Abstract
Background
The COMBINE Study evaluated the effects of acamprosate, naltrexone and the Combined Behavioral Intervention (CBI). In secondary analyses, our goals were to identify trajectories of any drinking prior to randomization, to characterize subjects in these trajectories, and to assess whether pre-randomization trajectories predict drinking outcomes and moderate treatment response.
Methods
We analyzed daily indicators of any drinking in 90 days prior to randomization using a trajectory-based approach. General linear models and generalized logistic regression assessed main and interactive effects of pre-randomization drinking trajectories and treatment on summary drinking measures during active treatment.
Results
We identified five trajectories of any drinking prior to randomization: “T1: frequent drinkers”, “T2: very frequent drinkers”, “T3: nearly daily drinkers”, “T4: consistent daily drinkers” and “T5: daily drinkers stopping early”. During treatment, “T3: nearly daily drinkers” and “T4: consistent daily drinkers” had significantly worse drinking outcomes than “T1: frequent drinkers” while “T5: daily drinkers stopping early” had comparable drinking outcomes to “T1: frequent drinkers”. Acamprosate significantly increased the chance of abstinence from heavy drinking for the “T2: very frequent drinking” trajectory but decreased the chance of abstinence from heavy drinking for “T5: daily drinkers stopping early”. Naltrexone differentially improved rates of continuous abstinence for very frequent drinkers.
Conclusions
Acamprosate benefited very frequent drinkers and contrary to expectations was associated with poorer response compared to placebo for consistent daily drinkers who had longer durations of pretreatment abstinence (e.g., ≥ 14 days). Baseline drinking trajectories also moderated naltrexone effects. These findings may help clinicians identify patients for whom acamprosate and naltrexone may be most beneficial.
Keywords: naltrexone, acamprosate, clinical trial, latent class, trajectory-based analysis
Introduction
The COMBINE Study investigated questions related to the benefits of combining behavioral and pharmacological interventions (Anton et al, 2006). Analyses of the primary endpoints, time to the first day of heavy drinking and percent days abstinent, revealed that either naltrexone or Combined Behavioral Intervention (CBI) improved outcome, but did not find significant effects of acamprosate either alone or in combination. In a secondary trajectory-based analyses (Gueorguieva et al, 2010), we showed that naltrexone alone, CBI alone and the combination of naltrexone and CBI decreased the likelihood of following different post-randomization drinking trajectories. Thus, we illustrated the advantages of such a modeling approach in revealing additional information about the potential mechanism of treatment effects. In this study, we apply the trajectory-based approach to identify trajectories of drinking prior to randomization and use these as a novel measure to predict treatment response and to evaluate trajectories as potential moderators of treatment response.
The failure to find an effect of acamprosate in the original analysis was puzzling given that most European studies had demonstrated its efficacy (Lhuintre et al., 1990; Mason and Ownby, 2000; Whitworth et al., 1996). Several methodological differences from the European trials have been pointed to including the short duration of abstinence required and the fact that very few patients in COMBINE were detoxified (Anton et al., 2006; Kiefer and Mann, 2006). One proposed mechanism for acamprosate’s efficacy is that it dampens glutamatergic hyperactivity related to alcohol acute and protracted withdrawal (De Witte et al., 2003; Heilig and Egli, 2006; Mann et al., 2008). In light of this mechanism, Kiefer and Mann (2006) have hypothesized that the COMBINE Study, by enrolling individuals who were able to achieve 4 days of pretreatment abstinence without detoxification, may have selected a less dependent sample that would not be responsive to acamprosate.
By evaluating baseline trajectories of drinking that capture frequency of drinking and lead-in abstinence simultaneously, we sought to identify a subgroup of patients for whom acamprosate would be effective. We hypothesized a priori that individuals whose baseline drinking trajectory was characterized by consistent daily drinking and recent cessation, compared to a trajectory of consistent daily drinking and earlier initiation of abstinence, may benefit from acamprosate. This hypothesis was based on the assumption that glutamatergic hyperactivity would be more pronounced during early abstinence rather than following extended abstinence. In the COMBINE Study, participants were required to have at least four days of abstinence and no more than 21 days, so our assessment of recency of cessation is within these confines. Most participants achieved this without outpatient or inpatient detoxification.
Kampman et al. (2009) compared the efficacy of acamprosate initiated during outpatient detoxification to that of acamprosate initiated immediately following successful detoxification lasting no more than 14 days during which at least 3 days of consecutive abstinence occurred. Their results indicated that acamprosate was more effective when initiated following successful detoxification and at least three days of abstinence. This study was unable to examine the influence of more extended periods of pretreatment abstinence.
We also explored whether baseline trajectories predict who benefits from naltrexone or from the CBI. One of the debates within the naltrexone literature is whether naltrexone works best in those who abstain prior to treatment or among those who drink on naltrexone (Sinclair, 2001; Ray et al., 2010). However, few studies have specifically examined how pretreatment abstinence influences treatment response. A secondary analyses of a study of extended release naltrexone (Garbutt et al., 2005; O’Malley et al., 2007) found that the effects of naltrexone were larger in participants who had achieved a period of abstinence prior to randomization (whether 4 or 7 days of abstinence) and that extended release naltrexone helped maintain abstinence in these patients.
Analysis of baseline drinking trajectories may also inform the design of future pharmacotherapy studies if we identify a trajectory that is associated with high rates of response regardless of treatment. Exclusion of such “placebo responders” in clinical trials could improve power to test study hypotheses.
In summary, the goals of this study were to identify trajectories of any drinking prior to randomization in the COMBINE clinical trial, to characterize subjects in these trajectories, and to assess whether pre-randomization trajectories predict post-randomization drinking outcomes and whether they moderate treatment effects on these outcomes. We hypothesized that a baseline trajectory of intensive drinking with recent cessation of alcohol prior to enrollment would predict an increased likelihood of abstinence in response to active acamprosate compared to placebo acamprosate. We did not have specific a priori hypotheses regarding trajectory subgroups that would benefit from naltrexone.
Methods
The COMBINE study enrolled 1,383 abstinent alcohol dependent patients. Eight groups received Medication Management (MM) and either placebos, naltrexone, acamprosate, or naltrexone + acamprosate for 16 weeks. Half of these groups also received the CBI. Participants on different treatments were comparable on seventy-six pretreatment characteristics (Anton et al, 2006). A ninth group that received CBI alone with no pills in order to examine placebo effects in secondary analyses is not included in this report.
The Form 90 (Miller, 1996; Miller and DelBoca, 1994) was used to retrospectively collect daily drinking data at intake and the Timeline Follow-back interview (Sobell and Sobell, 1992, 1995) was administered at each subsequent study appointment to obtain reports of daily drinking. Both are semi-structured interviews based on a day-by-day calendar method to reconstruct alcohol use, allowing collection of information on days of drinking as well as amount consumed per day. Both are comprehensive self-report measures and have good reliability and internal consistency on summary drinking measures (Sobell and Sobell, 1992, 1995; Tonnigan et al, 1997). In this trajectory-based reanalysis we focused on baseline daily binary indicator of drinking (1 if any drinks were consumed by the subject on that day, 0 otherwise).
Identification of baseline drinking trajectories
We used the approach of Nagin (1999) and Nagin and Tremblay (2001) to identify distinct trajectories of drinking patterns during the 90 days prior to randomization. The models assumed fixed polynomial trends over time within each trajectory class and the time origin was the day before the subjects started taking medication. The final models were obtained via model selection (number of trajectory classes and degree of the polynomial trends over time such as quadratic or cubic) based on the Schwartz Bayesian Criterion (BIC) and on having at least 5% of subjects in each trajectory class. BIC is a criterion of how well the model fits the data while keeping model complexity low. Our modeling strategy allowed the data to guide the choice of the number of trajectories that best fit the data and to determine the shape of each trajectory before randomization. It also allowed estimation of the proportion of the population whose treatment response corresponds most closely to each trajectory group. While drinking data are known to have strong 7-day periodicity, the public use COMBINE data set does not have such information and we were not able to incorporate periodicity into the models. For the analysis we used a customized SAS procedure (PROC TRAJ) developed by Jones et al (2001).
Based on the final trajectory models we calculated the posterior probabilities of membership in each trajectory class for each subject. We used categorical variables of trajectory class as response variables in the comparisons of baseline characteristics of the identified baseline trajectories. We also used such variables as predictors in the analyses of drinking outcomes. Classification accuracy was assessed using the entropy measure (Muthén, 2004) with values close to 1 indicating excellent classification of individuals to trajectory classes.
Comparison of baseline drinking trajectories on baseline characteristics
To understand the identified baseline trajectories, we compared baseline characteristics of the subjects classified in each trajectory using ANOVA, chi-square tests and non-parametric methods as needed. Since these analyses aimed to only characterize subjects in the different baseline trajectories we did not apply correction for multiple tests and report significant differences at 0.05, 0.01 and 0.001 level. Descriptive measures of drinking behavior included the following: peak BAC (averaged over the two heaviest drinking episodes in the 90 days prior to intake), drinks per drinking day, percent days abstinent, percent heavy drinking days and days of abstinence prior to randomization. Information about Alcoholics Anonymous attendance, inpatient treatment or alcohol detoxification medications, legal problems, and mental health problems for the 90 days prior to intake was obtained on the Form90. History of alcohol withdrawal symptoms was obtained from the SCID-IV Alcohol Module (First et al., 1997), and current symptoms were obtained using the Clinical Withdrawal Assessment Scale- AR (Sullivan et al., 1989) administered at intake. Other clinical assessments included the Alcohol Dependence Scale (ADS; Skinner and Allen, 1982), the total score of the Impulsive Drinking Subscale of the Drinker Inventory of Consequences (DrInC; Miller et al., 1995) and the total score of the Obsessive Compulsive Drinking Scale (Anton et al., 1995). Commitment to abstinence was determined from the treatment goal question from the Thoughts about Abstinence Scale (Hall et al., 1990). A binary variable was computed based on the response “I want to quit using alcohol once and for all, to be totally abstinent, and never use alcohol ever again for the rest of my life” versus all others. Participants completed these assessments at one of the intake appointments as described in the COMBINE methods paper (COMBINE Research Group, 2003).
Predictive and moderating effects of baseline trajectories of drinking on drinking outcomes
We modeled the effect of baseline trajectories and treatment (naltrexone, acamprosate, CBI and their interactions) on primary post-randomization drinking outcomes using general linear models for continuous variables and logistic regression models for binary outcomes. Percent abstinent days and percent heavy drinking days were based on the available drinking data from randomization through week 16. As reported in Anton et al. (2006), complete timeline data was available for 94% of the participants. Good Clinical Outcome was defined as abstinence or moderate drinking without problems based on responses the Form 90 and DrInC. Moderate drinking was defined as a maximum of 11 (women) or 14 (men) drinks per week, with no more than 2 days on which more than 3 drinks (women) or 4 drinks (men) were consumed. Problems were defined as endorsing 3 or more items on the DrInC assessing physical, social, and psychological consequences of drinking.
We also analyzed the following secondary outcome measures using the same approach: abstinence from heavy drinking in last two months of the trial and continuous abstinence using logistic regression. Abstinence from heavy drinking following a grace period has been proposed as an outcome measure for future clinical trials because it is associated with reduced risk of alcohol related consequences while allowing for improvements in drinking short of abstinence (Falk et al., 2009). We included continuous abstinence as an exploratory outcome because this was the primary outcome used in the approval of acamprosate by the FDA based on a reanalysis of data from three European clinical trials (Kranzler & Gage, 2008). For each drinking outcome, we performed backward elimination starting with a model with four-way and all lower order interactions among baseline trajectories and treatments, then dropping the four-way interaction if not significant at 0.05 level, and dropping the three-way interactions if they were not significant at 0.05 level. Mean differences and odds ratios were calculated for the significant interactions and main effects in the final models. Significant effects at 0.05, 0.01 and 0.001 levels are indicated in the tables presented in the Results section. Because this is exploratory analysis, we report all significant effects at 0.05 level. However, a more conservative approach would be to apply Bonferroni correction for the analysis of baseline trajectory effects on five outcome measures. With this correction, effects at the 0.01 level would be considered significant.
Results
Identification of baseline drinking trajectories
We identified five distinct trajectories of any drinking prior to randomization (Figure 1) with cubic polynomials describing the shapes over time. These trajectories can be referred to as “T1: frequent drinkers”, “T2: very frequent drinkers”, “T3: nearly daily drinkers”, “T4: consistent daily drinkers” and “T5: daily drinkers stopping early”. Entropy of the model was excellent (0.97) thus most subjects were clearly assigned to a particular trajectory.
Figure 1. Five baseline trajectories of any drinking. a.
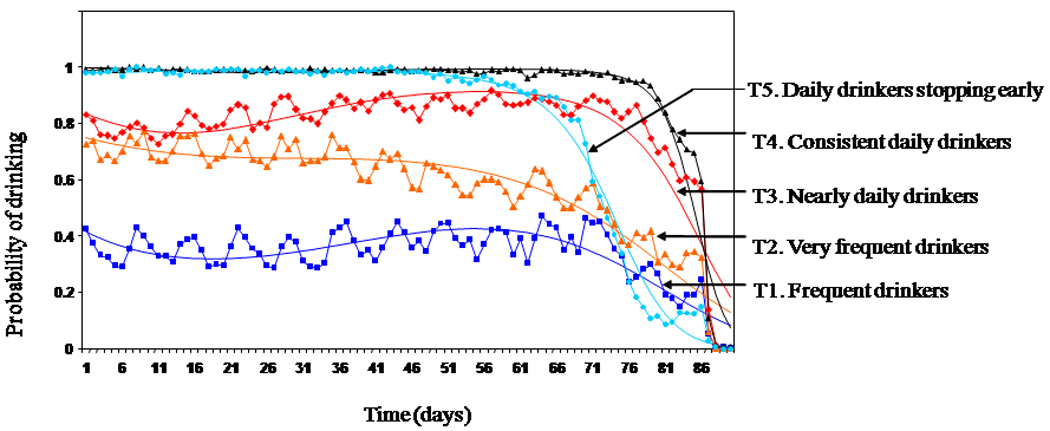
a Solid lines with symbols represent sample-based probabilities of drinking based on all subjects weighted by the posterior probability of trajectory membership. Solid lines without symbols represent model-based probabilities of drinking over time for each trajectory group.
In all trajectories drinking declined prior to randomization as required by the criteria for study entry but there were differences in the chance of drinking over the 90 day baseline period and the abruptness with which drinking was discontinued prior to study entry. “T1: frequent drinkers” (16.1% of the sample) had about a 40% chance of drinking on a particular day. “T2: very frequent drinkers” (20.8% of the sample) had about a 75% chance of drinking on a particular day. “T3: nearly daily drinkers” (17.9% of the sample) had between 80 and 90% chance of drinking on a particular day. “T4: consistent daily drinkers” (29% of the sample) had virtually 100% chance of drinking on a particular day and were barely able to stop drinking prior to study entry. In contrast “T5: daily drinkers stopping early” (16.2% of the sample) reduced their drinking substantially at least 15 days in advance of randomization. This might reflect repeated attempts to achieve the required four consecutive days of abstinence prior to study entry.
Comparison of baseline drinking trajectories on baseline characteristics
Table 1 compares the five trajectories on summary measures of alcohol consumption derived from the Form 90. The baseline characteristics of “T1: frequent drinkers” suggest a binge pattern with less frequent but more intense drinking (e.g., high peak blood alcohol level, more drinks per drinking day). Drinks per drinking day and peak BAC for Trajectories 2–3 were significantly lower than for “T1: frequent drinkers”. Participants in Trajectories 2–4 had significantly fewer days of abstinence prior to randomization than participants in “T1: frequent drinkers” (Mean = 9.5, SD=5.7) and participants in “T5: daily drinkers stopping early”. Subjects in “T5: daily drinkers stopping early” were drinking nearly every day, consumed more drinks per drinking day than the other trajectories except “T1: frequent drinkers” and had the longest duration of pre-randomization abstinence (Mean = 12.3, SD=6.3). Baseline percent days abstinent declined across trajectories 1 through 4.
Table 1.
Baseline Drinking Characteristics by Trajectories
| Descriptive statistics by drinking trajectory | |||||
|---|---|---|---|---|---|
| Baseline drinking summary measuresa | T1: Frequent (N=196) |
T2: Very frequent (N=259) |
T3: Nearly daily (N=219) |
T4: Daily (N=354) |
T5: Daily stopping early (N=198) |
| Peak BAC+++ | 0.38(0.16) | 0.33(0.16)*** | 0.28(0.15)*** | 0.28(0.16)*** | 0.36(0.17) |
| # of drinks/drinking day +++ | 15.42(10.11) | 11.96(8)*** | 11.08(6.13)*** | 11.22(6.87)*** | 14.48(8.09) |
| Percent days abstinent +++ | 59.64(16.53) | 43.6(17.18)*** | 13.94(10.05)*** | 3.93(5.63)*** | 17.87(20.87)*** |
| Percent heavy drinking days+++ | 35.88(16.1) | 47.06(21.24)*** | 74.19(21.47)*** | 85.22(20.86)*** | 74.4(26.95)*** |
| Days abstinent prior to the first day of treatment+++ | 9.45(5.69) | 8.11(5.73)** | 5.49(2.97)*** | 4.97(1.98)*** | 12.33(6.27)*** |
Peak BAC (Blood Alcohol content) is for the 90 day period prior to randomization; All other frequency measures are for the 30 baseline period prior to the initiation of abstinence prior to treatment.
NOTE: Significant overall test of differences among the five trajectories is denoted by the following superscripts next to the variable name:
<.05;
<.01;
<.001. Significant comparisons to T1 are denoted by superscripts next to the number for the corresponding trajectory:
<.05;
<.01;
<.001.
Table 2 compares the trajectory groups on demographic, treatment history, alcohol withdrawal and alcohol-related clinical characteristics. Patients in the five trajectories varied on numerous characteristics with the most pronounced differences observed between “T4: consistent daily drinkers” and “T5: daily drinkers stopping early”. Participants in “T5: daily drinkers stopping early” had higher ADS scores, higher rates of medical detoxification/inpatient treatment, more mental health problems, greater commitment to abstinence and attendance of Alcoholics Anonymous.
Table 2.
Baseline Characteristics by Trajectories
| T1: Frequent (N=196) |
T2: Very frequent (N=259) |
T3: Nearly daily (N=219) |
T4: Daily (N=354) |
T5: Daily stopping early (N=198) |
|
|---|---|---|---|---|---|
| Demographics, | n(%) | n(%) | n(%) | n(%) | n(%) |
| Age, Mean(SD)+++ | 41.18(9.38) | 43(9.42) | 44.33(9.77)** | 47.1(10.71)*** | 44.35(10.18)** |
| Female++ | 64(32.65%) | 81(31.27%) | 79(36.07%) | 114(32.2%) | 40(20.2%)** |
| White | 144(73.47%) | 201(77.61%) | 169(77.17%) | 286(80.79%)* | 141(71.21%) |
| Black++ | 12(6.12%) | 23(8.88%) | 15(6.85%) | 21(5.93%) | 29(14.65%)** |
| Hispanic | 30(15.31%) | 29(11.2%) | 24(10.96%) | 31(8.76%)* | 24(12.12%) |
| Other race | 10(5.1%) | 6(2.32%) | 11(5.02%) | 16(4.52%) | 4(2.02%) |
| High school or less education++ | 62(32.29%) | 73(28.63%) | 51(23.83%) | 91(26.15%) | 77(39.69%) |
| Not employed | 59(30.26%) | 66(25.68%) | 64(29.36%) | 93(26.27%) | 61(30.96%) |
| Not married+++ | 129(65.82%) | 158(61.24%) | 130(59.36%) | 168(47.46%)*** | 124(62.63%) |
| Weight | 181.06(35.08) | 180.90(38.25) | 181.65(40.80) | 180.20(40.64) | 173.53(32.20) |
| High GGT+++ | 37(19.27%) | 79(30.86%)** | 54(24.77%) | 127(35.98%)*** | 90(45.69%)*** |
| Current smoker+++ | 123(63.4%) | 143(56.3%) | 121(55.5%) | 147(42.73%)*** | 122(63.21%) |
| Prior treatment in past 90 days | |||||
| Overnight care/detox med.+++ | 28(14.29%) | 36(13.90%) | 35(15.98%) | 17(4.80%)*** | 50(25.25%)** |
| Any prior treatment+++ | 119(60.71%) | 145(55.98%) | 85(38.81%)*** | 134(37.85%)*** | 116(58.59%) |
| Attend AA+++ | 67(34.18%) | 80(30.89%) | 46(21%)** | 23(6.5%)*** | 46(23.23%)* |
| Withdrawal, n(%) | |||||
| History of ALC. withdrawal+++ | 124(63.27%) | 168(64.86%) | 131(59.82%) | 192(54.24%)* | 152(76.77%)** |
| CIWA Total Score, Mean(SD)+ | 1.45(1.87%) | 1.88(2.36%)* | 1.74(1.87%) | 2.07(2.44%)** | 2.08(2.24%)** |
| Alcohol related clinical characteristics, Mean(SD) | |||||
| Age of onset+++ | 27.64(10.54) | 29.49(10.66) | 31.62(11.16)*** | 32.03(11.86)*** | 31.15(11.55)** |
| ADS Score+++ | 18.81(7.53) | 17.96(6.93) | 16.11(7.18)*** | 14.12(6.46)*** | 18.19(8.07) |
| DrInC Total Score+++ | 49.07(20.29) | 50.08(19.84) | 49.02(21.41) | 42.46(19.23))*** | 52.07(20.55) |
| DrInC Impulsive Actions+++ | 8.07(4.39) | 7.73(3.97) | 7.67(4.28) | 6.63(4.13)*** | 8.46(4.66) |
| OCDS Total Score++ | 24.96(8.59) | 26.54(7.91)* | 27.57(7.73)** | 27.09(7.62)** | 27.48(9.36)** |
| Mental Health Problem, n(%)+++ | 112(57.14%) | 129(49.81%) | 102(46.58%)* | 130(36.72%)*** | 106(53.54%) |
| Legal problems (arrested/parole/probation), n(%)++ |
34(17.35%) | 36(13.9%) | 21(9.59%)* | 26(7.34%)*** | 31(15.66%) |
| Commitment to Abstinence, n(%)+++ | 86(43.88%) | 113(44.31%) | 74(34.91%) | 91(26.07%)*** | 94(48.21%) |
NOTE: Significant overall test of differences among the five trajectories is denoted by the following superscripts next to the variable name:
<.05;
<.01;
<.001. Significant comparisons to T1 are denoted by superscript s next to the number for the corresponding trajectory:
<.05;
<.01;
<.001.
Predictive and moderating effects of baseline trajectories of drinking on drinking outcomes
Interactions of baseline trajectories and acamprosate
We observed significant interactions between baseline trajectory and acamprosate for good clinical outcome and abstinence from heavy drinking in the last two months (Table 3). The acamprosate effects stayed significant after covarying for percent days abstinent at baseline and consecutive days abstinent prior to study entry. Thus, baseline trajectories appear to moderate acamprosate effects albeit the results were not consistent with our a priori hypothesis.
Table 3.
Significant Effects of Trajectories on Drinking Outcomes
| Descriptive statistics by drinking trajectory | ||||||
|---|---|---|---|---|---|---|
| Outcome, mean(SD) | Significant effect | T1: Frequent (N=196) |
T2: Very frequent (N=259) |
T3: Nearly daily (N=219) |
T4: Daily (N=354) |
T5: Daily stopping early (N=198) |
| Percent days abstinent+++ | Main effect of trajectory+++ | 87.4(16.6) | 80.8(22.3)* | 70.3(28.9)*** | 64.4(34.0)** | 84.8(22.9) |
| Percent heavy drinking days+++ | Main effect of trajectory+++ | 9.6(13.9) | 13.2(19.1) | 17.9(23.9)*** | 19.6(27.6)** | 10.4(19.4) |
| Good clinical outcome, n(%)++ | Main effect of trajectory++ | 129 (80.1%) | 149 (71.6%) | 115 (65.7%)** | 190 (64.6%)** | 126 (75.5%) |
| Interaction between trajectory and acamprosate+ |
Active acamprosate |
Active acamprosate |
Active acamprosate |
Active acamprosate |
Active acamprosate |
|
| 67 (83.8%) | 75 (68.8%)* | 61 (71.8%)* | 99 (67.8%)** | 58 (69.9%)* | ||
| Placebo acamprosate |
Placebo acamprosate |
Placebo acamprosate |
Placebo acamprosate |
Placebo acamprosate |
||
| 62 (76.5%) | 74 (74.8%) | 54 (60.0%)* | 91 (61.5%) | 68 (81.0%) | ||
|
Abstinence from heavy drinking in last two months |
Main effect of trajectory+ |
81 (45.0%) | 101 (41.1%) | 83 (40.5%) | 138 (41.4%) | 104 (55.9%)* |
| Interaction between trajectory and acamprosate+ |
Acamprosate | Acamprosate | Acamprosate | Acamprosate | Acamprosate | |
| 41 (46.6%) | 60 (47.6%) | 46 (45.5%) | 72 (43.9%) | 44 (48.4%) | ||
| Placebo | Placebo | Placebo | Placebo | Placebo | ||
| 40 (43.5%) | 41 (34.2%) | 37 (35.6%) | 66 (39.1%) | 60 (63.2%)** | ||
|
Continuous abstinence, n(%) |
Main effect of trajectory+++ |
36 (20.0%) | 56 (22.8%) | 30 (14.7%) | 59 (17.9%) | 65 (35.0%)** |
| Interaction between trajectory and naltrexone+ |
Naltrexone | Naltrexone | Naltrexone | Naltrexone | Naltrexone | |
| 15 (17.1%) | 36 (27.9%) | 13 (11.7%) | 33 (22.3%) | 35 (36.5%)** | ||
| Placebo | Placebo | Placebo | Placebo | Placebo | ||
| 21 (22.8%) | 20 (17.1%) | 17 (18.3%) | 26 (14.3%) | 30 (33.3%) | ||
NOTE: Significant overall test of differences among the five trajectories is denoted by the following superscripts next to the variable name:
<.05;
<.01;
<.001. Significant comparisons to T1 are denoted by superscript s next to the number for the corresponding trajectory:
<.05;
<.01;
<.001.
All other interactions involving baseline trajectories were not significant.
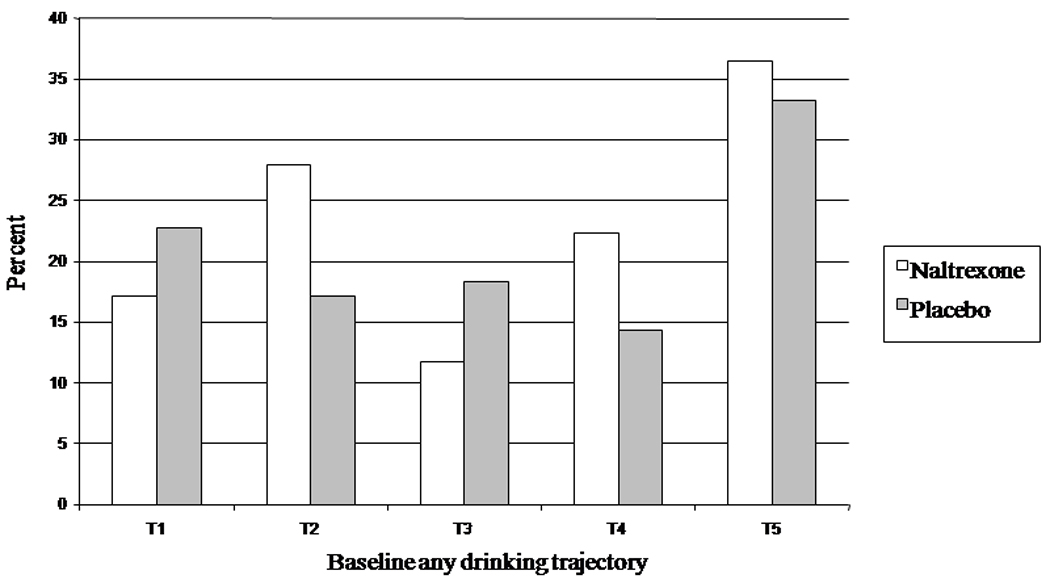
Comparisons of acamprosate and placebo within trajectory revealed that acamprosate significantly increased the chance of abstinence from heavy drinking for “T2: very frequent drinkers” (OR=1.74, 95% CI: (1.04, 2.91)) but decreased the chance of abstinence from heavy drinking for “T5: daily drinkers stopping early” (OR=0.54, 95% CI: (0.30, 0.98)). Figure 2 illustrates this comparison. The differences between active and placebo acamprosate were not significant for the remaining trajectories. Post-hoc tests for acamprosate effects for good clinical outcome were not statistically significant for any of the trajectories.
Figure 2.

Percent abstinent from heavy drinking in the last two months by baseline trajectory and acamprosate.
Given that acamprosate, compared to placebo, was associated with positive outcome for individuals in “T2: very frequent drinkers” and with poorer outcome in “T5: daily drinkers stopping early”, we conducted a logistic regression analysis via backward elimination to determine which patient characteristics most discriminated members of these two trajectories. Subjects in “T5: daily drinkers stopping early” were older (OR=1.03, 95% CI: (1.01, 1.05)), had higher rates of medical detoxification/inpatient treatment (OR=1.85, 95% CI: (1.03, 3.33)), and higher impulsive actions scores on the DrInC (OR=1.06, 95% CI: (1.01, 1.11)). They were also less likely to be female (OR=0.45, 95% CI: (0.27, 0.76)), weighed less (OR=0.99, 95% CI: (0.98, 1.00)) and were more likely to have elevated GGT levels (OR=1.57, 95% CI: (1.02, 2.41)).
Interactions of baseline trajectories and naltrexone
Baseline trajectories interacted with naltrexone in predicting continuous abstinence (Table 3 and Figure 3). Subjects in “T5: daily drinkers stopping early” had significantly higher rate of continuous abstinence than subjects in “T1: frequent drinkers” on naltrexone (36.5% compared to 17.1%) but not on placebo (33.3% compared to 22.8%). The effect of naltrexone was most pronounced for subjects in “T2: very frequent drinkers” as the odds of continuous abstinence in this trajectory were almost two times higher on naltrexone than on placebo (OR=1.88, 95% CI: (1.01, 3.48)).
Figure 3.
Percent continuously abstinent by baseline trajectory and naltrexone.
Interactions of baseline trajectories and CBI
There were no significant interactions between baseline trajectories and CBI on any of the outcomes.
Main effects of trajectories
Our models also revealed significant main effects of baseline trajectories of any drinking on all considered drinking outcomes (Table 3). Since there were significant interactions involving baseline trajectories for good clinical outcome and abstinence from heavy drinking during the last two months of treatment, we interpret only the significant main effects for the other three outcomes. “T2: very frequent drinkers” had significantly lower percent days abstinent than “T1: frequent drinkers”. “T3: nearly daily drinkers” and “T4: consistent daily drinkers” had significantly poorer outcomes on percent days abstinent, percent heavy drinking days compared to “T1: frequent drinkers”. “T5: daily drinkers stopping early” had comparable drinking outcomes to “T1: frequent drinkers” and had significantly better drinking outcomes than “T4: consistent daily drinkers” for all drinking outcomes.
Exploratory Analyses of Individual Summary Drinking Variables
The potential advantage of using trajectories rather than summary drinking measures in predicting outcome is that trajectories capture simultaneously different aspects of drinking behavior. In particular, the trajectories of baseline drinking identify differences in frequency of drinking and lengths of pre-randomization abstinence. The corresponding summary drinking variables that quantify these aspects of drinking behavior are percentage of days abstinent (PDA) and consecutive days of abstinence prior to randomization (CDA). Neither variable by itself was able to identify groups of subjects with positive, negative and neutral treatment effect of acamprosate.
Guided by the results from the trajectory analyses we defined a combined categorical variable based on CDA (CDA < 14 days vs. CDA ≥ 14 days) and PDA during the first 30 days of the 90 day pretreatment period (25% ≤ PDA < 55% vs. (PDA<25% or PDA≥55%). We selected the first 30 days because drinking frequency appeared more stable during this period based on visual inspection of the trajectory graphs. Furthermore, the cutoffs on both variables were also defined based on visual inspection of the data and on the comparison of the baseline characteristics of the subjects classified in the different trajectories. The idea behind the creation of this variable was to separate subjects in trajectory 2 who benefited from acamprosate, subjects in trajectory 5 for whom acamprosate was counterproductive and the remaining subjects so that we could clearly identify subgroups with different response to acamprosate. Then we included this categorical variable instead of trajectory membership in the model with no heavy drinking in the last two months as the response variable for acamprosate and continuous abstinence for naltrexone.
Using this variable we were able to observe a beneficial effect of acamprosate for subjects with between 25% and 55% PDA (OR = 2.00, 95% CI: 1.22, 3.31), an adverse effect of acamprosate for subjects outside of this PDA range with CDA ≥ 14 days (OR = 0.40, 95% CI: 0.20, 0.82) and non-significant acamprosate effect for subjects with CDA <14 days and with either small (<25%) or large (>55%) PDA (OR = 1.23, 95% CI: 0.92, 1.66). On the other hand, naltrexone was associated with significantly better chance for continuous abstinence than placebo for subjects with between 25% and 55% PDA (OR=1.88, 95% CI: (1.03, 3.43)) but not for subjects outside of this range (OR = 0.97, 95% CI: 0.51, 1.84 for subjects with PDA ≥ 14 days and OR=1.14, 95% CI: 0.76, 1.69 for subjects with PDA < 14 days).
Discussion
These exploratory trajectory-analyses identified specific patterns of drinking prior to randomization to treatment and confirmed the hypothesis that baseline trajectories influence post-randomization drinking outcomes. Of the five trajectories of drinking prior to randomization, the frequency of drinking increased from “T1: frequent drinkers” to “T4: consistent daily drinkers”. The fifth trajectory, while similar in frequency of drinking to “T4: consistent daily drinkers” was unique and associated with stopping drinking early prior to randomization.
As expected, baseline trajectories predicted primary and secondary outcomes during treatment. While outcomes were most positive for the trajectory associated with the lowest frequency of drinking (“T1: frequent heavy drinkers”) and declined across T2-T4, “T5: daily drinkers stopping early” had very good outcomes as well. Baseline trajectories of drinking also appear to moderate the efficacy of treatment primarily on secondary outcomes and most notably for acamprosate. Contrary to hypotheses, individuals in “T4: consistent daily drinkers” did not benefit from acamprosate and acamprosate decreased the odds of being abstinent from heavy drinking in the last two months of treatment for individuals in “T5: daily drinkers stopping early”. In contrast, acamprosate treatment was associated with significantly higher odds of abstinence from heavy drinking for subjects in “T2: very frequent drinkers”.
We had expected that individuals with a baseline trajectory characterized by consistent drinking and recent cessation of drinking might experience greater withdrawal and therefore benefit from acamprosate. However, members of “T4: consistent daily drinkers”, despite drinking nearly daily, had the lowest proportion of patients with a history of alcohol withdrawal, the lowest Alcohol Dependence Scale scores and were the least severe on a number of other baseline characteristics and did not differentially benefit from acamprosate on any of the primary or secondary outcomes. In contrast, acamprosate improved the chance for abstinence from heavy drinking for members of “T2: very frequent drinkers”. Although not significant, drinkers in “T3: nearly daily drinkers” experienced a similar benefit from acamprosate. Interestingly, these groups were intermediate in terms of frequency of baseline drinking and alcoholism severity. Although acamprosate is typically considered to promote abstinence, Chick et al (2010) reported that acamprosate lowered the percentage of nonabstinent drinkers who consumed 5 or more drinks per day in a secondary analysis of data from 15 placebo controlled trials.
More puzzling, however, was the finding that acamprosate was associated with worse drinking outcomes among patients in trajectory 5. “T5: daily drinkers stopping early” was distinctive among the trajectories for having among the best clinical trajectories with respect to drinking outcomes along with “T1: frequent drinkers”, despite exhibiting many other features of chronic severe alcohol dependence, i.e., more frequent drinking, prior medical detoxification and higher ADS scores. The capacity to initiate alcohol abstinence prior to the required date and to sustain this abstinence until the time of randomization also distinguished this trajectory. Stout (2000) previously demonstrated that a sustained period of abstinence following the end of a drinking episode during treatment predicts delayed return to drinking and heavy drinking. Our data suggest a similar relationship between pretreatment abstinence and drinking during treatment.
The question of why acamprosate might reduce the protective effect of membership in “T5: daily drinkers stopping early” remains open. Regardless of the mechanism, our exploratory analyses suggest that acamprosate may not be indicated for daily drinkers who have already achieved an extended period of pre-treatment abstinence (e.g., ≥14 days). However, the majority of participants attained abstinence without the aid of detoxification and so this finding may not apply to those who begin acamprosate directly from detoxification or through extended inpatient alcoholism treatment.
Baseline trajectories moderated naltrexone response only on the secondary outcome of continuous abstinence. In the latter case, the odds of complete abstinence was increased for “T2: very frequent drinkers” on naltrexone compared to placebo. Although acamprosate studies emphasize continuous abstinence as an outcome (Kranzler & Gage, 2008; Rösner et al, 2008) and naltrexone studies highlight effects on heavy drinking (e.g. Pettinati et al., 2006), oral naltrexone and extended release naltrexone have been shown to enhance abstinence in some studies (e.g., O’Malley et al., 2007; 2008). These data also suggest that the efficacy of naltrexone does not depend exclusively on sampling alcohol (Sinclair, 2001) at least for a subset of individuals defined on pretreatment drinking trajectories. Other research examining frequency of drinking during treatment suggest that there may be a subset of individuals in COMBINE who drink very regularly for whom naltrexone is associated with reduced risk of heavy drinking (Ray et al., 2010). Because all participants in COMBINE were required to have at least four days of abstinence, we are unable to evaluate the effects of a baseline trajectory in which there was no pretreatment abstinence.
A secondary objective of the current study was to identify baseline trajectories of drinking that might predict “placebo responders”. In this regard, trajectory 1, characterized by the least frequent pretreatment drinking and trajectory 5, characterized by daily drinking but earlier attainment of pretreatment abstinence, had the best overall outcomes. Participants in trajectory 1 appear to engage in more binge drinking (i.e., achieving higher peak blood alcohol levels with less frequent drinking) compared to members in trajectories 2–4. Participants in trajectory 5 differed substantially from members of all other trajectories and have more severe alcohol problems and higher probability of inpatient care and/or medical detoxification. Based on this pattern of results, future studies might consider including duration of pretreatment abstinence and/or the need for medications or hospitalization to achieve abstinence as a stratification variable or an exclusion variable.
The potential advantage of using trajectories rather than summary drinking measures in predicting outcome is that trajectories capture simultaneously different aspects of drinking behavior and provide an easily interpreted graphical summary of the patterns of drinking over time. These patterns could then be used to empirically derive combinations of summary drinking measures, most meaningful time periods for evaluation of these variables and most appropriate cutoffs on these variables so that subgroups with differential treatment effects could be specified. Our analyses identified differences in frequency of drinking during the first 30-days of the baseline period and lengths of pre-randomization abstinence among trajectories. Guided by the results of the trajectory analyses, we were able to define combinations of the two summary baseline variables (PDA and CDA) that serve as moderators of treatment effects. Thus, we believe our analyses show that trajectory-based approaches could provide useful information beyond what can be gleaned easily from analyses of the effects of single baseline variables or other limited exploration of baseline predictors. Other methods such as classification and regression trees can be used to assess whether the identified combinations of variables are indeed predicting treatment outcomes and moderating treatment effects and to explore additional combinations of summary drinking measures that might be predictive of good outcome.
The potential clinical application of our trajectory analysis for selection of patients for acamprosate could involve using the model we derived to estimate for a given individual the probability of their membership within each pre-randomization drinking trajectory based on their observed daily drinking. However, we realize that in a clinical setting it might be unrealistic to estimate trajectory membership precisely even if we provide computer code to do this. Thus, it may be more meaningful to consider the combined categorical variable that we derived based on the results of the trajectory analysis. Additional studies are required to replicate these results, which could be specific to the patient population and other design considerations.
A potential drawback of our analysis is that it is predicated on the assumption that different classes of trajectories exist. When no categorically different trajectories exist, a substantial percent of subjects will not be classified reliably into any one trajectory. Our good classification accuracy gives some reassurance that in this study categorically different classes do exist. Furthermore, the differential effects of trajectories on the outcomes and the dissimilarities of trajectories on baseline characteristics give further reassurance that the trajectories consist of categorically different subjects.
Sample size and population homogeneity limit the number and shape of considered trajectories. However, since COMBINE is the largest to date study of pharmacotherapies and behavioral therapies for alcoholism, power to detect distinct trajectories in this study is greater than in most other studies.
In conclusion, we identified baseline trajectories of drinking that influenced post-randomization drinking outcomes and interacted with acamprosate and with naltrexone on secondary outcomes. Although the overall results for the COMBINE study failed to find an effect of acamprosate, individuals with an intermediate trajectory of drinking frequency 60 to 90 days prior to beginning treatment (e.g., 25 – 55% days abstinent on an exploratory composite index) had higher rates of no heavy drinking at the end of treatment in these exploratory analyses. This same group had higher rates of continuous abstinence with naltrexone compared to placebo. Contrary to prevailing hypotheses, however, acamprosate appeared to weaken the otherwise positive prognosis of patients who had a trajectory of consistent daily drinking and earlier abstinence initiation (e.g., 14 days or more). These findings may help clinicians identify patients for whom acamprosate and naltrexone may be most beneficial.
Acknowledgments
The project described was supported by Grant Number R01AA017173 from the National Institute on Alcohol Abuse and Alcoholism. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism or the National Institutes of Health. This work was also supported by the Center for Translational Neuroscience of Alcoholism (P50 AA-012870), P50 DA09241, K05-AA014715, KO514906, K05 DA00089, the National Center for Research Resources, NCRR UL1RR024139, and the following U.S. Veterans Administration Centers: Mental Illness Research, Education and Clinical Center; Alcohol Research Center; Merit Review Program and National Center for PTSD.
Footnotes
Disclosure/Conflict of Interest:
During the period of 2008–2010, Dr. Krystal has served as a scientific consultant and/or on the Scientific Advisory Board to the following companies: Aisling Capital, LLC, Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly and Co, Gilead Pharmaceuticals, F Hoffman-La Roche Ltd, Forest Laboratories, GlaxoSmithKline, Janssen Pharmaceuticals, Lahocla Research Corporation, Merz Pharmaceuticals, Pfizer Pharmaceuticals, SK Holdings Co., Ltd, Takeda Industries, Teva Pharmaceutical Industries, and Transcept Pharmaceuticals. He holds less than $10,000 in exercisable warrant options with Transcept Pharmaceuticals. He is the principal investigator of a multicenter study in which Janssen Research Foundation has provided drug and some support to the Department of Veterans Affairs. He is a co-sponsor for two patents under review for glutamatergic agents targeting the treatment of depression. Over the past 2-years, Dr. O’Malley was a member of an ACNP Workgroup sponsored by Abbott Laboratories, Eli Lilly, Janssen, Schering Plough, Lundbeck, Glaxo-Smith Kline and Alkermes; partner in Applied Behavioral Research; consultant to Brown University and Gilead Pharmaceuticals; NABI pharmaceutical (research contract); and received travel reimbursement from Biological Psychiatry, the Controlled Release Society, the Drug Information Association and AMERSA. The other authors have no potential conflicts of interest to disclose.
References
- Adamson SJ, Sellman JD, Frampton CM. Patient predictors of alcohol treatment outcome: a systematic review. J Subst Abuse Treat. 2009;36:75–86. doi: 10.1016/j.jsat.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Anton RF, Moak DH, Latham P. The Obsessive Compulsive Drinking Scale: A self rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol Clin Exp Res. 1995;19:92–99. doi: 10.1111/j.1530-0277.1995.tb01475.x. [DOI] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo D, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence. The COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Bottlender M, Soyka M. Impact of craving on alcohol relapse during, and 12 months following, outpatient treatment. Alcohol Alcohol. 2004;39:357–361. doi: 10.1093/alcalc/agh073. [DOI] [PubMed] [Google Scholar]
- Brasser SM, McCaul ME, Houtsmuller EJ. Alcohol Clin Exp Res. 2004;28:1074–1083. doi: 10.1097/01.alc.0000130802.07692.29. [DOI] [PubMed] [Google Scholar]
- Chick J, Lehert P, Landron F. Does acamprosate improve reduction of drinking as well as aiding abstinence? J Psychopharmacology. 2010;17:397–402. doi: 10.1177/0269881103174017. [DOI] [PubMed] [Google Scholar]
- Ciraulo DA, Piechniczek-Buczek J, Iscan EN. Outcome predictors in substance use disorders. Psychiatr Clin North Am. 2003;26:381–409. doi: 10.1016/s0193-953x(02)00106-5. [DOI] [PubMed] [Google Scholar]
- The COMBINE Study Research Group. Testing combined pharmacotherapies and behavioral interventions in alcohol dependence: Rationale and methods. Alcohol Clin Exp Res. 2003;27:1107–1122. doi: 10.1097/00000374-200307000-00011. [DOI] [PubMed] [Google Scholar]
- De Witte P, Pinto E, Ansseau M, Verbanck P. Alcohol and withdrawal: from animal research to clinical issues. Neurosci Biobehav Rev. 2003;27:189–197. doi: 10.1016/s0149-7634(03)00030-7. [DOI] [PubMed] [Google Scholar]
- Falk M, Mattson M, Fertig J, Ryan M, Litten R. Efficacy of naltrexone in Combine trial using percentage of subjects with no heavy drinking days as treatment outcome measure. Alcohol Clin Exp Res. 2009;S33:114A. doi: 10.1111/j.1530-0277.2010.01290.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders-Clinician Version (SCID-CV) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Garbutt JC, Kranzler HR, O’Malley SS, et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: A randomized controlled trial. JAMA. 2005;293:1617–1625. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- Gueorguieva R, Wu R, Donnovan D, Rounsaville B, Couper D, Krystal J, O’Malley S. Naltrexone and combined behavioral intervention effects on trajectories of drinking in the COMBINE study. Drug Alcohol Depen. 2010;107:221–229. doi: 10.1016/j.drugalcdep.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SM, Havassy BE, Wasserman DA. Commitment to abstinence and acute stress in relapse to alcohol, opiates, and nicotine. J Consult Clin Psychol. 1990;58:175–181. doi: 10.1037//0022-006x.58.2.175. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M. Pharmacological treatment of alcohol dependence. Target symptoms and target mechanisms. Pharmacol Therapeut. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Jones B, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001;29:374–393. [Google Scholar]
- Kampman KM, Pettinati HM, Lynch KG, Xie H, Dackis C, Oslin DW, Sparkman T, Sharkoski T, O’Brien CP. Initiating acamprosate within detoxification in the treatment of alcohol dependence. Addict Behavior. 2009;34:581–586. doi: 10.1016/j.addbeh.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaskutas LA. Alcoholics anonymous effectiveness: faith meets science. J Addict Dis. 2009;28:145–157. doi: 10.1080/10550880902772464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer F, Mann K. Pharmacotherapy and behavioral intervention for alcohol dependence. JAMA, Letter to the Editor. 2006;296:1727–1728. doi: 10.1001/jama.296.14.1727-b. [DOI] [PubMed] [Google Scholar]
- Kranzler H, Gage G. Acamprosate efficacy in alcohol-dependent patients: Summary of results from three pivotal trials. American Journal of Addictions. 2008;717:70–76. doi: 10.1080/10550490701756120. [DOI] [PubMed] [Google Scholar]
- Lhuintre JP, Moore N, Tran G, Steru L, Langrenon S, Daoust M, et al. Acamprosate appears to decrease alcohol intake in weaned alcoholics. Alcohol Alcohol. 1990;25:613–622. doi: 10.1093/oxfordjournals.alcalc.a045057. [DOI] [PubMed] [Google Scholar]
- Mann K, Kiefer F, Spanagel R, Littleton J. Acamprosate: Recent findings and future research directions. Alcohol Clin Exp Res. 2008;32:1105–1110. doi: 10.1111/j.1530-0277.2008.00690.x. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Ownby RL. Acamprosate for the treatment of alcohol dependence: a review of double-blind, placebo controlled trails. CNS Spectr. 2000;5:58–69. doi: 10.1017/s1092852900012827. [DOI] [PubMed] [Google Scholar]
- Miller WR, Del Boca FK. Measurement of drinking behavior using the Form 90 family of instruments. J Stud Alcohol Suppl. 1994;12:112–118. doi: 10.15288/jsas.1994.s12.112. [DOI] [PubMed] [Google Scholar]
- Miller WR, Tonigan JS, Longabaugh R. Test manual. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 1995. Drinker Inventory of Consequences (DrInC): An instrument for assessing adverse consequences of alcohol abuse. [Google Scholar]
- Miller WR. NIAAA Project MATCH Monograph Series. Washington: Government Printing Office; 1996. Form 90: A structured assessment interview for drinking and related behaviors. [Google Scholar]
- Muthén BO. Mplus Technical Appendices. Los Angeles, CA: Muthén & Muthén; 1998–2004. Appendix 8. [Google Scholar]
- Nagin DS. Analyzing developmental trajectories: a semiparametric, group-based approach. Psychol Methods. 1999;4:139–157. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- Nagin DS, Tremblay RE. Analyzing developmental trajectories of distinct but related behaviors: a group-based method. Psychol Methods. 2001;6:18–34. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Garbutt JC, Gastfriend DR, Dong Q, Kranzler HR. Efficacy of extended-release naltrexone in alcohol-dependent patients who are abstinent prior to treatment. J Clin Psychopharm. 2007;27:507–512. doi: 10.1097/jcp.0b013e31814ce50d. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Robin RW, Levenson AL, et al. Naltrexone alone and with sertraline for the treatment of alcohol dependence in Alaska Natives and Non-Natives residing in rural settings: a randomized controlled trial. Alcohol Clin Exp Res. 2008;32:1–13. doi: 10.1111/j.1530-0277.2008.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelc I, Ansoms C, Lehert P, et al. The European NEAT Program: An integrated approach using acamprosate and psychosocial support for the prevention of relapse in alcohol-dependent patients with a statistical modeling of therapy success prediction.”. Alcohol Clin Exp Res. 2002;26:1529–1538. doi: 10.1097/01.ALC.0000029584.62149.22. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, O’Brien CP, Rabinowitz AR, Wortman SP, Oslin DW, Kampman KM, Dackis CA. The status of naltrexone in the treatment of alcohol dependence: specific effects on heavy drinking. J Clin Psychopharmacol. 2006;26(6):610–625. doi: 10.1097/01.jcp.0000245566.52401.20. [DOI] [PubMed] [Google Scholar]
- Project MATCH Research Group. Matching alcoholism treatments to client heterogeneity: Project MATCH posttreatment drinking outcomes. J Stud Alcohol. 1997;58:7–29. [PubMed] [Google Scholar]
- Project MATCH Research Group. Project MATCH secondary a priori hypotheses. Addiction. 1997;92:1671–1698. [PubMed] [Google Scholar]
- Ray L, Krull JL, Leggio L. The effects of Naltrexone among alcohol non-abstainers: results from the COMBINE Study. Frontiers of Psychiatry. 2010 doi: 10.3389/fpsyt.2010.00026. doi:10.3389/fpsyt.2010.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner S, Leucht S, Lehert P, Soyka M. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacology. 2008;22:11–23. doi: 10.1177/0269881107078308. [DOI] [PubMed] [Google Scholar]
- Sinclair JD. Evidence about the use of naltrexone and four different ways of using it in the treatment of alcoholism. Alcohol Alcoholism. 2001;36:2–10. doi: 10.1093/alcalc/36.1.2. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: Measurement and validation. J Abnorm Psychol. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-back: A technique for assessing self-reported ethanol consumption. In: Allen J, Litten RZ, editors. Measuring alcohol consumption: Psychosocial and biological methods. New Jersey: Human Press; 1992. [Google Scholar]
- Sobell LC, Sobell MB. Alcohol consumption measures. In: Allen JP, Columbus M, editors. Assessing alcohol problems: A guide for clinician and researchers. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 1995. pp. 55–73. [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: The revised Clinical Institute Withdrawal Assessment for Alcohol scale (CIWA-AR) Br J Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Tonigan JS, Miller WR, Brown JM. The reliability of Form 90: An instrument for assessing alcohol treatment outcome. J Stud Alcohol. 1997;58:358–364. doi: 10.15288/jsa.1997.58.358. [DOI] [PubMed] [Google Scholar]
- Weisman AM, Berman ME, Taylor S. Effects of clorazepate, diazepam, and oxazepam on a laboratory measurement of aggression in men. Int Clin Psychopharm. 1998;13:183–188. doi: 10.1097/00004850-199807000-00005. [DOI] [PubMed] [Google Scholar]
- Whitworth AB, Fischer F, Lesch OM, et al. Comparison of acamproste and placebo in long-term treatment of alcohol dependence. Lancet. 1996;347:1438–1442. doi: 10.1016/s0140-6736(96)91682-7. [DOI] [PubMed] [Google Scholar]


