Abstract
Naturally occurring regulatory T cells (nTregs; CD4+CD25+Foxp3+) are capable of suppressing the chronic inflammation observed in a variety of different animal models of autoimmune and chronic inflammatory diseases such as inflammatory bowel diseases, diabetes, and arthritis. A major limitation in exploring how and where nTregs exert their suppression in vivo is the relative paucity of these regulatory cells. Although several laboratories have described different methods to expand flow-purified nTregs or convert conventional/naïve T cells (CD4+Foxp3−) to Foxp3-expressing “induced” Tregs (iTregs; CD4+Foxp3+) ex vivo, we have found that many of these approaches are encumbered with their own limitations. Therefore, we sought to develop a relatively simple ex vivo method to generate large numbers of Foxp3-expressing iTregs that can be used to evaluate their trafficking properties, suppressive activity, and therapeutic efficacy in a mouse model of chronic gut inflammation in vivo. We present a detailed protocol demonstrating that polyclonal activation of conventional CD4+ T cells in the presence of IL-2, TGFβ, and all trans retinoic acid induces >90% conversion of these T cells to Foxp3-expressing iTregs as well as promotes a three- to fourfold increase in proliferation following a 4-day incubation period in vitro. This protocol enhances modestly the surface expression of the gut-homing adhesion molecule CCR9 but not α4β7. Furthermore, we provide preliminary data demonstrating that these iTregs are significantly more potent at suppressing T-cell activation in vitro and are equally effective as freshly isolated nTregs at attenuating chronic colitis in vivo. Finally, we report that this protocol has the potential to generate 30–40 million iTregs from one healthy mouse spleen.
Keywords: iTregs, TGFβ, Retinoic acid, Colitis, Inflammatory bowel disease
1. Introduction
Naturally occurring regulatory T cells (nTregs; CD4+CD25+Foxp3+) are known to suppress a wide range of immune responses via several different mechanisms including production of regulatory cytokines, competition for essential cytokines, and contact-dependent mechanisms (1–4). Because nTregs have been shown to suppress Th1 and Th17 autoimmune responses in vivo, a great deal of interest has been generated regarding the possible use of these cells to treat patients with chronic inflammatory disorders such as the inflammatory bowel diseases (IBD; Crohn’s disease, ulcerative colitis), diabetes, arthritis, and graft vs. host disease. A major limitation in defining how and where nTregs exert their suppression in vivo is the relative paucity of these regulatory cells as they constitute <10% of the peripheral CD4+ T-cell population in mice and only 1–2% of CD4+ T cells in humans. In an attempt to over-come this significant limitation, several laboratories have described different methods to expand flow-purified nTregs (5–8) or convert conventional/naïve T cells (CD4+Foxp3−) to Foxp3-expressing “induced” Tregs (iTregs; CD4+Foxp3+) ex vivo (9–13). However, we have found that many of these approaches are encumbered with their own limitations including loss of Foxp3 expression during expansion of flow-purified nTregs or lack of robust Foxp3 expression and/or proliferation during conversion of conventional T cells to iTregs. Because of these shortcomings, we developed a relatively simple ex vivo method to generate large numbers of Foxp3-expressing iTregs that can be used to evaluate their trafficking properties, suppressive activity, and therapeutic efficacy in a mouse model of chronic gut inflammation in vivo. We present a detailed protocol demonstrating that polyclonal activation of conventional CD4+ T cells in the presence of IL-2, TGFβ, and all trans retinoic acid induces >90% conversion of these T cells to Foxp3-expressing iTregs as well as promotes a 3-4-fold increase in proliferation following a 4-day incubation period in vitro. In addition, this protocol enhances modestly the surface expression of the gut-homing adhesion molecule CCR9 but not α4β7. Furthermore, we provide preliminary data demonstrating that these iTregs are significantly more potent at suppressing T-cell activation in vitro and are equally effective as nTregs at attenuating chronic colitis in vivo. Finally, we report that this protocol has the potential to generate 30–40 million iTregs from one healthy mouse spleen.
2. Materials
2.1. Animals
C57Bl/6 wild-type (WT) and recombinase activating gene-1-deficient (RAG-1−/−) mice were obtained from the Jackson Laboratory (Bar Harbor, Maine), whereas Foxp3GFP “knockin” mice were obtained from the LSUHSC breeding facility (originally obtained from Dr. Alexander Rudensky, University of Washington). All mice were housed under specific pathogen-free conditions in the LSUHSC-Shreveport animal care facility.
2.2. Tissue Culture Plastic Ware and Reagents
Costar® 24-Well Clear TC-Treated Microplates, Sterile (Corning).
Mouse CD3e-monoclonal antibody (mAb) (eBioscience).
Phosphate-buffered saline (PBS) pH 7.4.
2.3. Splenocyteand and CD4 + T-Cell Preparation
PBS with 4% fetal bovine serum (PBS/FBS).
Red blood cells lysis buffer (RBC-LB): 0.14 M NH4Cl and 0.0165 M Tris base in water with pH adjusted to 7.1–7.2.
Trypan Blue solution: 0.4% solution.
Dynal® Mouse CD4 Cell Negative Isolation Kit (Invitrogen).
Dynal buffer: 1× PBS with 0.1% bovine serum albumin (BSA) and 2 mM EDTA.
Fetal bovine serum.
2.4. T-Cell Conversion
RPMI-10 Complete medium: RPMI-1640 (Sigma) supplemented with l-glutamine, antibiotic/antimycotic solution, 50 μM β-mercaptoethanol, and 10% FBS.
Recombinant Human TGF-β1 (2 μg; R&D Systems) is first dissolved in 40 μl of a 4 mM HCl solution to which 360 μl of 0.1% BSA in PBS is then added to yield a 5 μg/ml stock solution. Aliquots of this solution are stored at −80°C (see Note 1).
Recombinant Human IL-2 (Chiron) is dissolved in distilled water to given a final concentration of 18 × 106 U/ml. This stock solution is kept at 4°C.
A 10 mM stock solution of all trans Retinoic acid (Acros) is made by dissolving in dimethyl sulfoxide (DMSO) and stored in small aliquots at −80°C. Subsequent dilutions can be made using RPMI-10 as described later.
2.5. Flow-Cytometric Analysis
Allophycocyanin (APC)-conjugated CD4 antibody (clone GK1.5), Phycoerythrin (PE)-conjugated Foxp3 antibody (clone FJK-16s), and Foxp3 staining buffer set (all from eBioscience).
3. Methods
The overall objective of the protocol described below is to generate large numbers of iTregs from a mouse splenocyte preparation in a relatively short period of time using common immunological methods and laboratory instrumentation. If performed carefully, this method does not require the use of fluorescence-activated cell sorting (FACS) and can be accomplished using a variety of different genetically engineered mutant mouse T cells. Indeed, we describe the use of genetically engineered Foxp3GFP knockin mice (14) in which expression of the green fluorescent protein (GFP) is driven by the Foxp3 promoter, thereby providing a “nonlethal” method to quantify (by flow cytometry) Foxp3 expression within T cells without permeabilizing/killing the lymphocytes. We compare conversion of these Foxp3GFP T cells with that obtained using T cells obtained from WT mice or mice deficient in specific selectins and/or integrins. Finally, we present a detailed protocol for assessing the suppressive activity of the iTregs in vitro and in a model of chronic gut inflammation in vivo.
3.1. Preparation of CD3 mAb-Coated, 24-Well Tissue Culture Plates
A 10 μg/ml solution of CD3 mAb is prepared by adding 120 μl of the stock CD3-antibody (1 mg/ml) into 12 ml of cold sterile PBS, which is then mixed by vortex (see Note 2).
500 μl aliquots of the 10 μg/ml CD3 mAb solution is pipetted into each well of the 24 wells.
The plate is sealed with Parafilm to prevent evaporation and stored overnight in refrigerator at 4°C (The CD3 mAb solution will be removed in step 4, Subheading 3.4).
3.2. Preparation of a Single Cell Suspension from Mouse Spleens
This section describes the preparation of splenocytes from either Foxp3GFP or WT spleens and is applicable for most mouse strains and phenotypes. All steps outlined in this section should be performed under sterile conditions using sterile reagents and plasticware.
Each spleen is aseptically dissected from the mouse (using sterile instruments) and placed in a small Petri dish containing 10 ml of ice-cold PBS/FBS (see Note 3).
Using the frosted sides of two microscope slides, press against the spleen and grind it into a homogenous pulp in the Petri dish.
Using a 10-ml syringe without the needle, aspirate the cell suspension, attach a 26-G needle, and expel the contents of the syringe through a 70-μm cell strainer and into a 15-ml sterile conical centrifuge tube.
Rinse the Petri dish with an additional 5 ml of PBS/FBS, aspirate the cell suspension into the 10-ml syringe, and repeat step 3 combining the contents into the same sterile 15-ml conical centrifuge tube from above.
Centrifuge the cells for 10 min at 400 × g in a cooled centrifuge (4°–8°C). Aspirate and discard most of the supernatant leaving approximately 500 μl of residual fluid to resuspend the cell pellet. Using a 1-ml micropipettor, gently resuspend the pellet in the residual fluid and measure this volume.
Remove 20 μl of the cell suspension and add it to a 500-μl microcentrifuge tube containing 60 μl of RBC-LB. Allow the cells to incubate at room temperature in the RBC-LB for 3 min to insure complete lysis of erythrocytes.
After the 3-min incubation in RBC-LB, remove 10 μl of the cell solution and add it to new microcentrifuge tube containing 90 μl of 0.4% trypan blue. Gently finger tap the tube to mix the cells (do not vortex) and remove 10 μl to count on a hemocytometer.
3.3. CD4 + T-Cell Enrichment Using Negative Selection
This section describes the steps required to enrich the splenocyte preparation for CD4+ T cells. Although the protocol described here was performed using the Dynal® Mouse CD4 Cell Negative Isolation Kit (Invitrogen), any negative selection protocol for enriching CD4+ T cells from a splenocyte preparation may be used.
Splenocyte concentration is adjusted to 1 × 107 cells per 100 μl using Dynal buffer (see Note 4).
For every 100 μl of cell suspension, 20 μl of FBS and 20 μl of antibody mix from the Invitrogen kit are added to the tube. The cell suspension is incubated at 4°C for 20 min on a gently rocking platform.
Following the incubation period, the tubes are filled with Dynal buffer, gently mixed by inverting the tubes and centrifuged at 400 × g for 10 min at 4°C. The supernatant is aspirated and discarded.
For every 1 × 107 cells present in the cell pellet, 800 μl of Dynal buffer and 200 μl of prewashed Dynabeads are added, and the cells are resuspended (instructions for washing beads can be found in the kit instructions) (see Note 5). The cell and bead suspension is then incubated at room temperature with gentle rocking for 15 min.
Following this 15-min incubation period, the cell and bead mixture is gently resuspended five times using a 1-ml micropi-pettor and an additional 1 ml of Dynal buffer for every 1 × 107 cells is added to the tube. The tube is then placed against the magnet and separation is allowed to proceed for 3 min at room temperature according the companies protocol. This step removes B-cells, CD8+ T cells, macrophages, PMNs, erythrocytes, monocytes, and dendritic cells by promoting the magnetic binding of these cells to wall of the tube leaving an enriched population of CD4+ T cells in the fluid phase.
The fluid from the tube is removed being careful not to disturb the beads bound to the sides of the tube by the magnet and then transferred into a new sterile 15-ml plastic conical tube. The tube is filled with Dynal buffer and centrifuged at 400 × g for 10 min at 4°C. The supernatant is aspirated and discarded. The pellet is resuspended in PBS/FCS. This fraction contains a highly enriched population of CD4+ T cells that will be used for Treg conversion. A small aliquot of these cells should be removed and analyzed for CD4-purity using flow cytometry. We routinely achieve CD4+ T-cell enrichment of 85–92% using the Dynal negative selection kit.
3.4. Conversion of CD4+ Foxp3− T Cells to iTregs
The protocol outlined in this section describes the methods required to convert 12 million negatively selected CD4+ T cells to iTregs in one 24-well tissue culture plate. We have found that negatively selected CD4+ T cells are routinely >85–92% conventional T cells (CD4+Foxp3− T cells) and will generate >90% CD4+Foxp3+ iTregs using the conversion protocol described below. If greater purity is required for, FACS may be used to generate iTreg purity of >98%.
In order to minimize well-to-well variability during the plating of large numbers of cells into multiple wells in a 24-well plate, we first prepare a 24-ml cell suspension in RPMI-10 containing 12 million CD4+ T cells, 135 U/ml of human recombinant IL-2, 20 ng/ml TGFβ, and 1 nM (1 pmol/ml) all trans retinoic acid (RA).
To do this, we add small aliquots (<100 μl) of concentrated stock solutions of IL-2, TGFβ and RA to approximately 23 ml of RPMI-10 in the absence of T cells. For example, we add 12 μl of an IL-2 stock solution (270,000 U/ml in RPMI-10), 96 μl of the TGFβ stock solution (5 μg/ml in RPMI-10), and 12 μl of the RA stock solution (2 μM in RPMI-10) to 22.88 ml of RPMI-10. The tube is gently mixed by rocking 2–3 times.
One ml of a CD4+ T-cell suspension containing 12 million cells per ml in RPMI-10 is then added to the 23-ml solution containing IL-2, TGFβ, and RA to give a final volume of 24 ml at a cell concentration of 0.5 × 106/ml. The tube is gently mixed by rocking and placed on ice until ready to plate.
The CD3 mAb precoated plate is removed from the refrigerator that was prepared the day before, and the antibody solution is removed from each of the wells by aspiration. The wells are then washed three times with 500 μl sterile PBS (room temperature) to remove excess antibody. After the last wash, be sure to thoroughly remove excess PBS.
Add 1 ml of the cell suspensions to each of the 24 wells. Incubate at 37°C with 5% CO2 for 4 days.
3.5. Flow Cytometry
To ensure that conversion has been successful, it is best to always remove a small portion of the cells to perform analysis using flow cytometry. For cells obtained from Foxp3GFP mice, analysis can be performed by first staining cells with a mAb to CD4 tagged with APC and then analyze for CD4+GFP+ cells. For cells obtained from WT mice, the cells must be permeabilized according to instructions with the Foxp3 antibody and the Foxp3 staining buffer. In brief, cells are stained with a mAb to CD4 tagged with APC and then permeabilized and stained with a mAb to Foxp3 tagged with PE, or an isotype control. For measurement of CCR9 and lymphocyte Peyer patch adhesion molecule (LPAM; α4β7), standard surface staining is performed using mAbs specific for these cell-associated adhesion molecules. Briefly, cells are labeled with PE-conjugated CCR9-mAb (clone XW-1.2; eBioscience) and APC-conjugated LPAM-1 antibody (clone DATK32; eBio-science) or their respectively isotype control Abs. Surface expression was determined using flow cytometry.
3.6. Anticipated Results
3.6.1. Importance of IL-2, TGF-β, and Retinoic Acid for Foxp3 Expression
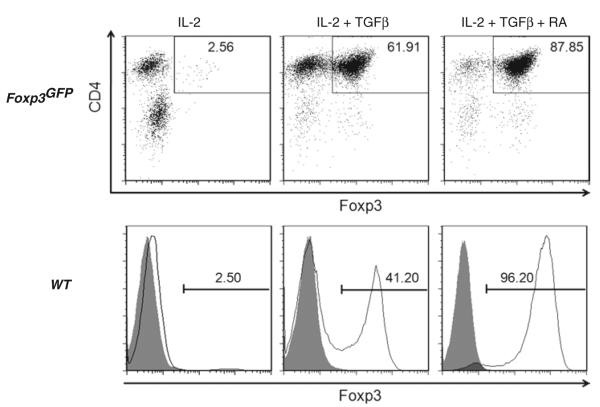
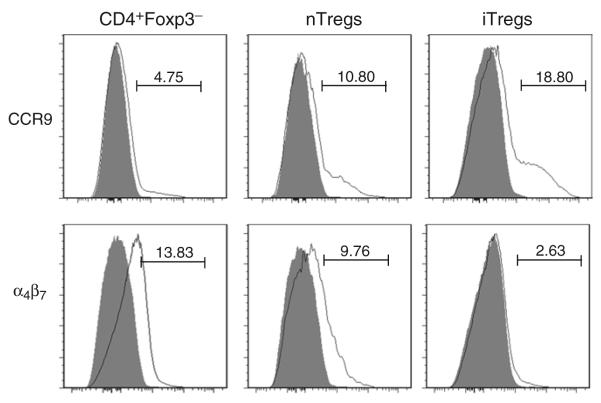
IL-2 and TGF-β have both been shown to be required for the conversion of conventional/naïve CD4+Foxp3− cells to iTregs ex vivo (9, 10, 15). In addition, retinoic acid (RA) has been shown enhance the conversion of naïve cells into iTregs (10, 12, 13, 16, 17). Therefore, we wished to ascertain how these different cytokines and RA affected conversion of negatively selected CD4+ T cells to iTregs in our standard protocol. Converted CD4+ T cells from Foxp3GFP mice have the advantage that they can then be analyzed by flow cytometry for the presence of Foxp3 (i.e., GFP) without the need for permeabilization. Converted WT cells must be permeabilized before staining with the Foxp3 mAb, since Foxp3 is an intracellular transcription factor. The permeabilization process can be performed in as little as 30 min, but results are not different if the cells are kept in the fixation/permeabilization solution for up to 18 h. The staining for Foxp3 with PE-conjugated antibody and the isotype control is then carried out for 30 min in the dark. The CD4+Foxp3+ T cells represent the iTreg population. In agreement with others, we found that addition of TGF-β (and RA) is required for maximal generation of iTregs whether the CD4+ were obtained from Foxp3GFP and WT animals (Fig. 1). Addition of TGF-β and IL-2 but omission of RA induced Foxp3 expression in 62 and 41% of CD4+ T cells from Foxp3GFP and WT mice, respectively (Fig. 1). Addition of RA to cells containing both IL-2 and TGFβ induced maximal expression of Foxp3 in agreement with other investigators (10, 12, 17).The reason for enhanced expression of Foxp3 by addition of exogenous RA is not known with certainty but may be due its inhibitory effect on the contaminating CD4+CD44hi T-cell population that counterregulates the expression of the Treg transcription factor (17) and/or by directly promoting TGFβ-mediated Foxp3 expression upon activation of naïve CD4+Foxp3− T cells (18). In agreement with other studies using similar, but not identical, culture conditions (9–13), we find that activation of CD4+ T cells in the presence of IL-2, TGFβ and RA induces modestly the expression of the gut-homing adhesion molecule CCR9 (Fig. 2). Surprisingly and in contrast to other studies (9–13), we do not observe enhanced expression of the gut-homing integrin LPAM (α4β7) (Fig. 2). The reason for these differences with other conversion studies is not apparent; however, it may be that α4β7 expression is dependent upon the presence of secondary signals provided by CD28 mAb or antigen-presenting cells that were used in previous studies (9–13). We omit CD28 mAb from our protocol because we find that it appears to enhance activation-induced cell death and reduces overall T-cell proliferation (data not shown). Despite the absence of α4β7 expression, our ex vivo-generated iTregs are very effective at suppressing chronic colitis in vivo suggesting that this “gut-homing” integrin is not required for iTreg trafficking to the colon and suppression of disease(see below).
Fig. 1.
Conversion of negatively-selected CD4+ T cells to Foxp3-expressing iTregs ex vivo. The top row illustrates Foxp3 expression in CD4+ T cells obtained from Foxp3GFP “knockin” mice that were activated with plate-bound CD3 mAb in the presence of IL-2 or the combinations of IL-2 and TGFβ or IL-2, TGFβ and all trans retinoic acid (RA) for 4 days at 37°C. The bottom row illustrates Foxp3 expression in wild type (WT) CD4+ T cells incubated in CD3-coated wells containing the same combinations of cytokines and RA for 4 days at 37°C. WT lymphocytes were gated on CD4+ T cells and then analyzed for Foxp3 expression. The shaded curves represent isotype control Ab staining.
Fig. 2.
Expression of CCR9 and α4β7 on naïve T cells, nTregs, and iTregs. Negatively selected CD4+ T cells from Foxp3GFP mice were sorted for naïve (CD4+GFP−) T cells and nTregs (CD4+GFP+ T cells) whereas iTregs (CD4+Foxp3+) were generated from CD4+GFP− T cells as described in Fig. 1. Cells were gated on CD4+ T cells and surface expression of CCR9 and α4β7 was quantified by flow cytometry. The shaded curves represent isotype control Ab staining.
3.6.2. Time-Dependent Expression of Foxp3 and Cell Proliferation
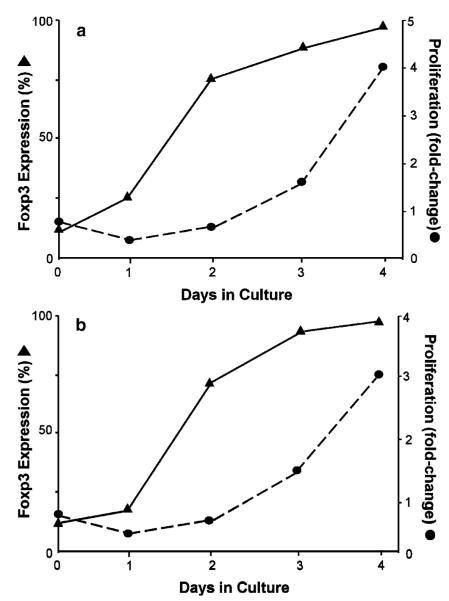
In order to determine the optimal incubation time for conversion of naïve T cells to iTregs and for maximal proliferation, CD4+ T cells obtained from Foxp3GFP or WT mice were removed at different times, washed, counted, resuspended in PBS/FCS, and stained for CD4 by labeling the cells with APC-tagged mAb for 15 min in the dark. We find that Foxp3 expression precedes T-cell proliferation in a time-dependent manner such that >90% of the viable CD4+ T cells express Foxp3 by day 4 (Fig. 3). Indeed, we observe an initial decrease in cell number lasting from 24–48 h. This decrease is then followed by a time-dependent increase in T-cell proliferation such that maximal proliferation is observed at day 4 reaching a three- to fourfold expansion from what was originally plated (Fig. 3).This conversion protocol produces very similar results using negatively selected CD4+ T cells obtained from Fopx3GFP or WT mice (Fig. 3) (see Note 6).
Fig. 3.
Time-dependent induction and expansion of Foxp3-expressing iTregs ex vivo. Foxp3 expression and proliferation were quantified at different times in vitro for CD4+Tcells obtained from Foxp3GFP “knockin” mice (a) or WT mice (b) activated with plate-bound CD3 mAb in the presence of IL-2, TGFβ, and RA. Proliferation was expressed as fold change from the original cell number.
3.6.3. In Vitro Suppression Assay
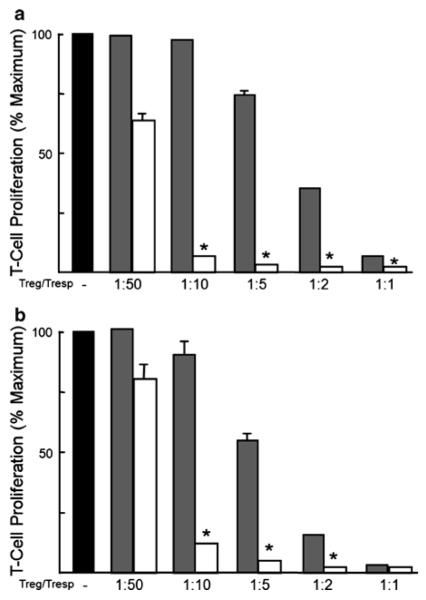
To compare the suppressive activity of iTregs with freshly isolated nTregs, we performed a standard in vitro suppression assay with minor modifications (19). Briefly, freshly isolated, negatively selected CD4+ T cells from Foxp3GFP were sorted for nTregs (CD4+GFP+ T cells) and CD4+GFP− (responder) T cells. Varying numbers of CD4+GFP+ T cells (nTregs) or iTregs (from Foxp3GFP mice) were cultured with 5 × 104 responder CD4+GFP− T cells, 105 irradiated antigen-presenting cells, and 1 μg/ml CD3 Ab. Antigen-presenting cells were prepared by irradiating (2,000 rad) splenocytes. The cells were cultured for 96 h, with the addition of 1 μCi/well of tritiated (3H)-thymidine for the final 24 h. These same suppression assays were also performed with flow-purified WT nTregs (CD4+CD25+ T cells) or iTregs in the presence of WT responder (CD4+CD25−) T cells, irradiated WT splenocytes, and CD3 Ab. Figure 4 demonstrates that iTregs generated from Foxp3GFP or WT T cells were significantly more suppressive than freshly nTregs.
Fig. 4.
Suppressive activity of freshly isolated nTregs or ex vivo-generated iTregs. (a) Freshly isolated, negatively selected CD4+ T cells from Foxp3GFP mice were sorted for nTregs (CD4+GFP+ T cells) and naïve (CD4+GFP−) T cells. Varying numbers of CD4+GFP+ T cells (nTregs) or ex vivo-generated iTregs (from Foxp3GFP mice) were cultured with 5 × 104 naïve (responder) CD4+GFP− T cells, 105 irradiated splenocytes (from Foxp3GFP mice), and 1 μg/ml CD3 mAb. The cells were incubated for 96 h at 37°C with 1 μCi/well of 3H-thymidine added during the final 24 h. (b) This same suppression assay was performed with flow-purified WT nTregs (CD4+CD25+ T cells) or ex vivo-generated iTregs in the presence of WT responder cells (CD4+CD25− T cells), irradiated WT splenocytes and CD3 mAb. Data represent the mean ± SEM for triplicate samples performed at least two different times. Black bars represent T-cell proliferation in the absence of Tregs; shaded bars represent T-cell proliferation in the presence of nTregs; and white bars represent T-cell proliferation in the presence of iTregs. *P < 0.05 compared to proliferation in the absence of Tregs.
3.6.4. Foxp3 Expression in CD4+ T Cells Deficient in L-Selectin, β7 Integrin, or Both
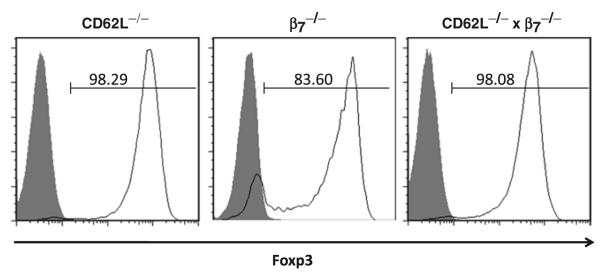
Because our laboratory is interested in T-cell trafficking, we wished to determine whether our conversion protocol could be used to convert CD4+ T cells obtained from mice deficient in different selectins and/or integrins. Therefore, we enriched for CD4+ T cells by negative selection of splenocytes obtained from C576Bl/6 mice that were deficient in L-selectin (CD62L−/−), β7 integrin (β7−/−), or both (CD62L−/− × β7−/−) and subjected these cells to our conversion protocol. We found, using our standard 4-day incubation period, that all three mutant T-cell populations converted to the iTreg phenotype were quantitatively similar to WT CD4+ T cells (Fig. 5). These data demonstrate the utility of this protocol for producing iTregs from several different mutant CD4+ T-cell populations.
Fig. 5.
Foxp3 expression in CD4+ T cells obtained from mice deficient in l-Selectin, β7 integrin, or both. Negatively selected CD4+ T cells obtained from mice deficient in l-Selectin (CD62L−/−), β7 integrin (β7−/−), or both (CD62L−/− × β7−/−) were plate into CD3 mAb-coated wells containing IL-2, TGFβ, and RA for 4 days at 37°C. Cells were permeabilized and stained for CD4 and Foxp3. Cells were gated on CD4+ T cells and Foxp3 expression was determined by flow cytometry. The shaded curves represent isotype control Ab staining.
3.6.5. Suppressive Activity of iTregs in a Mouse Model of Chronic Gut Inflammation
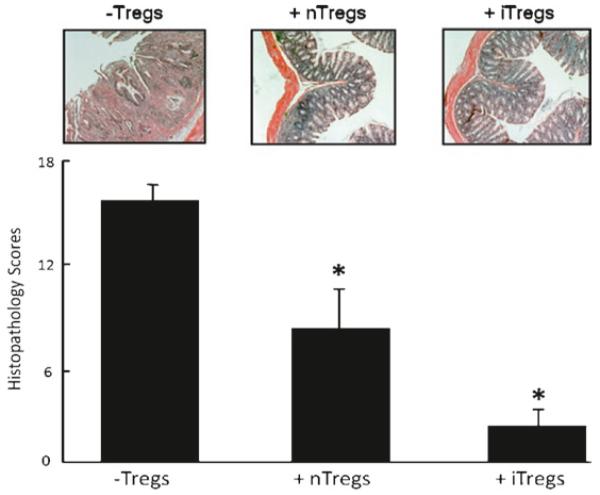
Having established the feasibility of producing large numbers of iTregs with potent suppressive activity in vitro, we next wished to assess the therapeutic efficacy of these ex vivo-generated iTregs in suppressing the development of chronic colitis in mice in vivo. To do this, we utilized the well-characterized T-cell transfer model of chronic colonic inflammation in mice (20). Although a detailed description of this model is beyond the scope of the current chapter, we refer the reader to the recently published protocol for inducing chronic colitis using this model (20). Briefly, chronic colitis is induced in lymphopenic recombinase activating gene-1 deficient mice (RAG−/−) by adoptive transfer of 0.5 × 106 WT CD4+CD45RBhigh(naïve) T cells. Chronic colitis develops within 6–8 weeks post T-cell transfer. To compare the suppressive activity of cultured iTregs with freshly isolated nTregs (CD4+CD25+ T cells) in this model of inflammatory bowel disease, we injected (i.p.) 1 × 105 iTregs or nTregs into RAG−/− recipients 10 days following the adoptive transfer of the disease-producing CD4+CD45RBhigh T cells. At 8-week post-transfer of naïve T cells, mice were euthanized, their colons removed and cleansed of fecal material, and fixed in PBS formalin. Colons were then embedded in paraffin, sectioned, and stained with H&E and scored by a pathologist unfamiliar with the treatment groups. Figure 6 demonstrates that iTregs are as effective as freshly isolated nTregs in suppressing the development of chronic colitis in this model of IBD.
Fig. 6.
Suppressive activity of freshly isolated nTregs or ex vivo-generated iTregs in a mouse model of chronic colitis. Chronic colitis was induced in recombinase activating gene-1 deficient (RAG−/−) mice by adoptive transfer of 5 × 105 WT CD4+CD45RBhigh(naïve) T cells. Freshly isolated nTregs (1 × 105 cells; CD4+CD25+ T cells) or ex vivo-generated iTregs (1 × 105 cells) from WT mice were injected (i.p.) into RAG−/− recipients 10 days following the adoptive transfer of the disease-producing CD4+CD45RBhigh T cells. At 8-week posttransfer of naïve T cells, mice were euthanized and their colons removed for blinded histopathological evaluation.
4. Conclusions
We describe a detailed protocol for generating large numbers of iTregs from a mouse splenocyte preparation in a relatively short period of time using common immunological methods and laboratory instrumentation. This method does not require cell sorting for most studies and is therefore less expensive and time-consuming than some protocols described elsewhere. Indeed, we have found no obvious advantage of using flow-purified CD4+GFP− or CD4+CD25− T cells for conversion to iTregs using our protocol (data not shown).
Conversion of negatively selected CD4+ T cells to iTregs can be performed with cells obtained from different populations of C57Bl/6 mice including WT and Foxp3GFP mice as well as mice with genetic ablation of different T-cell-associated selectins and/or integrins.
This conversion protocol induces modestly the expression of the gut-homing chemokine receptor CCR9 but not α4β7.
Ex vivo-generated iTregs possess potent suppressive activity that is equal to or exceeds that of freshly isolated nTregs in in vitro models of T-cell activation as well as in a mouse model chronic gut inflammation.
Data obtained using this protocol predict that one healthy mouse spleen has the potential to generate between 21 and 36 million iTregs following a 4-day incubation period.
5. Notes
When working with TGF-β, it should be kept in mind that repeated freeze–thaw cycles should be avoided for this protein. Therefore, we recommend making single-use aliquots of TGF-β.
This can be scaled down if the number of iTregs required is much less than will be generated with a conversion in a full 24-well plate.
Prior to dissection, the mouse abdomen should be wiped 2–3 times with 70% ethanol.
Up to 50 million cells can be processed in a single 15-ml sterile conical centrifuge tube, and up to 200 million cells can be processed in a single 50-ml conical tube.
When resuspending the cell pellet in the Dynal buffer, it is important to use only part of the final volume required to resuspend the pellet to achieve optimal resuspension. Once the pellet has been uniformly resuspended, the remainder of the Dynal buffer, followed by the beads, is added to the tube.
We have observed that culturing cells for longer periods of time and/or adding CD28 mAb does not increase iTreg yield and may actually induce cell death such that proliferative activity decreases. Therefore, all experiments are performed in the absence of CD28 mAb with a 4-day incubation period unless otherwise noted.
References
- 1.Sakaguchi S, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 2.Scheffold A, Murphy KM, Hofer T. Competition for cytokines: T(reg) cells take all. Nat. Immunol. 2007;8:1285–1287. doi: 10.1038/ni1207-1285. [DOI] [PubMed] [Google Scholar]
- 3.Uhlig HH, et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J. Immunol. 2006;177:5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat. Rev. Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Earle KE, et al. In vitro expanded human CD4+CD25+ regulatory T cells suppress effector T cell proliferation. Clin. Immunol. 2005;115:3–9. doi: 10.1016/j.clim.2005.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang Q, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J. Exp. Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 8.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 9.Chen W, et al. Conversion of peripheral CD4+ J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fantini MC, Dominitzki S, Rizzo A, Neurath MF, Becker C. In vitro generation of CD4+ CD25+ regulatory cells from murine naive T cells. Nat. Protoc. 2007;2:1789–1794. doi: 10.1038/nprot.2007.258. [DOI] [PubMed] [Google Scholar]
- 12.Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J. Immunol. 2007;179:3724–3733. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- 13.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 14.Fontenot JD, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Zheng SG, Wang JH, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4(+)CD25(−) cells to CD25(+)Foxp3(+) regulatory T cells and for expansion of these cells. J. Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 16.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill JA, et al. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mucida D, et al. Retinoic acid can directly promote TGF-beta-mediated Foxp3(+) Treg cell conversion of naive T cells. Immunity. 2009;30:471–472. doi: 10.1016/j.immuni.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostanin DV, et al. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G135–G146. doi: 10.1152/ajpgi.90462.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]