Abstract
Purpose
L-Arginine (ARG) is converted to nitric oxide (NO) and L-citrulline (CIT) by endothelial nitric oxide synthase which is competitively inhibited by asymmetric dimethylarginine (ADMA). We have developed a liquid chromatography-mass spectrometric method for the simultaneous determination of endogenous ARG, labeled ARG (15N4-ARG), CIT, ADMA, and its inactive isomer, symmetric dimethylarginine (SDMA) in biological samples.
Methods
Concentrations of unlabeled ARG, 15N4-ARG, CIT, ADMA, and SDMA in EA.hy926 human endothelial cell lysate, cell incubation media, rat plasma or rat urine were measured by hydrophilic-interaction liquid chromatography electrospray tandem mass spectrometry. 13C6-ARG, D4-CIT and D7-ADMA were used as internal standards for ARG, CIT and dimethylarginines, respectively.
Results
The calibration curves of ARG, 15N4-ARG, CIT, ADMA, and SDMA were linear and independent of several sample matrices. Intra- and inter-day variabilities for the quantification of all the compounds were below 15 % in quality control samples. Application of this method to determine the uptake as well as efflux of these compounds was illustrated through in vitro cell study by exposing human endothelial cells to 15N4-ARG, which allowed the observation of generation of 15N3-CIT and 15N3-ARG in the cell lyate. Use of these isotopes adds insights into the cellular handling of endogenous vs. exogenous ARG. Application of this method for rat plasma and rat urine assays was demonstrated after ARG oral supplementation in rats.
Conclusion
An LC-MS/MS method was developed to quantify 6 ARG-related compounds simultaneously, utilizing 3 separate internal standards. This assay allows concurrent monitoring of uptake, efflux and metabolic processes when isotope-labeled ARG and CIT are measured, and can be applied for determination of these compounds in rat plasma and rat urine.
Keywords: L-arginine, L-citrulline, asymmetric dimethylarginine, symmetric dimethylarginine, LC-MS/MS
1. INTRODUCTION
L-arginine (ARG) and its methylated metabolite, asymmetric dimethylarginine (ADMA), play an essential role in the regulation of nitric oxide (NO) production. ARG is the substrate for the enzyme nitric oxide synthase (NOS) to produce NO and L-citrulline (CIT) in various cell types, including endothelial cells [1]. On the other hand, ADMA is a competitive inhibitor of NOS [2] and its systemic concentration has been found to be elevated (and thus useful as a risk index) in a variety of diseases including chronic renal failure, hypercholesterolemia, preeclampsia, hypertension, type 2 diabetes mellitus, pulmonary hypertension, coronary artery disease [2–11]. Symmetric dimethylarginine (SDMA), an inactive isomer of ADMA, is shown to be an index of renal function [12–14]. Although SDMA does not inhibit NOS, it shares cellular transport and elimination processes with ARG and ADMA [15, 16]. Therefore, the availability of an accurate, simple, and reliable bioanalytical method for the simultaneous determination of these compounds would be useful for their biomedical investigations.
However, the quantification of ARG and its methylated metabolites faces substantial analytical challenges because of their physicochemical characteristics and their endogenous presence [17]. These compounds are polar, non-volatile and devoid of chromophores, so their analysis without derivatization by means of reversed phase HPLC is difficult. Even with derivatization, specificity and sensitivity represent challenges when using UV absorbance and fluorescence detection. Finally, their endogenous presence adds another level of complexity because the fates of exogenous added ARG and generated metabolites cannot be distinguished from those of their endogenous counterparts.
To address some of these methodological issues, a liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for measuring ARG and methylated arginines has been developed [18]. This method, however, needs further improvement in order for it to be useful for mechanistic studies concerning the cellular transport and metabolism of ARG and methylated arginines, particularly in view of the fact that discrete cellular compartments for ARG have been proposed [19, 20]. To distinguish the relative fates of exogenous vs. endogenous ARG, isotopically labeled ARG, e.g., 15N4-ARG, can be used as an exogenous source. The ability of the current method to provide simultaneous detection of 15N4-ARG and 14N4-ARG has not been established. Additionally, the available assay is not able to determine the concentration of CIT concurrently. CIT is a co-metabolite when ARG is metabolized by NOS to produce NO, and it has been utilized as an indicator of NOS activity in the traditional radioactive ARG to CIT conversion assay. CIT also acts as an endogenous source of ARG via the well-known process of “CIT-ARG recycling” [21, 22]. Thus, results obtained from simultaneous bioanalysis of CIT, ARG, and other ARG related compounds would provide valuable insights into the interlinking processes of ARG synthesis, metabolism and action.
Therefore, the aim of the present work is to improve the existing LC-MS/MS method so as to enable the simultaneous determination of ARG, 15N4-ARG, CIT, ADMA and SDMA in biological samples, utilizing stable isotope-labeled counter parts of ARG, CIT, and ADMA as internal standards. Validation studies were carried out to examine the accuracy, precision and robustness of this assay. The ability of this assay to determine the cellular and systemic changes of these compounds after in vitro and in vivo ARG supplementation was assessed.
2. MATERIALS AND METHODS
2.1. Chemicals and reagents
ARG (as L-arginine HCl), CIT (as L-citrulline), ADMA (as NG, NG-dimethylarginine dihydrochloride), and SDMA [as NG, NG′-dimethyl-L-arginine di(p-hydroxyazobenzene-p′-sulfonate) salt] were purchased from Sigma. 15N4-ARG [as ARG:HCl (U-15N4, 98%)] and the 3 internal standards, 13C6-ARG [as ARG:HCl (U-13C6, 98%)], D4-CIT [as L-citrulline (4,4,5,5-D4, 96.5 %)], and D7-ADMA [as ADMA:HCl:H2O (2,3,3,4,4,5,5-D7, 98%)] were obtained from Cambridge Isotope Laboratories, Inc. These compounds were used without further purification. Cell culture reagents were purchased from Invitrogen.
2.2. Cell culture
For the transport studies, EA.hy926 human vascular endothelial cells [23] were grown, according to literature conditions [24], i.e., in regular Dulbecco's modified Eagle's medium (DMEM) which contains 4.5 G/L of D-glucose and 84 mg/L of ARG supplemented with 10 % fetal bovine serum, and 100 U/mL penicillin and 100 μg/mL streptomycin at 37 °C in a 5% CO2 incubator. Preliminary studies in our laboratory (Mohan and Fung, unpublished data) indicated that the high concentrations of glucose and ARG (HGHA) in this medium inhibited eNOS activity. For the observation of 15N3-CIT and 15N3-ARG, cells were grown in a low glucose-low ARG modified DMEM (LGLA) containing 0.9 G/L of glucose and 21 mg/L of ARG.
2.3. In vitro cell study
After cells were grown to confluence in a 6-well plate for 7 days, they were washed twice with phosphate-buffered saline and equilibrated in Locke's solution (LS; 154 mM NaCl, 5.6 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 10 mM HEPES, 3.6 mM NaHCO3 and 5.6 mM glucose) for 1 hour before the experiment. Different concentrations of 15N4-ARG (0, 10, 50, 100, 200, 500 and 1000 μM) were added to the cells. After 2 hours, the cell incubation medium was collected to determine extracellular concentrations and cells were lysed and collected to determine cellular concentrations. Protein concentrations in the cell lysates were determined by Lowry assay.
2.4. In vivo animal study
To study the effect of oral ARG supplementation, adult male Sprague Dawley rats were divided into 2 groups, one of which received 1.25 % ARG in the drinking water, and the other group served as control. On day 2, rats were transferred to the metabolism cages and urine samples were collected for 20 hours before sacrifice for analysis. On day 3, rats were sacrificed and blood samples were collected from thoracic aorta to determine concentrations of ARG, CIT and methylated arginines in plasma. These studies were carried out under an approved protocol by the Institutional Animal Care and Use Committee at the University at Buffalo.
2.5. Calibration standards
Stock solutions containing ARG, 15N4-ARG, CIT, ADMA, or SDMA were prepared in water and then diluted with mobile phase B (acetonitrile containing 0.5% acetic acid and 0.025% trifluoroacetic acid). These solutions were added to various media to produce different calibration standards. The media include mobile phase B, Locke's solution, EA.hy926 cell lysates from HGHA and LGLA culture conditions (each at a protein concentration of 500 μg/mL), pooled rat plasma, and rat urine. Quality control samples at 3 concentration levels of each compound, based on the ranges of its calibration standard, were also prepared as described for calibration standards.
2.6. Sample preparation
Pooled rat plasma and rat urine samples were diluted with water 10 times and 5 times, respectively. An aliquot of 20 μL of each of the following matrix (mobile phase B, cell incubation media, cell lysate, diluted rat plasma and diluted rat urine) was mixed with 3 internal standards (20 μL of 13C6-ARG 1μM, 20 μL of D4-CIT 1 μM, and 20 μL of D7-ADMA 250 nM) and 120 μL of mobile phase B was added for protein precipitation. After centrifugation at 10,000 × g for 20 min, the supernatant was collected for analysis.
2.7. LC-MS/MS conditions
The liquid chromatography consists of Shimadzu LC-20AD delivery pump, SIL-20AC autosampler and CBM-20A system controller (Shimadzu Scientific Instruments; Columbia, MD). The analytes were separated on a 150 mm × 2.1 mm, Alltima HP HILIC 3 μm column by an isocratic elution with 15 % mobile phase A (water containing 0.5 % acetic acid and 0.025 % trifluoroacetic acid) and 85 % mobile phase B (acetonitrile containing 0.5% acetic acid and 0.025% trifluoroacetic acid) at a flow rate of 0.25 mL/min for 6 min.
[M+H]+ ions were analyzed in the multiple reaction monitoring mode of the ABI/Sciex API3000 triple quadrupole mass spectrometer (Applied Biosystems, Foster City, CA) equipped with an electrospray ion (ESI) source. The spray voltage was set at 5.5 kV. The flow rates of nebulizer gas (N2) and curtain gas (N2) were maintained at 10 Arb and 8 Arb, respectively. The auxiliary gas (N2) was heated up to 350°C with flow rate set at 4 Arb. Fragmentation took place at collision gas (N2) pressure of 4 mTorr. Quantification was performed using multiple reaction monitoring (MRM) under unit mass resolution for Q1 and low mass resolution for Q3. Each transition was monitored with 200 msec dwell time.
2.8. Statistical analysis
Statistical analyses were performed using Student's t-test or one-way ANOVA, followed by Tukey post-hoc test, with p<0.05 set as being significant.
3. RESULTS
3.1. Chromatography and detection
The assay parameters for the 5 analytes and the 3 internal standards are listed in Table 1 and the representative ion chromatograms of each analyte are shown in Supplementary Figure 1. Each complete chromatographic run took 6 min. MS/MS spectra gave unique signals of each analyte so that complete chromatographic separation was not necessary between ARG and 15N4-ARG, and between ADMA and SDMA. When neat solutions of ARG, 15N4-ARG, CIT, ADMA, and SDMA were individually injected onto the LC-MS/MS, no appreciable peaks were observed in the detection channels for the other compounds at their corresponding retention times. Two unidentified peaks were observed in the chromatograms for CIT and ADMA, but these were separated from the peaks of the designated analytes by retention time and they did not interfere with the quantification of these compounds.
Table 1.
Observed MRM transitions and MS settings.
| Compounds | MRM transition (m/z) | Retention time (min) | DPa (V) | FPb (V) | CEc (eV) | CXPd (V) |
|---|---|---|---|---|---|---|
| Analytes | ||||||
| ARG | 175.2 → 70.0 | 3.38 | 40 | 200 | 30 | 15 |
| 15N4-ARG | 179.2 → 71.1 | 3.38 | 40 | 200 | 30 | 15 |
| CIT | 176.2 → 70.1 | 2.82 | 28 | 200 | 30 | 12 |
| ADMA | 203.2 → 46.2 | 3.84 | 40 | 300 | 40 | 8 |
| SDMA | 203.2 → 172.2 | 3.66 | 40 | 300 | 20 | 8 |
| Internal Standards | ||||||
| 13C6-ARG | 181.2 → 74.2 | 3.38 | 40 | 200 | 30 | 15 |
| D4-CIT | 180.2 → 74.1 | 2.81 | 28 | 200 | 40 | 12 |
| D7-ADMA | 210.4 → 77.3 | 3.82 | 40 | 300 | 40 | 8 |
Declustering potential
Focusing potential
Collision energy
Collision cell exit potential.
3.2. Calibration
All calibration curves were linear with correlation coefficients of > 0.99. In general, the slopes of the calibration curves are similar among all the matrices (Supplementary Table 1). The similarity in the observed slopes of calibration curves in different matrices suggests that the use of isotope-labeled internal standard had minimized the matrix effect for the quantification for these compounds. In comparison, analysis of the two dimethylarginines using 13C6-ARG as an internal standard showed significant matrix effects (Supplementary Table 2, Supplementary Figures 2 and 3).
Substantial differences however were found in the intercept values among the various assay matrices (Supplementary Table 1). Biological matrices such as cell lysate, rat plasma and rat urine exhibited significantly different intercept values representing the basal concentrations of the analytes already present in the matrix. Consistently, in the case of 15N4-ARG, which is not an endogenous compound, the calibration curves are all parallel and the observed intercepts were zero regardless of the assay matrix.
3.3. Assay validation
In the present study, the lower limit of quantification (LLOQ) was defined as the lowest standard on the calibration curve where the analyte peak was at least 5 times the response compared to blank response with a precision of ≤20 % and an accuracy of 80 – 120 % [25]. The LLOQ values and calibration ranges for each analyte in different matrices are listed in Table 2. Except for 15N4-ARG, the LLOQ for all other analytes was dependent on the assay matrix. Non-biological matrices (i.e., mobile phase B and cell incubation medium) invariably gave rise to the lowest LLOQ, whereas rat plasma and rat urine samples yielded the highest LLOQ. These observed differences were primarily due to the presence of endogenous analyte in the cellular and biological samples.
Table 2.
Sensitivity and calibration ranges in different matrices
| ARG | 15N4-ARG | CIT | ADMA | SDMA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Matrix | LLOQ (μM) | Calibration range (μM) | LLOQ (μM) | Calibration range (μM) | LLOQ (μM) | Calibration range (μM) | LLOQ (μM) | Calibration range (μM) | LLOQ (μM) | Calibration range (μM) |
| Mobile Phase B |
0.1 | 0.1 – 20 | 0.1 | 0.1 – 20 | 0.02 | 0.02 – 1 | 0.010 | 0.010 – 0.500 | 0.010 | 0.010 – 0.500 |
| Cell incubation medium |
0.1 | 0.1 – 20 | 0.1 | 0.1 – 20 | 0.02 | 0.02 – 1 | 0.010 | 0.010 – 0.500 | 0.010 | 0.010 – 0.500 |
| Cell lysate, LGLAa |
5 | 5 – 200 | 0.1 | 0.1 – 20 | 0.1 | 0.1 – 5 | 0.025 | 0.025 – 1.000 | 0.010 | 0.010 – 0.500 |
| Cell lysate, HGHAb |
5 | 5 – 200 | 0.1 | 0.1 – 20 | 0.1 | 0.1 – 5 | 0.025 | 0.025 – 1.000 | 0.010 | 0.010 – 0.500 |
| Plasma | 5 | 5 – 200 | 0.1 | 0.1 – 20 | 2 | 2 – 100 | 0.025 | 0.025 – 1.000 | 0.010 | 0.010 – 0.500 |
| Urine | 5 | 5 – 200 | 0.1 | 0.1 – 20 | 0.2 | 0.2 – 10 | 0.025 | 0.025 – 1.000 | 0.100 | 0.100 – 5.00 |
Cell lysate obtained from EA.hy926 cells after grown in low glucose and low arginine condition
Cell lysate obtained from EA.hy926 cells after grown in high glucose and high arginine condition.
Stability of the various analytes in cell lysates, rat plasma and rat urine were assessed by comparing freshly made samples to thawed samples kept at room temperature for 5 hours. Statistical difference in concentration was not observed with any of the analytes. QC samples stored at −80 °C for 1 month did not show any degradation vs. freshly prepared samples. Collected samples were normally analyzed within the period.
The intra- and inter-day accuracy and precision of the quantification were evaluated by using quality control samples and the results at the lowest concentration are shown in Table 3 (n=5, Complete validation results are shown in Supplementary Table 3). Good accuracies were obtained for all target analytes with intra-day and inter-day bias less than 13.4 % which was the highest value found for SDMA. The intra-day and inter-day precision ranged from 0.59 % to 14.2 % and from 0.78 % to 14.4 %, respectively, which satisfy the criteria stated in the FDA guidance on bioanalytical methods validation [25].
Table 3.
Intra- and inter- day accuracy and precision of QC samples at the lowest concentration for 5 analytes in 6 media (n=5 each). Accuracy is determined as the percentage deviation of calculated concentrations from the nominal concentrations; Precision is determined as the coefficient of variation (CV) of the 5 measurements. Additional data using higher concentrations can be seen in Supplementary Table 3.
| A. Intraday variability | |||||||
|---|---|---|---|---|---|---|---|
| Matrix | Mobile phase B | Cell incubation medium | Cell lysate, LGLAa | Cell lysate, HGHAb | Rat plasma | Rat urine | |
| ARG | Concentration (μM) | 0.2 | 0.2 | 8 | 10 | 8 | 8 |
| Accuracy (%) | −8.30 | −1.80 | 8.80 | 6.20 | −0.33 | 12.4 | |
| Precision (%) | 3.42 | 11.1 | 3.62 | 4.18 | 2.59 | 1.77 | |
|
| |||||||
| 15N4-ARG | Concentration (μM) | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Accuracy (%) | −1.90 | −8.80 | 2.20 | −3.60 | −9.00 | 10.9 | |
| Precision (%) | 2.68 | 4.11 | 2.60 | 6.16 | 2.46 | 5.73 | |
|
| |||||||
| CIT | Concentration (μM) | 0.04 | 0.04 | 0.15 | 0.2 | 4 | 0.4 |
| Accuracy (%) | −2.5 | 3.15 | −5.20 | 7.40 | 9.70 | 10.8 | |
| Precision (%) | 5.46 | 6.44 | 8.66 | 2.19 | 2.38 | 4.98 | |
|
| |||||||
| ADMA | Concentration (μM) | 0.02 | 0.02 | 0.04 | 0.05 | 0.04 | 0.04 |
| Accuracy (%) | −7.50 | 4.30 | 10.10 | −6.40 | 2.05 | −12.8 | |
| Precision (%) | 4.67 | 5.72 | 4.70 | 10.4 | 8.50 | 9.57 | |
|
| |||||||
| SDMA | Concentration (μM) | 0.02 | 0.02 | 0.02 | 0.025 | 0.02 | 0.2 |
| Accuracy (%) | −3.4 | −1.30 | 8.70 | 4.96 | 0.50 | 7.30 | |
| Precision (%) | 5.4 | 6.33 | 2.55 | 10.8 | 8.56 | 11.2 | |
| B. Interday variability | |||||||
|---|---|---|---|---|---|---|---|
| Matrix | Mobile phase B | Cell incubation medium | Cell lysate, LGLAa | Cell lysate, HGHAb | Rat plasma | Rat urine | |
| ARG | Concentration (μM) | 0.2 | 0.2 | 8 | 10 | 8 | 8 |
| Accuracy (%) | 4.60 | 3.50 | −1.78 | −4.88 | 6.90 | 7.90 | |
| Precision (%) | 13.6 | 11.7 | 8.35 | 8.50 | 9.27 | 5.09 | |
|
| |||||||
| 15N4-ARG | Concentration (μM) | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Accuracy (%) | 4.30 | −5.50 | 0.70 | 3.60 | −1.50 | 9.70 | |
| Precision (%) | 7.97 | 6.89 | 3.59 | 5.60 | 7.11 | 4.52 | |
|
| |||||||
| CIT | Concentration (μM) | 0.04 | 0.04 | 0.15 | 0.2 | 4 | 0.4 |
| Accuracy (%) | 0.85 | 7.30 | −1.73 | 7.10 | 8.30 | 4.10 | |
| Precision (%) | 6.34 | 5.51 | 9.26 | 2.32 | 3.81 | 9.60 | |
|
| |||||||
| ADMA | Concentration (μM) | 0.02 | 0.02 | 0.04 | 0.05 | 0.04 | 0.04 |
| Accuracy (%) | −3.30 | −7.00 | 1.20 | −1.12 | 3.25 | −12.1 | |
| Precision (%) | 2.09 | 4.11 | 11.3 | 14.4 | 8.90 | 4.59 | |
|
| |||||||
| SDMA | Concentration (μM) | 0.02 | 0.02 | 0.02 | 0.025 | 0.02 | 0.2 |
| Accuracy (%) | −2.60 | −7.00 | −0.20 | 5.52 | 1.30 | 10.3 | |
| Precision (%) | 9.74 | 4.81 | 11.1 | 9.24 | 11.4 | 10.5 | |
Cell lysate obtained from EA.hy926 cells after grown in low glucose and low arginine condition
Cell lysate obtained from EA.hy926 cells after grown in high glucose and high arginine condition.
Carry-over was assessed by injecting blank sample after a high concentration standard (upper limit of quantitation) in each matrix. Analyte peaks were not observed in the blank sample which was injected right after the standard solution. Biological blank matrix such as cell lysate, rat plasma, and rat urine showed peaks of ARG, CIT, ADMA, and SDMA. However, these peaks represented the endogenous level of these compounds, not from carry-over. No appreciable carry-over peaks of 15N4-ARG, D4-CIT, and D7-ADMA were observed after injection of their standard solutions in any of the blank matrices.
Ion suppression was assessed by the ratios of the analyte peak response in the presence of matrix ions to that in the absence of matrix ions. Significant reduction in the peak response of 15N4-ARG, 13C6-ARG, and D4-CIT in the presence of each matrix ions was observed (Supplementary Table 4). For D7-ADMA, ionization were suppressed in the presence of cell incubation medium and rat urine, but were enhanced in the presence of cell lysate and rat plasma matrix. Due to the endogenous presence of ARG, CIT, ADMA, and SDMA in the biological matrices, ion suppression was assessed for these analytes.
3.4. 15N3-CIT and 15N3-ARG
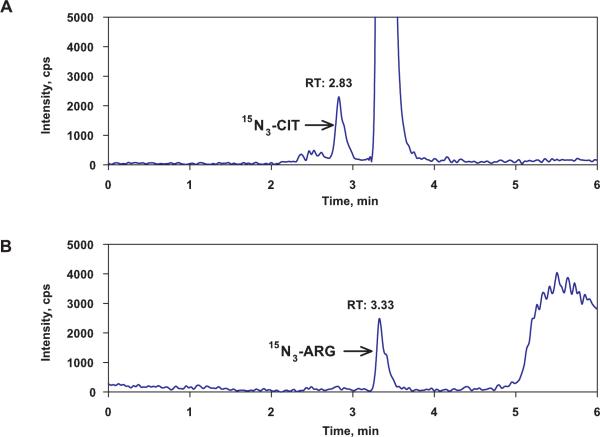
Figure 1A shows that a peak with m/z of 179.2→71.1 and a retention time of 2.83 minutes was observed in the chromatogram. The peak was tentatively assigned as that for 15N3-CIT, but cannot be unequivocally identified at present, pending the availability of an authentic standard. Although the theoretical m/z transition of this peak is the same as that for 15N4-ARG, its retention is identical to that of CIT and D4-CIT (2.82 and 2.81 min, respectively, see Table 1). This 15N3-CIT peak was observed only in the cell lysate samples which were incubated with 15N4-ARG suggesting that it was formed from 15N4-ARG by cellular enzymes. An additional peak, with m/z of 178.2→71.1 and a retention time of 3.33 minutes (Figure 1B) was tentatively assigned as 15N3-ARG, based on its theoretical m/z and retention time. This peak also could be observed only in cellular samples, and mostly has arisen from conversion of 15N3-CIT through the cellular CIT-ARG recycling process.
Figure 1.
The ion chromatograms of (A) 15N3-CIT and (B) 15N3-ARG in cell lysate samples after cells were incubated with 100 μM of 15N4-ARG. A peak with m/z of 179.2→71.1 and a retention time of 2.83 min (panel A) was assigned as that for 15N3-CIT. A peak with m/z of 178.2→71.1 and a retention time of 3.33 minutes (panel B) was assigned as 15N3-ARG based on their theoretical m/z and retention times.
3.5. Application to a human plasma sample
When the present method was applied to analyze a plasma sample from a healthy individual, the concentrations were found 60.4 ± 3.8 μM for ARG, 32.1 ± 2.2 μM for CIT, 0.519 ± 0.068 μM for ADMA, and 0.648 ± 0.067 μM for SDMA based on 3 separately prepared samples measured 5 times (n=15). These values were within the known ranges of these compounds in human plasma, i.e., 60.6 – 94.0 μM for ARG, 30.5 – 40.0 μM for CIT, 0.124 – 0.600 μM for ADMA, and 0.164 – 0.690 μM for SDMA [18, 26–35].
3.6. Application of 15N4-ARG, ARG, CIT, ADMA and SDMA assay to in vitro cell study
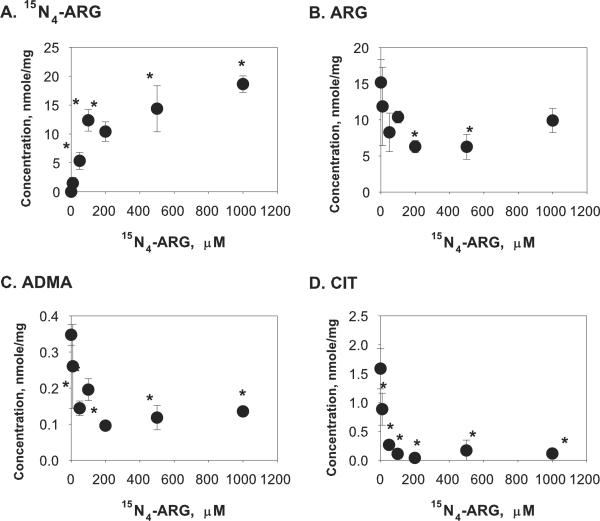
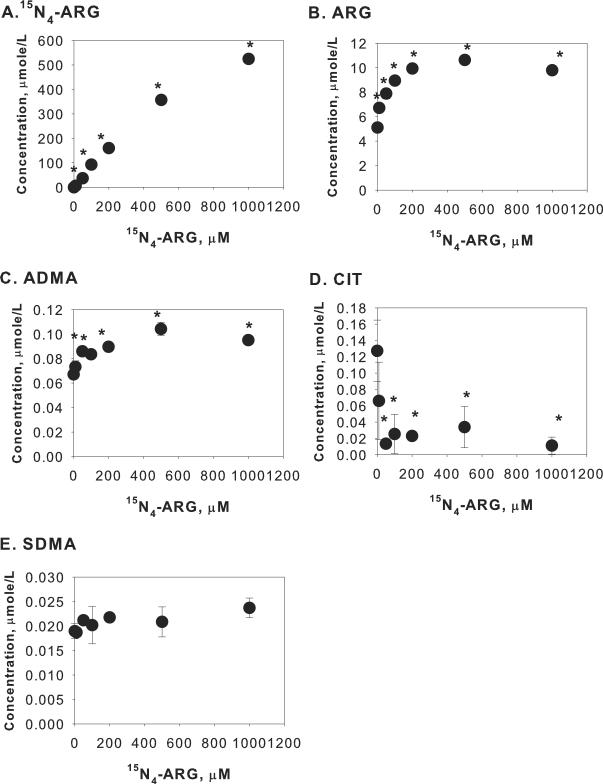
To evaluate the capability of this assay to discern complex cellular events associated with exogenous ARG supplementation, concentrations of ARG, CIT and methylated arginines were determined in the cell lysate and in the incubation medium after endothelial cells were incubated with different concentrations of 15N4-ARG for 2 hours. As shown in Figure 2A, cellular 15N4-ARG concentration was significantly increased when EA.hy926 cells were incubated with increasing extracellular 15N4-ARG concentration. This influx of 15N4-ARG was however accompanied by efflux of cellular ARG and ADMA, as evident from increased extracellular ARG and ADMA (Figure 3B and 3C) while their cellular concentrations decreased (Figure 2B and 2C). Interestingly, unlabeled CIT concentrations were reduced in both cell lysate and incubation medium (Figure 2D and Figure 3D). Cellular SDMA was not detected and there was no change in extracellular SDMA concentrations (Figure 3E) upon 15N4-ARG challenge.
Figure 2.
Cellular concentrations of (A) 15N4-ARG, (B) ARG, (C) ADMA, and (D) CIT after incubation with different concentrations of 15N4-ARG for 2 hours. Each point represents mean ± SD (n=3). *p<0.05 vs Control (i.e. 15N4-ARG = 0 μM).
Figure 3.
Extracellular concentrations of (A) 15N4-ARG, (B) ARG, (C) ADMA, (D) CIT, and (E) SDMA after incubation with different concentrations of 15N4-ARG for 2 hours. Each point represents mean ± SD (n=3). *p<0.05 vs Control (i.e. 15N4-ARG = 0 μM).
3.7. Application of ARG, CIT, ADMA and SDMA assay to in vivo animal study
The ability of the present assay to monitor changes in plasma concentrations and urinary secretion of ARG-related compounds after ARG supplementation was also examined by in vivo animal study. Table 4 shows that after 3 days of ARG oral supplementation, plasma ARG concentration was significantly increased in the ARG group compared to the control group, while plasma CIT concentration was unchanged. Plasma ADMA concentration increased slightly while SDMA did not. The volume of urine collected over 20 hours varied substantially among individual rats, from 4.0 mL to 15 mL (15 mL was the limit of the container), but there was no difference between control and ARG group. The urinary amount of ARG was found to be increased and ADMA was decreased in ARG supplemented rats. The amount of CIT and SDMA was not changed significantly. Urine pH was not changed due to ARG dosing.
Table 4.
Plasma concentrations and urinary amounts after ARG supplementation for 3 days in rats.
| Plasma (μM) | Urine (nmoles) | |||
|---|---|---|---|---|
| CONTROL (n=8) | 1.25% ARG (n=8) | CONTROL (n=6) | 1.25% ARG (n=6) | |
| ARG | 140 ± 28 | 312 ± 140** | 349 ± 173 | 1042 ± 561*+ |
| CIT | 90.2 ± 16.8 | 99.8 ± 36.6 | 62.9 ± 10.3 | 88.5 ± 52.3 |
| ADMA | 0.831 ± 0.098 | 1.05 ± 0.20* | 17.9 ± 13.9 | 3.03 ± 2.73* |
| SDMA | 0.982 ± 0.243 | 1.10 ± 0.23 | 149 ± 65 | 72.2 ± 103.7 |
Data are presented as the mean ± SD
p<0.05
p<0.01 vs control group.
Reported value (n =5) excluded an outlier which has a value of 9945 nmoles.
4. DISCUSSION
In this work, we developed and validated an LC-MS/MS assay for the simultaneous determination of ARG, CIT and isomeric dimethylarginines in six different matrices, including cell lysates, rat plasma and rat urine. Three stable isotope-labeled internal standards were used to reduce matrix effects and to improve assay accuracy and precision. Application of this assay was demonstrated through in vitro cell study and in vivo animal studies.
The use of hydrophilic-interaction liquid chromatography in this method led to substantial retention of the polar substances without the need for derivatization. The applied HPLC mobile phase, acetonitrile: water: acetic acid: trifluoroacetic acid (85: 15: 0.5: 0.025, v/v) fostered ion formation in an ESI source and the addition of a weak acid, acetic acid, reduced sensitivity loss caused by trifluoroacetic acid [17, 36, 37]. The use of tandem mass spectrometric detection overcame the problem in the reported HPLC or LC-MS assay of incomplete chromatographic separation of ADMA from SDMA [30, 38–40]. The different fragmentation patterns observed for these two isomers in the current method (m/z of 203.2 → 46.2 for ADMA and 203.2 →172.2 for SDMA) allow for separate and accurate determination of both ADMA and SDMA in biological samples. When injected individually, there was no appreciable peak of SDMA in the sample which is enriched with ADMA and vice versa.
The use of three separate stable isotope-labeled compounds as internal standards overcame the matrix effect which is known to be susceptible in ESI sources [18], as evident by the nearly identical slopes in the calibration curves observed for each analyte (ARG, 15N4-ARG, CIT, or ADMA) in all tested biological matrices (cell lysates, rat plasma and rat urine). These results are well contrasted with the substantial matrix-dependent differences in the calibration slopes of ADMA and SDMA when homoarginine was used as an internal standard [41]. We also observed different calibration slopes of ADMA and SDMA in different matrices when 13C6-ARG was used instead of D7-ADMA as an internal standard (Supplementary Figures 2 and 3).
A strict comparison with the previous literature reported HILIC LC-MS/MS assay [18] was not possible because the biological matrices used there (human plasma and human urine) were not included in this study. Nevertheless, the present assay has comparable or improved lower limit of quantification (shown in Table 2) vs. the literature assay. The LLOQ of 5.0 μM for ARG in rat plasma, cell lysates, and rat urine in the method is similar to the reported LLOQ of 7.5 μM, 3.75 μM, and 2.5 μM in human plasma, cell culture supernatant, and in urine, respectively. The literature assay reported a LLOQ of 0.15 μM for ADMA and 0.2 μM for SDMA in human plasma; 0.075 μM for ADMA and 0.1 μM for SDMA in cell culture supernatants; 5 μM for ADMA and SDMA in urine; the present assay showed a LLOQ of 0.025 μM for ADMA and 0.01 μM for SDMA in all the biological matrices (except 0.1 μM for SDMA in rat urine). The differences in LLOQ values observed from various biological matrices arose in part from the dissimilar baseline concentrations of the analytes. Application of the current method to a stored human plasma sample showed that the values obtained for ARG, CIT, ADMA and SDMA were within the normal physiological limits reported for these compounds [17, 34].
Although we did not include a stable isotope-labeled SDMA as an internal standard, it appeared that D7-ADMA provided a satisfactory substitute. Compared to 13C6-ARG, the better performance of D7-ADMA as an internal standard for SDMA quantification (Supplementary Table 2) was found especially in the urine samples which exhibited a high endogenous SDMA level. Unlike ADMA, which can be eliminated by either metabolism via dimethylarginine dimethylaminohydrolase or renal secretion, SDMA is known to be exclusively eliminated into the urine. Recently, a close correlation among serum creatinine, GFR, and SDMA were shown [12] and SDMA has been suggested to be a useful marker of renal function [14].
In addition to ARG and isomeric dimethylarginines, the current assay allows for the simultaneous assay of CIT, which is intricately involved in ARG synthesis and metabolism. Concurrent determination of CIT and ARG is impossible in ARG assays employing HPLC fluorescence with cation exchange solid phase extraction, wherein CIT was removed during sample cleanup [28]. HPLC separation of the ortho-phthalaldehyde and 2-mercaptoethanol derivatives of CIT along with ARG, ADMA, and SDMA was achieved by gradient elution with a formic acid/ ammonium formate buffer and methanol on a C18 column and ESI-MS detection [41]. However, complete chromatographic separation required a long run time, i.e., 27 min with an additional 5 min to reequilibrate the column. Moreover, homoarginine was used as an internal standard for CIT quantification, and therefore the assay is susceptible to matrix related effects. Recently, stable isotope-labeled CITs had been applied as an internal standard in the determination of underivatized CIT by LC-MS or UPLC-MS/MS [34, 35]. Here, we used D4-CIT as an internal standard for CIT quantification and were able to measure CIT, ARG and isomeric dimethylarginines without any derivatization, and in the same run within 6 min.
Analysis of 15N4-ARG, an exogenous ARG, was verified in the present assay as well. The mass unit selectivity of the LC-MS/MS method allows separation between ARG and 15N4-ARG. Because 15N4-ARG is absent endogenously, its determination in the present assay was associated with higher sensitivity. The LLOQ of 15N4-ARG was, regardless of the matrix, 0.1 μM, which is 50 times lower than that for ARG in cell lysate, rat plasma and rat urine (5 μM). The higher sensitivity observed for 15N4-ARG would favor its use in metabolic and transport studies that explore the relative roles of exogenous vs. endogenous ARG.
To illustrate this point, we showed that exposure of endothelial cells to 15N4-ARG could reveal some of the complex cellular processes that affect the cellular concentrations of ARG-related compounds. The influx of exogenous 15N4-ARG not only increased total cellular ARG (unlabeled ARG + 15N4-ARG), but induced the efflux of endogenous cellular ARG and ADMA. It has been known that the ARG transporter, cationic amino acid transporter 1 (CAT1), is sensitive to trans-stimulation [24] and ADMA has a high affinity to CAT1 as well [42, 43]. Therefore, it is possible that the influx of 15N4-ARG stimulated the efflux of cellular ARG and ADMA by CAT1 trans-stimulation. On the other hand, unlabeled CIT concentrations were intriguingly reduced in both the cell and in the incubation medium. We showed in subsequent experiments that when cells were exposed to unlabeled ARG, unlabeled CIT concentrations were not changed (data not shown), suggesting that when 15N4-ARG exposure led to the formation of 15N-CIT, and endogenous CIT was being replaced by 15N-labeled CIT. This finding suggested that endogenous CIT was preferably metabolized, vs. formed CIT, in EA.hy926 cells.
In the course of the present work, we found that it was possible to use our assay to monitor the presence of 15N3-CIT and 15N3-ARG. 15N3-CIT and 15NO are formed from the metabolism of 15N4-ARG via nitric oxide synthase, and its back conversion to ARG, via the CIT-ARG cycle [21, 22], forms 15N3-ARG. At the present time, authentic standards of 15N3-CIT and 15-ARG are not available, so quantitative assessments of the concentrations of these metabolic products of 15N4-ARG are not yet feasible. Nevertheless, the use of relative peak intensities of these compounds may allow additional insights into ARG metabolism when 15N4-ARG is used.
Application of this method was also illustrated by an in vivo animal study. We observed an increase in the mean plasma ARG concentration to 312 μM after ARG supplementation compared with 140 μM in control normal rats, consistent with literature results [44]. ARG supplementation also led to elevated plasma ADMA concentration compared to the control group. This elevation may be a result of stimulated efflux of cellular ADMA by the elevated plasma ARG concentration, as we have observed in vitro using EA.hy926 cells (Figure 3C). It is also possible that the elimination of ADMA is reduced after ARG supplementation, because ARG could inhibit dimethylarginine dimethylaminohydrolase, the metabolizing enzyme for ADMA in HepG2 cells [45]. Further studies are required to clarify the mechanisms responsible for the alteration of ADMA pharmacokinetics after ARG supplementation.
5. CONCLUSIONS
A simple, sensitive and reproducible method capable for the simultaneous bioanalysis of endogenous ARG, exogenous 15N4-ARG, CIT, ADMA, and SDMA was developed and validated. This assay can be applied to various transport, metabolism, and pharmacokinetic studies associated with either in vitro or in vivo ARG supplementation.
Supplementary Material
Acknowlegements
We thank Donna Ruszaj and David Soda for their excellent technical assistance. This work was supported in part by NIH grant HL81580.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Palmer RM, Ashton DS, Moncada S. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- [2].Vallance P, Leone A, Calver A, Collier J, Moncada S. Lancet. 1992;339:572–575. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- [3].Ueda S, Yamagishi S, Kaida Y, Okuda S. Nephrology (Carlton) 2007;12:582–590. doi: 10.1111/j.1440-1797.2007.00840.x. [DOI] [PubMed] [Google Scholar]
- [4].Bode-Boger SM, Boger RH, Galland A, Tsikas D, Frolich JC. Br J Clin Pharmacol. 1998;46:489–497. doi: 10.1046/j.1365-2125.1998.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pettersson A, Hedner T, Milsom I. Acta Obstet Gynecol Scand. 1998;77:808–813. [PubMed] [Google Scholar]
- [6].Surdacki A, Nowicki M, Sandmann J, Tsikas D, Boeger RH, Bode-Boeger SM, Kruszelnicka-Kwiatkowska O, Kokot F, Dubiel JS, Froelich JC. J Cardiovasc Pharmacol. 1999;33:652–658. doi: 10.1097/00005344-199904000-00020. [DOI] [PubMed] [Google Scholar]
- [7].Abbasi F, Asagmi T, Cooke JP, Lamendola C, McLaughlin T, Reaven GM, Stuehlinger M, Tsao PS. Am J Cardiol. 2001;88:1201–1203. doi: 10.1016/s0002-9149(01)02063-x. [DOI] [PubMed] [Google Scholar]
- [8].Gorenflo M, Zheng C, Werle E, Fiehn W, Ulmer HE. J Cardiovasc Pharmacol. 2001;37:489–492. doi: 10.1097/00005344-200104000-00016. [DOI] [PubMed] [Google Scholar]
- [9].Antoniades C, Shirodaria C, Leeson P, Antonopoulos A, Warrick N, Van-Assche T, Cunnington C, Tousoulis D, Pillai R, Ratnatunga C, Stefanadis C, Channon KM. Eur Heart J. 2009;30:1142–1150. doi: 10.1093/eurheartj/ehp061. [DOI] [PubMed] [Google Scholar]
- [10].Boger RH. Ann Med. 2006;38:126–136. doi: 10.1080/07853890500472151. [DOI] [PubMed] [Google Scholar]
- [11].Boger RH, Maas R, Schulze F, Schwedhelm E. Pharmacol Res. 2009;60:481–487. doi: 10.1016/j.phrs.2009.07.001. [DOI] [PubMed] [Google Scholar]
- [12].Fliser D, Kronenberg F, Kielstein JT, Morath C, Bode-Boger SM, Haller H, Ritz E. J Am Soc Nephrol. 2005;16:2456–2461. doi: 10.1681/ASN.2005020179. [DOI] [PubMed] [Google Scholar]
- [13].Bode-Boger SM, Scalera F, Kielstein JT, Martens-Lobenhoffer J, Breithardt G, Fobker M, Reinecke H. J Am Soc Nephrol. 2006;17:1128–1134. doi: 10.1681/ASN.2005101119. [DOI] [PubMed] [Google Scholar]
- [14].Kielstein JT, Salpeter SR, Bode-Boeger SM, Cooke JP, Fliser D. Nephrol Dial Transplant. 2006;21:2446–2451. doi: 10.1093/ndt/gfl292. [DOI] [PubMed] [Google Scholar]
- [15].Closs EI, Basha FZ, Habermeier A, Forstermann U. Nitric Oxide. 1997;1:65–73. doi: 10.1006/niox.1996.0106. [DOI] [PubMed] [Google Scholar]
- [16].Al Banchaabouchi M, Marescau B, Possemiers I, D'Hooge R, Levillain O, De Deyn PP. Pflugers Arch. 2000;439:524–531. doi: 10.1007/s004249900220. [DOI] [PubMed] [Google Scholar]
- [17].Martens-Lobenhoffer J, Bode-Boger SM. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851:30–41. doi: 10.1016/j.jchromb.2006.07.038. [DOI] [PubMed] [Google Scholar]
- [18].Martens-Lobenhoffer J, Bode-Boger SM. Clin Chem. 2006;52:488–493. doi: 10.1373/clinchem.2005.060152. [DOI] [PubMed] [Google Scholar]
- [19].Closs EI, Scheld JS, Sharafi M, Forstermann U. Mol Pharmacol. 2000;57:68–74. [PubMed] [Google Scholar]
- [20].Simon A, Plies L, Habermeier A, Martine U, Reining M, Closs EI. Circ Res. 2003;93:813–820. doi: 10.1161/01.RES.0000097761.19223.0D. [DOI] [PubMed] [Google Scholar]
- [21].Hecker M, Sessa WC, Harris HJ, Anggard EE, Vane JR. Proc Natl Acad Sci U S A. 1990;87:8612–8616. doi: 10.1073/pnas.87.21.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Phillips GB, Buckman BO, Davey DD, Eagen KA, Guilford WJ, Hinchman J, Ho E, Koovakkat S, Liang A, Light DR, Mohan R, Ng HP, Post JM, Shaw KJ, Smith D, Subramanyam B, Sullivan ME, Trinh L, Vergona R, Walters J, White K, Whitlow M, Wu S, Xu W, Morrissey MM. J Med Chem. 1998;41:3557–3562. doi: 10.1021/jm980280h. [DOI] [PubMed] [Google Scholar]
- [23].Edgell CJ, McDonald CC, Graham JB. Proc Natl Acad Sci U S A. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kakoki M, Kim HS, Edgell CJ, Maeda N, Smithies O, Mattson DL. Am J Physiol Renal Physiol. 2006;291:F297–304. doi: 10.1152/ajprenal.00417.2005. [DOI] [PubMed] [Google Scholar]
- [25].Food and Drug Administration . Guidance for Industry: Bioanalytical Method Validation, US Department of Health and Human Services. Center for Drug Evaluation and Research; Rockville, MD: 2001. [Google Scholar]
- [26].Terrlink T, van Leeuwen PA, Houdijk A. Clin Chem. 1994;40:245–249. [PubMed] [Google Scholar]
- [27].Vishwanathan K, Tackett RL, Stewart JT, Bartlett MG. J Chromatogr B Biomed Sci Appl. 2000;748:157–166. doi: 10.1016/s0378-4347(00)00399-6. [DOI] [PubMed] [Google Scholar]
- [28].Teerlink T, Nijveldt RJ, de Jong S, van Leeuwen PA. Anal Biochem. 2002;303:131–137. doi: 10.1006/abio.2001.5575. [DOI] [PubMed] [Google Scholar]
- [29].Crenn P, Vahedi K, Lavergne-Slove A, Cynober L, Matuchansky C, Messing B. Gastroenterology. 2003;124:1210–1219. doi: 10.1016/s0016-5085(03)00170-7. [DOI] [PubMed] [Google Scholar]
- [30].Huang LF, Guo FQ, Liang YZ, Li BY, Cheng BM. Anal Bioanal Chem. 2004;380:643–649. doi: 10.1007/s00216-004-2759-y. [DOI] [PubMed] [Google Scholar]
- [31].Martens-Lobenhoffer J, Krug O, Bode-Boger SM. J Mass Spectrom. 2004;39:1287–1294. doi: 10.1002/jms.684. [DOI] [PubMed] [Google Scholar]
- [32].Kirchherr H, Kuhn-Velten WN. Clin Chem. 2005;51:249–252. doi: 10.1373/clinchem.2004.042663. [DOI] [PubMed] [Google Scholar]
- [33].Schwedhelm E, Tan-Andresen J, Maas R, Riederer U, Schulze F, Boger RH. Clin Chem. 2005;51:1268–1271. doi: 10.1373/clinchem.2004.046037. [DOI] [PubMed] [Google Scholar]
- [34].Demacker PN, Beijers AM, van Daal H, Donnelly JP, Blijlevens NM, van den Ouweland JM. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:387–392. doi: 10.1016/j.jchromb.2008.12.041. [DOI] [PubMed] [Google Scholar]
- [35].Wang H-Y, Hu P, Jiang J. Chromatographia. 2010;71:933–939. [Google Scholar]
- [36].Guo Y, Gaiki S. J Chromatogr A. 2005;1074:71–80. doi: 10.1016/j.chroma.2005.03.058. [DOI] [PubMed] [Google Scholar]
- [37].Shou WZ, Naidong W. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;825:186–192. doi: 10.1016/j.jchromb.2005.01.011. [DOI] [PubMed] [Google Scholar]
- [38].Pettersson A, Uggla L, Backman V. J Chromatogr B Biomed Sci Appl. 1997;692:257–262. doi: 10.1016/s0378-4347(96)00525-7. [DOI] [PubMed] [Google Scholar]
- [39].Pi J, Kumagai Y, Sun G, Shimojo N. Journal of Chromatography B: Biomedical Sciences and Applications. 2000;742:199–203. doi: 10.1016/s0378-4347(00)00145-6. [DOI] [PubMed] [Google Scholar]
- [40].Huang L-F, Guo F-Q, Liang Y-Z, Hu Q-N, Cheng B-M. Analytica Chimica Acta. 2003;487:145–153. [Google Scholar]
- [41].Martens-Lobenhoffer J, Bode-Boger SM. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;798:231–239. doi: 10.1016/j.jchromb.2003.09.050. [DOI] [PubMed] [Google Scholar]
- [42].Bogle RG, MacAllister RJ, Whitley GS, Vallance P. Am J Physiol. 1995;269:C750–756. doi: 10.1152/ajpcell.1995.269.3.C750. [DOI] [PubMed] [Google Scholar]
- [43].Teerlink T. Vasc Med. 2005;10(Suppl 1):S73–81. doi: 10.1191/1358863x05vm597oa. [DOI] [PubMed] [Google Scholar]
- [44].Pieper GM, Siebeneich W, Dondlinger LA. Eur J Pharmacol. 1996;317:317–320. doi: 10.1016/s0014-2999(96)00831-x. [DOI] [PubMed] [Google Scholar]
- [45].Wang J, Sim AS, Wang XL, Wilcken DE. Cell Mol Life Sci. 2006;63:2838–2846. doi: 10.1007/s00018-006-6271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.