Abstract
The inducible isoform of nitric-oxide synthase (iNOS) is involved in neuropathogenesis associated with infection and disease in the brain. Hence, there is considerable interest in the identification of therapeutic interventions to prevent iNOS-mediated pathology. Astroglia are a major site of iNOS expression during neuropathogenesis. To mimic a key component of neuroinflammation, human A172 astroglial cells were exposed in vitro to a cytokine mixture containing interferon γ, tumor necrosis factor α, and interleukin-1β, resulting in significant iNOS expression. Next, we assessed the effects of the mu opioid receptor antagonist, β-funaltrexamine (β-FNA), on cytokine induced iNOS expression in human astroglia. β-FNA dose-dependently inhibited iNOS expression. β-FNA transcriptionally (or pre-transcriptionally) inhibited cytokine-induced iNOS activation as indicated by a significant decrease in NOS2 messenger RNA expression. Further characterization of the novel, anti-inflammatory actions of β-FNA may provide insights for pharmacologic strategies to treat or prevent brain pathologies associated with neuroinflammation.
Keywords: neuroinflammation, opioids, astrocytes, nitric oxide, fentanyl, morphine
Introduction
Increasing evidence suggests that the inducible isoform of nitric-oxide synthase (iNOS) is instrumental in the neuropathogenesis associated with infection (Poluektova et al. 2005) trauma (Louin et al. 2006) and neurodegenerative diseases (Marchetti et al. 2005). While not typically expressed in the central nervous system (CNS) under physiological conditions, iNOS is transcriptionally induced by various stimuli associated with pathologic insults including, hypoxia, bacterial components, viral proteins, and cytokines. Once expressed, iNOS catalyzes the nicotinamide adenine dinucleotide phosphate-dependent oxidation of l-arginine to nitric oxide (NO) and citrulline. Unlike other NOS isoforms, iNOS has continuously high and long-lasting activity and, depending on the temporal expression and cellular context, can either be neurotoxic or neuroprotective. Increased iNOS activation associated with infection is typically neuroprotective given the role of NO in the destruction of infectious organisms. Whereas, sustained iNOS activation in the CNS is predominantly detrimental resulting in neurotoxicity and cognitive deficit. Therefore, there is an interest in identifying therapeutic strategies to attenuate excessive iNOS expression and activity, thereby reducing further neuropathogenesis (Jafarian-Tehrani et al. 2005; Louin et al. 2006). For instance, administration of 1400W (a selective inhibitor of iNOS) within 18 h of traumatic brain injury reduces neuropathogenic insult (Jafarian-Tehrani et al. 2005). More recently, opioids such as morphine have gained attention for their potential neuroprotective actions (Zhao et al. 2006). However, very little is known about the specific effects of opioids on iNOS activation in astroglia. Additionally, the clinical use of mu opioid receptor (MOR) agonists such as morphine is associated with numerous adverse effects including respiratory depression, nausea, and dependence. Herein, we have assessed the effects of a non-addictive opioid agent, the MOR-selective antagonist, β-funaltrexamine (β-FNA), on human astroglial iNOS expression. We have demonstrated that cytokine-induced iNOS expression in human astroglial cells is dose-dependently inhibited by β-FNA. Evidence for a pretranslational mechanism of action is presented and discussed; to our knowledge, this is the first report of iNOS inhibition by β-FNA.
Materials and methods
Cell culture
The human astrocytoma cell line (A172, ATCC #CRL-1620; American Type Culture Collection, Manassas, VA, USA) was used to model a key component of neuroinflammation. A172 cells were maintained as previously described (Davis et al. 2002), and experimental cultures were seeded at a cell density (1×104/cm2) to provide 80–90% confluence at the time of treatment.
iNOS induction
Optimal induction of iNOS expression in A172 cells requires co-exposure to a mixture of cytokines (Davis et al. 2002). Thus, iNOS was induced by 8–24 h exposure to a cytokine mixture containing 100 ng/ml human recombinant IFNγ (Peprotech Inc., Rocky Hill, NJ), 30 ng/ml human recombinant TNFα (Peprotech), and 5 ng/ml human recombinant IL-1β (Peprotech). To assess the effects of β-FNA on iNOS expression, β-FNA (0.05–33 μM; Sigma, St. Louis, MO) was added to cell cultures at the time of cytokine stimulation. Cell viability was monitored using the 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium (MTT) assay as previously described (Davis et al. 2007).
To quantitate iNOS activity, nitrite accumulation in the culture media was determined spectrophotometrically 24 h post-stimulation using the Griess reagent as previously described (Davis et al. 2002). Quantitative analysis of NOS2 gene expression was evaluated using the quantitative real-time polymerase chain reaction (qRT-PCR) and the SYBR green I reporter assay. Total cellular RNA was collected according to an established procedure (Davis and Syapin 2004). Primers, forward (5′ GGAGCCAGCTCTGCATTATC 3′) and reverse (5′ TTTTGTCTCCAAGGGACCAG 3′) were then developed for amplification of a 195-bp region of the iNOS gene (GenBank accession no. NM_000625) using Primer 3 software. The PCR product generated was sequenced to confirm that the amplicon was iNOS. Two-step reverse transcriptase–polymerase chain reactions (RT-PCR) were performed. Complementary DNA (cDNA) synthesis was performed using 1 μg of total RNA using the QuantiTec reverse transcription kit (Qiagen, Valencia, CA, USA), which includes a DNA removal step to eliminate genomic contamination. Manufacturer’s protocol was followed with one modification where the cDNA synthesis reaction was incubated for 30 min at 42°C instead of the 15 min described in the protocol. The quantitative gene expression analysis was performed using the FastStart SYBR green master kit (Roche Applied Science, Indianapolis, IN, USA) in a Biorad MyiQ detection system (Biorad, Hercules, CA, USA). A total reaction volume of 15 μl contained 400 nM forward primer, 400 nM reverse primer, 1× master mix, and 40 ng of cDNA. Thermal cycling conditions were 95°C for 10 min, followed by 45 additional cycles of 95°C for 30 s, 58°C for 30 s, and 72°C for 30 s, and a melting curve to ensure the amplification of a single product. Ribosomal 18S RNA (18S, RNA control kit, Eurogenetec, Philadelphia, PA, USA) was assayed according to the manufacturer’s protocol for each sample for normalization. Relative quantification of gene expression was evaluated using the comparative computed tomography (CT) method as described previously (Hettinger et al. 2001). The ΔCT value was determined by subtracting the gene CT value of each sample from the corresponding sample ribosomal 18S CT value. Calculation of ΔΔCT was carried out by subtracting the mean ΔCT value obtained for the unstimulated samples from all other ΔCT mean values. Fold changes in gene expression were then calculated from the ΔΔCT values using the formula 2−ΔΔCt.
Statistical analysis
Prism™ version 4.0 software (GraphPad Inc., San Diego, CA) was used for figure presentation, curve fitting, percent transformations, and statistical analysis. Analyses included one-way analysis of variance (ANOVA) with Neuman–Kuels multiple comparison post hoc test as well as nonlinear regression in which the Hill slope was not constrained. Data are presented as mean + SEM or mean IC50 values. For analysis of iNOS gene expression, data were analyzed by the least square ANOVA using the Proc Mixed procedure of the Statistical Analysis Software (SAS 1988). The fixed effect of treatment was analyzed for iNOS gene expression using ΔCT values. Results are presented as least square means ± standard error of the mean. A probability (P) of <0.05 was accepted as demonstrating statistically significant differences between groups. The number of replicate measures and independent experiments from which the data were obtained are provided in the individual figure legends.
Results
β-FNA effects on iNOS activity
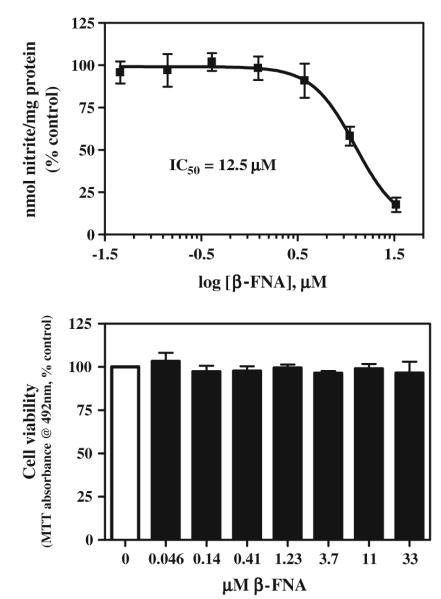
β-FNA potently inhibited cytokine-induced iNOS activity in A172 cells (Fig. 1). The reduction in iNOS activity mediated by β-FNA was not a consequence of cytotoxicity, as no differences in viability were detected among the concentrations tested (Fig. 1). Also, control experiments confirmed that β-FNA did not interfere with the nitrite assay and had no effect on nitrite detection (data not shown).
Fig. 1.
β-Funaltrexamine dose-dependently inhibits cytokine-stimulated nitrite production A172 cells. Astroglial cells were exposed for 24 h to a cytokine mixture containing IFNγ (100 ng/ml), IL-1β (5 ng/ml), and TNFα (30 ng/ml) in the presence or absence of 0.05–33 μM β-FNA. Nitrite accumulation (top panel) in the culture media was determined spectrophotometrically using the Griess reagent. Cell viability (bottom panel) was assessed using the MTT assay. The curve was generated by nonlinear regression in which the Hill slope was not constrained. Data points represent the mean ± SEM of duplicate measures from four to eight independent experiments
β-FNA effects on NOS2 mRNA expression
Minimal NOS2 mRNA expression was detected in unstimulated control cells as revealed by qRT-PCR (Fig. 2). Conversely, 8 h cytokine exposure resulted in a marked induction of NOS2 mRNA expression. Cytokine-induced NOS2 mRNA expression was significantly inhibited in the presence of 11 μM β-FNA (Fig. 2). Exposure to β-FNA alone for 8 h had no effect on NOS2 mRNA expression in A172 cells.
Fig. 2.
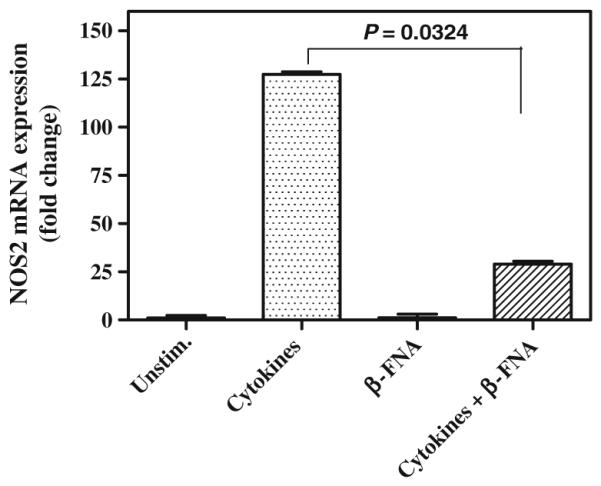
β-Funaltrexamine inhibits cytokine-stimulated NOS2 mRNA expression in A172 cells. Astroglial cells were exposed for 8 h to a cytokine mixture containing IFNγ (100 ng/ml), IL-1β (5 ng/ml) and TNFα (30 ng/ml) in the presence or absence of 11 μM β-FNA. NOS 2 mRNA expression was measured using qRT-PCR and is expressed as fold change. qRT-PCR was performed using SYBR green reporter assay. The mean ΔCT value obtained after normalization with 18S for unstimulated cells was used as a baseline for comparing NOS2 gene expression among treatments. Results are presented as least square means + standard error of the mean
Discussion
Astroglia are a major source of iNOS expression and NO production in the brain, and extensive and/or prolonged iNOS expression is presumably instrumental in neuropathogenesis (Louin et al. 2006). In the present study, iNOS activation was assessed through measurement of nitrite accumulation in the media (which only occurs in these cells after iNOS expression) and at the level of NOS2 mRNA expression. Details on the time course of cytokine-induced iNOS expression in A172 cells, at the level of mRNA and protein, have been discussed in a previous report (Davis and Syapin 2004). Likewise, cytokine-stimulated iNOS activity in A172 cells as measured by nitrite accumulation and the citrulline conversion assay has been characterized by our group (Davis et al. 2002; Davis and Syapin 2004).
Ablation of iNOS induction prevents neuropathogenesis (Jafarian-Tehrani et al. 2005; Louin et al. 2006). In particular, opioids such as morphine have neuroprotective properties (Zhao et al. 2006). While the mechanism for the neuroprotective effects of morphine are not completely understood, under certain conditions, morphine attenuates oxidative and proinflammatory stress (Lee et al. 2004). In our human astroglial model, we found that morphine (≥1 mM) and the more potent MOR agonist, fentanyl (≥0.2 mM) only inhibited iNOS expression at relatively high, biologically irrelevant concentrations (data not shown). Interestingly, the MOR antagonist, β-FNA failed to block the inhibitory actions of fentanyl (data not shown). However, this is the first report to demonstrate that β-FNA dose-dependently inhibits iNOS activity in astroglial cells. Inhibition of NOS2 mRNA expression by β-FNA suggests that iNOS activation is inhibited transcriptionally or pre-transcriptionally, yet the details remain to be elucidated. Given that the MOR selective antagonist, β-FNA, did not block fentanyl-mediated inhibition of iNOS activity suggests a MOR-independent mechanism.
The anti-inflammatory actions of β-FNA identified in this study support and add to the findings of others (Liu et al. 2000), which have identified MOR-independent, anti-inflammatory actions of the non-specific opioid receptor antagonist, naloxone. For example, both the MOR inactive stereoisomer (+)-naloxone and (−)-naloxone reduced inflammatory-induced damage of rat dopaminergic neurons, suggesting a potential therapeutic role in the treatment of Parkinson’s disease (Liu et al. 2000). More recently, this same group determined that Aβ (1–42)-induced neurotoxicity was reduced by naloxone in an opioid receptor-independent mechanism (Liu et al. 2002). It was determined that the neuroprotective effects of naloxone were due to reduced microglial superoxide production (Liu et al. 2002).
Astroglia express all four opioid receptor types (mu, kappa, delta, and nociceptin/orphanin FQ), and these receptors are differentially expressed among brain regions (Ruzicka et al. 1995; Zhao et al. 2002). We recently determined that human MOR (hMOR) is expressed in A172 cells after TNFα exposure (preliminary data). Thus, it is possible that inhibition of iNOS induction results from β-FNA action at a receptor other than MOR. For instance, depending on the opioid receptor type, β-FNA can exhibit initial KOR agonist activity and then irreversibly bound antagonist activity at MOR (Qi et al. 1990).
Maximal induction of iNOS in A172 cells requires NF-κB activation, as demonstrated via pharmacologic inhibition of iNOS activation with two mechanistically distinct NF-κB inhibitors (MG-132 and helenalin, data not shown). We recently found that translocation of the p65 subunit of the transcription factor NF-κB into the nucleus is inhibited by β-FNA (Davis et al. 2007). Thus, it may be that β-FNA inhibits iNOS induction through modulation of NF-κB expression or function; however, further investigation is needed.
In summary, the neuroprotective properties of opioid compounds are not completely understood, but both MOR-dependent and MOR-independent mechanisms are likely involved. Regarding therapeutic potential, β-FNA is particularly attractive given it is a safe, non-addictive opioid agent that readily enters the CNS. A better understanding of the mechanism governing the anti-inflammatory actions of β-FNA may provide clues for development of therapeutic strategies to combat pathologies in the CNS associated with neuroinflammation.
Acknowledgment
This work was supported in part by NIH grants AA 014955 (RLD) and DA 012448 (CWS).
Contributor Information
Randall L. Davis, Department of Pharmacology/Physiology, Oklahoma State University Center for Health Sciences, 1111 West 17th Street, Tulsa, OK 74107, USA
Daniel J. Buck, Department of Pharmacology/Physiology, Oklahoma State University Center for Health Sciences, 1111 West 17th Street, Tulsa, OK 74107, USA
Neda Saffarian, Department of Pharmacology/Physiology, Oklahoma State University Center for Health Sciences, 1111 West 17th Street, Tulsa, OK 74107, USA.
Shekher Mohan, Department of Pharmacology/Physiology, Oklahoma State University Center for Health Sciences, 1111 West 17th Street, Tulsa, OK 74107, USA.
Udaya DeSilva, Division of Agricultural Sciences and Natural Resources, Oklahoma State University, Stillwater, OK 74078, USA.
Samodha C. Fernando, Division of Agricultural Sciences and Natural Resources, Oklahoma State University, Stillwater, OK 74078, USA
Craig W. Stevens, Department of Pharmacology/Physiology, Oklahoma State University Center for Health Sciences, 1111 West 17th Street, Tulsa, OK 74107, USA
References
- Davis RL, Syapin PJ. Acute ethanol exposure modulates expression of inducible nitric-oxide synthase in human astroglia: evidence for a transcriptional mechanism. Alcohol. 2004;32:195–202. doi: 10.1016/j.alcohol.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Davis RL, Dertien J, Syapin PJ. Ethanol-induced modulation of inducible nitric-oxide synthase activity in human A172 astrocytoma cells. Alcohol Clin Exp Res. 2002;26:1404–1411. doi: 10.1097/01.ALC.0000030841.92766.80. [DOI] [PubMed] [Google Scholar]
- Davis RL, Buck DJ, Saffarian N, Stevens CW. The opioid antagonist, beta-funaltrexamine, inhibits chemokine expression in human astroglial cells. J Neuroimmunol. 2007;186:141–149. doi: 10.1016/j.jneuroim.2007.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettinger AM, Allen MR, Zhang BR, Goad DW, Malayer JR, Geisert RD. Presence of the acute phase protein, bikunin, in the endometrium of gilts during estrous cycle and early pregnancy. Biol Reprod. 2001;65:507–513. doi: 10.1095/biolreprod65.2.507. [DOI] [PubMed] [Google Scholar]
- Jafarian-Tehrani M, Louin G, Royo NC, Besson VC, Bohme GA, Plotkine M, Marchand-Verrecchia C. 1400W, a potent selective inducible NOS inhibitor, improves histopathological outcome following traumatic brain injury in rats. Nitric Oxide. 2005;12:61–69. doi: 10.1016/j.niox.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Lee J, Kim MS, Park C, Jung EB, Choi DH, Kim TY, Moon SK, Park R. Morphine prevents glutamate-induced death of primary rat neonatal astrocytes through modulation of intracellular redox. Immunopharmacol Immunotoxicol. 2004;26:17–28. doi: 10.1081/iph-120029941. [DOI] [PubMed] [Google Scholar]
- Liu B, Du L, Hong JS. Naloxone protects rat dopaminergic neurons against inflammatory damage through inhibition of microglia activation and superoxide generation. J Pharmacol Exp Ther. 2000;293:607–617. [PubMed] [Google Scholar]
- Liu Y, Qin L, Wilson BC, An L, Hong JS, Liu B. Inhibition by naloxone stereoisomers of beta-amyloid peptide (1–42)-induced superoxide production in microglia and degeneration of cortical and mesencephalic neurons. J Pharmacol Exp Ther. 2002;302:1212–1219. doi: 10.1124/jpet.102.035956. [DOI] [PubMed] [Google Scholar]
- Louin G, Marchand-Verrecchia C, Palmier B, Plotkine M, Jafarian-Tehrani M. Selective inhibition of inducible nitric oxide synthase reduces neurological deficit but not cerebral edema following traumatic brain injury. Neuropharmacology. 2006;50:182–190. doi: 10.1016/j.neuropharm.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Marchetti B, Serra PA, Tirolo C, L’Episcopo F, Caniglia S, Gennuso F, Testa N, Miele E, Desole S, Barden N, Morale MC. Glucocorticoid receptor-nitric oxide crosstalk and vulnerability to experimental parkinsonism: pivotal role for glia-neuron interactions. Brain Res Brain Res Rev. 2005;48:302–321. doi: 10.1016/j.brainresrev.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Poluektova L, Meyer V, Walters L, Paez X, Gendelman HE. Macrophage-induced inflammation affects hippocampal plasticity and neuronal development in a murine model of HIV-1 encephalitis. Glia. 2005;52:344–353. doi: 10.1002/glia.20253. [DOI] [PubMed] [Google Scholar]
- Qi JA, Heyman JS, Sheldon RJ, Koslo RJ, Porreca F. Mu antagonist and kappa agonist properties of beta-funaltrexamine (beta-FNA) in vivo: long-lasting spinal analgesia in mice. J Pharmacol Exp Ther. 1990;252:1006–1011. [PubMed] [Google Scholar]
- Ruzicka BB, Fox CA, Thompson RC, Meng F, Watson SJ, Akil H. Primary astroglial cultures derived from several rat brain regions differentially express mu, delta and kappa opioid receptor mRNA. Brain Res Mol Brain Res. 1995;34:209–220. doi: 10.1016/0169-328x(95)00165-o. [DOI] [PubMed] [Google Scholar]
- Zhao H, Huang HW, Wu GC, Cao XD. Effect of orphanin FQ on interleukin-1beta mRNA transcripts in the rat CNS. Neuroscience. 2002;114:1019–1031. doi: 10.1016/s0306-4522(02)00233-6. [DOI] [PubMed] [Google Scholar]
- Zhao P, Huang Y, Zuo Z. Opioid preconditioning induces opioid receptor-dependent delayed neuroprotection against ischemia in rats. J Neuropathol Exp Neurol. 2006;65:945–952. doi: 10.1097/01.jnen.0000235123.05677.4b. [DOI] [PubMed] [Google Scholar]