Abstract
Antimicrobial susceptibilities and toxin types were determined for 275 Clostridium perfringens isolates collected in Ontario in the spring of 2005. Minimal inhibitory concentrations (MICs) of C. perfringens isolates for 12 antimicrobials used in therapy, prophylaxis, and/or growth promotion of cattle (n = 40), swine (n = 75), turkeys (n = 50), and chickens (n = 100) were determined using the microbroth dilution method. Statistical analyses and MIC distributions showed reduced susceptibility to bacitracin, clindamycin, erythromycin, florfenicol, and tetracycline for some isolates. Reduced susceptibility to bacitracin was identified in chicken (64%) and turkey (60%) isolates. Swine isolates had predominantly reduced susceptibility to clindamycin (28%) and erythromycin (31%), whereas bovine isolates had reduced susceptibility to clindamycin (10%) and florfenicol (10%). Reduced susceptibility to tetracycline was spread across all species. No clear reduced susceptibility, but elevated MIC50 for virginiamycin was found in chicken isolates in comparison with isolates from other species. Toxin typing revealed that C. perfringens type A is the dominant toxin type isolated in this study across all 4 host species.
Résumé
La sensibilité aux antibiotiques et les types de toxines ont été déterminés pour 275 isolats de Clostridium perfringens amassés en Ontario au printemps 2005. Les concentrations minimales inhibitrices (MICs) envers C. perfringens de 12 antimicrobiens utilisés en thérapie, en prophylaxie et/ou comme promoteur de croissance chez les bovins (n = 40), porcs (n = 75), les dindes (n = 50) et les poulets (n = 100) ont été déterminées à l’aide de la méthode de micro-dilution en bouillon. Les analyses statistiques et les distributions de MIC ont montré une sensibilité réduite à la bacitracine, clindamycine, érythromycine, florfénicol, et la tétracycline pour certains isolats. Une sensibilité réduite à la bacitracine a été identifiée pour les isolats de poulet (64 %) et de dinde (60 %). Les isolats porcins avaient une sensibilité réduite à la clindamycine (28 %) et à l’érythromycine (31 %), alors que les isolats bovins avaient une sensibilité réduite à la clindamycine (10 %) et au florfénicol (10 %). Une réduction de sensibilité à la tétracycline était répandue parmi toutes les espèces. Aucune sensibilité évidente mais une MIC50 élevée envers la virginiamycine a été observée parmi les isolats de poulet comparativement aux isolats des autres espèces. Le typage de toxine a révélé que le type A de C. perfringens est le type dominant isolé chez les 4 espèces hôtes faisant l’objet de cette étude.
(Traduit par Docteur Serge Messier)
Introduction
Clostridium perfringens is commonly found in the environment and in the gastrointestinal tract of a variety of mammals and birds where it is considered a part of the normal bacterial flora (1,2). It is also recognized as an important pathogen in domestic animals, wildlife, and humans. Clostridium perfringens can cause gas gangrene and food poisoning in humans; necrotic enteritis in poultry; enterotoxemia in lambs and calves; and enteritis in pigs, cattle, dogs, and horses (1,3). Clostridium perfringens isolates can be grouped into 5 types (A to E) based on the presence of 4 major toxins; α, β, ɛ, and ι (3). Type A isolates produce α-toxin only; type B isolates produce α-, β-, and ɛ-toxins; type C isolates produce α- and β-toxins; type D isolates produce α- and ɛ-toxins; and type E isolates produce α- and ι-toxins. While the role of the β-, ɛ-, and ι-toxins in the pathogenesis of enteric diseases has been well-documented, the role of the α-toxin is still controversial (1,4,5).
Regardless of the type, C. perfringens isolates can also produce β2-toxin and enterotoxin (CPE). The β2-toxin has been associated with the onset of enteritis in pigs, horses, and cattle, and appears to have similar, but weaker, biological activity as the β-toxin (6). Enterotoxin has been associated with diarrheal disease in some animal species, and, more importantly, with food poisoning in humans (1,7).
Antimicrobial growth promoters (AGPs) are still widely used in Canada even though most of them are banned in the European Union (2). The wide use of antimicrobial agents for growth promotion and disease prevention in Canada raises an important question regarding the emergence and spread of antimicrobial resistance primarily in the resident normal enteric flora, including C. perfringens. Resistance of C. perfringens isolates from animals to bacitracin, tetracycline, clindamycin, lincomycin, and erythromycin has been reported in several countries (8–12). Nevertheless, there is a lack of more comprehensive information on C. perfringens isolates from food animals in Canada.
The purpose of this study was to evaluate the susceptibility of bovine, chicken, porcine, and turkey C. perfringens isolates to antimicrobial agents used in therapy, prophylaxis, and/or growth promotion in Ontario. In addition, genotyping of C. perfringens isolates was performed to gain insight into the distribution of toxin types in isolates from the 4 animal species included in this study.
Materials and methods
Bacterial isolates and growth conditions
A total of 275 C. perfringens isolates of bovine (n = 40), chicken (n = 100), porcine (n = 85), and turkey (n = 50) origin were tested. The isolates were collected from specimens submitted to the Animal Health Laboratory (AHL) over the period of January to March of 2005. During that period, all clinical cases of bovine and porcine diarrhea were cultured for C. perfringens. However, no attempts were made to determine the clinical significance of C. perfringens in these cases. The majority of poultry isolates were recovered from increased mortality cases, the remaining few were isolated from cases of necrotic enteritis confirmed by necropsy (5 in total). The bovine, porcine, chicken, and turkey samples were from 31, 38, 9, and 6 commercial operations, respectively.
The isolates were obtained from animals of different ages. Bovine isolates were recovered from 3 major age groups: ≤ 2 mo (21 isolates), > 2 mo to 24 mo (7 isolates), and > 24 mo (10 isolates). Porcine isolates were isolated from animals ≤ 2 wk (45 isolates), > 2 wk to 8 wk (14 isolates), and > 8 wk (16 isolates). Among chicken isolates, 95 of them originated from animals < 4 wk of age and only 5 from animals > 4 wk of age. The majority of turkey isolates were recovered from > 2-week-old animals (30 isolates). The rest of the turkey isolates were from ≤ 2-week-old animals (20 isolates). No age information was available for 2 bovine and 10 porcine isolates.
Initially, specimens (intestine or fecal samples) were plated on phenylethylalcohol (PEA) plates and incubated in anaerobic jars for 48 h at 37°C. Clostridium perfringens colonies were identified by using a double zone of hemolysis followed by Gram staining. Further confirmation was done using the inverse CAMP reaction (13). Only one isolate with confirmed C. perfringens identification was investigated further for each sample. All isolates were stored at −70°C in 20% glycerol in brain heart infusion (BHI) until further use. Clostridium perfringens ATCC 13124 (Inverness Medical, Ottawa, Ontario) was used as a control. The strain was stored under the same conditions as the clinical isolates.
Antimicrobial susceptibility testing
Twelve different antimicrobials were selected for susceptibility testing using the microbroth dilution method (Table I). The antimicrobial dilution ranges (Table I) were established based on either the earlier published studies by Watkins et al (14), Marks and Kather (15), Johansson et al (9), and Martel et al (10), or on the expected susceptibility ranges published in Prescott and Baggot (16). The microbroth dilution panels were purchased in a frozen 96-well format (Trek Diagnostic Systems, Cleveland, Ohio, USA). Each well contained 50 μL of 2 × antimicrobial dilution in supplemented brucella broth for anaerobes (Trek Diagnostic Systems). The panels were stored at −20°C and thawed immediately before use.
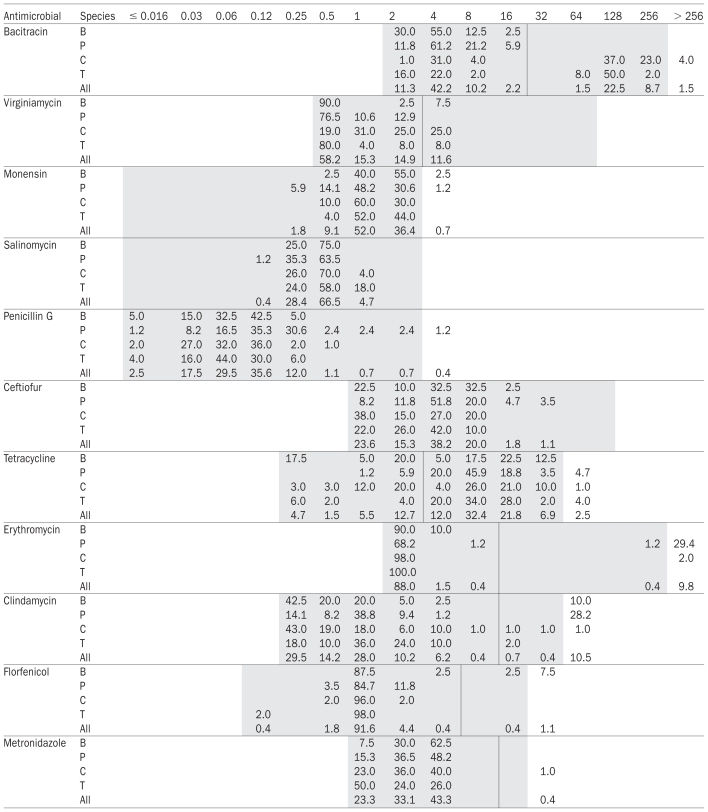
Table I.
Distribution of minimal inhibitory concentrations (MICs) obtained by broth microdilution for 12 antimicrobial agents on Clostridium perfringens isolates from cattle, swine, chickens, and turkeys from Ontario
B — bovine isolates (n = 40); P — porcine isolates (n = 85); C — chicken isolates (n = 100); T — turkey isolates (n = 50); All — isolates from all 4 species together (n = 275); the epidemiological cut-off values are indicated by the vertical lines shown; the grey cells in the table indicate the range of serial dilutions used for testing.
For MIC testing, 2 to 3 colonies of C. perfringens, from a 24 h culture grown at 37°C on blood agar plates (BAP) under anaerobic conditions, were resuspended into 5 mL of demineralized water (Trek Diagnostic Systems) to achieve a 0.5 McFarland turbidity. One hundred microliters of this suspension was then transferred to 11 mL of supplemented brucella broth for anaerobes (Trek Diagnostic Systems). This suspension was thoroughly mixed and 50 μL aliquots were dispensed into individual wells on MIC microplates using the Sensititre Automated Inoculation System for a final volume of 100 μL (Trek Diagnostic Systems). The microplates were sealed with perforated sealers (Trek Diagnostic Systems) and incubated in square GENbox anaerobic jars (BioMérieux Canada, St-Laurent, Quebec) for 24 h at 37°C. Clostridium perfringens ATCC 13124 was included as a control with every batch tested. The MICs were read manually using a light box (Sensititre; Trek Diagnostic Systems).
Reproducibility of C. perfringens MIC testing with strain ATCC 13124
Two different technologists tested MICs for this strain on 3 different days in triplicate yielding a total of 18 test replications and readings. A fresh bacterial suspension was prepared for each replicate in 5 mL of demineralized water from overnight bacterial growth on BAP. To assess the number of bacteria in the wells, bacterial counts were performed. Briefly, after the susceptibility test panels were inoculated with the bacterial suspension, 100 μL was withdrawn from 1 of the 3 antimicrobial-free growth control wells and serially diluted in phosphate buffered saline (PBS) solution. Aliquots of these dilution series were plated on BAP and bacterial counts were recorded after 24 h incubation in an anaerobic atmosphere at 37°C. In addition, a sterility test was done by inoculating a panel with supplemented brucella broth only, followed by incubation.
Genotyping
All C. perfringens isolates, including ATCC 13124, were examined by multiplex polymerase chain reaction (PCR) for the presence of α-, β-, ɛ-, ι-toxin, β2-toxin (cpb2), and enterotoxin (cpe) genes (17,18). The PCR for the β2-toxin was used to detect both consensus and atypical β2-toxin genes in the isolates tested. Briefly, a few colonies from overnight BAP cultures were resuspended into 200 μL of Instagene Matrix (BioRad Laboratories, Mississauga, Ontario). This suspension was incubated at 56°C for 20 min, 100°C for 10 min, and on ice for 5 min. The samples were then centrifuged at 16 000 × g for 5 min. Two microliters of the supernatant was used as a template for the PCR. The PCR was done using a kit (Qiagen Multiplex PCR kit; Qiagen, Mississauga, Ontario).
Data management and statistical analyses
Data management and all statistical analyses were done using computer software (SAS/STAT software, Version 9.1 for Windows; SAS Institute Inc., Cary, North Carolina, USA). Epidemiological cut-off values were determined by visual inspection of the MIC distributions (bacitracin, erythromycin, tetracycline, clindamycin, florfenicol, and metronidazole) or by setting the MIC closest to 2 standard deviations above the geometric mean MIC (virginiamycin), unless there was insufficient evidence to determine an epidemiological cut-off value (monensin, salinomycin, ceftiofur, and penicillin G). The MIC50 is the median MIC. The MIC90 is the value for which 90% of the isolates have MICs below or equal to it and 10% of the isolates have MICs above it. Differences in resistance rates between host species were tested using Fisher’s exact test. For significant results, pairwise comparisons of resistance rates between species were calculated using the 2-sided Fisher’s exact test (19). The nonparametric Kruskal-Wallis method was used to compare MIC distributions among the isolates from the different host species (20). Pairwise associations between antimicrobial resistances were determined using Pearson correlation coefficients and the exact Pearson chi-square test for significance; Spearman’s rank order correlation was used to measure the strength of association between antimicrobials’ MIC values with chi-squared tests of association to determine significance (21). Testing for overall association between the presence of the β2-toxin gene and resistance to antimicrobials was done using the Mantel-Haenszel chi-squared test (19); significant associations, within host species, were further investigated using the Fisher’s exact test. All significance tests were done using a cut-off value of 5%, without adjustment for multiple testing.
Results
Reproducibility of MICs
The reproducibility of MICs with C. perfringens ATCC 13124 was established by testing 18 separate panels. Identical MIC values were obtained with all replicates for tetracycline, erythromycin, clindamycin, virginiamycin, bacitracin, sulfamethazine, and salinomycin (data not shown). A variation of one doubling dilution step was observed for penicillin, monensin, and metronidazole, whereas a variation of two doubling dilutions was recorded for results obtained with ceftiofur (the second doubling dilution was obtained only once out of 18 trials).
The average colony forming units (cfu) of C. perfringens ATCC 13124 per mL of growth media was 1.0 × 105, as assessed in 9 independent experiments. The lowest value was 9.6 × 104 cfu/mL and the highest was 1.3 × 105 cfu/mL. These values were slightly below the range recommended by the plate manufacturer (2 to 7 × 105 cfu/mL), but likely consistent throughout the study.
Susceptibility testing results
The MIC distributions for each antimicrobial agent and animal host species are presented in Table I. The MICs for sulfamethazine were all above the dilution range (> 256 μg/mL) and, therefore, can not be interpreted further. For most of the other antimicrobials, the MICs of the isolates covered most of the dilution range tested.
Overall, bimodal distributions of MICs were detected for bacitracin, erythromycin, and clindamycin (Table I). Multimodal distributions were also visible among isolates from only some host species for tetracycline (cattle, chicken) and florfenicol (cattle). The MIC distributions for penicillin were relatively broad and contained some outliers, but were not clearly multimodal (Table I). For metronidazole, a major mode was observed between 1 and 4 μg/mL with the exception of a single chicken isolate, which presented a clearly elevated MIC suggestive of a bimodal distribution.
For those antimicrobial agents with bimodal distributions overall or in some species only (bacitracin, erythromycin, clindamycin, tetracycline, florfenicol, and metronidazole), the epidemiological cut-off values between susceptible and resistant were placed between the 2 major modes (Table I). For salinomycin, the monomodal MIC distributions are clearly indicative of a lack of resistance determinant and all the isolates can be considered as susceptible to this antimicrobial agent. For virginiamycin the cut-off value was placed 2 standard deviations above the MIC geometric mean (Table I), since this value was within the dilution testing range. When this value was outside the testing range (monensin, penicillin G, ceftiofur), no cut-off was defined and the isolates were not further classified as resistant or susceptible.
When separating the results by animal species, statistical analysis suggested significant differences in the distribution of MICs between host species for all the antimicrobials, except tetracycline (Table II). This was not obvious in every case on the sole basis of MIC50, MIC90, or both (Table II), or of visual analysis of MIC distributions (Table I). For bacitracin, a clear bimodal distribution and elevated MIC50 and MIC90 were evident in isolates from chicken and turkey, but not in those from cattle and swine. For erythromycin, a secondary MIC mode and an elevated MIC90 were evident in swine isolates, but not in those from other species, although 2 isolates from chicken also presented very high MICs (Table I). For clindamycin MICs, a clear secondary mode and an elevated MIC90 were identified in swine isolates only. Elevated MICs similar to those from porcine isolates were seen in 4 cattle isolates (Table I). However, contrary to the majority of porcine isolates, these 4 bovine isolates did not have elevated MICs for erythromycin. For virginiamycin, chicken isolates showed a shift toward higher MICs (81% of isolates with MICs 1 μg/mL) when compared with the other species (19% of isolates with MICs ≥ 1 μg/mL). Ten percent of the cattle isolates had clearly elevated florfenicol MICs, but none were found in the other host species (Tables I and II).
Table II.
Comparison of antimicrobial susceptibility between animal species
| Antimicrobial agent | Bovine | Porcine | Chickens | Turkeys | Statistical significance (P) |
|---|---|---|---|---|---|
| Bacitracin | 0.0a | 0.0a | 64.0%b | 60.0%b | < 0.001 |
| MIC50/MIC90 | 4/8 | 4/8 | 128/256 | 128/128 | < 0.001 |
| Virginiamycin | 7.5%a | 0.0b | 25.0%c | 8.0%a | < 0.001 |
| MIC50/MIC90 | 0.5/0.5 | 0.5/2 | 1/4 | 0.5/2 | < 0.001 |
| Monensin | n/a | n/a | n/a | n/a | — |
| MIC50/MIC90 | 2/2 | 1/2 | 1/2 | 1/2 | 0.002 |
| Salinomycin | 0.0 | 0.0 | 0.0 | 0.0 | — |
| MIC50/MIC90 | 0.5/0.5 | 0.5/0.5 | 0.5/0.5 | 0.5/1 | 0.03 |
| Penicillin G | n/a | n/a | n/a | n/a | — |
| MIC50/MIC90 | 0.06/0.12 | 0.12/0.25 | 0.06/0.12 | 0.06/0.12 | < 0.001 |
| Ceftiofur | n/a | n/a | n/a | n/a | — |
| MIC50/MIC90 | 4/8 | 4/8 | 2/8 | 4/4 | < 0.001 |
| Tetracycline | 57.5%a | 92.9%b | 62.0%a | 88.0%b | < 0.001 |
| MIC50/MIC90 | 8/32 | 8/16 | 8/32 | 8/16 | 0.16 |
| Erythromycin | 0.0a | 30.6%b | 2.0%a | 0.0a | < 0.001 |
| MIC50/MIC90 | 2/2 | 2/> 256 | 2/2 | 2/2 | < 0.001 |
| Clindamycin | 10.0%a | 28.2%b | 2.0%a | 0.0c | < 0.001 |
| MIC50/MIC90 | 0.5/4 | 1/64 | 0.5/4 | 1/4 | < 0.001 |
| Florfenicol | 10.0%a | 0.0b | 0.0b | 0.0b | < 0.001 |
| MIC50/MIC90 | 1/4 | 1/2 | 1/1 | 1/1 | 0.02 |
| Metronidazole | 0.0 | 0.0 | 1.0% | 0.0 | 1.000 |
| MIC50/MIC90 | 4/4 | 2/4 | 2/4 | 1/4 | < 0.001 |
For each antimicrobial, the numbers in the first line represent the percentage of isolates classified as resistant using the epidemiological cut off values shown in Table I.
Animal species with prevalence of resistance labeled with different letters were statistically significantly different in pairwise comparisons (performed when homogeneity of all prevalences was rejected).
MIC50 and MIC90 values for each antimicrobial’s MIC distribution.
The P-values were obtained by comparing the overall MIC distributions between host species. The statistics were obtained using Fisher’s exact tests for the comparison of prevalences and Kruskal-Wallis tests for the comparisons of MIC distributions.
n/a — Not applicable because no cut-off value could be defined.
Other significant differences between species were less consistent or less clear and more difficult to interpret (Table II). Because only 1 isolate was classified as resistant, no significant difference was seen between animal species in resistance rates for metronidazole. A large proportion of isolates were classified as resistant to tetracycline across all animal species (Table II).
Many significant pairwise associations were seen between MICs for different antimicrobials (Table III), but their exact relevance is generally difficult to evaluate. Strong associations (such as a relatively high Spearman correlation coefficient supported by a highly significant P-value) between antimicrobial agents of the same class were seen between penicillin G and ceftiofur, between erythromycin and clindamycin, and between monensin and salinomycin. Other relatively strong associations were seen between tetracycline and clindamycin, as well as between florfenicol and erythromycin (Table III). Although usually weaker, most negative associations were seen between bacitracin resistance and resistance to other antimicrobial agents (Table III).
Table III.
Pairwise associations between antimicrobial minimal inhibitory concentration (MIC) values in 275 Clostridium perfringens isolates from cattle, swine, chickens, and turkeys
| BAC | VIR | MON | SAL | PEN | XNL | TET | ERY | CLI | FLO | MET | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BAC | NA | ||||||||||
| VIR | 0.23 (P < 0.001) | NA | |||||||||
| MON | — | — | NA | ||||||||
| SAL | — | — | 0.43 (P < 0.001) | NA | |||||||
| PEN | −0.31 (P < 0.001) | −0.18 (P = 0.002) | −0.19 (P = 0.002) | — | NA | ||||||
| XNL | −0.32 (P < 0.001) | — | — | — | 0.66 (P < 0.001) | NA | |||||
| TET | — | 0.28 (P < 0.001) | — | 0.15 (P = 0.015) | — | 0.12 (P = 0.04) | NA | ||||
| ERY | −0.27 (P < 0.001) | 0.18 (P = 0.003) | 0.19 (P = 0.002) | — | — | — | 0.18 (P = 0.003) | NA | |||
| CLI | −0.14 (P = 0.023) | 0.21 (P < 0.001) | — | — | — | — | 0.50 (P < 0.001) | 0.50 (P < 0.001) | NA | ||
| FLO | — | 0.15 (P = 0.015) | 0.20 (P = 0.001) | — | — | 0.14 (P = 0.020) | — | 0.49 (P < 0.001) | 0.28 (P < 0.001) | NA | |
| MET | −0.38 (P < 0.001) | — | — | 0.13 (P = 0.030) | 0.34 (P < 0.001) | 0.34 (P < 0.001) | — | — | — | 0.14 (P = 0.023) | NA |
— no statistically significant association detected; the first number in each cell is the Spearman correlation coefficient for the respective specific pairwise association; NA — not applicable; BAC — bacitracin; VIR — virginiamycin; MON — monensin; SAL — salinomycin; PEN — penicillin; XNL — ceftiofur; TET — tetracycline; ERY — erythromycin; CLI — clindamycin; FLO — florfenicol; MET — metronidazole.
The resistance profiles observed for each species are listed in Table IV. Overall, they confirm what was seen for each antimicrobial separately. One hundred and nine isolates were multiresistant (4 resistant to 4 antimicrobials, 35 resistant to 3 antimicrobials, and 70 resistant to 2 antimicrobials). Many of these multiresistance profiles included tetracycline (n = 107) and the combination of resistance to erythromycin and clindamycin (n = 26).
Table IV.
Resistance profile distribution among Clostridium perfringens isolates from cattle, swine, chickens, and turkeys
| Number of resistances | BAC | VIR | TET | ERY | CLI | FLO | MET | Cattle | Swine | Chickens | Turkeys | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 42.5% | 5.9% | 2.0% | 12.0% | 10.9% (n = 30) | |||||||
| 1 | X | 35.0% | 12.7% (n = 35) | |||||||||
| 1 | X | 47.5% | 63.5% | 14.0% | 28.0% | 36.7% (n = 101) | ||||||
| 2 | X | X | 22.0% | 52.0% | 17.5% (n = 48) | |||||||
| 2 | X | X | 19.0% | 6.9% (n = 19) | ||||||||
| 2 | X | X | 2.4% | 0.7% (n = 2) | ||||||||
| 2 | X | X | 1.2% | 0.4% (n = 1) | ||||||||
| 3 | X | X | X | 5.0% | 8.0% | 3.3% (n = 9) | ||||||
| 3 | X | X | X | 1.0% | 0.4% (n = 1) | |||||||
| 3 | X | X | X | 27.1% | 1.0% | 8.7% (n = 24) | ||||||
| 3 | X | X | X | 2.5% | 0.4% (n = 1) | |||||||
| 4 | X | X | X | X | 1.0% | 0.4% (n = 1) | ||||||
| 4 | X | X | X | X | 7.5% | 1.1% (n = 3) |
BAC — bacitracin; VIR — virginiamycin; TET — tetracycline; ERY — erythromycin; CLI — clindamycin; FLO — florfenicol; MET — metronidazole; Ceftiofur and penicillin G are not included as resistance to these antimicrobials was determined to be n/a.
The numbers in the last 5 columns represent percentages of isolates with this specific resistance profile and the numbers in parentheses represent the actual number of isolates.
Genotyping
From 275 clinical isolates tested all but 1 were type A (data not shown). A single type D isolate, also positive for the cpe gene, was recovered from a turkey. The β2 gene was detected in the type D isolate, as well as in a significant proportion of type A isolates. The highest prevalence of this gene was found in turkey isolates (90%) followed by chicken isolates (88%). The prevalence of this gene was lower in porcine isolates (57.6%) and it was detected in only 7.5% of bovine isolates.
A significant negative association was detected between the β2-toxin gene and resistance to erythromycin (P < 0.001) and clindamycin (P < 0.001) (data not shown). Porcine isolates resistant to these 2 antimicrobials were significantly less likely (P < 0.001) to carry the β2-toxin gene than susceptible ones. This association was not detected in any other species and no significant association was seen between the toxin and resistance to any other antimicrobial.
Discussion
When assessed with the C. perfringens ATCC 13124 strain, the microbroth dilution method used in this study appears to be a reproducible way of determining MICs for a variety of antimicrobials. The slight variations of results for some antimicrobials were all within the acceptable range of CLSI quality control limits, with the exception of ceftiofur (22). Since the assessment of growth was done manually, the observed discrepancy likely resulted from differences in individual readings.
Optimal testing ranges are difficult to define on the basis of the sparse descriptions of antimicrobial susceptibility ranges for C. perfringens available in the literature. For most of the antimicrobials, the MICs of the isolates tested covered most of the dilution range used in this study with the exception of sulfamethazine. Since MICs for all isolates including C. perfringens ATCC 13124 were > 256 μg/mL it is tempting to speculate that C. perfringens isolates may be intrinsically resistant to sulfonamides. Some slight adjustments may also be needed in the testing ranges for virginiamycin, monensin, and salinomycin in order to better capture the whole range of susceptibilities present in C. perfringens populations.
Decreased susceptibility to several antimicrobial agents of practical relevance for therapy and prevention of animal diseases caused by C. perfringens was observed. Decreased susceptibilities were not distributed evenly across the 4 animal species investigated and suggested a potential relation with the specific antimicrobial use practices in each one of these species. For example, bovine and porcine C. perfringens isolates showed a monomodal distribution of MIC values for bacitracin, whereas bimodal distributions were observed in both chicken and turkey isolates. These findings are in agreement with the published reports on bimodal distribution of MIC values for bacitracin in poultry C. perfringens isolates (11,14,23). However, although clear bimodal distributions of MICs were noted in all these studies, there were slight differences in actual MIC values depending on the testing method. According to Benning and Mathers (24), MIC values for bacitracin can be at least 2 dilutions higher when a broth microdilution method is used instead of an agar dilution method. Thus, MIC50 and MIC90 values can vary accordingly and it is important to establish which method of susceptibility testing was used when comparing bacitracin MICs between studies. At present, however, it is not clear if these differences in MICs have any clinical significance. The correlation of in vitro data with clinical efficacy of bacitracin needs to be evaluated.
Decreased susceptibility to bacitracin in poultry isolates is not surprising given the frequent use of bacitracin for prevention of necrotic enteritis (25). The exact mechanism for bacitracin resistance in C. perfringens, however, remains to be elucidated and further investigations should be conducted on our isolates to assess if they carry the transferable plasmid-borne bacitracin resistance genes in C. perfringens isolates recently identified in C. perfringens from Quebec (26).
A clear bimodal MIC distribution was also observed in porcine C. perfringens isolates for erythromycin and clindamycin as the representatives of the macrolide-lincosamide-streptogramin (MLS) antimicrobial family. This type of resistance is likely due to the presence of the ermQ gene encoding for an erythromycin resistance methylase, which provides resistance to most members of the MLS group (8,14,24,27). Therefore, the significant pairwise association detected between erythromycin and clindamycin MIC values and, thus, resistances, was not surprising. A selective pressure imposed by the use of antimicrobial agents from the MLS group, such as erythromycin, tylosin, lincomycin, or clindamycin, is likely a contributing factor. Rood et al (8) showed that a higher percentage of C. perfringens isolates resistant to erythromycin-lincomycin-clindamycin were recovered from porcine farms with regular antimicrobial use compared with farms with no antimicrobial use.
In bovine C. perfringens isolates, bimodal distributions were observed for clindamycin and florfenicol but not erythromycin. At present nothing is known about resistance determinants to florfenicol in C. perfringens. The lack of a bimodal distribution for erythromycin, however, indicates the existence of a resistance mechanism to lincosamides that is different from ermQ gene. It remains to be established if decreased susceptibility to lincosamides in our study is conferred by a recently described lincosamide nucleotidyltransferase (28).
No bimodal distribution of MIC values was observed for virginiamycin, but slightly higher MIC50/MIC90 values were detected in chicken (1/4 μg/mL) isolates in comparison with isolates from other species. Although no antimicrobial use data were collected, it is tempting to speculate that these higher virginiamycin MICs are the result of higher selection pressure exerted in chicken by the use of virginiamycin for the prevention of necrotic enteritis (29). Slightly higher MIC50/MIC90 values for virginiamycin in chicken (2/16 μg/mL) and turkey (2/16 μg/mL) isolates were also reported previously by Watkins et al (14) using the microbroth dilution method and a larger number of farms (n = 48) than in the present study (n = 15). It is likely that mechanisms other than the classical MLS determinants are involved in the observed virginiamycin resistance, since no co-resistance was detected between virginiamycin-erythromycin-clindamycin (Table IV).
Reduced susceptibility to tetracycline detected in C. perfringens isolates across all host species is consistent with the results of several studies from other countries. Using a microbroth dilution method and a breakpoint of ≥ 4 μg/mL, Johansson et al (9) found that 76%, 10%, and 29% of avian isolates from Sweden, Denmark, and Norway, respectively, had decreased susceptibility to tetracycline. Similar frequencies of decreased susceptibility to tetracycline were also reported in Belgium using the agar dilution method. Despite using a higher breakpoint of ≥ 8 μg/mL in this latter study, 80% of porcine, 61% of bovine, and 74% of avian isolates still showed decreased tetracycline susceptibility (23). At present there are numerous genes associated with resistance to tetracycline in Gram-positive bacteria and it remains to be established which ones are present in C. perfringens in Ontario.
Clostridium perfringens type A was the predominant type isolated in our study and most of the isolates carried the β2-toxin gene. No attempts, however, were made to differentiate between the consensus and atypical β2-toxin gene. Distribution frequency of the β2-toxin gene was dependent on the animal host species. It was most frequent among avian isolates. This finding is in agreement with a previous study by Crespo et al (30), in which more than 74% of avian isolates carried the β2-toxin gene. Isolates from necrotic enteritis cases are less likely to carry this gene (38.5%) in comparison with isolates from healthy birds (90%) (8,30). Since most of our avian isolates were recovered from birds with no necrotic enteritis, the high percentage of β2-positive avian isolates found here is not surprising.
The reasons for the negative association detected between clindamycin-erythromycin resistance in porcine isolates and the β2-toxin gene are not clear. Since both the β2-toxin gene and macrolide-lincosamide resistance genes are usually located on plasmids, one could suspect that these 2 kinds of plasmids belong to the same incompatibility group and exclude one another. Such phenomena have been cited repeatedly in other circumstances to explain negative associations between plasmid-borne genes (31). However, other hypotheses including interactions between antimicrobial use practices and disease control strategies may also explain such observations.
In summary, this study provides a baseline for antimicrobial susceptibilities of C. perfringens isolated in Ontario from 4 different animal species. Our results show widespread decreased susceptibility to antimicrobials in C. perfringens. Decreased susceptibility is not equally distributed among isolates from different animal host species, likely as a consequence of differential antimicrobial use. Some decreased susceptibility to multiple antimicrobials commonly present in other bacterial species was observed in this study, thus suggesting the potential for therapeutic challenges in the future if care is not taken to avoid the selection of multiresistant organisms. It is advisable to periodically monitor the trends in susceptibility patterns of C. perfringens isolates, considering the possibility that this organism can be a source of resistance genes that can be transferred to other bacterial species (28), including animal and human pathogens. A more detailed study to assess the susceptibility patterns of avian C. perfringens isolates is currently underway in our laboratory.
References
- 1.Songer JG. Clostridial enteric diseases of domestic animals. Clin Microbiol Rev. 1996;9:216–234. doi: 10.1128/cmr.9.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Immerseel F, de Buck J, Pasmans F, Huyghebaert G, Haesebrouck F, Ducatelle R. Clostridium perfringens in poultry: An emerging threat for animal and public health. Avian Pathol. 2004;33:537–549. doi: 10.1080/03079450400013162. [DOI] [PubMed] [Google Scholar]
- 3.Petit L, Gilbert M, Popoff MR. Clostridium perfringens: Toxinotype and genotype. Trends Microbiol. 1999;7:104–110. doi: 10.1016/s0966-842x(98)01430-9. [DOI] [PubMed] [Google Scholar]
- 4.Van Immerseel F, Rood JI, Moore RJ, Titball RW. Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends Microbiol. 2008;17:32–36. doi: 10.1016/j.tim.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Timbermont L, Lanckriet A, Cholamiandehkordi AR, et al. Origin of Clostridium perfringens isolates determines the ability to induce necrotic enteritis in broilers. Com Immun Microbiol Infec Dis. 2009;32:503–512. doi: 10.1016/j.cimid.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Schotte U, Truyen U, Neubauer H. Significance of β2-toxigenic Clostridium perfringens infections in animals and their predisposing factors-A review. J Vet Med. 2004;51:423–426. doi: 10.1111/j.1439-0450.2004.00802.x. [DOI] [PubMed] [Google Scholar]
- 7.Lahti P, Heikinheimo A, Johansson T, Korkeala H. Clostridium perfringens type A strains carrying a plasmid-borne enterotoxin gene (genotype IS1151-cpe or IS1470-like-cpe) as a common cause of food poisoning. J Clin Microbiol. 2008;46:371–373. doi: 10.1128/JCM.01650-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rood JI, Maher EA, Somers EB, Compos E, Duncan CL. Isolation and characterization of multiply antibiotic-resistant Clostridium perfringens strains from porcine feces. Antimicrob Agents Chemother. 1978;13:871–880. doi: 10.1128/aac.13.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansson A, Greko C, Engstrom BE, Karlsson M. Antimicrobial susceptibility of Swedish, Norwegian, and Danish isolates of Clostridium perfringens from poultry, and distribution of tetracycline resistance genes. Vet Microbiol. 2004;99:251–257. doi: 10.1016/j.vetmic.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Martel A, Devriese LA, Cauwerts K, de Gussem K, Decostere A, Haesenbrouck F. Susceptibility of Clostridium perfringens strains from broiler chickens to antibiotics and anticoccidials. Avian Pathol. 2004;33:3–7. doi: 10.1080/0307945031000163291. [DOI] [PubMed] [Google Scholar]
- 11.Chalmers G, Bruce HL, Parreira VR, et al. Multilocus sequence typing analysis of Clostridium perfringens isolates from necrotic enteritis outbreaks in broiler chicken populations. J Clin Microbiol. 2008;46:3957–3964. doi: 10.1128/JCM.01548-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gholamiandehkordi A, Eeckhaut V, Lancriet A, et al. Antimicrobial resistance in Clostridium perfringens isolates from broilers in Belgium. Vet Res Commun. 2009;33:1031–1037. doi: 10.1007/s11259-009-9306-4. [DOI] [PubMed] [Google Scholar]
- 13.Hansen MV, Elliott LP. New presumptive identification test for Clostridium perfringens: Reverse CAMP test. J Clin Microbiol. 1980;12:617–619. doi: 10.1128/jcm.12.4.617-619.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watkins KL, Shryock TR, Dearth RN, Saif YM. In-vitro antimicrobial susceptibility of Clostridium perfringens from commercial turkey and broiler chicken origin. Vet Microbiol. 1997;54:195–200. doi: 10.1016/s0378-1135(96)01276-x. [DOI] [PubMed] [Google Scholar]
- 15.Marks SL, Kather EJ. Antimicrobial susceptibilities of canine Clostridium difficile and Clostridium perfringens isolates to commonly utilized antimicrobial drugs. Vet Microbiol. 2003;94:39–45. doi: 10.1016/s0378-1135(03)00061-0. [DOI] [PubMed] [Google Scholar]
- 16.Prescott JF, Baggot JD. Antimicrobial therapy in veterinary medicine. 2nd ed. Ames, Iowa: Iowa State Univ Pr; 1993. [Google Scholar]
- 17.Meer RR, Songer JG. Multiplex polymerase chain reaction assay for genotyping Clostridium perfringens. Am J Vet Res. 1997;58:702–705. [PubMed] [Google Scholar]
- 18.Baums CG, Schotte U, Amtsberg G, Goethe R. Diagnostic multiplex PCR for toxin genotyping of Clostridium perfringens isolates. Vet Microbiol. 2004;100:11–16. doi: 10.1016/S0378-1135(03)00126-3. [DOI] [PubMed] [Google Scholar]
- 19.Fleiss JL, Levin B, Paik MC. Statistical methods for rates and proportions. 3rd ed. Hoboken, New Jersey: John Wiley & Sons; 2003. p. 760. [Google Scholar]
- 20.Holander M, Wolfe DA. Nonparametric Statistical Methods New York. New York: John Wiley & Sons; 1973. p. 503. [Google Scholar]
- 21.Stokes ME, Davis CS, Koch GG. Categorical data analysis using SAS system. Cary North Carolina: SAS Institute Inc; 1995. p. 499. [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals: Approved standard M31-A2. NCCLS, Wayne: Pennsylvania, USA; 2002. [Google Scholar]
- 23.Dutta GN, Devriese LA. Susceptibility of Clostridium perfringens of animal origin to fifteen antimicrobial agents. J Vet Pharmacol Therap. 1980;3:227–236. [Google Scholar]
- 24.Benning VR, Mathers JJ. Comparison of agar dilution and broth microdilution methods of anaerobic antimicrobial susceptibility testing using several veterinary antibiotics against Clostridium perfringens strains originating from porcine and avian sources. Anaerobe. 1999;5:561–569. [Google Scholar]
- 25.Prescott JF, Sivendra R, Barnum DA. The use of bacitracin in the prevention and treatment of experimentally-induced necrotic enteritis in the chicken. Can Vet J. 1978;19:181–183. [PMC free article] [PubMed] [Google Scholar]
- 26.Jalbert L, Tremblay C, Harel J, Archambault M. Characterization of new genes encoding for acquired bacitracin resistance in Clostridium perfringens. Abstract S10–2 ASM Conferences. Antimicrob Res Zoonotic Bacteria Foodborne Pathol. 2008:25. [Google Scholar]
- 27.Berryman DI, Lyristis M, Rood JI. Cloning and sequence analysis of ermQ, the predominant macrolide-lincosamide-streptogramin B resistance gene in Clostridium perfringens. Anti Agents Chemo. 1994;35:1041–1046. doi: 10.1128/aac.38.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyras D, Adams V, Ballard SA, et al. tISCpe8, an IS1595-family lincomycin resistance element located on a conjugative plasmid in Clostridium perfringens. J Bacteriol. 2009;191:6345–6351. doi: 10.1128/JB.00668-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giguère S. Lincosamides, pleuromutilins, and streptogramins. In: Giguère S, Prescott JF, Baggot JD, Walker RD, Dowling PM, editors. Antimicrobial Therapy in Veterinary Medicine. Ames, Iowa: Blackwell Publ; 2006. pp. 179–190. [Google Scholar]
- 30.Crespo R, Fisher DJ, Shivaprasad HL, Fernandez-Miyakawa ME, Uzal FA. Toxinotypes of Clostridium perfringens isolated from sick and healthy avian species. J Vet Diagn Invest. 2007;19:329–333. doi: 10.1177/104063870701900321. [DOI] [PubMed] [Google Scholar]
- 31.Monti-Bragadin C, Samer L. Compatibility of pTM89, a new F-like R factor, and of derivative plasmids. J Bacteriol. 1975;124:1132–1136. doi: 10.1128/jb.124.3.1132-1136.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]