odor as more intense 25 minutes after exposure to alternative odor presentations of weak concentration (Pol, Hijman, Baaré, & van Ree, 1998). Olfactory bulb injections of NMDA or bicuculline (a GABAa receptor antagonist) and even odor preexposure can affect later olfactory perception in a nonspecific manner (Mandairon, Stack, Kiselycznyk, & Linster, 2006; Okutani, Zhang, Yagi, & Kaba, 2002). Data from single unit recordings in the main olfactory bulb of rabbits suggest that dendrodentritic interactions between the mitral/tufted cells and granule cells underlie lateral inhibition, which regulates the specificity of odor responses (Yokoi et al., 1995). Odor perception and discrimination seem to be highly dependent on the structural similarity and neural pattern overlap between two odors (Mandairon et al., 2006; Yokoi et al., 1995). The neural pattern evoked by odors used in the current experiments remains to be tested in the neonatal rat.
Obtaining results that lack stimulus specificity early in ontogeny is not without precedent. Young infants tend to respond to amodal characteristics of their environment such as intensity, duration or affect more so than older or adult organisms, which focus on modality specific information (see Spear & Hyatt, 1993; Spear & Molina, 1987; Turkewitz & Mellon, 1989). Encoding their odor exposure by intensity of the stimulus versus the specific quality (lemon versus cineole odor) of the odorant could help explain the lack of odor specificity seen in Exp 4. For example, pups’ responding at test may indicate recognition not of the lemon odor per se but rather the intensity of this prior odor experience and thus they respond to similarly intense cineole odor at test. Nevertheless, this amodal processing does not at present explain the lack of generality of these odor-nonspecific effects. For instance, in Exp 4, pups previously given cineole odor exposure responded very little to either lemon or cineole odors at test. The idea of amodal processing could nevertheless guide future experiments to further characterize immediate olfactory learning. A first step may be to explicitly vary odor intensity, using dilutions, during exposure and testing.
One theory of mere exposure learning is that it is not non-associative but rather associative learning, wherein the US is the absence of a negative consequence (Zajonc, 2001). Novelty is generally avoided initially but eventually no longer poses a threat and therefore signals safety. Explicit associative conditioning in Experiment 5 resulted in increased motor activity to lemon odor (compared to controls), which had predicted milk. This finding, along with the fact that milk is appetitive to infant rats, allows one to infer that increased motor activity indicates a preference (Cheslock, Varlinskaya, Petrov, & Spear, 2000). Unfortunately, in pups incapable of locomotion, motor activity could signify approach or withdrawal just as kicking can indicate interest or impatience (Thelen, 1981). Although behavioral activation does not reveal the pup’s affective response to the odor, numerous past studies with various species have reported that mere exposure increases preference for that odor (Balogh & Porter, 1986; Hepper, 1988; Porter & Etscorn, 1974; Zajonc, 1968). Unfortunately, our attempts to obtain satisfactorily reliable measures of preference in the first hours after birth have so far been unsuccessful.
Of course learning does not start immediately after birth. Many studies have been conducted on fetal olfactory learning. Similar to what we see with olfactory learning soon after birth, learning about an odor prenatally changes responsiveness to that same odor whether tested in utero (Smotherman & Robinson, 1985) or as a neonate (Chotro & Molina, 1992) or older (Abate, Pueta, Spear, & Molina, 2008). Work on prenatal olfactory learning suggests that these experiences serve to ease the transition from fetal to neonatal life in rabbits (Coureaud, Schaal, Hudson, Orgeur, & Coudert, 2002) and rats (Pedersen & Blass, 1982) as well as humans (Schaal, Marlier, & Soussignan, 1998). The effect of early olfactory learning on later behavior has been an extensively studied topic. This is likely due to its ecological relevance for subsequent nutrition, protection, warmth, food preference, home orientation and even mate choice (Alberts & May, 1984; Fillion & Blass, 1986; Hepper, 1988; Rosenblatt, 1983; Teicher & Blass, 1977). Studying the rat pup within minutes or a few hours after birth is a vital step in understanding the neurobiology of early learning and memory.
Acknowledgments
The research presented in this article was supported by grants from National Institute of Mental Health (RO1MH035219) and the National Institute on Alcohol Abuse and Alcoholism (RO1AA013098, R01AA015992 and R01AA011960) to Norman E. Spear. We express our appreciation to Teri Tanenhaus for assistance with the manuscript.
References
- Abate P, Pueta M, Spear NE, Molina JC. Fetal learning about ethanol and later ethanol responsiveness: Evidence against “safe” amounts of prenatal exposure. Experimental Biology and Medicine. 2008;233:139–154. doi: 10.3181/0703-MR-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts JR. Huddling by rat pups: ontogeny of individual and group behavior. Developmental Psychobiology. 2007;49:22–32. doi: 10.1002/dev.20190. [DOI] [PubMed] [Google Scholar]
- Alberts JR, May B. Nonnutritive, thermotactile induction of filial huddling in rat pups. Developmental Psychobiology. 1984;17:161–181. doi: 10.1002/dev.420170207. [DOI] [PubMed] [Google Scholar]
- Balogh RD, Porter RH. Olfactory preferences resulting from mere exposure in human neonates. Infant Behavior and Development. 1986;9:395–401. [Google Scholar]
- Bordner KA, Spear NE. Olfactory learning in the one-day old rat: Reinforcing effects of isoproterenol. Neurobiology of Learning and Memory. 2006;86:19–27. doi: 10.1016/j.nlm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Peck CA. Spontaneous recovery in cross-motivational transfer (counterconditioning) Animal Learning and Behavior. 1992;20:313–321. [Google Scholar]
- Bowlby J. Attachment. Basic Books; New York: 1965. [Google Scholar]
- Brumley MR, Robinson SR. Facial wiping in the rat fetus: variation of chemosensory stimulus parameters. Developmental Psychobiology. 2004;44:219–229. doi: 10.1002/dev.20005. [DOI] [PubMed] [Google Scholar]
- Cheslock SJ, Sanders SK, Spear NE. Learning during the newborn’s first meal: special resistance to retroactive interference. Developmental Science. 2004;7:581–598. doi: 10.1111/j.1467-7687.2004.00382.x. [DOI] [PubMed] [Google Scholar]
- Cheslock SJ, Varlinskaya EI, Petrov ES, Spear NE. Rapid and robust olfactory conditioning with milk before suckling experience: promotion of nipple attachment in the newborn rat. Behavioral Neuroscience. 2000;114:484–495. [PubMed] [Google Scholar]
- Chotro MG, Molina JC. Bradycardiac responses elicited by alcohol odor in rat neonates: influence of in utero experience with ethanol. Psychopharmacology (Berl) 1992;106(4):491–496. doi: 10.1007/BF02244820. [DOI] [PubMed] [Google Scholar]
- Coureaud G, Schaal B, Hudson R, Orgeur P, Coudert P. Transnatal olfactory continuity in the rabbit: behavioral evidence and short-term consequence of its disruption. Developmental Psychobiology. 2002;40:372–390. doi: 10.1002/dev.10038. [DOI] [PubMed] [Google Scholar]
- Eilam D, Smotherman WP. How the neonatal rat gets to the nipple: common motor modules and their involvement in the expression of early motor behavior. Developmental Psychobiology. 1998;32:57–66. doi: 10.1002/(sici)1098-2302(199801)32:1<57::aid-dev7>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Fillion TJ, Blass EM. Infantile experience with suckling odors determines adult sexual behavior in male rats. Science. 1986;231:729–731. doi: 10.1126/science.3945807. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Harlow MK. The affectional systems. In: Schrier A, Harlow HF, Stollnitz F, editors. Behavior of Nonhuman Primates. Vol. 2. Academic Press; New York: 1965. [Google Scholar]
- Hepper PG. Adaptive fetal learning: prenatal exposure to garlic affects postnatal preferences. Animal Behaviour. 1988;36:935–936. [Google Scholar]
- Herlenius E, Lagercrantz H. Neurotransmitters and neuromodulators during early human development. Early Human Development. 2001;65:21–37. doi: 10.1016/s0378-3782(01)00189-x. [DOI] [PubMed] [Google Scholar]
- Hofer MA, Shair H, Singh P. Evidence that maternal ventral skin substances promote suckling in infant rats. Physiology & Behavior. 1976;17:131–136. doi: 10.1016/0031-9384(76)90279-1. [DOI] [PubMed] [Google Scholar]
- Hudson R. Olfactory Imprinting. Current Opinion in Neurobiology. 1993;3:548–552. doi: 10.1016/0959-4388(93)90054-3. [DOI] [PubMed] [Google Scholar]
- Kraebel KS, Spear NE. Infant rats are more likely than adolescents to orient differentially to amodal (intensity-based) features of single-element and compound stimuli. Developmental Psychobiology. 2000;36:49–66. [PubMed] [Google Scholar]
- Leon M. Catecholaminergic contributions to early learning. Advances in Pharmacology. 1998;42:961–964. doi: 10.1016/s1054-3589(08)60907-2. [DOI] [PubMed] [Google Scholar]
- Lorenz K. The conception of instinctive behavior. In: Schiller CH, editor. Instinctive behavior. International Universities Press; New York: 1937. pp. 129–175. [Google Scholar]
- Mandairon N, Stack C, Kiselycznyk C, Linster C. Broad activation of the olfactory bulb produces long-lasting changes in odor perception. Proceedings of the National Academy of Sciences. 2006;103:13543–13548. doi: 10.1073/pnas.0602750103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SS. Unpublished master’s thesis. Binghamton University; Binghamton, NY: 2006. Nonassociative and associative learning in the neonatal rat and parallel changes in neurohormone and brain monoamine levels. [Google Scholar]
- Miller SS, Spear NE. Olfactory learning in the rat neonate soon after birth. Developmental Psychobiology. 2008;50:554–565. doi: 10.1002/dev.20318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SS, Spear NE. Mere odor exposure learning is more effective immediately after birth than 24 hours later. 2009 doi: 10.1002/dev.20456. Manuscript in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Wilson DA, Levine S, Sullivan RM. Dual circuitry for odor-shock conditioning during infancy: corticosterone switches between fear and attraction via amygdala. The Journal of Neuroscience. 2006;26:6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Schwarze D, Muller-Schwarze C. Olfactory imprinting in a precocial mammal. Nature. 1971;229:55–56. doi: 10.1038/229055a0. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health U.S. Government Printing Office; Washington, DC: Guide for the care and use of laboratory animals. 1986 DHEW Publication No. 86-23.
- Okutani F, Zhang J-J, Yagi F, Kaba H. Non-specific olfactory aversion induced by intrabulbar infusion of the GABAA receptor antagonist bicuculline in young rats. Neuroscience. 2002;112:901–906. doi: 10.1016/s0306-4522(02)00117-3. [DOI] [PubMed] [Google Scholar]
- Parsons PJ, Fagan T, Spear NE. Short-term retention of habituation in the rat: a developmental study from infancy to old age. Journal of Comparative and Physiological Psychology. 1973;84:545–553. doi: 10.1037/h0034889. [DOI] [PubMed] [Google Scholar]
- Pedersen PE, Blass EM. Prenatal and postnatal determinants of the 1st suckling episode in albino rats. Developmental Psychobiology. 1982;4:349–355. doi: 10.1002/dev.420150407. [DOI] [PubMed] [Google Scholar]
- Petrov ES, Varlinskaya EI, Spear NE. Self-administration of ethanol and saccharin in newborn rats: Effects on suckling plasticity. Behavioral Neuroscience. 2001;115:1318–1331. [PubMed] [Google Scholar]
- Pol HEH, Hijman R, Baaré WFC, van Ree JM. Effects of context on judgements of odor intensities in humans. Chemical Senses. 1998;23:131–135. doi: 10.1093/chemse/23.2.131. [DOI] [PubMed] [Google Scholar]
- Porter RH, Etscorn F. Olfactory imprinting resulting from brief exposure in Acomys cahirinus. Nature. 1974;250:732–733. doi: 10.1038/250732a0. [DOI] [PubMed] [Google Scholar]
- Romantshik O, Porter RH, Tillmann V, Varendi H. Preliminary evidence of a sensitive period for olfactory learning by human newborns. Acta Paediatrica. 2007;96:372–376. doi: 10.1111/j.1651-2227.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- Ronca AE, Alberts JR. Simulated uterine contractions facilitate fetal and newborn respiratory behavior in rats. Physiology & Behavior. 1995;58:1035–1041. doi: 10.1016/0031-9384(95)00155-c. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS. Olfaction mediates developmental transition in the altricial newborn of selected species of mammals. Developmental Psychobiology. 1983;16:347–375. doi: 10.1002/dev.420160502. [DOI] [PubMed] [Google Scholar]
- Schaal B, Hummel T, Soussignan R. Olfaction in the fetal and premature infant: functional status and clinical implications. Clinics in Perinatology. 2004;31:261–285. vi–vii. doi: 10.1016/j.clp.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Schaal B, Marlier L, Soussignan R. Olfactory function in the human fetus: evidence from selective neonatal responsiveness to the odor of amniotic fluid. Behavioral Neuroscience. 1998;12:1438–1449. doi: 10.1037//0735-7044.112.6.1438. [DOI] [PubMed] [Google Scholar]
- Sluckin W. Imprinting in guinea-pigs. Nature. 1968;220:1148. doi: 10.1038/2201148a0. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. The rat fetus in its environment: behavioral adjustments to novel, familiar, aversive, and conditioned stimuli presented in utero. Behavioral Neuroscience. 1985;99:521–530. doi: 10.1037//0735-7044.99.3.521. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. Habituation in the rat fetus. The Quarterly Journal of Experimental Psychology B Comparative and Physiological Psychology. 1992;44:215–230. doi: 10.1080/02724999208250613. [DOI] [PubMed] [Google Scholar]
- Spear LP, Specht SM, Kirstein CL, Kuhn CM. Anterior and posterior, but not cheek, intraoral cannulation procedures elevate serum corticosterone levels in neonatal rat pups. Developmental Psychobiology. 1989;22:401–411. doi: 10.1002/dev.420220407. [DOI] [PubMed] [Google Scholar]
- Spear NE. Forgetting as retrieval failure. In: Honig WK, James PHR, editors. Animal memory. Academic Press; New York: 1971. [Google Scholar]
- Spear NE, Hyatt L. How the timing of experience can affect the ontogeny of learning. In: Turkewitz G, Devenny D, editors. Developmental Time and Timing. Erlbaum; Hillsdale, NJ: 1993. [Google Scholar]
- Spear NE, McKinzie DL. Intersensory integration in the infant rat. In: Lewkowicz DJ, Lickliter R, editors. The Development of Intersensory Perception: Comparative Perspectives. Erlbaum; Hillsdale, NJ: 1995. pp. 133–161. [Google Scholar]
- Spear NE, Molina JC. The role of sensory modality in the ontogeny of stimulus selection. In: Krasnegor NA, Blass EM, Hofer MA, Smotherman WP, editors. Perinatal development: A psychobiological perspective. Academic Press; New York: 1987. [Google Scholar]
- Spear NE, Molina JC. Fetal or infantile exposure to ethanol promotes ethanol ingestion in adolescence and adulthood: a theoretical review. Alcoholism: Clinical & Experimental Research. 2005;29:909–929. doi: 10.1097/01.alc.0000171046.78556.66. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, McGaugh J, Leon M. Norepinephrine induced plasticity and one-trial olfactory learning in neonatal rats. Developmental Brain Research. 1991;60:219–228. doi: 10.1016/0165-3806(91)90050-s. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Taborsky-Barba S, Mendoza R, Itano A, Leon M, Cotman CW, et al. Olfactory classical conditioning in neonates. Pediatrics. 1991;87:511–518. [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Toubas P. Clinical usefulness of maternal odor in newborns: soothing and feeding preparatory responses. Biology of the Neonate. 1998;74:402–408. doi: 10.1159/000014061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA. The locus coeruleus, norepinephrine, and memory in newborns. Brain Research Bulletin. 1994;35:467–472. doi: 10.1016/0361-9230(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Blass EM. First suckling response of the newborn albino rat: the roles of olfaction and amniotic fluid. Science. 1977;198:635–636. doi: 10.1126/science.918660. [DOI] [PubMed] [Google Scholar]
- Thelen E. Kicking, rocking, and waving: contextual analysis of rhythmical stereotypies in normal human infants. Animal Behavior. 1981;29:3–11. doi: 10.1016/s0003-3472(81)80146-7. [DOI] [PubMed] [Google Scholar]
- Turkewitz G, Mellon RC. Dynamic organization of intersensory function. Canadian Journal of Psychology. 1989;43:286–301. doi: 10.1037/h0084214. [DOI] [PubMed] [Google Scholar]
- Varendi H, Porter RH, Winberg J. The effect of labor on olfactory exposure learning within the first postnatal hour. Behavioral Neuroscience. 2002;116:206–211. doi: 10.1037//0735-7044.116.2.206. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Stevenson RJ. Learning to smell: Olfactory perception from neurobiology to behavior. The Johns Hopkins University Press; Baltimore, MD: 2006. The relationship between stimulus intensity and perceptual quality. [Google Scholar]
- Wilson DA, Sullivan RM, Leon M. Odor familiarity alters mitral cell response in the olfactory bulb of neonatal rats. Brain Research. 1985;354:314–317. doi: 10.1016/0165-3806(85)90186-5. [DOI] [PubMed] [Google Scholar]
- Winberg J, Porter RH. Olfaction and human neonatal behaviour: clinical implications. Acta Paediatrica. 1998;87:6–10. doi: 10.1080/08035259850157787. [DOI] [PubMed] [Google Scholar]
- Yokoi M, Mori K, Nakanishi S. Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proceedings of the National Academy of Sciences. 1995;92:3371–3375. doi: 10.1073/pnas.92.8.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajonc RB. Attitudinal effects of mere exposures. Journal of Personality and Social Psychology. 1968;9:1–27. [Google Scholar]
- Zajonc RB. Mere Exposure: A Gateway to the Subliminal. Current Directions in Psychological Science. 2001;10:224–228. [Google Scholar]