Abstract
The objectives of this study were to determine the effect of bacterial culture conditions on adherence of enterohemorrhagic Escherichia coli (EHEC) O157:H7 strain 86-24 in vivo to pig enterocytes and to compare the results with adherence in vitro to cultured HEp-2 and IPEC-J2 cells. Growth of O157:H7 in MacConkey broth (MB) resulted in almost no adherence to both HEp-2 and IPEC-J2 cells; prior exposure of the bacteria to pH 2.5 reduced adherence. There was greater adherence by bacteria from static cultures than by those from shaken cultures and by bacteria cultured in brain–heart infusion (BHI) plus NaHCO3 (BHIN) than by bacteria cultured in BHI. In contrast, in pig ileal loops, bacteria cultured in MB adhered well to enterocytes, and prior exposure to pH 2.5 had no effect on adherence. Among several media tested for their effect on bacterial adherence in the pig intestine, MB and BHIN proved to be the best. Bacterial adherence was dose-dependent and was more extensive in the ileum than in the colon. This study demonstrated that there are remarkable differences between culture conditions that promote adherence of an EHEC O157:H7 strain in vitro and in vivo, that culture conditions profoundly affect adherence to epithelial cells in vitro and in vivo, and that pig ileal loops are better suited to adherence studies than are colon loops.
Résumé
La présente étude avait comme objectifs de déterminer l’effet des conditions de culture sur l’adhérence d’Escherichia coli entéro-hémorragique (EHEC) O157:H7 souche 86-24 in vivo aux entérocytes porcins et de comparer les résultats avec l’adhérence in vitro à des cellules Hep-2 et IPEC-J2. La croissance de O157:H7 dans le bouillon MacConkey (MB) a eu comme résultat que presqu’aucune adhérence n’a été notée aux cellules Hep-2 et IPEC-J2; une exposition préalable des bactéries à un pH de 2,5 a réduit l’adhérence. Il y avait une plus grande adhérence par les bactéries provenant d’une culture statique comparativement à celles obtenues de cultures agitées et de bactéries cultivées en bouillon cœur-cerveau (BHI) additionné de NaHCO3 (BHIN) que celles cultivées en BHI. Dans les anses intestinales de porc, les bactéries cultivées en MB adhéraient bien aux entérocytes et une exposition préalable à pH 2,5 n’avait aucun effet sur l’adhérence. Parmi plusieurs milieux testés pour leur effet sur l’adhérence des bactéries dans l’intestin de porc, le MB et le BHIN se sont avérés les meilleurs. L’adhérence bactérienne était dose-dépendante et était plus extensive dans l’iléon que dans le côlon. Cette étude a démontré qu’il y avait des différences marquées entre les conditions de culture qui favorisaient l’adhérence d’une souche EHEC O157:H7 in vitro et in vivo, que les conditions de culture affectaient grandement l’adhérence aux cellules épithéliales in vitro et in vivo, et que les anses intestinales porcines sont mieux adaptées aux études sur l’adhérence que les anses de côlon.
(Traduit par Docteur Serge Messier)
Introduction
Enterohemorrhagic Escherichia coli (EHEC), represented by the protoserotype O157:H7, causes large outbreaks and sporadic cases of diarrhea, hemorrhagic colitis (HC), and hemolytic–uremic syndrome (HUS), the most serious sequela of EHEC O157:H7 infection (1). Verotoxin (VT) secreted by EHEC is responsible for the development of HC and HUS (2,3).
The sequence of events involved in EHEC O157:H7 infection is poorly understood; however, the first step is colonization of the intestinal mucosa. This process is mediated by proteins that are required for attaching and effacing (AE) lesions (4) and several putative adherence factors. The locus for enterocyte effacement (LEE) encodes these proteins and is critical for adherence to and colonization of the intestine. The AE lesion is characterized by actin-rich pedestal formation on the host cell surface around the bacteria, destruction of brush-border microvilli, and intimate adhesion of the pathogen to the enterocyte surface (5). The LEE encodes a major outer membrane surface adhesin, intimin, and its receptor, Tir (translocated intimin receptor), which is inserted into the host cell membrane by the type III secretion system (TTSS) (6,7). Intimate adherence is mediated by the interaction between Tir and intimin (8). Many potential adherence factors found in various serotypes of EHEC may contribute to the disease and outbreaks caused by this organism (9). Such factors include the putative adhesins Efa1 (EHEC factor for adherence 1), Iha (IrgA homolog adhesin), and AIDA-1 (the adhesin involved in diffuse adherence), as well as Lpf (long polar fimbriae) (10,11).
Expression of the LEE genes and potential adherence factors are under complex regulation and respond to changing environmental stimuli, such as temperature, pH, and nutrient availability (12,13). Such regulation allows coordinated and timely expression of the factors critical for bacterial growth, survival, and infection in the environment of the intestine. Expression of the LEE genes and secretion of their products are affected by bacterial growth conditions; for example, EHEC will adhere more efficiently to epithelial cells in vitro when grown in Dulbecco’s modified Eagle’s essential medium (DMEM) than when grown in Luria-Bertani (LB) broth (14), and growth with aeration inhibits AE lesion formation (15). Effects of culture conditions on adherence of EHEC to intestinal epithelial cells in vivo had not been investigated, and the failure of some animal infection studies may have been due to unfavorable culture conditions. This prompted the current investigation of the effects of bacterial growth conditions on adherence of E. coli O157:H7 to intestinal epithelial cells both in vitro and in vivo in order to identify those conditions that are suited for use in bacterial adherence investigation.
Pigs are a highly relevant animal model and are extensively used for the investigation of EHEC O157:H7 pathogenesis. Although EHEC O157:H7 causes typical AE lesions in the pig intestine (16–18), most studies have involved gnotobiotic pigs, which lack normal microflora (19–21). To better reflect the intestinal environment that EHEC O157:H7 encounters after entering the gastrointestinal tract, this study used a gut-loop model in conventional pigs with intact microflora.
Materials and methods
Bacteria, tissue cells, media, and reagents
Strain 86-24, a clinical isolate of EHEC O157:H7, was used for this study along with the mutant O157:H7 strain 86-24ΔescN, which lacks the capacity to adhere (22). Cultured HEp-2 cells (American Type Culture Collection CCL23) were maintained in Eagle’s Minimal Essential Medium (EMEM) (Invitrogen, Carlsbad, California, USA), and IPEC-J2 pig jejunal epithelial cells (22) were maintained in DMEM (Invitrogen). Both media were supplemented with 10% fetal bovine serum (FBS), penicillin (100 IU/mL), and streptomycin (100 μg/mL).
Preliminary studies showed that EHEC O157:H7 strain 86-24 grown overnight in LB with shaking adhered poorly to HEp-2 cells: on average 19% of the HEp-2 cells had adherent bacteria, most having clusters of only 5 to 9 bacterial cells. In a search for improved levels of adherence, the effects of various culture conditions, including various culture media, addition of NaHCO3, pretreatment by exposure to low pH, and incubation with shaking versus without shaking, were assessed. The effect of adding NaHCO3 was examined, as it was reported that bicarbonate ion enhanced bacterial adherence (23). Sodium bicarbonate was added to the medium at a final concentration of 44 mM. To mimic the low pH environment of the human stomach before bacteria encounter intestinal epithelial cells, O157:H7 strain 86-24 was cultured in LB at pH 2.5 at 37°C with shaking for 3 h, pelleted by centrifugation, resuspended in BHIN or MB, and incubated without shaking at 37°C overnight before it was used in the adherence assays.
Fisher Scientific, Nepean, Ontario, was the source of LB broth, brain–heart infusion (BHI) broth, trypticase soy broth (TSB), and MacConkey broth (MB).
In vitro adherence assay
Bacteria were grown for 16 to 18 h in 3 mL of each of the media, which included LB, EMEM, BHI, TSB, MB, and BHI plus NaHCO3 (BHIN), in a 12-mL sterile plastic tube (Fisher Scientific) either capped tightly and incubated without shaking or capped loosely and incubated with shaking. Unless otherwise indicated, cultures were grown without shaking. The density of the cell cultures was adjusted photometrically so that cultures contained approximately 5 × 108 CFU (colony forming units)/mL before their use in the assay.
The HEp-2 and IPEC-J2 cell adherence assays were conducted as described previously (22). Briefly, approximately 2 × 105 HEp-2 or IPEC-J2 cells were dispensed into each well of 6-well cell culture plates (Costar, Corning, New York, USA) and grown in EMEM and DMEM, respectively, overnight in the presence of 5% CO2. The assays and quantification of adherence were conducted as previously detailed (24), with approximately 107 bacteria from each overnight culture added and the mixture incubated at 37°C in 5% CO2. After 3 h of incubation the medium was removed by pipetting, fresh medium was added, and the cells were incubated under the same conditions for another 3 h. The cells were then washed with phosphate-buffered saline to remove unbound bacteria, fixed with 70% methanol for 10 min, and stained with 1:40 Giemsa (Sigma, St. Louis, Missouri, USA) for 30 min. Adherence was examined by light microscopy and quantified by examining 100 consecutive cells per well and recording the percentage of cultured cells with clusters of 5 to 9, 10 to 19, or > 19 bacteria.
Pig gut-loop experiments
Preliminary studies were conducted, using bacteria grown in LB with shaking, to determine the age of pigs to be used in the experiments and to assess whether the tests should be done in the terminal ileum or the colon. No adherence of O157:H7 strain 86-24 to intestinal epithelial cells was observed in any of the six 4-week-old pigs that were tested, whereas there was adherence among the 2-week-old pigs tested: ileal loops from 3 of the 17 showed some adherence, the mean proportion of villi with clusters of bacteria being 8.7% (standard deviation 11.7%). Therefore, the effects of culturing in selected media and of initial exposure to low pH were investigated only in ileal loops. Selection of the culture media and growth conditions was according to publications commonly used.
The experimental protocols and care of the animals were approved by the University of Guelph Animal Care Committee. Bacteria were grown in different culture media at 37°C overnight, concentrated by centrifugation, and resuspended in EMEM containing 10% FBS to prepare approximately 5 × 108, 5 × 109, and 5 × 1010 CFU/mL of bacteria as inocula.
The 63 pigs used were female and 12 to 14 d old; 2 or 3 pigs from the same litter were used each time. Preparation of the gut loops was as previously described (24). With a 25-gauge needle, a 2-mL inoculum containing 109 to 1011 CFU of the test organisms was injected into the lumen of the ileal loops and a 1.0-mL volume of inoculum into the colon loops. For each pig the treatments were assigned randomly, 1 loop receiving strain 86-24 and the other loop the negative-control mutant O157:H7 strain 86-24ΔescN. After inoculation the ileum and colon were replaced in the abdomen, and the laparotomy incision was closed.
The pigs were euthanized by an overdose of pentobarbital 15 to 16 h after inoculation of the loops, and pieces of the intestinal loops were quickly excised for histopathologic, immunohistochemical, and electron microscopic (EM) examination. Sample preparation and procedures were as described previously (24). Fluid accumulation in the ileal loops was measured as the volume per loop.
Statistical analysis
All analyses were performed with SAS for Windows, version 8.02 (SAS Institute, Cary, North Carolina, USA). In vitro adherence of bacteria to the cultured cells was compared by analysis of variance of the percentage of cells with adherent clusters of 5 to 9, 10 to 19, and > 19 bacteria, as well as the total adherence (the percentage of cells with ≤ 5 adherent bacteria), with the use of PROC GLM. Data were expressed as the mean of at least 3 separate experiments ± the standard deviation. In vivo adherence was compared similarly by analysis of variance of the mean percentage of villi with ≥ 5 adherent bacteria for the total number of loops tested. P-values ≤ 0.05 were considered significant.
Results
Effects of culture conditions on bacterial adherence in vitro
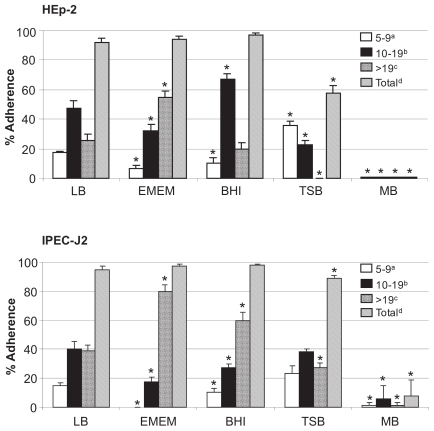
There was almost no adherence to HEp-2 cells when EHEC O157:H7 was grown in MB (Figure 1). Among the other culture media, TSB was associated with the least total adherence to HEp-2 cells (P < 0.008), the most small clusters (P < 0.0001), and the fewest large clusters (P < 0.01). Similar patterns of adherence were observed for IPEC-J2 cells (Figure 1). Total bacterial adherence to HEp-2 cells was significantly lower with shaken overnight cultures in LB and BHI than with static cultures in those media (Figure 2). Similar results were observed with bacteria grown in TSB and EMEM and tested for adherence to HEp-2 cells (data not shown).
Figure 1.
Effects of culture media on adherence of enterohemorrhagic Escherichia coli (EHEC) O157:H7 strain 86-24 to HEp-2 and IPEC-J2 cells. Adherence was quantified by examining 100 cells for each assay and recorded as the mean percentage of cells (+ standard deviation) with clusters of 5 to 9, 10 to 19, or > 19 adherent bacteria and the total percentage of cells with a cluster of 5 or more adherent bacteria. * — P < 0.05; LB — Luria-Bertani broth; EMEM — Eagle’s Minimal Essential Medium; BHI — brain–heart infusion (BHI) broth; TSB — trypticase soy broth; MB — MacConkey broth.
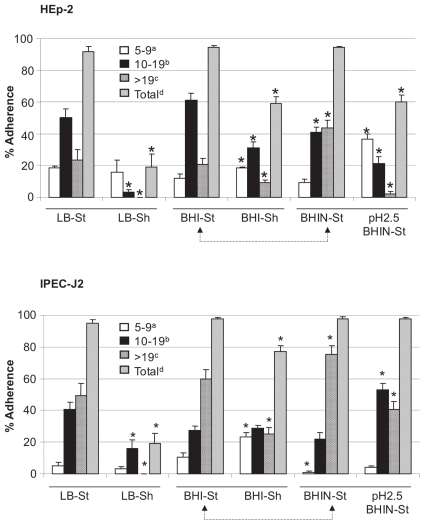
Figure 2.
Effects of culture media, shaking (Sh), and exposure to low pH before culture on adherence of EHEC O157:H7 strain 86-24 to HEp-2 and IPEC-J2 cells. Adherence was quantified and recorded as described for Figure 1. * — P < 0.05; St — static culture; BHIN — BHI plus NaHCO3. Arrow connections indicate that BHI and BHIN were also compared.
The addition of NaHCO3 to the medium at a final concentration of 44 mM increased the pH by only 0.2 units, but in tests with HEp-2 cells this addition increased the proportion of cells with large clusters of bacteria cultured in BHI (Figure 2) and increased both total adherence (from 56.0% ± 3.7% to 77.0% ± 4.0%; P < 0.05) and the proportion of cells with large clusters of bacteria cultured in TSB (from 18.8% ± 3.7% to 60.0% ± 4.0%; P < 0.05) (data not shown). Despite the addition of NaHCO3 to MB, bacteria grown in this medium failed to adhere (data not shown). Similar results were observed with IPEC-J2 cells with the addition of NaHCO3 to BHI, TSB, and MB (Figure 2 and data not shown).
Exposure to a pH of 2.5 resulted in a reduction in total adherence and large cluster formation on HEp-2 cells (Figure 2) and a reduction in large cluster formation on IPEC-J2 cells (Figure 2).
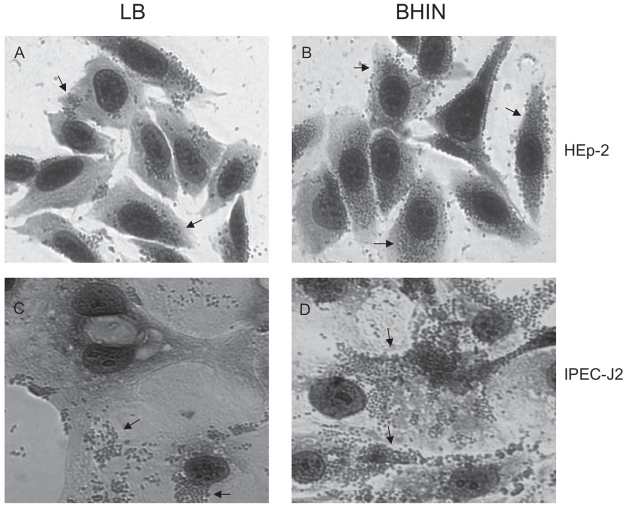
The overall pattern of adherence to IPEC-J2 cells was similar to that for HEp-2 cells, but there was a trend towards greater adherence and more large clusters of bacteria with IPEC-J2 cells than with HEp-2 cells (Figures 1, 2, and 3; P < 0.024). The pattern of adherence of the bacteria to IPEC-J2 cells was mainly localized (Figure 3). Growth of the bacteria in the commonly used medium LB resulted in less bacterial adherence than did growth in BHIN (P < 0.05) (Figure 3).
Figure 3.
Pattern of adherence to HEp-2 (A and B) and IPEC-J2 (C and D) cells of EHEC O157:H7 strain 86-24 grown in LB (A and C) and BHIN (B and D). The cells were stained with Giemsa and viewed by light microscopy (×400) after the 6-h adherence assay. Bacteria adhered to IPEC-J2 cells in clusters that appeared localized but not in the classic pattern (arrows). Adherence was more extensive for bacteria cultured in BHIN than for those cultured in LB for both HEp-2 and IPEC-J2 cells.
Effects of loop location on bacterial adherence
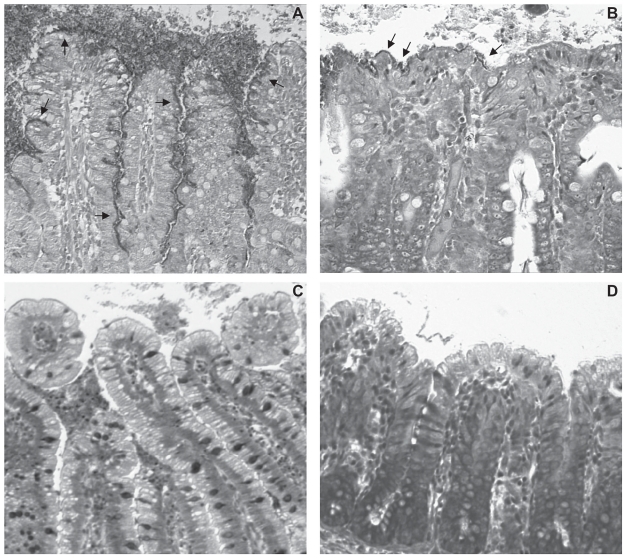
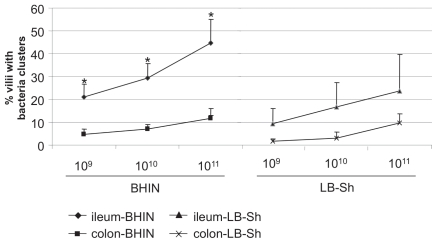
The typical light microscopic appearance of clusters of EHEC O157:H7 strain 86-24 on villi in the ileum and in the colon is shown in Figures 4A and 4B, respectively. The negative-control strain 86-24ΔescN failed to adhere to the ileum or the colon (Figures 4C and 4D, respectively). As shown in Figures 4 and 5, adherence was significantly more extensive in the ileum than in the colon (P = 0.0435), and there was a dose-related adherence response to EHEC O157:H7 grown in BHIN or in LB with shaking in both the ileum and the colon (P = 0.0001). In the ileal loops the EHEC O157:H7 inoculum of 1011 CFU resulted in significantly more accumulated fluid than did the inoculum of 109 CFU (P = 0.0495; data not shown). Examination by EM of a sample of the bacterial clusters showed characteristic AE lesions, and the adherent bacteria were identified as being O157 by immunohistochemical study (data not shown). These findings suggested that ileal loops were better suited for studies of bacterial adherence and fluid accumulation induced by EHEC O157:H7 than were colon loops, and that the dose of 1011 CFU was suitable for studies of the frequency of adherence by EHEC O157:H7. Therefore, for the subsequent experiments in the 2-wk-old pigs, ileal loops and a dose of 1011 CFU were used.
Figure 4.
Bacterial adherence on villi in pig ileal (A) and colon (B) loops inoculated with wild-type EHEC O157:H7 strain 86-24 grown in BHIN; the adherence is more extensive in the ileal loops than in the colon loops. Arrows point to clusters of bacteria. Villi in control ileal (C) and colon (D) loops, inoculated with the mutant strain 86-24ΔescN, show no adherence. Giemsa; ×200.
Figure 5.
Effects of bacterial dose (109, 1010, or 1011 colony-forming units) on adherence in the ileum and the colon of EHEC O157:H7 strain 86-24 grown in BHIN or in LB with shaking. Adherence was quantified as the percentage of villi with 5 or more adherent bacterial clusters. * — significant difference (P < 0.05) between the ileum and the colon.
Effects of culture conditions on bacterial adherence in pig ileal loops
Of the 63 pigs tested, 40 (64%) had intimately adherent bacterial clusters on their villi after inoculation of 1011 CFU of EHEC O157:H7 strain 86-24. Table I summarizes the effects of various culture conditions on the number of ileal loops with adherent bacterial clusters and the percentage of villi with bacterial clusters. Growth of the bacteria in BHIN (with or without prior exposure to pH 2.5) resulted in the highest percentages of loops with adherent clusters (100% and 84%, respectively). Growth in MB caused massive accumulation of fluid in the loops; therefore, a dose of 1010 CFU of bacteria was used in subsequent experiments. With the reduced dose, growth in MB (with or without prior exposure to pH 2.5) resulted in lower percentages of loops with adherent bacterial clusters (42% and 52%, respectively) but a mean percentage of villi with bacterial clusters similar to that with growth in BHIN and testing at a dose 10 times higher. Static growth in LB, LB plus NaHCO3 (LBN), and EMEM was much less effective, inducing adherence to 0% to 40% of the loops.
Table I.
Effect of culture conditions on adherence of enterohemorrhagic Escherichia coli O157:H7 strain 86-24 inoculated [in a dose of 1011 colony-forming units (CFU)] into pig ileal loops
| Culture conditions | Number of loops tested | Number (and %) of loops with clusters of bacteria | % of villi with clusters of bacteria (mean ± standard deviation) |
|---|---|---|---|
| BHIN, pH 7.4a | 31 | 26 (84) | 30.2 ± 20.2 |
| BHIN, pH 2.5b | 14 | 14 (100) | 23.4 ± 15.5 |
| MB, pH 7.4a | 21 | 11 (52) | 21.5 ± 20.6 |
| MB, pH 2.5b | 12 | 5 (42) | 23.1 ± 12.3 |
| LB | 5 | 1 (20) | 0.3 |
| LB-Sh | 21 | 7 (33) | 19.9 ± 22.12 |
| LBN | 5 | 2 (40) | 5.2 |
| EMEM | 5 | 0 (0) | n/ac |
Without prior exposure to pH 2.5.
Exposed to pH 2.5 for 3 h before culture.
BHIN — brain–heart infusion broth plus NaHCO3; MB — MacConkey broth (a dose of 1010 CFU was used because a dose of 1011 CFU elicited a massive fluid response); LB — Luria-Bertani broth; LB-Sh — LB with shaking; LBN — LB plus NaHCO3; EMEM — Eagle’s Minimal Essential Medium.
There was no adherence.
Considerable pig-to-pig variation was observed. When pigs from the same litter were compared for their responses to inoculation with EHEC O157:H7 strain 86-24, some pigs showed no adherence, some had adherence in a small percentage of loops, and some had adherence in a high percentage of villi. There were no significant differences in the mean percentage of villi with bacterial clusters after inoculation of bacteria cultured in BHIN, MB, or LB with shaking, whereas growth in LB without shaking, LBN, and EMEM was associated with significantly lower (P < 0.05) percentages of villi with adherent bacteria (Table I).
Adherence of bacteria grown in LB with and without shaking was compared in the same 5 pigs: adherence was observed in only 1 pig, in which 1 loop with LB static culture had attachment to 0.3% of the villi and 1 loop with LB-shaken culture had attachment to 1.7% of the villi. Exposure to pH 2.5 before culture in BHIN resulted in intimate bacterial adherence in the highest percentage of loops; however, the mean percentage of villi with bacterial clusters was not significantly different from that for bacteria not exposed to pH 2.5 before culture. Similarly, with growth in MB, prior exposure to pH 2.5 did not result in a significant difference in the mean percentage of villi with bacterial clusters.
The distance of the ileal loops from the ileocecal junction appeared not to affect the extent of AE lesion development (data not shown).
Discussion
This study has shown that EHEC O157:H7 strain 86-24 tends to bind more to IPEC-J2 cells than to HEp-2 cells, in agreement with a previous report that cells of porcine intestinal origin support a high level of adherence of EHEC O157:H7 (25). Since HEp-2 cells, which are immortalized human laryngeal epithelial cells, have been used extensively for studies on adherence of enteropathogenic E. coli and EHEC (26,27), it was valuable to use these cells so that comparisons could be made with other studies. However, IPEC-J2 cells, which are undifferentiated and not immortalized porcine intestinal epithelial cells (28), are a more relevant cell for studies of the intestinal pathogen E. coli O157:H7. This is particularly the case when comparisons are being made between adherence in vitro and in the ligated pig intestine. The slightly larger estimated size of the IPEC-J2 cells, compared with the HEp-2 cells, could have contributed to the higher frequency of adherence of the bacteria to the IPEC-J2 cells. The present observations strongly suggest that IPEC-J2 cells may be a suitable model for studying some aspects of EHEC O157:H7 pathogenesis.
The more extensive adherence to epithelial cells by bacteria grown under static as opposed to shaken culture conditions supports reports that oxygen-enriched environments impair the ability of EHEC O157:H7 to adhere (15). High oxygen levels may negatively regulate the expression of proteins such as Tir, EspA, and EspB that are involved in AE lesion formation (29). With restriction of oxygen, maturation of a functional TTSS was accelerated in EHEC O157:H7 (30). Limited oxygen may better mimic the host intestinal environment, which induces expression of the LEE-encoded proteins.
Addition of NaHCO3 to BHI and TSB enhanced adherence. This result supports previous findings that bicarbonate ion stimulates expression of LEE and certain non-LEE encoded genes and results in greater adherence of EHEC O157:H7 to Caco-2 cells and more shedding from 14-week-old conventional pigs (23,25). Sodium bicarbonate was also required for maximum secretion of EspB by EPEC (12); however the effect of NaHCO3 appears to be related to the medium, as its addition to MB in the present study did not result in increased adherence.
The reason for the lack of adherence in vitro by the bacteria cultured in MB is not known. Bacteria grown in MB adhered relatively well in the ileal loops. Some factors required for adherence and AE lesion development may have been repressed by culture in MB but activated by the host intestinal environment. Bile salts may act as an environmental signal in vivo, regulating bacterial gene expression and contributing to bacterial survival and colonization of the intestine (31,32). It may be worth while to investigate the effect of lactose, a major component of MB, on adherence both in vitro and in vivo.
Since low pH is a feature of the gastric environment, the effect on EHEC O157:H7 adherence of prior exposure to pH 2.5 for 3 h was examined. This treatment caused a reduction in the pathogen’s attachment to the epithelial cells in vitro. The mechanism for the reduced adherence is not known. However, the 2 acid-resistance pathway regulators rpoS and gadE in EHEC O157 are induced by a low pH (33). GadE was reported to be a negative regulator of LEE-encoded gene expression, reducing the adherence of EHEC O157 to Caco-2 cells (34). Overnight incubation at pH 7.4 after the low pH treatment did not restore the adherence phenotype. In contrast with the in vitro adherence assay, exposure to pH 2.5 had essentially no effect on cluster development in pig gut loops. After the 3 h of low-pH treatment, the number of live bacteria was a small percentage of the inoculum (2.9 × 102 compared with 8.5 × 108 cells). This might suggest that exposure to the low pH selectively enriched the acid-resistant bacteria that were more virulent in the host intestine. Clearly, in vitro conditions differ from the conditions in the intestine, where the environment might favor the expression and secretion of virulence factors required for infection (12). The effects of culture in MB on adherence in vivo support this hypothesis.
The present experiments revealed that EHEC O157:H7 strain 86-24 caused adherence more frequently in the distal ileum than in the spiral colon. These results are consistent with recent findings with in vitro organ culture of the human colon: EHEC O157:H7 had a tropism for follicle-associated epithelium (FAE) of Peyer’s patches in the distal ileum (35). These patches may represent the initial colonization site for EHEC O157:H7, after which the bacteria spread to other regions of the gut, including the proximal intestine and colon (35). Colonic colonization is the subsequent major event and leads to colonic disorders. The FAE may be important in mediating colonization and the initial stage of infection, during which bacteria are selected for subsequent colonic colonization or genes are induced to mediate subsequent adherence in other regions of the intestine (35).
Fluid accumulation in the ileal loops in response to EHEC O157:H7, estimated as the volume per loop, was dose-dependent. It is difficult to accurately measure length of intestine owing to contraction and relaxation, and this activity slows the collection of tissue samples at postmortem examination. The loops were all of the same approximate length, and no adjustment was made for slight differences in length.
The loop experiments were terminated after 15 to 16 h so that the pigs’ intestinal ligations would be maintained no longer than was necessary. The preliminary studies had determined that AE lesions were visible as early as 4 h in some pigs and that 15 to 16 h was a satisfactory period for detection of AE lesions. It is possible that later examination of the loops might have resulted in different observations, such as reversal of adherence or delayed adherence. It is one of the limitations of the gut-loop model that observations are usually made at a single time point and that, for humane reasons, the duration of the study must be limited.
In conclusion, our study showed that culture conditions play an important role in the ability of EHEC O157:H7 to adhere to cultured cells and to epithelial cells in the pig terminal ileum. Bacteria cultured in BHIN adhered well both in vitro and in vivo, whereas those grown in MB adhered to ileal loops but showed little or no adherence in vitro. Pre-exposure of the bacteria to pH 2.5 reduced adherence in vitro but had no effect on the frequency of adherence in the ileal loops. Interestingly, EHEC O157:H7 strain 86-24 adhered more extensively in the distal ileum than in the spiral colon. Investigation of the genes involved in the influence of culture conditions on adherence in vivo is under way in our laboratory.
Acknowledgments
We thank Dr. Hai Yu for assistance with statistical analysis and Dr. Parviz Sabour for critical review of the manuscript. The research was supported by the Canadian Institutes of Health Research. Yanni Feng was a visiting scholar supported by the China Scholarship Council. Xianhua Yin was financially supported by Agriculture and Agri-Food Canada.
References
- 1.Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johannes L, Romer W. Shiga toxins — from cell biology to biomedical applications. Nat Rev Microbiol. 2010;8:105–116. doi: 10.1038/nrmicro2279. Epub 2009 Dec 21. [DOI] [PubMed] [Google Scholar]
- 3.Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet. 2005;365:1073–1086. doi: 10.1016/S0140-6736(05)71144-2. [DOI] [PubMed] [Google Scholar]
- 4.Frankel G, Phillips AD, Rosenshine I, Dougan G, Kaper JB, Knutton S. Enteropathogenic and enterohaemorrhagic Escherichia coli: More subversive elements. Mol Microbiol. 1998;30:911–921. doi: 10.1046/j.1365-2958.1998.01144.x. [DOI] [PubMed] [Google Scholar]
- 5.LeBlanc JJ. Implication of virulence factors in Escherichia coli O157:H7 pathogenesis. Crit Rev Microbiol. 2003;29:277–296. doi: 10.1080/713608014. [DOI] [PubMed] [Google Scholar]
- 6.Kenny B, DeVinney R, Stein M, Reinscheid DJ, Frey EA, Finlay BB. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 7.Jarvis KG, Giron JA, Jerse AE, McDaniel TK, Donnenberg MS, Kaper JB. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci U S A. 1995;92:7996–8000. doi: 10.1073/pnas.92.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 9.Toma C, Martinez Espinosa E, Song T, et al. Distribution of putative adhesins in different seropathotypes of Shiga toxin-producing Escherichia coli. J Clin Microbiol. 2004;42:4937–4946. doi: 10.1128/JCM.42.11.4937-4946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor DE, Rooker M, Keelan M, et al. Genomic variability of O islands encoding tellurite resistance in enterohemorrhagic Escherichia coli O157:H7 isolates. J Bacteriol. 2002;184:4690–4698. doi: 10.1128/JB.184.17.4690-4698.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perna NT, Glasner JD, Burland V, Plunkett G., III . The genomes of Escherichia coli K-12 and pathogenic E. coli. In: Donnenberg MS, editor. Escherichia coli: Virulence Mechanisms of a Versatile Pathogen. San Diego: Academic Press; 2002. pp. 3–53. [Google Scholar]
- 12.Kenny B, Abe A, Stein M, Finlay BB. Enteropathogenic Escherichia coli protein secretion is induced in response to conditions similar to those in the gastrointestinal tract. Infect Immun. 1997;65:2606–2612. doi: 10.1128/iai.65.7.2606-2612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beltrametti F, Kresse AU, Guzman CA. Transcriptional regulation of the esp genes of enterohemorrhagic Escherichia coli. J Bacteriol. 1999;181:3409–3418. doi: 10.1128/jb.181.11.3409-3418.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebel F, Deibel C, Kresse AU, Guzman CA, Chakraborty T. Temperature- and medium-dependent secretion of proteins by Shiga toxin-producing Escherichia coli. Infect Immun. 1996;64:4472–4479. doi: 10.1128/iai.64.11.4472-4479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girard F, Dziva F, van Diemen P, Phillips AD, Stevens MP, Frankel G. Adherence of enterohemorrhagic Escherichia coli O157, O26, and O111 strains to bovine intestinal explants ex vivo. Appl Environ Microbiol. 2007;73:3084–3090. doi: 10.1128/AEM.02893-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moxley RA, Francis DH. Overview of animal models. In: Kaper J, O’Brien AD, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington, DC: ASM Press; 1998. pp. 249–260. [Google Scholar]
- 17.Francis DH, Collins JE, Duimstra JR. Infection of gnotobiotic pigs with an Escherichia coli O157:H7 strain associated with an outbreak of hemorrhagic colitis. Infect Immun. 1986;51:953–956. doi: 10.1128/iai.51.3.953-956.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzipori S, Wachsmuth IK, Chapman C, et al. The pathogenesis of hemorrhagic colitis caused by Escherichia coli O157:H7 in gnotobiotic piglets. J Infect Dis. 1986;154:712–716. doi: 10.1093/infdis/154.4.712. [DOI] [PubMed] [Google Scholar]
- 19.Tzipori S, Gunzer F, Donnenberg MS, de Montigny L, Kaper JB, Donohue-Rolfe A. The role of the eaeA gene in diarrhea and neurological complications in a gnotobiotic piglet model of enterohemorrhagic Escherichia coli infection. Infect Immun. 1995;63:3621–3627. doi: 10.1128/iai.63.9.3621-3627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moxley RA, Berberov EM, Francis DH, et al. Pathogenicity of an enterotoxigenic Escherichia coli hemolysin (hlyA) mutant in gnotobiotic piglets. Infect Immun. 1998;66:5031–5035. doi: 10.1128/iai.66.10.5031-5035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordan DM, Sperandio V, Kaper JB, Dean-Nystrom EA, Moon HW. Colonization of gnotobiotic piglets by a luxS mutant strain of Escherichia coli O157:H7. Infect Immun. 2005;73:1214–1216. doi: 10.1128/IAI.73.2.1214-1216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin X, Wheatcroft R, Chambers JR, Liu B, Zhu J, Gyles CL. Contributions of O island 48 to adherence of enterohemorrhagic Escherichia coli O157:H7 to epithelial cells in vitro and in ligated pig ileal loops. Appl Environ Microbiol. 2009;75:5779–5786. doi: 10.1128/AEM.00507-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abe H, Tatsuno I, Tobe T, Okutani A, Sasakawa C. Bicarbonate ion stimulates the expression of locus of enterocyte effacement-encoded genes in enterohemorrhagic Escherichia coli O157:H7. Infect Immun. 2002;70:3500–3509. doi: 10.1128/IAI.70.7.3500-3509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin X, Chambers JR, Wheatcroft R, et al. Adherence of Escherichia coli O157:H7 mutants in vitro and in ligated pig intestines. Appl Environ Microbiol. 2009;75:4975–4983. doi: 10.1128/AEM.00297-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Best A, La Ragione RM, Clifford D, Cooley WA, Sayers AR, Woodward MJ. A comparison of Shiga-toxin negative Escherichia coli O157 aflagellate and intimin deficient mutants in porcine in vitro and in vivo models of infection. Vet Microbiol. 2006;113:63–72. doi: 10.1016/j.vetmic.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 26.Scaletsky IC, Michalski J, Torres AG, Dulguer MV, Kaper JB. Identification and characterization of the locus for diffuse adherence, which encodes a novel afimbrial adhesin found in atypical enteropathogenic Escherichia coli. Infect Immun. 2005;73:4753–4765. doi: 10.1128/IAI.73.8.4753-4765.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iyoda S, Watanabe H. Positive effects of multiple pch genes on expression of the locus of enterocyte effacement genes and adherence of enterohaemorrhagic Escherichia coli O157:H7 to HEp-2 cells. Microbiology. 2004;150(Pt 7):2357–2571. doi: 10.1099/mic.0.27100-0. [DOI] [PubMed] [Google Scholar]
- 28.Koh SY, George S, Brozel V, Moxley R, Francis D, Kaushik RS. Porcine intestinal epithelial cell lines as a new in vitro model for studying adherence and pathogenesis of enterotoxigenic Escherichia coli. Vet Microbiol. 2008;130:191–197. doi: 10.1016/j.vetmic.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 29.DeVinney R, Stein M, Reinscheid D, Abe A, Ruschkowski S, Finlay BB. Enterohemorrhagic Escherichia coli O157:H7 produces Tir, which is translocated to the host cell membrane but is not tyrosine phosphorylated. Infect Immun. 1999;67:2389–2398. doi: 10.1128/iai.67.5.2389-2398.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ando H, Abe H, Sugimoto N, Tobe T. Maturation of functional type III secretion machinery by activation of anaerobic respiration in enterohaemorrhagic Escherichia coli. Microbiology. 2007;153(Pt 2):464–473. doi: 10.1099/mic.0.2006/000893-0. [DOI] [PubMed] [Google Scholar]
- 31.Olive AJ, Kenjale R, Espina M, Moore DS, Picking WL, Picking WD. Bile salts stimulate recruitment of IpaB to the Shigella flexneri surface, where it colocalizes with IpaD at the tip of the type III secretion needle. Infect Immun. 2007;75:2626–2629. doi: 10.1128/IAI.01599-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Begley M, Sleator RD, Gahan CG, Hill C. Contribution of three bile-associated loci, bsh, pva, and btlB, to gastrointestinal persistence and bile tolerance of Listeria monocytogenes. Infect Immun. 2005;73:894–904. doi: 10.1128/IAI.73.2.894-904.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhagwat AA, Tan J, Sharma M, et al. Functional heterogeneity of RpoS in stress tolerance of enterohemorrhagic Escherichia coli strains. Appl Environ Microbiol. 2006;72:4978–4986. doi: 10.1128/AEM.02842-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tatsuno I, Nagano K, Taguchi K, Rong L, Mori H, Sasakawa C. Increased adherence to Caco-2 cells caused by disruption of the yhiE and yhiF genes in enterohemorrhagic Escherichia coli O157:H7. Infect Immun. 2003;71:2598–2606. doi: 10.1128/IAI.71.5.2598-2606.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chong Y, Fitzhenry R, Heuschkel R, Torrente F, Frankel G, Phillips AD. Human intestinal tissue tropism in Escherichia coli O157:H7 — initial colonization of terminal ileum and Peyer’s patches and minimal colonic adhesion ex vivo. Microbiology. 2007;153(Pt 3):794–802. doi: 10.1099/mic.0.2006/003178-0. [DOI] [PubMed] [Google Scholar]