Abstract
Regulation of gene transcription by neuronal activity is thought to be key to the translation of sensory experience into long-term changes in synaptic structure and function. Here we show that cpg15, a gene encoding an extracellular signaling molecule that promotes dendritic and axonal growth and synaptic maturation, is regulated in the somatosensory cortex by sensory experience capable of inducing cortical plasticity. Using in situ hybridization, we monitored cpg15 expression in 4-week-old mouse barrel cortex after trimming all whiskers except D1. We found that cpg15 expression is depressed in the deprived barrels and enhanced in the barrel column corresponding to the spared D1 whisker. Changes in cpg15 mRNA levels first appear in layer IV, peak 12 h after deprivation, and then decline rapidly. In layers II/III, changes in cpg15 expression appear later, peak at 24 h, and persist for days. Induction of cpg15 expression is significantly diminished in adolescent as well as adult CREB knockout mice. cpg15's spatio-temporal expression pattern and its regulation by CREB are consistent with a role in experience-dependent plasticity of cortical circuits. Our results suggest that local structural and/or synaptic changes may be a mechanism by which the adult cortex can adapt to peripheral manipulations.
Keywords: barrel cortex, cpg15, deprivation, experience-dependent plasticity
Introduction
The rodent vibrissae system is a useful model for studying activity-dependent plasticity in the adult cortex. Each vibrissa projects to a discrete aggregate of neurons in layer IV of the somatosensory cortex, known as a barrel (Woolsey and Van der Loos, 1970). Neurons in a particular barrel column are maximally excited by a single principal whisker and to a weaker extent by surround whiskers (Simons, 1978; Armstrong-James and Fox, 1987). Neuronal receptive fields in the barrel cortex are plastic and change in response to sensory experience even in the adult brain (reviewed in Fox, 2002). Simply trimming whiskers on the mystacial pad results in a profound depression of responses in the contralateral barrel cortex corresponding to the deprived whiskers (Diamond et al., 1993; Armstrong-James et al., 1994). When all the large whiskers are trimmed except a single (“spared”) whisker, responses to the spared vibrissa are potentiated in the neighboring, deprived barrels following the initial depression caused by trimming of their primary whiskers (Glazewski and Fox, 1996). Both synapse formation and elimination (Trachtenberg et al., 2002), and long term potentiation (LTP)-like mechanisms (Hardingham et al., 2003) have been implicated in barrel receptive field plasticity. Yet, the molecular mechanisms underlying cortical receptive field plasticity are largely unknown.
Molecules involved in learning and memory, LTP, long-term depression, and in visual system developmental plasticity, have also been implicated in receptive field plasticity of barrel cortex (Glazewski et al., 1996; Glazewski et al., 1999). One such molecule is the cAMP-responsive element binding protein (CREB), a transcription factor that regulates gene expression by binding to Ca2+/cAMP-responsive element (CRE) promoter sites (Shaywitz and Greenberg, 1999). In response to single whisker experience, potentiation of the spared vibrissa response in adult α, δ CREB knockout mice is reduced relative to wild-type animals (Glazewski et al., 1999). In transgenic mice carrying a lacZ reporter gene driven by a promoter containing CRE sites, single whisker experience induces lacZ expression in the barrel corresponding to the spared whisker (Barth et al., 2000). CREB activation during single whisker experience is likely to be one of the first steps in a transcriptional program leading to long-term changes in synaptic properties. cpg15 is a CREB target gene and a potential downstream effector in an activity-dependent transcriptional program. cpg15 (also known as Nrn1) was isolated in a screen for seizure-induced genes in the hippocampal dentate gyrus (Nedivi et al., 1993; Hevroni et al., 1998) and was subsequently shown to be regulated by light in the visual system, where it is expressed in correlation with critical periods for activity-dependent plasticity (Corriveau et al., 1999; Lee and Nedivi, 2002). cpg15 contains CRE sites in its promoter region and its activity-dependent regulation in cultured cortical neurons is partially regulated by CREB (Fujino et al., 2003). CPG15 (also known as neuritin-1) encodes a small highly conserved protein that in membrane-bound form coordinately regulates growth of apposing dendritic and axonal arbors, and promotes synaptic maturation (Nedivi et al., 1998; Cantallops et al., 2000).
Here we use in situ hybridization to monitor experience-dependent expression of cpg15 in barrel cortex of wild type and CREB knockout mice. We find that cpg15 expression is modulated by sensory experience capable of inducing receptive field plasticity and that this expression is regulated by CREB.
Materials and Methods
Animal Manipulations and Tissue Isolation
All animal work was approved by the Massachusetts Institute of Technology and Cold Spring Harbor Laboratory Committees on Animal Care and conforms to NIH guidelines for the use and care of vertebrate animals. C57BL/6 (+/+) (Charles River, Wilmington, MA) or CREB α, δ knockout mice (−/−) (Bourtchuladze et al., 1994; Hummler et al., 1994) were used at 4–5 weeks or 6 months of age. For the single whisker experience paradigm, mice were gently restrained by hand and all the large whiskers (A1–A4, B1–B4, C1–C5, D2–D5, E1–E5, α, β, γ, and δ) except D1 on the right side of the muzzle were trimmed close to the face (<1 mm) using microspring scissors. Whiskers on the left muzzle were left intact, and their corresponding hemispheres served as controls. After 12 h of sensory deprivation, mice were sacrificed by guillotine decapitation, and brains immediately removed. At 4–5 weeks, n = 8 brains per group (+/+ or −/−). At 6 months, n = 6 for +/+ and n = 5 for −/−. For a time course of the cpg15 response to the single whisker experience, additional 4–5-week-old mice were harvested after 3 h, 6 h, 24 h, 3 days (d), or 7 d of sensory deprivation (n = 2 brains per time point). For long deprivation periods, whiskers were trimmed every 2 d. Cortical hemispheres were rapidly dissected from the freshly removed brains and flattened between two clean microscope slides, rapidly frozen in dry ice, and stored at −80°C. Flattened cortices were tangentially sectioned by cryostat (12 μm), thaw-mounted, dried, fixed, dehydrated, and stored as previously described (Lee and Nedivi, 2002).
In Situ Hybridizations
In situ hybridizations on brains of age-matched wild type and mutant animals were always carried out in parallel. Brains from the 4-week wild type and mutant animals with 12 h of single whisker experience were processed for in situ hybridizations in four different experiments containing two brains from each group. Brains from the 6-month wild type and mutant animals were processed in four different experiments. Brains harvested from mice after 3 h, 6 h, 24 h, 3 d, or 7 d of sensory deprivation were processed in two experiments, each containing one brain from each time point. Prehybridization treatments, hybridizations, posthybridization washes, processing for autoradiography, counterstaining and mounting were done as previously described (Lee and Nedivi, 2002). In all experiments, alternate slides were stained by cytochrome oxidase to provide a clear anatomical map of the barrel field in layer IV (Wong-Riley, 1979). On the in situ hybridized slides, two to three sections from each brain contained barrel fields sufficiently intact for quantitative analysis. Dark-field images of these layer IV sections were imported into Adobe Photoshop 5.0 (Adobe Systems, San Jose, CA) with a Diagnostic Instruments (Sterling Heights, MI) Spot 2 digital camera mounted on a Nikon (Tokyo, Japan) Eclipse E600 using a 1×/0.04 Plan ultra-wide objective. Images were saved as gray-scale TIFFs and imported in NIH Image (version 1.62). Mean pixel density measurements were taken from four areas on each slide: the area representing the D1 whisker (barrel and septa), the area representing the other large whiskers (A1–A4, B1–B4, C1–C5, D2–D5, E1–E5, α, β, γ and δ) (deprived in single whisker experience), the area corresponding to the anterolateral small vibrissa (spared in single whisker experience), and the background outside the section. Pixel density was measured on a 0–255 scale, in which 255 is white. To ensure that measurements were unbiased, image files from wild type and mutant animals were coded and measured blind to age and genotype. The background served as a zero labeling negative control and was subtracted from the mean pixel densities of the D1, large barrel (Deprived), or small barrel (Spared) regions to yield the net mean pixel density for each region. The net mean pixel density of the D1 barrel was divided by the net mean density of the large or small barrel regions on the same section. Measurements from 2–3 sections per layer were averaged for each brain. Statistical significance was determined by unpaired Student's t test for all experiments except the time course. The time course data was compared using ANOVA post-hoc analysis with the Bonferroni/Dunn method. In all experiments, n represents number of brains.
Results
Experience-Dependent Expression of cpg15 in Barrel Cortex
We investigated whether cpg15 is expressed in barrel cortex and whether this expression is regulated by sensory experience known to induce plasticity. in situ hybridizations with a cpg15 probe were conducted on tangential sections through the barrel cortex of 4-week-old mice with normal whisker experience or after unilateral deprivation of all the large whiskers (A1–A4, B1–B4, C1–C5, D2–D5, E1–E5, α, β, γ and δ) except D1 on the right side of the muzzle (referred to as single whisker experience). Previous studies have shown that, at this age, single whisker experience induces significant experience-dependent plasticity in extragranular layers (Fox, 1994; Glazewski and Fox, 1996).
The patterned distribution of cpg15 mRNA in barrel-like compartments was readily apparent in tangential sections through layer IV of somatosensory cortex in mice with intact whisker fields [Fig. 1(A)]. cpg15 mRNA distribution was characterized by regions of low intensity signal corresponding to cell sparse barrels, separated by regions of high intensity signal corresponding to the relatively cell dense septa surrounding each barrel [Fig. 1(A)]. A quantitative comparison showed that, in control animals, mRNA signal intensity in the D1 barrel and surrounding septa was similar to the mRNA signal in other barrels (large and small) and their surround septa [Fig. 1(D), blue bars]. Twelve hours of single whisker experience resulted in increased cpg15 signal intensity within the D1 barrel and its septal rim as compared to the small barrels with septa corresponding to the spared anterolateral small vibrissae [Fig. 1(B) aligned with 1(C); quantified in 1(D), D1/Spared]. There was no apparent change in cpg15 expression in these small barrels located three to four arcs away from the D1 barrel. Most barrels and their surrounding septa corresponding to the deprived whisker rows showed a marked decrease in cpg15 signal intensity when compared to the small spared barrels and their septal rims [Fig. 1(D), Deprived/Spared]. Since changes in cpg15 expression in the barrels and their septal rims were qualitatively similar, we continued to measure expression in each barrel and its septal rim as a single unit (henceforth referred to as barrel unit). The difference in the D1/Deprived ratio between the control and single whisker experience mice was large and highly significant [Fig. 1(D), D1/Deprived]. The D1/Deprived ratio provides a measure of net change in cpg15 expression by combining the increased cpg15 mRNA levels in the D1 barrel unit with the diminished cpg15 expression in the adjacent deprived barrel units. These results demonstrate that cpg15 expression in barrel cortex can be regulated by changes in sensory experience known to induce receptive field plasticity.
Figure 1.
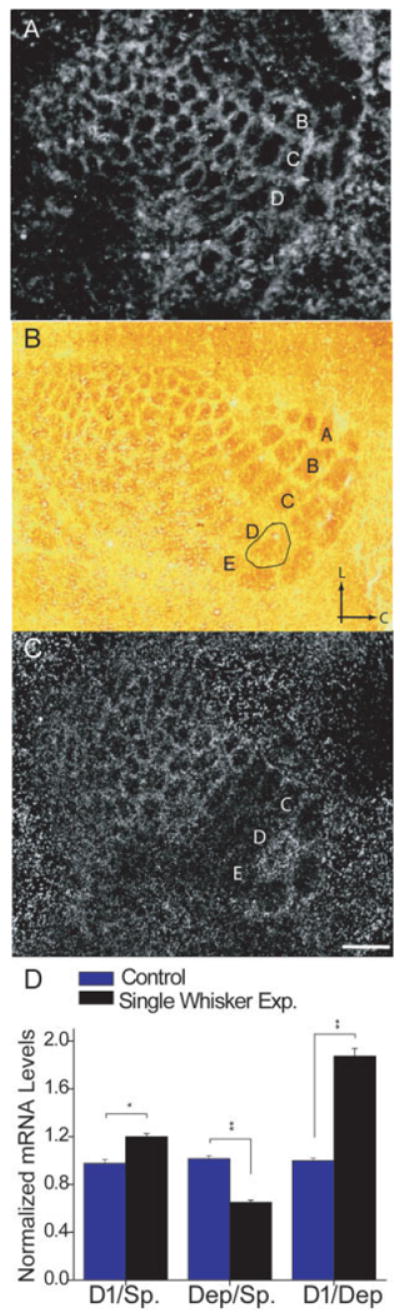
cpg15 expression in barrel cortex is regulated by single whisker experience. Representative dark-field photomicrographs of in situ hybridizations for cpg15 mRNA in mice: (A) with normal whisker experience, (C) after 12 h of single whisker experience. (B) Cytochrome oxidase staining of a section adjacent to (C) with the region representing the spared D1 whisker outlined. Arrows mark lateral (L) and caudal (C) orientation. (D) A quantitative comparison of sections such as shown in (A) and (C) (n = 8 brains per group, 2–3 sections per brain). In response to single whisker experience, cpg15 levels are increased in the barrel and septal region (barrel unit) representing the D1 whisker when compared with barrel units of the small (untrimmed in single whisker experience) whiskers in the same section (Spared), and decreased in the barrel units of the large whiskers (trimmed in single whisker experience) (Deprived). The D1/Deprived ratio represents the net change in cpg15 levels, combining the increase in the D1 barrel with the decrease in the surrounding deprived barrels. *p < 0.05, **p < 0.001. Scale bar, 350 μm.
Layer Localization of Activity-Dependent cpg15 Expression in Barrel Cortex
During adolescence and in the adult, receptive field plasticity in barrel cortex is manifested mainly in the superficial layers (reviewed in Fox, 2002). To determine if changes in cpg15 expression correspond with the location of electrophysiologically measured receptive field plasticity, we analyzed cpg15 distribution within serial sections tangentially cut through barrel cortex of 4-week-old mice after unilateral single whisker experience. We found that after 12 h of single whisker experience, cpg15 expression was highest in layer IV and layers II/III [Fig. 2(B–F, I)]. Although barrels were not anatomically apparent in layers II/III, cpg15 expression in these layers was similar to the pattern seen in layer IV, with increased expression in the region corresponding to the spared D1 barrel unit and decreased levels in the surrounding area representing the deprived whiskers. cpg15 expression in layers I, V and VI was low and remained relatively unchanged by whisker manipulation [Fig. 2(A, G–I)]. These results show that changes in cpg15 expression due to single whisker experience are localized to layers of barrel cortex that undergo electrophysiological changes during receptive field plasticity.
Figure 2.
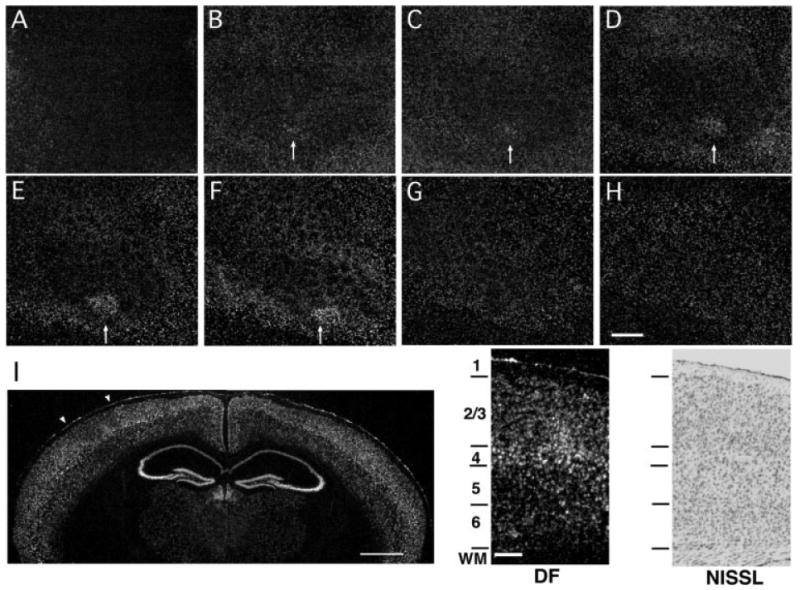
Activity-dependent cpg15 expression is localized to layers II/III and IV of barrel cortex. Representative dark-field photomicrograph of in situ hybridization for cpg15 mRNA on serial tangential sections through flattened cortex of a 4-week-old mouse after 12 h single whisker experience. The sections progress from layer I (A) to layer VI (H). Arrows mark the cortical region representing the spared whisker in layers II through IV (B–F). Scale bar, 0.5 mm. (I) cpg15 mRNA localization is shown in a coronal section through the brain of a mouse treated as above. The contralateral hemisphere to the spared whisker shows cpg15 up-regulation in the region representing the D1 whisker, with a concomitant down-regulation in the surrounding barrel field. White arrowheads indicate the region of the barrel field shown at high magnification on the right. Scale bar, 1 mm. Dark-field view shown side by side with a bright-field view of an adjacent Nissl stained section delineating cortical layers. Scale bar, 0.1 mm.
Time Course of Activity-Dependent cpg15 Expression in Barrel Cortex
Single unit studies have shown that changes in whisker responses can begin after 16 h of single whisker experience and continue for weeks thereafter (Barth et al., 2000). If these long-term changes result from alterations in molecular constituents within cortical neurons, one would expect that physiological changes be preceded by changes in gene expression. To investigate the onset and time course of cpg15 expression, we monitored mRNA levels through all cortical layers of mice after single whisker experience of 6, 12, 24 h, 3 d, or 7 d. In layer IV, the typical cpg15 pattern in response to single whisker experience could be seen as soon as 3 h after whisker trimming and was clearly visible until 12 h after onset of single whisker experience (Fig. 3, left). In layers II/III, onset of the cpg15 response was first evident at 6 h after whisker trimming, but was apparent up to 3 d after onset of single whisker experience (Fig. 3, right). To quantify these results and to asses whether cpg15 elevation in D1 and depression in the surrounding barrel field follow a similar time course, we compared the D1/Spared, Deprived/Spared, and D1/Deprived whisker ratios at each time point (Fig. 4). cpg15 induction in the D1 barrel (D1/Spared) peaked after 12 h of single whisker experience in layers II/III as well as IV [Fig. 4(A, B)] cpg15 depression in the surrounding barrel field (Deprived/Spared) showed a similar trend, but was significant only in layer IV at 12 h [Fig. 4(C, D)]. Although cpg15 induction in D1 [Fig. 4(A, B)] and depression in the deprived barrels [Fig. 4(C, D)] are significant only at 12 h (likely due to the small sample size at other time points), the patterns are somewhat different for the two layers, suggesting that with additional sampling it may be possible to discriminate whether cpg15 induction in the spared D1 barrel unit and depression in the surrounding deprived barrel units differ in their time course between layer IV and the superficial layers. When the net change in cpg15 expression was quantified by combining the increased cpg15 mRNA levels in the D1 barrel unit with the diminished cpg15 expression in the adjacent deprived barrel units (D1/Deprived), the difference in the time course of cpg15 expression between layers II/III and layer IV became evident, and was significant despite the small sample size. In layer IV, onset of change in cpg15 expression was after 3 h of single whisker experience, with levels markedly elevated only until 12 h [Fig. 4(E)]. In layers II/III, onset of the cpg15 response occurred later. A significant increase in the D1/Deprived signal ratio was detectable only after 12 h of single whisker experience [Fig. 4(F)]. However, in contrast to the rapid return to basal expression seen in layer IV, change in cpg15 levels in the layer II/III region corresponding to the D1 barrel unit was maintained for at least 3 d after onset of single whisker experience [Fig. 4(F)]. These laminar differences in the cpg15 response to single whisker experience are consistent with an initial activity-dependent response in layer IV, the primary thalamic input layer, subsequently progressing to the superficial layers where changes in receptive field properties then persist for days (Glazewski and Fox, 1996; Huang et al., 1998).
Figure 3.
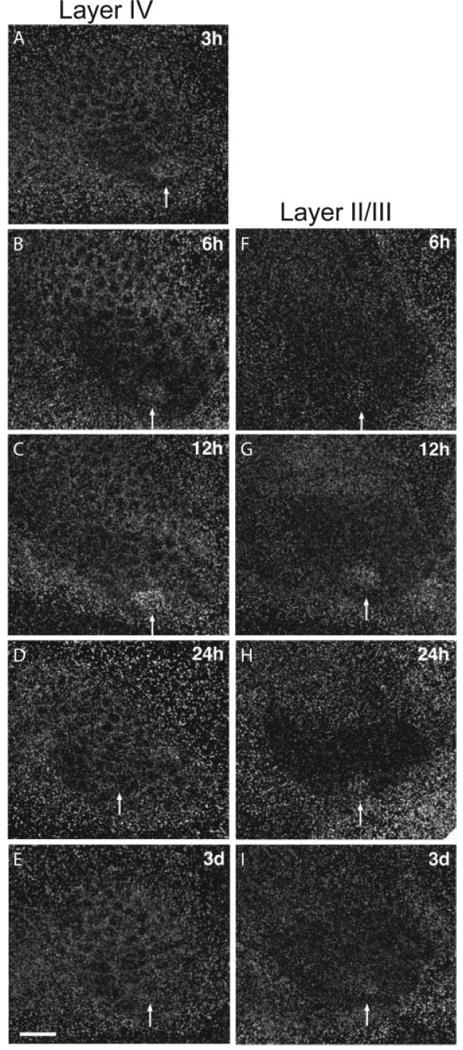
Time course of cpg15 expression in barrel cortex after spared whisker experience. Representative dark-field photomicrographs of cpg15 expression in layer IV (left) and layers II/III (right) of the barrel field after different lengths of spared whisker experience. Scale bar, 0.5 mm. Arrows mark the spared D1 barrel unit.
Figure 4.
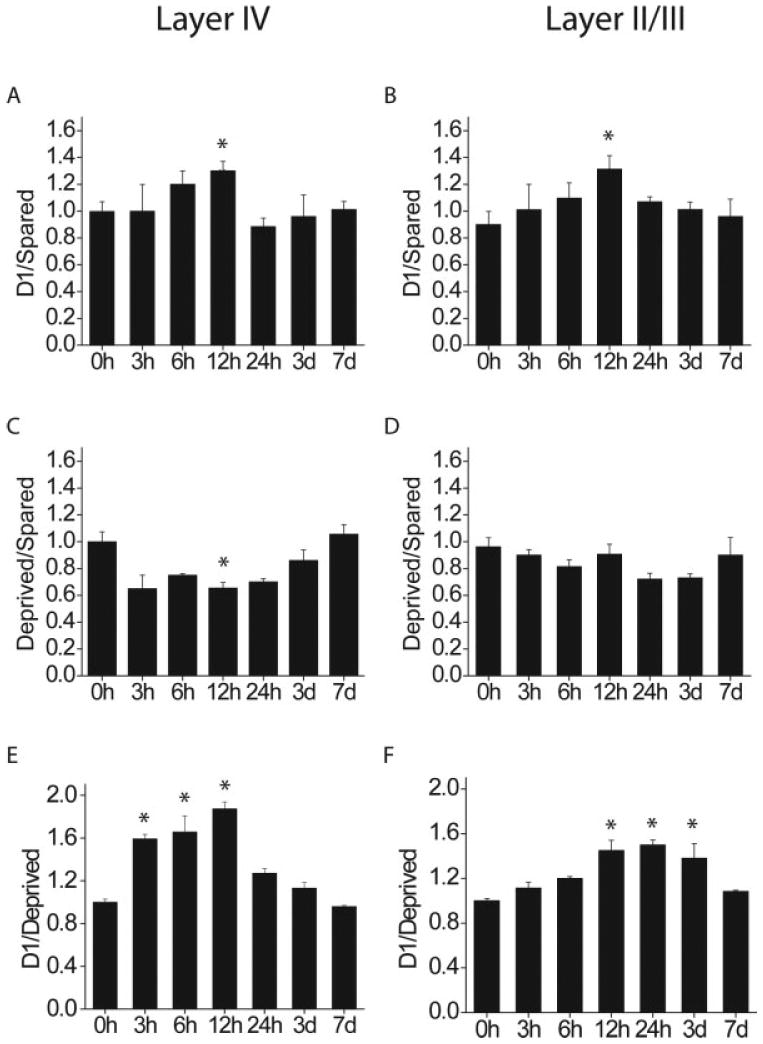
Quantification of the D1/Spared, Deprived/Spared, and D1/Deprived ratios in layers IV (A, C, E), and II/III (B, D, F) as a function of single whisker experience time (n = 2 per time point, 2–3 sections per brain). Compared to 0 h controls, *p < 0.0024 (5% significance level). Although cpg15 induction in D1 (A, B) and depression in the deprived barrels (C, D) are significant only at 12 h (likely due to the small sample size at other time points), the net change in cpg15 expression, combining induction in D1 and depression in the deprived neighboring barrel units (D1/Deprived) shows a significantly different time course in layers IV and II/III.
Decreased cpg15 Induction in α/δ CREB Knockout Mice
The transcription factor CREB is known to be required for experience-dependent receptive field plasticity in response to whisker manipulation (Glazewski et al., 1999). The requirement for CREB is likely due to its regulation of downstream effector genes that mediate changes in synaptic structure and function. Studies of cpg15 regulation in cultured cortical neurons indicate that CREB is a mediator of cpg15 activity-dependent expression in vitro (Fujino et al., 2003). To determine if cpg15 is a CREB-regulated effector gene that is induced in vivo during receptive field plasticity, we examined cpg15 expression in barrel cortex of α/δ CREB knockout mice after 12 h of single whisker experience. Since previous studies using the spared whisker paradigm have shown that plasticity in layers II/III of these mutants is relatively unaffected in adolescent animals (1–2 months) but is significantly diminished in adult (>6 months) mice (Glazewski et al., 1999), we tested both adolescent and adult knockout α/δ CREB mice.
When comparing cytochrome oxidase staining of layer IV sections from cortices of the CREB mutants with those of their wild-type counterparts, it was apparent that the size of the barrel field was smaller in the mutants [Fig. 5(A, B)]. CREB mutants were previously found to have a thalamus significantly smaller than normal (Pham et al., 2001), suggesting that CREB may have a role in development of sensory brain structures. However, organization of the individual barrels and their relative size and position within the field is normal. The CREB mutants show no overt behavioral abnormalities and the mutation does not seem to cause sensory or motor performance deficits (Bourtchuladze et al., 1994).
Figure 5.
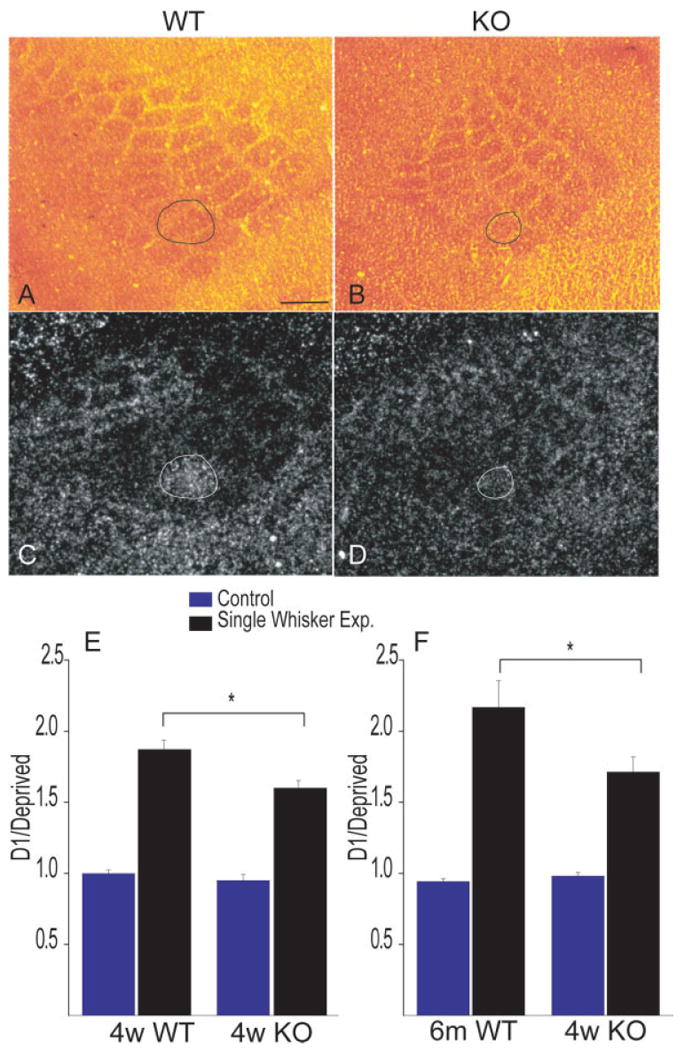
Induction of cpg15 is diminished in CREB mutant mice. Cytochrome oxidase staining of the barrel field of WT (A) and α/δ CREB knockout mice (B) with the D1 barrel unit outlined. Corresponding in situ hybridizations for cpg15 mRNA after 12 h single whisker experience in WT (C) and α/δ CREB knockout mice (D). (E) Quantification of the D1/Deprived ratios shows that cpg15 regulation in response to single whisker experience is significantly reduced in both 4-week-old (n = 8 +/+; n = 8 −/−; *p < 0.05) and 6-month-old animals (n = 6 +/+; n = 5 −/−; *p < 0.01).
In response to single whisker experience, the qualitative pattern of changes in cpg15 expression was similar in the wild-type and CREB mutants: cpg15 levels in the spared D1 barrel unit were increased while levels in the surrounding barrel field were decreased [Fig. 5(C, D)]. However, the magnitudes of the increase in the D1 barrel unit and the decrease in barrels representing the large deprived whiskers were both less in the α/δ CREB mutants relative to wild-type mice. To quantify the difference in cpg15 expression between wild-type and α/δ CREB knockouts, we compared the ratio of cpg15 signal intensity in D1 to its level in the surrounding deprived barrel units in control hemispheres or after single whisker experience. We found that cpg15 levels in control hemispheres of both adolescent 4-week-old mice and 6-month-old adults were not significantly different between the wild type and α/δ CREB knockouts [Fig. 5(E, F)]. The change in the D1/Deprived ratio in response to single whisker experience was 16% smaller in the 4-week-old α/δ CREB knockouts relative to their control counterparts [Fig. 5(E), *p < 0.05], and 23% smaller when 6-month-old mice were compared [Fig. 5(F), *p < 0.01]. These results show that lack of α/δ CREB isoforms diminishes activity-dependent changes in cpg15 expression in response to the spared whisker experience.
Discussion
We measured the experience-dependent expression of cpg15, a gene encoding a signaling molecule that promotes growth of dendritic and axonal arbors and synaptic maturation in developing neurons, in the adult barrel cortex. We found that the spatial and temporal patterns of cpg15 expression, and its regulation by CREB, are consistent with a role in mediating adult receptive field plasticity.
Spatial and Temporal Features of Cortical Plasticity
Layer II/III neurons are the primary substrate for receptive field plasticity in the adult barrel cortex (Glazewski and Fox, 1996; Glazewski et al., 1998). After deprivation, depression of deprived whisker responses, likely due to changes at synapses of layer IV onto layer II/III neurons (Allen et al., 2003; Shepherd et al., 2003), occurs rapidly (Glazewski and Fox, 1996). Potentiation of the response to the spared whisker in deprived barrels happens later. Previous studies in the Xenopus and feline visual systems have suggested that cpg15 expression is presynaptic to the connections undergoing activity-dependent plasticity (Corriveau et al., 1999; Nedivi et al., 2001). Thus, the spatio-temporal patterns of cpg15 expression we observe are consistent with experience-dependent physiological plasticity in barrel cortex. Early up-regulation (down-regulation) of cpg15 in layer IV may relate to strengthening (weakening) of layer IV to layer II/III synapses, while its subsequent expression in the superficial layers relates to modification of horizontal connections within these layers. It is interesting to note that cpg15 expression is regulated in both the barrel and its surrounding septal region since these structures receive input through parallel subcortical pathways (Koralek et al., 1988; Lu and Lin, 1993), and give rise to two distinct intracortical circuits (Kim and Ebner, 1999). The layer IV barrel columns receive input from the ventral posteromedial nucleus of the thalamus (VPM), and within somatosensory cortex project short distances to superficial layers of the same barrel or immediately neighboring barrels (Koralek et al., 1988; Lu and Lin, 1993; Kim and Ebner, 1999). Their corticocortical projections reach second somatosensory cortex (S-II). Neurons in the septal regions receive input from the posterior medial nucleus (PoM), and project to septa of more distant barrel cortex, as well as other cortical regions such as posteriomedial parietal cortex (PM) (Koralek et al., 1988; Lu and Lin, 1993; Kim and Ebner, 1999). These observations indicate that modifications in both types of circuits occur, or that they may be required for physiological changes in barrel representation.
Molecular Analysis of Receptive Field Plasticity in Barrel Cortex
Only a handful of studies have addressed the immediate effect (on the scale of hours) of sensory experience in the adult on expression of candidate proteins (Rocamora et al., 1996; Barth et al., 2000; Filipkowski et al., 2000; Bisler et al., 2002; Staiger et al., 2002). Here we show that, by altering sensory experience for a few hours, we can produce significant changes in cpg15 expression within barrel cortex. Transcriptional activation is usually a good indicator of a subsequent increase in protein levels. Indeed, following kainic acid seizure in the hippocampus, both the cpg15 transcript and the CPG15 protein are dramatically induced (Naeve et al., 1997; Nedivi et al., 1998). Other than transcription factors, cpg15 and BDNF are the only genes shown to be rapidly regulated by activity in a manner spatially and temporally correlated with experience-dependent synaptic modifications in adult barrel cortex (Rocamora et al., 1996). Moreover, cpg15 is the first activity-dependent effector gene demonstrated to be regulated downstream of CREB in the context of barrel cortex plasticity in vivo.
Previous studies have shown that barrel plasticity in α/δ CREB knockout mice is significantly reduced, although not eliminated (Glazewski et al., 1999). Our findings regarding cpg15 expression in these mice are consistent with these studies, in that activity-dependent cpg15 induction within the spared whisker barrel unit is diminished, but not completely abolished. In vitro studies of cpg15 regulation in cultured cortical neurons indicate that CREB is a mediator of cpg15 activity-dependent expression, but is by no means the only transcriptional activator involved (Fujino et al., 2003). Thus CREB likely works in concert with other transcription factors to modify expression of cpg15 and other genes that contribute to synaptic plasticity. Another possible explanation for the partial effect of α/δ CREB isoform deletion on both receptive field plasticity and cpg15 expression in barrel cortex is that in the mutants there is compensation for loss of the α/δ CREB isoforms by up-regulation of the beta isoform (Glazewski et al., 1999), or other CREB-like transcription factors (Hummler et al., 1994). These factors may act to partially rescue cortical receptive field plasticity.
Potential Role of cpg15 in Adult Receptive Field Plasticity
In the developing Xenopus tectum (Nedivi et al., 1998; Cantallops et al., 2000), cpg15 functions as an intercellular signaling molecule that promotes process outgrowth and synaptogenesis. While the role of cpg15 in adult barrel cortex has not been defined, it would be intriguing to consider that it performs a similar function. Recent in vivo imaging studies have not revealed changes in dendritic arbor morphology of barrel cortex neurons during receptive field plasticity (Trachtenberg et al., 2002). Yet these same studies provide evidence for increased synapse turnover in response to experience-dependent receptive field plasticity and demonstrate that synapse formation and elimination is associated with sprouting and retraction of dendritic spines (Trachtenberg et al., 2002). Expression of cpg15 in the context of receptive field plasticity could be related to its involvement in small-scale synaptic changes that consolidate adaptive cortical responses to peripheral manipulation.
Acknowledgments
Contract grant sponsor: NEI (E.N.).
Contract grant sponsor: Ellison Medical Foundation (E.N.).
Contract grant sponsor: Contract grant sponsor: NIH (K.S.).
Contract grant sponsor: Ford Foundation Fellowship (C.H.).
References
- Allen CB, Celikel T, Feldman DE. Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nature Neurosci. 2003;6:291–299. doi: 10.1038/nn1012. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M, Diamond ME, Ebner FF. An innocuous bias in whisker use in adult rats modifies receptive fields of barrel cortex neurons. J Neurosci. 1994;14:6978–6991. doi: 10.1523/JNEUROSCI.14-11-06978.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong-James M, Fox K. Spatiotemporal convergence and divergence in the rat S1 “barrel” cortex. J Comp Neurol. 1987;263:265–281. doi: 10.1002/cne.902630209. [DOI] [PubMed] [Google Scholar]
- Barth AL, McKenna M, Glazewski S, Hill P, Impey S, Storm D, Fox K. Up regulation of cAMP response element-mediated gene expression during experience-dependent plasticity in adult neocortex. J Neurosci. 2000;20:4206–4216. doi: 10.1523/JNEUROSCI.20-11-04206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisler S, Schleicher A, Gass P, Stehle JH, Zilles K, Staiger JF. Expression of c-Fos, ICER, Krox-24 and JunB in the whisker-to-barrel pathway of rats: time course of induction upon whisker stimulation by tactile exploration of an enriched environment. J Chem Neuroanat. 2002;3:187–198. doi: 10.1016/s0891-0618(01)00155-7. [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva A. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Cantallops I, Haas K, Cline HT. Postsynaptic CPG15 promotes synaptic maturation and presynaptic axon arbor elaboration in vivo. Nat Neurosci. 2000;3:1004–1011. doi: 10.1038/79823. [DOI] [PubMed] [Google Scholar]
- Corriveau R, Shatz CJ, Nedivi E. Dynamic regulation of cpg15 during activity-dependent synaptic development in the mammalian visual system. J Neurosci. 1999;19:7999–8008. doi: 10.1523/JNEUROSCI.19-18-07999.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond ME, Armstrong-James M, Ebner FF. Experience-dependent plasticity in adult rat barrel cortex. Proc Natl Acad Sci USA. 1993;90:2082–2086. doi: 10.1073/pnas.90.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipkowski RK, Rydz M, Berdel B, Morys J, Kaczmarek L. Tactile experience induces c-fos expression in rat barrel cortex. Learn Mem. 2000;7:116–122. doi: 10.1101/lm.7.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. The cortical component of experience-dependent synaptic plasticity in the rat barrel cortex. J Neurosci. 1994;14:7665–7679. doi: 10.1523/JNEUROSCI.14-12-07665.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. Anatomical pathways and molecular mechanisms for plasticity in the barrel cortex. Neuroscience. 2002;111:799–814. doi: 10.1016/s0306-4522(02)00027-1. [DOI] [PubMed] [Google Scholar]
- Fujino T, Lee WA, Nedivi E. Regulation of cpg15 by signaling pathways that mediate synaptic plasticity. Mol Cell Neurosci. 2003;24:538–554. doi: 10.1016/s1044-7431(03)00230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazewski S, Barth AL, Wallace H, McKenna M, Silva A, Fox K. Impaired experience-dependent plasticity in barrel cortex of mice lacking the alpha and delta isoforms of CREB. Cereb Cortex. 1999;9:249–256. doi: 10.1093/cercor/9.3.249. [DOI] [PubMed] [Google Scholar]
- Glazewski S, Chen CM, Silva A, Fox K. Requirement for α-CaMKII in experience-dependent plasticity of the barrel cortex. Science. 1996;272:421–423. doi: 10.1126/science.272.5260.421. [DOI] [PubMed] [Google Scholar]
- Glazewski S, Fox K. Time course of experience-dependent synaptic potentiation and depression in barrel cortex of adolescent rats. J Neurophysiol. 1996;75:1714–1729. doi: 10.1152/jn.1996.75.4.1714. [DOI] [PubMed] [Google Scholar]
- Glazewski S, Herman C, McKenna M, Chapman PF. Long-term potentiation in vivo in layers II/III of rat barrel cortex. Neuropharm. 1998;37:581–592. doi: 10.1016/s0028-3908(98)00039-2. [DOI] [PubMed] [Google Scholar]
- Hardingham N, Glazewski S, Pakhotin P, Mizuno K, Chapman PF, Giese KP, Fox K. Neocortical long-term potentiation and experience-dependent synaptic plasticity require α-calcium/calmodulin-dependent protein kinase II autophosphorylation. J Neurosci. 2003;23:4428–4436. doi: 10.1523/JNEUROSCI.23-11-04428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevroni D, Rattner A, Bundman M, Lederfein D, Gbarah A, Mangelus M, Silverman M, Kedar H, Naor C, Kornuc M, Hanoch T, Seger R, Theill L, Nedivi E, Richter Levin G, Citri Y. Hippocampal plasticity involves extensive gene induction and multiple cellular mechanisms. J Mol Neurosci. 1998;10:75–98. doi: 10.1007/BF02737120. [DOI] [PubMed] [Google Scholar]
- Huang W, Armstrong-James M, Rema V, Diamond ME, Ebner FF. Contribution of supragranular layers to sensory processing and plasticity in adult rat barrel cortex. J Neurophysiol. 1998;80:3261–3271. doi: 10.1152/jn.1998.80.6.3261. [DOI] [PubMed] [Google Scholar]
- Hummler E, Cole TJ, Blendy JA, Ganss R, Aguzzi A, Schmid W, Beermann F, Schutz G. Targeted mutation of the cAMP response element binding protein (CREB) gene: compensation within the CREB/ATF family of transcription factors. Proc Natl Acad Sci USA. 1994;91:5647–5651. doi: 10.1073/pnas.91.12.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U, Ebner FF. Barrels and septa: separate circuits in rat barrel field cortex. J Comp Neurol. 1999;408:489–505. [PubMed] [Google Scholar]
- Koralek KA, Jensen KF, Killackey HP. Evidence for two complementary patterns of thalamic input to the rat somatosensory cortex. Brain Res. 1988;463:346–351. doi: 10.1016/0006-8993(88)90408-8. [DOI] [PubMed] [Google Scholar]
- Lee WCA, Nedivi E. Extended plasticity of visual cortex in dark-reared animals may result from prolonged expression of genes like cpg15. J Neurosci. 2002;22:1807–1815. doi: 10.1523/JNEUROSCI.22-05-01807.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SM, Lin RC. Thalamic afferents of the rat barrel cortex: a light- and electron-microscopic study using Phaseolus vulgaris leucoagglutinin as an anterograde tracer. Somatosens. Mot Res. 1993;10:1–16. doi: 10.3109/08990229309028819. [DOI] [PubMed] [Google Scholar]
- Naeve GS, Ramakrishnan M, Rainer K, Hevroni D, Citri Y, Theill LE. Neuritin: a gene induced by neural activity and neurotrophins that promotes neuritogenesis. Proc Natl Acad Sci USA. 1997;94:2648–2653. doi: 10.1073/pnas.94.6.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedivi E, Hevroni D, Naot D, Israeli D, Citri Y. Numerous candidate plasticity-related genes revealed by differential cDNA cloning. Nature. 1993;363:718–722. doi: 10.1038/363718a0. [DOI] [PubMed] [Google Scholar]
- Nedivi E, Javaherian A, Cantallops I, Cline HT. Developmental regulation of CPG15 expression in Xenopus. J Comp Neurol. 2001;435:464–473. doi: 10.1002/cne.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedivi E, Wu GY, Cline HT. Promotion of dendritic growth by CPG15, an activity-induced signaling molecule. Science. 1998;281:1863–1866. doi: 10.1126/science.281.5384.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TA, Rubenstein JLR, Silva AJ, Storm DR, Stryker MP. The CRE/CREB pathway is transiently expressed in thalamic circuit development and contributes to refinement of retinogeniculate axons. Neuron. 2001;31:409–420. doi: 10.1016/s0896-6273(01)00381-6. [DOI] [PubMed] [Google Scholar]
- Rocamora N, Welker E, Pascual M, Soriano E. Up regulation of BDNF mRNA expression in the barrel cortex of adult mice after sensory stimulation. J Neurosci. 1996;16:4411–4419. doi: 10.1523/JNEUROSCI.16-14-04411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- Shepherd GMG, Pologruto TA, Svoboda K. Circuit analysis of experience-dependent plasticity in the developing rat barrel cortex. Neuron. 2003;38:277–289. doi: 10.1016/s0896-6273(03)00152-1. [DOI] [PubMed] [Google Scholar]
- Simons DJ. Response properties of vibrissa units in rat SI somatosensory neocortex. J Neurophysiol. 1978;41:798–820. doi: 10.1152/jn.1978.41.3.798. [DOI] [PubMed] [Google Scholar]
- Staiger JF, Masanneck C, Bisler S, Schleicher A, Zuschratter W, Zilles K. Excitatory and inhibitory neurons express c-Fos in barrel-related columns after exploration of a novel environment. Neuroscience. 2002;109:687–699. doi: 10.1016/s0306-4522(01)00501-2. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979;17:11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebal cortex. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]


