Abstract
A 22-year-old woman presented with generalised peritonitis from a gastric perforation due to erosion by an intragastric balloon inserted abroad 22 months previously in an attempt to help her lose weight. These balloons are of uncertain long-term benefit in obesity and should be removed after 6 months to avoid complications. This did not happen in this case; thus, leading to this life-threatening complication, which was treated with the removal of the balloon and omental patch repair of the perforation.
Background
Obesity is a major problem and intragastric balloon is a commonly performed procedure in Europe. In our case, it caused a life-threatening complication in a young woman that was avoidable. It is a highly unusual cause of peritonitis. The manufacturer's recommendation was ignored with regards to removal at the appropriate time and this highlights the dangers of no follow-up with procedures performed overseas.
Case presentation
A 22-year-old woman presented with a 1-day history of generalised abdominal pain with anorexia and nausea. She was febrile and tachycardic. Examination confirmed generalised peritonitis. She had a raised C reactive protein and white cell count with a neutrophilia of 37.8. Her body mass index was 38.3. She gave a history having had an intragastric balloon inserted in Estonia 22 months previously to help her lose weight and was apparently told that it would be safe to leave it in situ for 3 years.
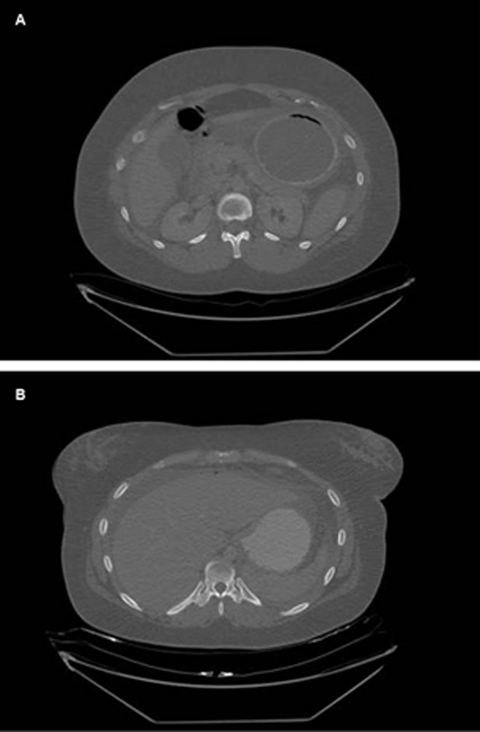
A CT scan showed the inflated balloon in the stomach but also revealed free air and free fluid in the peritoneal cavity suggestive of visceral perforation (figure 1). A diagnostic laparoscopy confirmed large amounts of intraperitoneal bilious fluid but the site of perforation was not obvious due to adhesions. A laparotomy revealed that the gastric balloon has eroded through the posterior wall of the stomach causing a perforation (figure 2). The gastric balloon was punctured and extracted via the perforation and the perforation repaired using a patch of omentum. Postoperatively the patient had to have an intra-abdominal collection drained percutaneously under CT guidance but otherwise made an uneventful recovery.
Figure 1.
CT abdomen (A) showing intragastric balloon within the stomach. (B) Showing intraperitoneal free air and free fluid.
Figure 2.
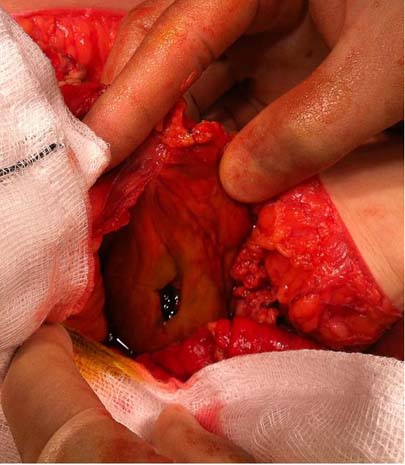
Perforation of the posterior wall of the stomach.
Outcome and follow-up
Complete recovery.
Discussion
We have identified 16 reported cases of gastric perforation, but the majority have occurred immediately or shortly after balloon insertion in contrast to our case, which occurred 22 months afterwards that could have been avoided by following the manufacturer's instructions.
Intragastric balloons have been used as a minimally invasive solution to obesity since 1985.1 Rarely used in the UK but more commonly used in Europe, these are silicon balloons that are inserted endoscopically and, thus, reducing the high risk of invasive surgery in obese patients and are used either on their own or as a preparation before major bariatric surgery. They act like artificial bezoars to provide a sense of premature satiety, which hypothetically causes weight loss.2 In general, the incidence of both minor complications (vomiting, balloon deflation, oesophagitis) and major complications (bowel obstruction, perforation) are low but these are well documented.1
The efficacy of these balloons in causing weight loss is questionable. While they may be effective in causing short-term weight loss,1 3 4 they are not recommended to stay in place for more than 6 months and the studies that evaluated efficacy at 24 months are either retrospective5 or had small numbers of patients.6 Indeed, a Cochrane review7 published in 2009 was unable to determine the efficacy of the intragastric balloon due to ‘heterogeneous and partially incomplete data’. This has also led to the omission of this procedure from national and international guidelines on obesity surgery such as that of the UK National Institute for Health and Clinical Excellence and the Food and Drug Administration (FDA) in USA. An original FDA approval was subsequently withdrawn due to complications requiring surgical intervention.8 Since then, as the modern balloons apparently cause fewer complications, the FDA has commissioned a randomised trial comparing the balloon with conventional management of obesity9 projected to finish in March 2012. Even in Europe where the insertion of an intragastric balloon is one of the most commonly performed bariatric procedures,10 there are no guidelines that we could find to support its use. The European Association for Endoscopic Surgery Guidelines published in 2005 concluded that five out of seven trials they reviewed showed no additional benefit for the intragastric balloon compared to diet modification, and when compared to obesity surgery, the balloon produced insufficient and non-durable weight loss.11
As the procedure has its attractions (relatively low cost and no risks of surgery or anaesthesia involved) the procedure may become more popular with obese people not currently eligible for National Health Service funded obesity surgery despite the lack of evidence. Having the procedure done abroad in this case meant that there was no follow-up. As opposed to the general recommendation that the balloon is removed after 6 months to avoid the risk of complications, this patient seems to have been wrongly advised that it could remain in situ for up to 3 years. This advice, if this were true, is both contrary to the available evidence and the product information,12 which warns of the dangers of leaving a balloon for longer than 6 months and caused this life-threatening complication of gastric perforation.
Learning points.
-
▶
When coming across patients who had this procedure performed in Europe, both general practitioners and hospital doctors should be vigilant that these should be removed after 6 months to prevent complications.
-
▶
Patients who are considering having this procedure done abroad may have to be counselled appropriately in the UK as they may not get the correct advice at the centre where this is done.
-
▶
Except perhaps as a method of short-term weight loss prior to conventional bariatric surgery, the evidence base is weak for an intragastric balloon to be considered as a solution to obesity.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Imaz I, Martínez-Cervell C, García-Alvarez EE, et al. Safety and effectiveness of the intragastric balloon for obesity. A meta-analysis. Obes Surg 2008;18:841–6 [DOI] [PubMed] [Google Scholar]
- 2.Nieben OG, Harboe H. Intragastric balloon as an artificial bezoar for treatment of obesity. Lancet 1982;1:198–9 [DOI] [PubMed] [Google Scholar]
- 3.Genco A, Bruni T, Doldi SB, et al. BioEnterics intragastric balloon: the Italian experience with 2515 patients. Obes Surg 2005;15:1161–4 [DOI] [PubMed] [Google Scholar]
- 4.Dumonceau JM. Evidence-based review of the Bioenterics intragastric balloon for weight loss. Obes Surg 2008;18:1611–17 [DOI] [PubMed] [Google Scholar]
- 5.Genco A, Balducci S, Bacci V, et al. Intragastric balloon or diet alone? A retrospective evaluation Obes Surg 2008;18:989–92 [DOI] [PubMed] [Google Scholar]
- 6.Mathus-Vliegen EM, Tytgat GN. Intragastric balloon for treatment-resistant obesity: safety, tolerance, and efficacy of 1-year balloon treatment followed by a 1-year balloon-free follow-up. Gastrointest Endosc 2005;61:19–27 [DOI] [PubMed] [Google Scholar]
- 7.Fernandes MAP, Atallah ÁN, Soares B, et al. Intragastric balloon for obesity. Cochrane Database Syst Rev 2007;1:CD004931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Classen M, Tytgat GNJ, Lightdale C. Gastroenterological Endoscopy. Germany: Georg Thieme Verlag, 2010 [Google Scholar]
- 9.Clinical Trials.gov Safety and Effectiveness of the BioEnterics Intragastric Balloon (BIB) System to Assist in the Weight Management of Obese Subjects, Study NCT00730327, 25/6/2010: http://clinicaltrials.gov/ct2/show/record/NCT00730327?term=intragastric+balloon&rank=5 (accessed 17 November 2010)
- 10.Śmigielski JA, Szewczyk T, Modzelewski B, et al. Gastric perforation as a complication after bioenterics intragastric balloon bariatric treatment in obese patients – synergy of endoscopy and videosurgery. Obes Surg 2010;20:1597–99 [DOI] [PubMed] [Google Scholar]
- 11.Sauerland S, Angrisani L, Belachew M, et al. Obesity surgery: evidence-based guidelines of the European Association for Endoscopic Surgery (EAES). Surg Endosc 2005;19:200–21 [DOI] [PubMed] [Google Scholar]
- 12.BioEnterics Corporation BioEnterics Intragastric Balloon System – Product information. http://www.bodiesbeautiful.co.uk/pdf/BIB_Product_Data_Sheet_EN.pdf (accessed 17 November 2010)