Abstract
Aims
The precise neurochemical perturbations through which perinatal (gestation/lactation) lead exposure modifies the reinforcement efficacy of various psychoactive drugs (e.g., cocaine, opiates) are unknown. The present study considers the role of altered serotonin and dopamine functionality in perinatal lead-psychostimulant interactions.
Main Methods
Female rats were administered a 16-mg lead or a control solution (p.o.) for 30 days prior to breeding with non-exposed males. Lead exposure was discontinued at weaning (postnatal day [PND] 21). Starting at PND 120, male rats born to control or lead-exposed dams were injected with either PAL-287 or PAL-353, at doses of 0, 2, 4, 8, or 16 umol/kg (ip) with each dose given prior to an acute (45 min) locomotion test. Whereas PAL-287 is a potent releaser of serotonin, PAL-353 is not. Each drug induces comparable release of norepinephrine (NE) and of dopamine (DA).
Key Findings
Control and lead rats exhibited minimal locomotion to PAL-287. PAL-353 produced a dose-dependent activation of locomotion in control rats relative to the effects of PAL-287 in control rats. Lead-exposed rats exhibited a subsensitivity to PAL-353 at doses of 4 and 8 umol/kg.
Significance
The subsensitivity of lead rats to PAL-353 is consistent with a lead-induced diminution of dopamine function, an effect noted earlier for the reuptake inhibitor cocaine (Nation et al. 2000). The similar response of lead and control rats to PAL-287 is inconsistent with diminished serotonin function.
Keywords: Body Weight, Monoamine, Amphetamine, Lead, Locomotion
Introduction
In spite of regulations aimed at reducing lead contamination, concern remains that certain populations, especially in the inner city, continue to be exposed to significant levels of lead. Of particular concern is the observation that the deleterious effects of lead appear to begin in utero. Pregnant women with elevated blood lead levels give birth to children who have correspondingly high concentrations of lead in their bloodstream. In fact, during pregnancy, lead previously stored in bone appears to be liberated and redistributed to the vascular system (Gulson et al. 1997). Blood lead levels reach a peak during the second trimester at which point the metal is is absorbed by the placenta, crosses the underdeveloped blood–brain barrier, and penetrates the soft bone structure. Although lead in blood may have a biologic half-life approximating 1 month, the half-life of lead in bone is estimated to be 20–30 years (Weizaecker 2003). Accordingly, possible health consequences associated with early lead exposure may be long lasting.
Developmental lead exposure seems to potentiate the behavioral effects associated with repeated administration of cocaine. That is, perinatal lead exposure increases the stimulatory properties of cocaine when animals are tested in a locomotor chamber at either postnatal day (PND) 30 or PND 90 and repeatedly dosed with a fixed dose of cocaine (Nation et al. 2000). In other studies, rats developmentally exposed to lead self-administered cocaine at doses too low to sustain responding in untreated controls (Nation et al. 2004; Valles et al. 2005). In contrast, perinatal lead exposure can result in an initial subsensitivity to cocaine in acute tests of locomotion (Nation et al. 2000).
The neurochemical underpinnings for the effects of perinatal lead on psychostimulant function are unknown. Drugs such as cocaine act to block the transporters for dopamine (DAT), norepinehrine (NET), and serotonin (SERT) (Howell and Kimmel 2008). There is evidence that perinatal lead exposure alters dopamine neurochemistry. Whereas dopamine levels in striatum and cortex are not altered, dopamine turnover is enhanced by prenatal lead exposure (Szczerbac et al. 2007). Additional studies suggest changes in presynaptic dopamine autoreceptors (Lasley 1992), diminished release of dopamine by potassium or amphetamine (Devoto et al. 2001) as well as enhanced D2 postsyanptic receptor function (Szczerbac et al. 2007) in lead as opposed to control animals. Cocaine, however, is also an antagonist for SERT which raises the issue of the extent to which lead-induced changes in SERT function contribute to augmented reactivity to cocaine (Nation et al. 2000). There are relatively few studies that bear on perinatal lead exposure and brain serotonin function. Whereas perinatal lead exposure was without effect on striatal serotonin level (Devoto et al. 2001), adult lead exposure reduces striatal serotonin (Virgolini et al. 2005).
In order to assess the possible roles of dopamine and serotonin function for the effect of perinatal lead on psychostimulant function, we employed two amphetamine-like analogs that vary in SERT activity. Whereas PAL-287 is a potent serotonin releaser, PAL-353 is not (see Table 1). PAL-287 and PAL-353 induce similar effects on release of norepinephrine (NE) and dopamine (DA) (Rothman et al. 2001; Wee et al. 2005). Behaviorally, PAL-287 induces less hyperlocomotion than does PAL-353, in part because the the serotonergic properties of PAL-287 normally attenuate the stimulant effects mediated by the impact of PAL-287 on release of DA/NE (Hall et al. 2008; Roberts et al. 1999; Rothman et al. 2005; Rothman et al. 2002; Wee et al. 2005). In the present study, adult male control and lead rats were dosed with these two drugs, at equivalent molecular amounts, in acute locomotion tests commencing at PND 120. Our guiding hypotheses were that if perinatal lead impairs serotonin function, then lead treated rats should exhibit enhanced locomotion relative to control rats since the serotonergic aspect of PAL-287 normally offsets the stimulatory properties associated with release of DA and NE. Secondly, if perinatal lead impairs dopamine function, then perinatal lead rats should exhibit diminished locomotion to PAL-353, a drug that releases DA/NE.
Table 1.
In vitro potency values of PAL compounds for monoamine release. Values are means for three experiments (methods are described in Rothman et al., 2001; Wee et al., 2005).
| Releasing Activity (IC50, in nMol) | |||
|---|---|---|---|
| DRUG: | Dopamine | Serotonin | Norepinephrine |
| PAL-287 | 12.6 | 3.4 | 11.1 |
| PAL-353 | 24.2 | 1937.0 | 16.1 |
Materials and Methods
Lead Exposure Regimen
For 30 consecutive days, 16 adult female (200–225) Sprague-Dawley rats (Harlan; Houston, TX) were exposed once a day to 0-mg lead (sodium acetate) or 16-mg lead (as lead acetate) daily using a 18 ga gavage needle to administer the respective solutions in a volume of 1.0 ml deionized water. This procedure has been used in previous developmental lead studies to ensure stable blood/tissue levels (cf. Nation et al. 2000, 2003, 2004; Rocha et al. 2004). The present lead concentration was selected based on previous studies that found it produces differential behavioral effects, while not altering dam body weights or the locomotor ability of pups (see Nation et al. 2000). Following this 30-day lead exposure period, females were bred with non-exposed males. Once females tested positive for copulatory plugs, the males were removed from the home cage. Females continued to receive their daily doses of the control solution or lead acetate solution throughout the gestation and lactation periods. Standard rat chow (Teklad; Madison, WI) and tap water were continuously available for dams in the home cage. Litters were culled to eight pups on PND 1.
Blood/Tissue Analyses
For control and lead-exposed dams, 100–150 µl of tail-blood was drawn at breeding, parturition (PND 2), and weaning (PND 21) and analyzed for lead levels. A single littermate for each of the test animals was sacrificed on PND 2, and blood samples were collected for subsequent analyses. Dams were sacrificed at weaning with blood and tissue (brain, kidney, liver and bone) samples collected for subsequent analyses.
Lead residues in blood and tissue samples were measured by inductively coupled plasma - mass spectroscopy on a Perkin Elmer DRC 2 instrument following acid digestion in a microwave. The 208Pb isotope and 209Bi were used as internal standards. Weighted linear calibration was performed with a blank and three external standards (0.05, 20, and 200 parts per billion) and was verified by analyzing NIST SRM 1640 (trace elements in water). Data were acquired in peak hopping mode, using the autolens feature and three replicate reads per determination. Verification of the calibration and baseline were performed after every group of 10 samples and at the end of the analytical run.
Rate of pregnancy did not differ between groups (p>0.05). On PND 21, pups used for testing were weaned and housed individually. All animals were maintained on a 12-hour light/dark cycle. Testing commenced on PND 120, at approximately 10:00 hrs, two hrs into the 12-hr light cycle.
Apparatus
The assessment of locomotion was made in a set of 8 automated optical beam activity monitors (Model RXYZCM-16; Accuscan Instruments, Columbus, OH, USA). Each monitor is housed within a 40 × 40 × 30.5 cm acrylic cage. Activity monitors and cages were located in a sound-proof room with a 40 dB [SPL] white noise generator operating continuously. A multiplexor-analyzer monitored beam breaks from the optical beam activity monitors and tracked the simultaneous interruption of beams. The multiplexor-analyzer updated the animal's position in the acrylic cage every 10 msec using a 100% real-time conversion system. Computerized integration of the data obtained from the monitor afforded the recording of general activity using total distance traveled scores (in cm) as the primary dependent measure (cf. Sandberg et al. 1987).
Drugs
A vehicle saline solution was prepared as 0.9% sodium chloride in distilled water. The amphetamine-like drugs used in the present study were synthesized by Dr. Bruce E. Blough. PAL-287 is 1-napthyl-aminopropane while PAL-353 is meta-fluoramphetamine (Wee et al. 2005). The profile of activity of these drugs at DAT, NET, and SERT is given in Table 1. Each molecule exerts a similar releasing effect at DAT and at NET. Of these, PAL-287 exerts the greatest SERT releasing activity, whereas PAL-353 exert substantially less SERT releasing activity. Drug doses (umol/kg) were calculated as the base (with equivalent numbers of molecules) and all injections were administered i.p. in a volume of 1 ml/kg.
Procedure
Test animals were randomly selected from a given control or lead litter and then these groups were in turn randomly assigned to drug treatment condition. Thus four test groups (n=8 each) were created by interacting lead-exposure condition (0, 16 mg) and drug type (PAL-287 or PAL-353). In the initial phase of the project, animals were tested during 1 hr sessions each day for 7 successive days, in squads of eight rats, counterbalancing by group. With the room lights off, animals were placed in their respective test chambers for a 15-min baseline-recording period. On the last two days of the baseline period, each rat received an i.p. injection of saline at the end of the 15 min period. After injection, each rat was placed back in the chamber immediately for an additional 45 min period. On Days 8, 11, 14, 17, and 20, all animals within each of the four groups received successive daily ip injections of 0, 2.0, 4.0, 8.0 and 16.0 umol/kg of their respective PAL drug. Each rat received each drug dose once and drug doses were given in random order. On the two days in between each drug dose, no injections were given and tests were conducted as above. The rats were run in four squads to yield a group size of n=8 per drug condition for the present study.
Data Analyses
The overall design of the study was a split-plot (mixed) factorial design consisting of the between-group factors of lead exposure (control versus lead) and PAL drug (PAL-287 versus PAL-353) and within-group factors of PAL dose (0, 2.0, 4.0, 8.0, and 16.0 umol/kg) and time after injection (three 15 min bins over a 45 min period). Because the treatment means and variances were proportional, the total distance traveled scores were subjected to a log transformation (Kirk 1982). The body weight and blood lead level data were analyzed using a complete factorial consisting of the factors of lead exposure (control versus lead). Statistical significance was deemed to be p < 0.05 and the Bonferroni procedure was used to examine mean group differences.
Results
Blood/Tissue Lead Levels
As expected, dams exposed to lead exhibited a considerable body lead burden (Table 2). At the time of weaning, dams exhibited significant levels of lead accumulation in tibia ((F1,14) = 47.9, p < 0.0001), in kidney ((F1,14) = 31.9, p < 0.0001), In liver ((F1,14) = 15.4, p < 0.002), in brain ((F1,14) = 280.1, p < 0.0001), as well as blood ((F1,14) = 20.5, p < 0.0001). Similarly, littermates showed significant blood levels of lead at PND 21((F1,14) = 95.7, p < 0.0001). In the present study, blood lead levels were not determined in PND 120 rats, inasmuch as in earlier studies, lead is cleared from the blood so that by PND 70 (and beyond), blood lead levels in both control and lead groups are similar (Nation et al. 2000). Thus, the behavioral disturbances associated with perinatal lead exposure occurred at a time point far beyond that at which the lead burden had cleared from blood.
Table 2.
Mean (SEM) blood and tissue lead concentration values for dams and littermates of test animals.
| Blood lead concentration (µg/dl) | ||
| Group 0-mg | Group 16-mg | |
| Dams | ||
| Postnatal Day 2 | 2.34 (1.10) | 86.58 (18.6) * |
| Postnatal Day 21 | 1.01 (0.36) | 43.45 (7.19) * |
| Littermates | ||
| Postnatal Day 2 | 0.87 (0.41) | 53.51 (5.38) * |
| Tissue Concentrations of Dams at Weaning (µg/g) | ||
| Brain | 0.01 (0.001) | 0.56 (0.03) * |
| Kidney | 0.08 (0.02) | 14.19 (2.50) * |
| Liver | 0.02 (0.001) | 0.93 (0.23) * |
| Tibia | 0.38 (0.07) | 121.34 (17.54) * |
The symbol * indicates that control and lead-exposed animals were significantly different (p < 0.05). N=8 per control/lead treatment condition.
Total Distance Traveled Scores
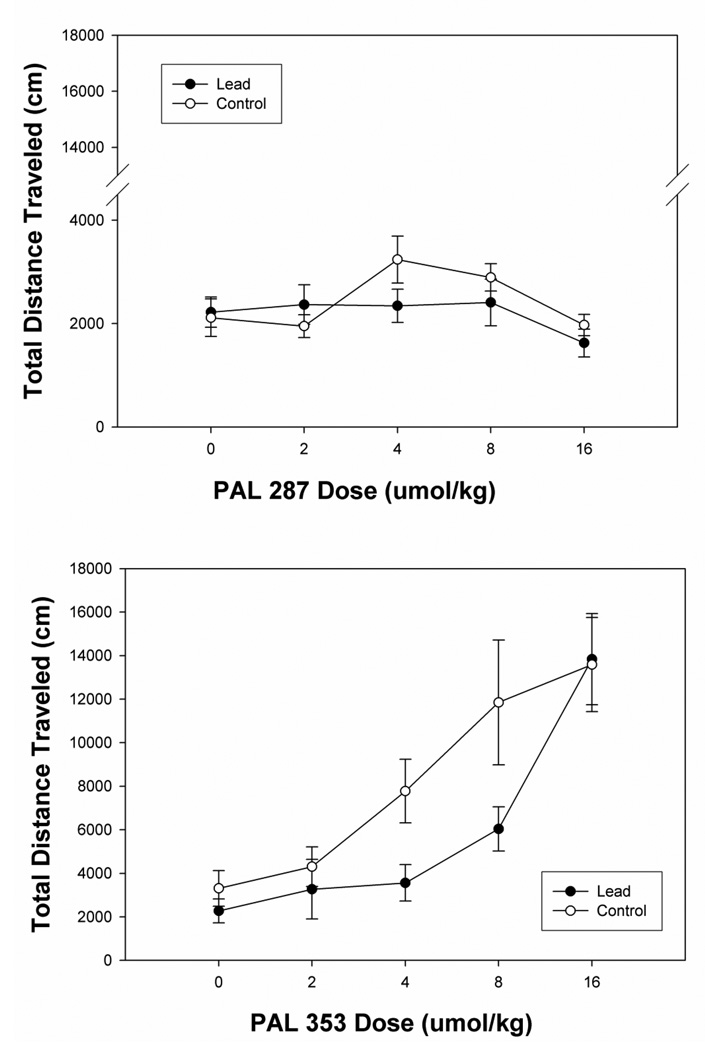
The impact of PAL-283 and of PAL-353 on total distance traveled scores in lead and control rats is depicted in Figure 1. Analysis of the impact of PAL-287 and PAL-353 in control rats only revealed a significant effect of drug (F(1, 14) = 21.1, p < 0.0001), a significant effect of dose (F(4,56) = 15.88, p < 0.0001) and a significant interaction between drug and dose (F(4,56) = 9.347, p < 0.0001). The interaction reflected the fact that PAL-353 markedly stimulated forward locomotion as dose increased, whereas PAL-287 did not. The overall ANOVA revealed a significant effect of dose (F(4, 112) = 19.01, p < 0.0001), a significant effect of drug (F(1, 28) = 21.1, p < 0.0001) and a nearly significant effect of lead (F(1, 28) = 3.938, p < 0.057). The latter outcome is attributed to the observation that trend analyses revealed a significant linear trend for dose (F(1,28) = 65.992, p < 0.0001) whereas there was a significant quadratic trend for the interaction of dose and lead (F(1,28) = 4.338, p < 0.045) as well as a significant quadratic trend for the interaction of dose and drug (F(1,28) = 12.18, p < 0.002). This trend reflected control versus lead differences at the intermediate doses of PAL-353, but not PAL-287.
Fig.1.
Panel A. Mean group total changes in total distance traveled scores (cm/45 min) for control and lead exposed rats injected with a dose series of 0, 2, 4, 8 and 16 umol/kg PAL-287. Panel B: Mean group total changes in total distance traveled scores (cm/45 min) for control and lead exposed rats injected with a dose series of 0, 2, 4, 8 and 16 umol/kg PAL-353. The lines above and below each symbol represent the S.E.M.
Additional analyses were computed for each drug separately comparing lead and control responses as a function of dose. Analyses of data from PAL-353 treatment conditions revealed a significant effect of dose (F(4,56) = 35.38, p < 0.0001) as well as a significant quadratic trend for the interaction of lead and dose (F(1,14) = 5.04, p < 0.041). The changes in locomotion induced by PAL-353 for each level of dose were used to compute a regression curve for each rat and that curve was used to generate individual ED50 values for each rat. The average group ED50 value (+ SEM) for the lead group was 5.79 (± 0.88) umol/kg, which was significantly higher than the 3.53 (+ 0.73) umol/kg value noted for control rats treated with PAL-353 (t(14) = 2.85, p < 0.013). In contrast, analyses of lead and control data for PAL-287 treatment only revealed a significant effect of dose (F(4,56) = 2.94, p < 0.028), but no effect of lead treatment nor was there an interaction between lead and dose in the PAL-287 conditions. Because of the shape of the dose-effect curves for PAL-287, no efforts were made to compute ED50 values for the control and lead groups. Overall, these analyses suggest no differences between lead and control responses to PAL-287, whereas PAL-353 induced less locomotion in lead rats than in control rats, in effect shifting the dose-effect curve to the right.
Discussion
In the present experiment, PAL-287 induced minimal changes in locomotion in either control or lead treated rats across a range of doses (2 umol/kg through 16 umol/kg). In contrast PAL-353 induced marked locomotion in control rats that peaked at 16 umol/kg. Each of these two drugs exerts a similar degree of releasing activity at NET and at DAT, but have widely varying releasing activity at SERT (Rothman et al. 2001; Wee et al. 2005; Negus et al. 2007). PAL-353 has minimal releasing activity at SERT in contrast to PAL-287. The reduced stimulation of locomotion in control rats in response to PAL-287, relative to PAL-353, confirm and extend earlier studies in which PAL-287 induced less motor activation than did PAL-353 in rats (Rothman et al. 2005; Wellman et al. 2009). These results are consistent with the view that activity at SERT may interact so as to diminish or attenuate drug reinforcement associated with activity at DAT/NET (Hall et al. 2008; Roberts et al. 1999; Rothman et al. 2005; Rothman et al. 2002; Wee et al. 2005).
In the present study, rats exposed to lead did not show an altered profile of locomotion to PAL-287, a drug that only weakly stimulates locomotion. Lead expsoure did, however, alter the locomotor responses of rats treated with PAL-353, a drug that markedly stimulates locomotion. The interaction of lead with PAL-353, however, was a function of dose. Although lead rats showed a subsensitivity to PAL-353 at doses of 4 and 8 umol/kg, these rats showed locomotor stimulation at 16 umol/kg PAL-353 that was similar to that of control rats. Earlier studies of psychostimulant function in perinatally lead exposed rats have noted a biphasic response. In rats perinatally exposed to lead and tested at PND 90, 7 daily treatments with cocaine resulted in a locomotor subsensitivity to cocaine in lead rats (Nation et al. 2000). When the duration of daily cocaine exposure was increased to 14 days, perinatally lead treated rats showed a supersensitivity to the locomotor effects of cocaine. Our present data, in which rats treated with PAL-353 showed a subsensitivity are similar to the 7 day data point of the Nation et al. (2000) study. It should be noted that the Nation et al. (2000) study employed cocaine, a psychostimulant that acts via blockade of DAT, NET and SERT. The present study is the first to examine the impact of drugs that are substrates for these transporters and thus act as releasers of dopamine, of norepinephrine, and of serotonin in perinatal lead rats.
The overall profile of effects of the present study do not support the view that the capacity of lead to alter psychostimulant function is modulated by diminished serotonin function. When challenged with PAL-287, a drug that is a substrate for SERT, there were no differences between lead and control groups. Were lead to impair serotonin function, our prediction was that lead rats would show greater behavioral activation to PAL-287. This was based on the view that lead rats would fail to react to the serotonergic property of PAL-287, which normally offsets the motor activation noted in control rats associated with release of DA and NE. In contrast, when challenged with PAL-353, lead rats showed a subsensitivity similar to that noted earlier by Nation et al. (2000) for cocaine. This outcome is most consistent with the view that lead impairs dopamine function. Future studies are required to consider the impact of repeated dosing of lead and control rats with PAL-353 and perhaps PAL-287.
Acknowledgements
The present study was supported by NIDA R21 DA017230-01 (P.J.W.), by NIDA R01 DA012970 (B.E.B.) and by the Intramural Research Program, NIDA, NIH and DHHS (R.B.R.). The authors thank Sam Buckman for his valuable technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Devoto P, Flore G, Ibba A, Fratta W, Pani L. Lead intoxication during intrauterine life and lactation but not during adulthood reduces nucleus accumbens dopamine release as studied by brain microdialysis. Toxicology Letters. 2001;121:199–206. doi: 10.1016/s0378-4274(01)00336-8. [DOI] [PubMed] [Google Scholar]
- Gulson BL, Jameson CW, Mahaffey KR, Mizon KJ, Korsch MJ, Vimpani G. Pregnancy increases mobilization of lead from maternal skeleton. Journal of Laboratory and Clinical Medicine. 1997;130:51–62. doi: 10.1016/s0022-2143(97)90058-5. [DOI] [PubMed] [Google Scholar]
- Hall DA, Stanis JJ, Avila HM, Gulley JM. A comparison of amphetamine- and methamphetamine-induced locomotor activity in rats: Evidence for qualitative differences in behavior. Psychopharmacology. 2008;195:469–478. doi: 10.1007/s00213-007-0923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Kimmel HL. Monoamine transporters and psychostimulant addiction. Biochemical Pharmacology. 2008 Jan 1;75(1):196–217. doi: 10.1016/j.bcp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Kirk RE. Experimental Design: Procedures for the Behavioral Sciences. second ed. California: Wadsworth; 1982. p. 83. [Google Scholar]
- Lasley SM. Regulation of dopaminergic activity, but not tyrosine hydroxylase, is diminished after chronic inorganic lead exposure. Neurotoxicology. 1992 Fall;13(3):625–635. [PubMed] [Google Scholar]
- Nation JR, Cardon AL, Heard HM, Valles R, Bratton GR. Perinatal lead exposure and relapse to drug-seeking behavior in the rat: A cocaine reinstatement study. Psychopharmacology. 2003;168:236–243. doi: 10.1007/s00213-003-1405-2. [DOI] [PubMed] [Google Scholar]
- Nation JR, Miller DK, Bratton GR. Developmental lead exposure alters the stimulatory properties of cocaine at PND 30 and PND 90 in the rat. Neuropsychopharmacology. 2000;23:444–454. doi: 10.1016/S0893-133X(00)00118-4. [DOI] [PubMed] [Google Scholar]
- Nation JR, Smith KR, Bratton GR. Early developmental lead exposure increases sensitivity to cocaine in a self-administration paradigm. Pharmacology Biochemistry and Behavior. 2004;77:127–135. doi: 10.1016/j.pbb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB. Monoamine releasers with varying selectivity for dopamine/norepinephrine versus serotonin release as candidate "agonist" medications for cocaine dependence: studies in assays of cocaine discrimination and cocaine self-administration in rhesus monkeys. Journal of Pharmacology and Experimental Therapeutics. 2007;320:627. doi: 10.1124/jpet.106.107383. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Phelan R, Hodges LM, Hodges MM, Bennett B, Childers S, Davies H. Self-administration of cocaine analogs by rats. Psychopharmacology. 1999 Jun;144(4):389–397. doi: 10.1007/s002130051022. [DOI] [PubMed] [Google Scholar]
- Rocha A, Valles R, Cardon AL, Bratton GR, Nation JR. Self-administration of heroin in rats: effects of low-level lead exposure during gestation and lactation. Psychopharmacology. 2004;174:203–210. doi: 10.1007/s00213-003-1742-1. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;479:23. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Appetite suppressants as agonist substitution therapies for stimulant dependence. Annals of the New York Academy of Sciences. 2002 Jun;965:109–126. doi: 10.1111/j.1749-6632.2002.tb04155.x. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Woolverton WL, Anderson KG, Negus SS, Mello NK, Roth BL, Baumann MH. Development of a rationally designed, low abuse potential, biogenic amine releaser that suppresses cocaine self-administration. Journal of Pharmacology and Experimental Therapeutics. 2005 Jun;313(3):1361–1369. doi: 10.1124/jpet.104.082503. [DOI] [PubMed] [Google Scholar]
- Sandberg PR, Zoloty SA, Willis R, Ticarich CD, Rhoads K, Nagy RP, Mitchell SG, Laforest AR, Jenks JA, Harkabus LJ, Gurson D, Finnefrock JA, Bednerik EJ. Digiscan activity: automated measurement of thigmotactic and stereotypic behavior in rats. Pharmacology Biochemistry and Behavior. 1987;27:569–572. doi: 10.1016/0091-3057(87)90369-8. [DOI] [PubMed] [Google Scholar]
- Szczerbak G, Nowak P, Kostrzewa RM, Brus R. Maternal lead exposure produces long-term enhancement of dopaminergic reactivity in rat offspring. Neurochemical Research. 2007 Oct;32(10):1791–1798. doi: 10.1007/s11064-007-9306-0. [DOI] [PubMed] [Google Scholar]
- Valles R, Rocha A, Cardon AL, Bratton GR, Nation JR. The effects of the GABAA antagonist bicuculline on cocaine self-administration in rats exposed to lead during gestation/lactation. Pharmacology Biochemistry and Behavior. 2005;80:611–619. doi: 10.1016/j.pbb.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Virgolini MB, Chen K, Weston DD, Bauter MR, Cory-Slechta DA. Interactions of chronic lead exposure and intermittent stress: consequences for brain catecholamine systems and associated behaviors and HPA axis function. Toxicological Sciences. 2005 Oct;87(2):469–482. doi: 10.1093/toxsci/kfi269. [DOI] [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL. Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. Journal of Pharmacology and Experimental Therapeutics. 2005;313:848. doi: 10.1124/jpet.104.080101. [DOI] [PubMed] [Google Scholar]
- Weizaecker K. Lead toxicity during pregnancy. Primary Care in Obstetrics and Gynecology. 2003;10:304–309. [Google Scholar]
- Wellman PJ, Davis KW, Clifford PS, Rothman RB, Blough BE. Changes in feeding and locomotion induced by amphetamine analogs in rats. Drug and Alcohol Dependence. 2009;100:234–239. doi: 10.1016/j.drugalcdep.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]