Abstract
The objective of this study was to describe the estimated within-herd prevalence (WHP) of Mycobacterium avium subsp. paratuberculosis (Map) in a sample of infected dairy herds in Minnesota (N = 66) using test results from bacterial culture of pooled fecal samples. Fecal samples were collected from up to 100 cows in each herd and were tested using bacterial culture in pools of 5 cows based on age order. The mean herd size was 222 (44 to 1500) milking cows; the cows were predominantly Holstein. Using a frequentist approach, the within-herd mean individual fecal prevalence was 10% [95% confidence interval (CI) = 4% to 16%] assuming 70% test sensitivity and 99.5% test specificity. Using Bayesian methods, the estimated true within-herd individual cow prevalence was 14% (95% CI = 7% to 27%). Within-herd prevalence was higher in larger dairy herds than in herds with fewer cows. As Map is the causative agent of Johne’s disease (JD), the results of this study could contribute to the success of a nationwide control program for this disease.
Résumé
L’objectif de la présente étude était de décrire la prévalence intra-troupeau estimée (WHP) de Mycobacterium avium ssp. paratuberculosis (Map) dans un échantillon de troupeaux infectés du Minnesota (N = 66) à l’aide des résultats de test de la culture bactérienne de pools d’échantillons fécaux. Des échantillons de fèces ont été prélevés de jusqu’à 100 vaches dans chaque troupeau et ont été testés par culture bactérienne de pools de 5 vaches regroupées en ordre d’âge. La taille moyenne d’un troupeau était de 222 (44 à 1500) vaches laitières; les vaches étaient en prédominance des Holstein. Utilisant une approche fréquentiste, la prévalence fécale individuelle moyenne intra-troupeau était de 10 % [intervalle de confiance 95 % (CI) = 4 % à 16 %] en assumant une sensibilité du test de 70 % et une spécificité du test de 99,5 %. En utilisant de méthodes Bayesienne, la vraie prévalence individuelle estimée intra-troupeau était de 14 % (95 % CI = 7 % à 27 %). La prévalence intra-troupeau était plus élevée dans les troupeaux laitiers plus gros que dans les troupeaux avec moins de vaches. Étant donné que Map est l’agent étiologique de la maladie de Johne (JD), les résultats de la présente étude pourrait contribuer au succès d’un programme national de maîtrise de cette maladie.
(Traduit par Docteur Serge Messier)
Introduction
Fecal culture is considered the gold standard for detecting Mycobacterium avium subsp. paratuberculosis (Map), the causative agent of Johne’s disease (JD). Due to its high cost and long turnaround, however, estimation of within-herd prevalence (WHP) has typically been limited to seroprevalence estimates. These estimates (where available) are imprecise due to the low sensitivity of the test as well as the low number of cows tested in many seroprevalence studies. For example, using both fecal culture and serum enzyme-linked immunosorbent assay (ELISA) tests in parallel, 1 study of 7 dairy herds in Michigan, USA reported an apparent prevalence that varied from 16% to 81% (1). These estimates, however, were obtained from only 7 herds and a total of 533 cows. A study in Colorado, which used only 15 herds but a total of 10 280 cows, found that the apparent WHP varied from 0% to 7.8% (2). In contrast, a large seroprevalence study in the Netherlands, which used a random sample of 378 dairy herds and 15 882 animals older than 36 months, reported that the overall average WHP was 2.54% (95% CI = 2.22 to 2.87) and 4.72 (95% CI = 4.31 to 4.76) among the 77 seropositive herds (3). Thus, apparent WHP seems to vary significantly among studies, depending on the number of herds and the total number of animals tested.
It is therefore evident that in order to more accurately estimate WHP, additional information is urgently needed not only from a large number of cows and herds, but also by using fecal culture, which is known to have a higher sensitivity (Se) and specificity (Sp) than serologic tests. Knowledge of WHP is important because it is a key parameter in developing strategies for herd testing and control. Implementing specific management practices on dairy farms to decrease within-herd transmission of JD depends on estimating within-herd prevalence and information is useful in assessing progress made in control programs. For example, a study that used within-herd prevalence as the outcome of a simulation model concluded that positive herds can have economic benefits by applying management practices such as contract rearing of heifers and improved calf hygiene (4). On the other hand, without any control efforts, the mean prevalence gradually increased to > 50% after 20 y. Within-herd prevalence is thus used as the measure of success for any control program.
While improving the sensitivity and specificity of diagnostic tests has been the focus of much research, a different approach aims to develop more cost-effective testing strategies using available diagnostic tests. It has been suggested that a pooled fecal culture method, which combines fecal samples from several cows into 1 culture unit, is a good alternative strategy for lowering the costs of procedures in herd-screening programs for dairy cattle and sheep (5–9). In a simulation study (10), it was found that the use of fecal pools reduced testing costs by 43% in a 100-cow herd with a high prevalence of Map and by up to 71% in a 1000-cow herd with a low prevalence. Nevertheless, a possible trade-off for the reduced cost is a decrease in test sensitivity of up to 60% (11). Additional information is needed about the use of pooled fecal culture to estimate WHP, so that this method could become well-established among veterinary authorities that aim to control the disease.
The objective of this study was to describe the estimated within-herd prevalence of Map in a sample of infected dairy cattle herds in Minnesota, using test results from bacterial culture of pooled fecal samples.
Materials and methods
This study was a part of a larger study to evaluate the association between Map in dairy cows and their environment that used bacterial culture of pooled fecal samples to assess herd infection status (12). In summer 2002, 108 Minnesota dairy herds were sampled, including 80 herds known to be infected and 28 herds shown to be noninfected by previous testing. Herds were selected from the database available for 2 Johne’s disease control programs run by the Minnesota Board of Animal Health (MBAH): herds known to be infected and herds known to be noninfected based on previous testing in the JD Control Program and the Voluntary Johne’s Disease Herd Status Program. Herd owners were contacted by letter to request their voluntary participation in the study.
A 40-g fecal sample was collected from up to 100 cows in each herd by rectal retrieval using a disposable obstetric glove and placed in a 90-mL plastic container. In herds with more than 100 milking cows, cows were selected as randomly as possible, given the limitation of working on a commercial farm with a very tight schedule. All samples were stored in an iced cooler while being transported to Minnesota Veterinary Diagnostic Laboratory (MVDL) for further processing. The age of each cow sampled was recorded. As the objective of this study was to describe within-herd prevalence, data analysis was restricted to herds with at least 1 test-positive fecal pool. All negative herds from either program were therefore excluded from the analysis.
Bacterial culture of fecal samples
Fecal samples were sorted at the MVDL based on the cows age, with feces from 5 cows per pooled sample. A 2-g sample of feces from each cow was mixed at the laboratory with similar samples from other cows within the pool and a 2-g sample of the resulting pooled fecal sample was processed using the bacterial culture method described in a previous study (7). Briefly, a sedimentation culture procedure was used (13) with 72 h of sedimentation before inoculation of 4 tubes containing HEY medium. Colony counts were recorded weekly for 16 wk and final results were reported as negative, light bacterial load (BL) (mean of 0.25 to 9 colonies per tube, CPT), moderate BL (mean of 10 to 49 CPT), and heavy BL (mean of 〉 50 CPT). Herds were defined as positive if at least 1 pool was culture positive to Map.
Statistical Analysis
Apparent and true within-herd prevalence of the fecal pool were calculated using standard formulas (14). Apparent prevalence (AP) was defined as the number of test positive pools/total number of pools tested and true prevalence (TP) was defined as AP-(1-Sp)/Se-(1-Sp), where pooled sensitivity = 70% and pooled specificity = 99.5% (15). Pool sensitivity was defined as the proportion of pooled samples with positive results of all the pools with at least 1 positive cow. Pool specificity was defined as 1 minus the proportion of false positive pools. A false positive pool was defined as a fecal pool that cultured positive from 5 fecal culture negative cows.
Individual cow fecal prevalence was estimated among herds with positive pools using 3 approaches described in a previous study (16), including the following:
-
a frequentist approach in which Se and Sp each equal 100%,
Equation 1 -
a frequentist approach in which Se = 70% and Sp = 99.5%,
Equation 2 where: Pr = individual fecal prevalence, Se = pool sensitivity, Sp = pool specificity, k = number of animals per pool, and P = proportion of positive pools; and
The Bayesian approach requires input for prior estimates of prevalence and test Se and Sp based on expert knowledge or previous data (16). As priors for animal level prevalence for the Bayesian method, herd ELISA prevalence obtained from previous test results of the participating herds was used. As priors for herd Se and Sp, values were used that are similar to those published in recent literature. For both Se and Sp, it was assumed that a beta distribution was the prior distribution with the following parameters [Se = Beta (22.51, 10.22), median = 0.69, left (lower limit, 2.5th percentile) = 0.52, right X (upper limit, 97.5th percentile) = 0.83, Sp = Beta (151.2, 1), median = 0.995, left X = 0.9759, right X = 0.998]. For methods A and B, exact 95% CI were calculated, assuming a binomial distribution for the number of positive pools so that prevalence lower limit (PL) ≥ 0 and prevalence upper limit (PU) ≤ 1 (16). For the Bayesian method, 95% probability intervals were obtained from the distribution of the posterior as the 2.5th and 97.5th percentiles (15).
Results
Samples were collected from 8695 cows from 108 herds and 1739 fecal pools were tested using bacterial culture. Of the 108 herds tested, 66 (61%) had at least 1 fecal pool positive to Map. The following analysis is therefore restricted to these positive herds. A total of 1227 pools were tested from the 66 herds and 304 pools (25%) were test-positive. The mean herd size was 222 (44 to 1500) milking cows, the cows were predominantly Holsteins, and average milk production per lactation was approximately 10 500 kg. Of the 66 herds, 86% used freestall housing for milk cows. Of the 66 herds, 26%, 32%, and 42% had < 100, 100 to 200, and > 200 milking cows, respectively. In 10 of the herds (15%), all cows were sampled. On average, 54% of the cows were sampled in the other 56 herds (7% to 96%). On average, the number of cows and pools tested in each herd was 91 and 18, respectively.
The Map mean fecal pool apparent prevalence (AP) was 26% (minimum 5%, maximum 67%) and the mean fecal pool true prevalence (TP) was 37% (minimum 8%, maximum 100%). The proportion of high prevalence herds (> 30% positive pools) was significantly higher among herds with > 200 cows than among herds with < 100 cows (P = 0.02) and the proportion of low prevalence herds (5% to 9%) was inversely associated with herd size category (P = 0.03). Pearson correlation between the number of milk cows and the percent of positive pools was 0.17 (P < 0.01). Of the 66 positive herds, 65% had at least 1 pool with high bacterial concentration and in 33% and 1.5% (1 herd) of the herds, the highest pool bacterial load was moderate and low concentration, respectively.
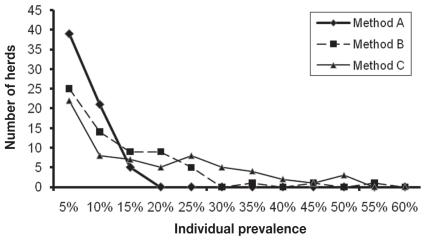
The median within-herd individual animal fecal prevalence (IP) was similar in method B and C (13% and 12%, respectively) compared to a lower median IP for method A (4.6%) (Table I). The frequency distribution of within-herd IP based on methods B and C was similar (Figure 1).
Table I.
Comparison of 3 methods for estimating individual prevalence (IP) of Map based on fecal pool samples in 66 Minnesota dairy herds
| Method | Mean IP | 95% CI | Median IP | Min. to Max. |
|---|---|---|---|---|
| A | 6% | 2% to 14% | 4.6% | 1% to 20% |
| B | 10% | 4% to 16% | 13% | 3% to 70% |
| C | 14% | 7% to 27% | 12% | 2% to 49% |
A — Frequentist approach in which sensitivity and specificity each equal 100%.
B — Frequentist approach in which sensitivity = 70% and specificity = 99.5%.
C — Bayesian approach in which sensitivity = 70% and specificity = 99.5%.
CI — Confidence interval.
Figure 1.
Comparison of 3 methods for estimating within-herd individual prevalence of Mycobacterium paratuberculosis based on fecal pool samples in 66 Minnesota dairy herds.
Method A — Frequentist approach in which sensitivity and specificity each equal 100%.
Method B — Frequentist approach in which sensitivity = 70% and specificity = 99.5%.
Method C — Bayesian approach in which sensitivity = 70% and specificity = 99.5%.
Discussion
The objective of this study was to characterize the estimated within-herd prevalence of Map in a sample of infected Minnesota dairy herds. The strength of the study is the large sample size in terms of the number of herds and cows. In addition, different types of housing were represented in this study. While average herd size and lactation milk production in the participating herds are above the average for dairy herds in the US and Minnesota due to their general management practices as well as housing, participating herds are representative for midsize and high producing dairies of the Midwest and the US. Therefore, the results of this study can be referred to this dairy population. Owners of these herds are usually more aware of JD and are therefore willing to invest resources in controlling the disease (18). Information about within-herd prevalence in these herds could therefore contribute to the success of a nationwide control program for Johne’s disease.
The current study used 5 cows in pools based on a study that found that the sensitivity of detection for Map was greater with a smaller pool size (5 versus 10 samples per pool) and in pools with at least 1 of 5 cows shedding high BL (94%), compared to low BL (44%) (7). In contrast, another study (9) recommended 10 cows per pool since its sensitivity was found to be very similar to the sensitivity when 5 cows per pool were used. Another study (19) evaluated the use of fecal pools by comparing it to individual fecal culture. As the current study used this knowledge as part of a large-scale field study, not using individual cow culture is not a major limitation. This is because the main focus of the study was to describe the distribution of Map in a variety of dairy herds using the fecal pool approach which has been shown previously to reflect individual culture prevalence.
In the current study, a non-perfect pool Sp of 99.5% was used because the pooling approach requires additional processing procedures that can result in cross-contamination between pools. Consequently, while possibly very rare, a fecal pool can be tested positive while all individual samples are tested negative.
One limitation of this study is related to fecal culture sensitivity constraints. In the current study, the limited pool test sensitivity for light shedders (1 to 10 CPT), possibly due to the dilution effect, may have failed to detect Map in some of the fecal pools and as a result may have underestimated the true WHP. The use of sedimentation as a concentration method for fecal culture as in the current study has previously been reported as being less sensitive than the centrifugation method at detecting cows that are low shedders (20,21).
The literature provides several methods for estimating the prevalence of infection from pooled results, most of which are frequentist in nature (16,22). While frequentist methods do not take existing information about population prevalence into account, Bayesian methods allow such information to be incorporated (23). The estimation of the individual prevalence of JD based on fecal pool prevalence for Map was first described (using the Bayesian method) in a California study with a 4% animal level prevalence (95% CI, 2% to 8%) (17). This difference from our results is likely due to differences in climate, housing, management, and herd size in dairy herds between California and those in Minnesota. When evaluating the results obtained by the 3 methods, it is important to consider that they assume random sampling and random allocation of the cows into the pools. In the current study, fecal pools were formed based on age order. It is expected that any bias due to violation of the random allocation assumption was minimal because of lack of significance of the age order of cows within the fecal pools (data not presented).
The results obtained by the methods that used non-perfect test sensitivity (70%) were expected to differ from the method that assumed perfect test sensitivity. The primary advantage of using the Bayesian approach is that it takes into consideration the uncertainty related to the test sensitivity, specificity, and prevalence. The large (95%) CI of mean within-herd prevalence probably reflects this uncertainty. Since the test sensitivity also depends on the distribution of stages of infection in the test population (23), the estimates using the Bayesian approach are most likely to reflect the real individual prevalence. A recent study (24) used similar methods to estimate the prevalence of ovine JD infection from pooled fecal samples and found that the Bayesian methods produced more variable mean estimates and narrower credible intervals than the frequentist methods, which is in contrast to our findings.
Using a stochastic model, a previous study (10) found that herd sensitivity increased as the proportion of heavy shedders increased. In the current study, the high proportions of herds with at least 1 pool with heavy bacterial load (65%) support this finding. Furthermore, similar to our findings, the previous study (10) found that the probability of detecting at least 1 pool of moderate or heavy shedders was almost 100% in larger and higher prevalence herds. While light fecal shedding cows may represent 70% of the infected animals in heavily infected herds (25), in the current study at least 1 pool was likely to have heavy BL in 65% of the herds.
The greater proportion of high prevalence herds among larger herds (> 100 cows) suggests that common management practices on these farms in areas such as cattle housing and feeding, cow flows, calf rearing, and introduction of cattle from other herds may contribute to the within-herd transmission of JD and therefore to higher prevalence.
The current study provides an estimate of WHP of Map infection from a sample of Minnesota dairy herds. These study results are useful for the JD control program nationwide since there is a need for initial WHP in order to predict future prevalence after JD control programs have been implemented.
References
- 1.Johnson-Ifearulundu YJ, Kaneene JB, Sprecher DJ, Gardiner JC, Lloyd JW. The effect of subclinical Mycobacterium paratuberculosis infection on days open in Michigan, USA, dairy cows. Prev Vet Med. 2000;46:171–181. doi: 10.1016/s0167-5877(00)00145-8. [DOI] [PubMed] [Google Scholar]
- 2.Hirst HL, Garry FB, Morley PS, et al. Seroprevalence of Mycobacterium avium subsp. paratuberculosis infection among dairy cows in Colorado and herd-level risk factors for seropositivity. J Am Vet Med Assoc. 2004;225:97–101. doi: 10.2460/javma.2004.225.97. [DOI] [PubMed] [Google Scholar]
- 3.Muskens J, Barkema HW, Russchen E, van Maanen K, Schukken YH, Bakker D. Prevalence and regional distribution of paratuberculosis in dairy herds in The Netherlands. Vet Microbiol. 2000;77:253–261. doi: 10.1016/s0378-1135(00)00310-2. [DOI] [PubMed] [Google Scholar]
- 4.Groenendaal H, Nielen M, Jalvingh AW, Horst SH, Galligan DT, Hesselink JW. A simulation of Johne’s disease control. Prev Vet Med. 2002;54:225–245. doi: 10.1016/s0167-5877(02)00027-2. [DOI] [PubMed] [Google Scholar]
- 5.Kalis CHJ, Hesselink JW, Barkema HW, Collins MT. Culture strategically pooled bovine fecal samples as a method to screen herds for paratuberculosis. J Vet Diagn Invest. 2000;12:547–551. doi: 10.1177/104063870001200609. [DOI] [PubMed] [Google Scholar]
- 6.Sergeant ES, Whittington RJ, More SJ. Sensitivity and specificity of pooled faecal culture and serology as flock-screening tests for detection of ovine paratuberculosis in Australia. Prev Vet Med. 2002;52:199–211. doi: 10.1016/s0167-5877(01)00261-6. [DOI] [PubMed] [Google Scholar]
- 7.Wells SJ, Whitlock RH, Lindeman CJ, Fyock T. Evaluation of bacteriologic culture of pooled fecal samples for detection of Mycobacterium paratuberculosis. Am J Vet Res. 2002;63:1207–1211. doi: 10.2460/ajvr.2002.63.1207. [DOI] [PubMed] [Google Scholar]
- 8.Whittington RJ, Fell S, Walker D, et al. Use of pooled fecal culture for sensitive and economic detection of Mycobacterium avium subsp. paratuberculosis infection in flocks of sheep. J Clin Microbiol. 2000;38:2550–2556. doi: 10.1093/benz/9780199773787.article.b00008347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Schaik G, Pradenas FM, Mella NA, Kruze VJ. Diagnostic validity and costs of pooled fecal samples and individual blood or fecal samples to determine the cow- and herd-status for Mycobacterium avium subsp. paratuberculosis. Prev Vet Med. 2007;82:159–165. doi: 10.1016/j.prevetmed.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 10.van Schaik G, Stehman SM, Schukken YH, Rossiter CR, Shin SJ. Pooled fecal culture sampling for Mycobacterium avium subsp. paratuberculosis at different herd sizes and prevalence. J Vet Diagn Invest. 2003;15:233–241. doi: 10.1177/104063870301500304. [DOI] [PubMed] [Google Scholar]
- 11.Vialard J, Lacheretz A, Thiercy-Richard Y, Prave M. Détection de l’infection paratuberculeuse chez les bovins par une technique de coproculture de groupe. Revue Méd Vét. 1993;144:527–533. [Google Scholar]
- 12.Raizman EA, Wells SJ, Godden SM, et al. The distribution of Mycobacterium avium ssp. paratuberculosis in the environment surrounding Minnesota dairy farms. J Dairy Sci. 2004;87:2959–2966. doi: 10.3168/jds.S0022-0302(04)73427-X. [DOI] [PubMed] [Google Scholar]
- 13.Whipple DL, Callihan DR, Jarnagin JL. Cultivation of Mycobacterium paratuberculosis from bovine fecal specimens and a suggested standardized procedure. J Vet Diagn Invest. 1991;3:368–373. doi: 10.1177/104063879100300424. [DOI] [PubMed] [Google Scholar]
- 14.Martin SW, Meek AH, Willeberg P. Veterinary Epidemiology, Principles and Methods. 1st ed. Ames, Iowa: Iowa State University Press; 1987. p. 343. [Google Scholar]
- 15.Collins MT, Gardner IA, Garry FB, Roussel AJ, Wells SJ. Consensus recommendations on the diagnostic testing for the detection of the paratuberculosis in cattle in the United States. J Am Vet Med Assoc. 2006;229:1912–1919. doi: 10.2460/javma.229.12.1912. [DOI] [PubMed] [Google Scholar]
- 16.Cowling DW, Gardner IA, Johnson WO. Comparison of methods for estimation of individual level prevalence based on pooled samples. Prev Vet Med. 1999;39:211–225. doi: 10.1016/s0167-5877(98)00131-7. [DOI] [PubMed] [Google Scholar]
- 17.Tavornpanich S, Gardner IA, Anderson RJ, et al. Evaluation of microbial culture of pooled fecal samples for detection of Mycobacterium avium subsp. paratuberculosis in large dairy herds. Am J Vet Res. 2004;65:1061–1070. doi: 10.2460/ajvr.2004.65.1061. [DOI] [PubMed] [Google Scholar]
- 18.Raizman EA, Wells SJ, Godden SM, Fetrow J, Friendshuh K, Oakes MJ. Characterization of Minnesota dairy herds participating in a Johne’s disease control program and evaluation of the program risk assessment tool. Prev Vet Med. 2006;75:22–33. doi: 10.1016/j.prevetmed.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Wells SJ, Godden SM, Lindman CJ, Collins JE. Evaluation of bacteriologic culture of individual and pooled fecal samples for detection of Mycobacterium paratuberculosis in dairy cattle herds. J Am Vet Med Assoc. 2003;223:1022–1025. doi: 10.2460/javma.2003.223.1022. [DOI] [PubMed] [Google Scholar]
- 20.Whitlock RH, Rosenberger AE. Fecal culture protocol of mycobacterium paratuberculosis. A recommended procedure. Proc Ann Meetin US Anim Hlth Assoc. 1990;94:280–285. [Google Scholar]
- 21.Whitlock RH, Rosenberger AE, Sweeney RW, Spencer PA. National survey of diagnostic laboratories; the performance of fecal culture and serologic testing for Johne’s disease. Proceedings of the Fifth International Colloquium on Paratuberculosis. 1992:242–249. [Google Scholar]
- 22.Sacks JM, Bolin SR, Crowder SV. Prevalence estimation from pooled samples. Am J Vet Res. 1989;50:205–206. [PubMed] [Google Scholar]
- 23.Gardner IA. The utility of Bayes’ theorem and Bayesian inference in veterinary clinical practice and research. Aust Vet J. 2002;80:758–761. doi: 10.1111/j.1751-0813.2002.tb11347.x. [DOI] [PubMed] [Google Scholar]
- 24.Toribio JA, Sergeant ES. A comparison of methods to estimate the prevalence of ovine Johne’s infection from pooled faecal samples. Aust Vet J. 2007;85:317–324. doi: 10.1111/j.1751-0813.2007.00188.x. [DOI] [PubMed] [Google Scholar]
- 25.Whitlock RH, Wells SJ, Sweeney RW, Van Tiem J. ELISA and fecal culture for paratuberculosis (Johne’s disease): Sensitivity and specificity of each method. Vet Microbiol. 2000;77:387–398. doi: 10.1016/s0378-1135(00)00324-2. [DOI] [PubMed] [Google Scholar]