Abstract
Foam drying, a modified freeze drying process, was utilized to produce a heat-stable, live attenuated Salmonella Typhi ‘Ty21a’ bacterial vaccine. Ty21a vaccine was formulated with pharmaceutically approved stabilizers, including sugars, plasticizers, amino acids, and proteins. Growth media and harvesting conditions of the bacteria were also studied to enhance resistance to desiccation stress encountered during processing as well as subsequent storage at elevated temperatures. The optimized Ty21a vaccine, formulated with trehalose, methionine, and gelatin, demonstrated stability for approximately 12 weeks at 37°C (i.e., time required for the vaccine to decrease in potency by 1log10 CFU) and no loss in titer at 4 and 25°C following storage for the same duration. Furthermore, the foam dried Ty21a elicited a similar immunogenic response in mice as well as protection in challenge studies compared to Vivotif™, the commercial Ty21a vaccine. The enhanced heat stability of the Ty21a oral vaccine, or Ty21a derivatives expressing foreign antigens (e.g. anthrax), could mitigate risks of vaccine potency loss during long term storage, shipping, delivery to geographical areas with warmer climates or during emergency distribution following a bioterrorist attack. Because the foam drying process is conducted using conventional freeze dryers and can be readily implemented at any freeze drying manufacturing facility, this technology appears ready and appropriate for large scale processing of foam dried vaccines.
Keywords: Salmonella, foam drying, freeze drying, oral vaccine, live bacterial vaccine, temperature-stability
1. Introduction
There are only a few licensed live attenuated, enteric bacterial vaccines currently on the U.S. market, including Bacillus Calmette-Guerin (BCG) vaccines, Theracys® and TICE® BCG, and Salmonella enterica serovar Typhi strain Ty21a (Vivotif®). S. Typhi Ty21a serves as an oral typhoid vaccine and was constructed by random chemical mutagenesis resulting in a gal E deficient and Vi capsule-nonexpressing mutant of strain Ty2 [1]. While Ty21a is used mainly as a travellers’ vaccine for the US and EU markets, Vivotif® is a very important vaccine for the developing world because typhoid infections are very common and often life-threatening. Ty21a engenders long term (>7 years) protection against typhoid fever [2] and cross-protects against serovars Paratyphi A and B [3]. Worldwide there are about 21 million cases of typhoid fever per year, leading to >200,000 deaths, which could largely be avoided with better vaccination coverage [4]. Additionally, the proven safety record of S. Typhi Ty21a is the basis for its selection as a vaccine delivery vector for several heterologous targets including Shigella sonnei and Shigella dysenteriae antigens [5, 6], anthrax PA antigen [7], and anti-cancer siRNAs [8]. Vivotif® is sold in an enteric-coated capsule formulation or in a liquid formulation consisting of a lyophilized vaccine that requires reconstitution with buffer and diluent. Both formulations demonstrate good efficacy and produce few and minor adverse reactions [9–12]. The liquid formulation is reported to be superior immunologically to the enteric-coated capsules [13]. These vaccines, however, possess low thermal stability, require refrigeration, and lose viability quickly when exposed to elevated temperatures [14]. The shelf life of lyophilized Ty21a is dependent on residual water, excipients, and processing temperatures and conditions during manufacturing, as well as temperature of product storage [15].
Compared to liquid formulations, solid formulations have multiple advantages such as superior storage stability, reduced molecular mobility and unwanted chemical reactions, and less package weight for increased ease in shipping and distribution. Furthermore, as all currently available commercial vaccines require low temperature storage, the goal is to utilize solid state stabilization techniques to enhance their room temperature or high temperature stability and to reduce the reliance on cold-chain to maintain efficacy and ensure safety. Unlike freeze drying and spray drying, which expose the drug substance to low and high temperatures, respectively, drying processes have been developed which can be conducted at room temperature. Annear described a drying process, involving foaming, whereby Salmonella ndolo and Vibrio cholerae were dried under high vacuum, while the ampoules containing the bacterial suspensions were immersed in a water bath at 20°C [16]. Another process described by Annear, introduced a secondary drying step, in which the dried vaccine was immersed in a water bath at 100°C and placed under high vacuum, during which the partially dried suspension expanded rapidly into a glassy foam cake structure [17]. Another Cambridge University microbiologist, Lord Stamp, also described a similar ‘foam drying’ technique in which Chromobacterium prodigiosum was successfully stabilized by drying the bacterial suspension in a desiccator containing P2O5 at a pressure of 100–300mm Hg for 2–3 days at room temperature [18]. The bacterial titer obtained using this method was approximately 3 times higher compared to that obtained from lyophilization, perhaps due to the avoidance of freezing-associated stresses. In fact, the creation of ice-water interface during the freezing step of lyophilization has been reported to lead to denaturation of proteins [19, 20]. Temperature and pressure control involved in the above studies are possible using a freeze drying equipment available today. More recently, Bronshtein described a method to dry biologically active materials at a pressure sufficient to cause the solution to ‘boil’ [21] while Roser described a method to dry biological macromolecules at a temperature above freezing in the presence of trehalose [22]. Both methods are similar to the process originally described in detail by Annear more than 40 years prior, in that the drying is conducted under non-freezing conditions, with the dehydration process being driven by the lowered hydrostatic pressure. The boiling process described by Bronshtein is a consequence of the decreased pressure, e.g. boiling point depression of the solvent, which results in expanded foam, as described by Annear [17]. Furthermore, samples dried according to Annear’s process exhibited a wide range of temperature profiles during dehydration; depending on the drying temperature, the rate of pressure decrease, and the solution composition, some of the samples likely underwent freezing while others did not [23]. A similar method was described by Truong-Le, whereby the bioactive material, which was being dried at a shelf temperature that was actually above freezing, was dried through the steps of freezing followed by sublimation, as a result of evaporation-induced cooling under low chamber pressure [24]. The methods that followed Annear, namely those described by Stamp and Lord, appear to involve similar process ranges in pressure and temperature regimes, wherein the formulation would have undergone similar chemical-physical transitions. The cell wall of Salmonella, as with other gram negative bacteria, contains a thin peptidoglycan layer and an outer membrane, composed of phospholipids and lipopolysaccharides, which are adjacent to the cytoplasmic membrane [25–27]. The challenge in drying live pathogens with a lipid membrane structure is in preserving the membrane integrity and avoiding rupture. The hydrostatic pressure across the bacterial membrane increases dramatically during desiccation, which could affect membrane integrity [28]. The rate with which water removal affects the hydrostatic pressure and membrane stability could differ depending on the drying processes employed. Furthermore, the substitution of lost hydrogen bonding using pharmaceutical stabilizers could impact the preservation of membrane structures (water replacement hypothesis) [29–31].
The structural complexity and thermal instability of bacteria such as Salmonella Typhi Ty21a suggested that a successful stabilization effort may require not only a combination of effective stabilizers and drying process methods, but also examination of growth media and harvesting conditions to derive a starting material that exhibit improved ability to withstand stresses associated with pharmaceutical dehydration processes.
The current study presents unique formulations and a foam drying process to produce high temperature-resistant, live typhoid vaccine. The foam drying process described in this manuscript is conducted at a non-freezing condition, similar to the work described above. However, it is differentiated by its enhanced capability to control and monitor system pressure and temperature through the use of a lyophilizer. Furthermore, as part of the bacterial stabilization efforts, the growth media as well as the harvesting conditions were optimized to enhance the stability of bacteria to desiccation and to subsequent high-temperature storage. The stabilized vaccine can be further fabricated into an easy to administer dosage format (e.g., quick dissolving wafers or thin films) for oral delivery, allowing for a convenient method of mass vaccination.
2. Material and methods
2.1. Vaccine vectors
Live attenuated Salmonella enterica serovar Typhi vaccine strain Ty21a (or ‘Ty21a’), and other Ty21a-vectored vaccine strains were obtained from the laboratory of Dennis Kopecko (CBER, FDA).
2.2. Growth media
Trypticase soy broth (TSB) and brain heart infusion (BHI) growth media were obtained from BD Biosciences. Ty21a grown in BHI broth provided a more robust titer than those grown in traditional TSB (data not shown). Ty21a grown in BHI consistently reached titers of 10log10 compared to 9.3log10 for those grown in TSB under similar growth conditions. The effects of processing stress from various drying methods, including foam drying, were found to be comparable between the two growth media (data not shown). In certain conditions, NaCl was added to modify the osmolarity of the growth media.
2.3. Formulation components
Trehalose was obtained from Pfanstiehl Laboratories (Waukegan, IL). Dimethyl sulfoxide (DMSO), galactose, glycerol, L-methionine, Pluronic F68, and sodium chloride were purchased from Sigma-Aldrich (St. Louis, MO). Special LE200 SOL-KKA gelatin was purchased from Gelita (Sioux City, IA). Potassium phosphate dibasic and monobasic salts were purchased from EMD (Gibbstown, NJ).
2.4. Growth Conditions
Ty21a vaccine was cultured in either TSB or BHI broth supplemented with 0.005 % galactose for 8 hours in a 1L glass bottle at 37°C while being shaken at 220 rpm. In some cases, the osmolarity of the growth media was modified by the addition of NaCl, up to a concentration of 400mM. Log phase bacteria were harvested at 2 hours (1.1 OD600nm) while stationary phase bacteria were harvested at 6 hours (1.7 OD600nm). Vaccine potency (CFU/ml) was determined by plating out the Ty21a vaccine on tryptic soy agar plates overnight at 37°C.
2.5. Foam Drying
Ty21a was cultured by incubation in BHI broth overnight and harvested at stationary phase, unless indicated otherwise, followed by pelleting at 2500× g for 10 minutes. The bacterial pellet was resuspended in an equal volume of formulation containing various combinations of stabilizers. 1mL of the formulated Ty21a vaccine was aliquoted into each 10mL vial and then foam dried. The water contents of all formulations used in the studies were kept between 2–4%. Unless stated otherwise, the pressure of the freeze dryer chamber (Virtis Advantage XL-70, Gardiner, NY) was decreased in a step-wise manner from atmospheric to 100mTorr, while maintaining the shelf temperature at 15°C. Low pressure was maintained for approximately 40 hours, followed by additional 20 hours of drying at 20°C. The vials were sealed under slight vacuum (650 torr) in argon gas, crimped, and stored at various temperatures for stability analysis.
2.6. Spray Drying
Formulated Ty21a was spray dried using a Büchi B-190 Mini Spray Dryer (Flawil, Switzerland) assembled inside of an enclosure that allows for humidity and temperature control, typically below 10% relative humidity (RH) and 30°C, respectively. The spray dryer was equipped with a custom-built Sonicair nozzle (IVEK, North Springfield, VT) designed to atomize the feed solution at a low pressure using sonic energy. Spray drying process condition consisted of: 0.75 mL/min feed rate, 45°C outlet temp (Tout), and 15 psi atomization pressure (Patm), provided by nitrogen gas.
2.7. Freeze drying
Formulated Ty21a was freeze dried using a Virtis 25EL freeze dryer (Gardiner, NY). Samples were lyophilized in 10mL vials with a fill volume of 1mL. The samples were quenched in liquid nitrogen prior to being placed in the lyophilizer with its shelf temperature pre-set to −32°C. Primary drying was conducted at −32°C for 24 hours and secondary drying was conducted at 10°C for 8 hours. Vials were sealed under sterile argon gas at the end of the drying cycle prior to being removed from the chamber.
2.8. Plating Conditions
Foam dried Ty21a vaccine preparations were reconstituted using nanopure water and serially diluted using Hanks’ Balanced Salt Solutions (HBSS) purchased from Invitrogen (San Diego, CA). Dilutions of the reconstituted Ty21a vaccine were plated out onto tryptic soy agar (TSA) plates warmed to room temperature. The plates were incubated at 37°C for 20 hours, and the number of colonies was counted.
2.9. Immunogenicity Study
Measurement of serum antibody titers specific for Salmonella Typhi (Sigma Salmonella typhosa Lipopolysaccharide; Catalog # L6386) was done by a capture enzyme-linked immunosorbent assay (ELISA) using standard procedures. Serum samples from vaccinated and control mice were assayed for Salmonella Typhi-specific total IgG antibody responses in mice. Immunolon® 2 flat bottom microtiter plates (ThermoLabsystems, Franklin, MA) were coated with 1 μg/mL of lipopolysaccharide (LPS) and incubated overnight at 4°C. The next day, the plates were washed three times with wash buffer (PBS containing 0.05% Tween-20). The plates were blocked with 5% bovine serum albumin in PBS for 1 hr at 37°C. Each serum sample was serially diluted 1:3 in blocking buffer typically from 1:100 to 1:300,000. The samples were incubated for 1 hr at 37°C. Total IgG antibody titers were detected by using an affinity purified, peroxidase-labeled, goat anti-mouse IgG (KPL, Gaithersburg, MD) diluted 1:1000 in blocking buffer and incubated for 30 minutes at room temperature. The plates were developed with 100μL of 2,2′-azino-di-(3-ethylbenzthiazoline-6-sulfonic acid) substrate (ABTS; KPL). After incubating for 15 minutes at room temperature, the reaction was stopped by adding 100μL of ABTS Peroxidase Stop Solution (KPL). Absorbance at 405nm (A405) was measured using a spectrophotometer. All samples were assayed in triplicate. End-point antibody titers were expressed as the maximum dilution of sample giving an absorbance of greater than 0.2 A405. The results are presented as the reciprocal of the dilution multiplied by the absorbance value.
2.10. Mice and Immunizations
Balb/cAnN mice, purchased from the National Cancer Institute-Division of Cancer Treatment (Frederick, MD) or Taconic (Germantown, NY), were maintained in the Small Animal Facility of the Food and Drug Administration (Bethesda, MD) and used with the approval of the Food and Drug Administration’s Institutional Animal Care and Use Committee. For immunizations, freshly grown Salmonella Typhi was given either intranasally (i.n.) at a dose of 1×108 CFU or intraperitoneally (i.p.) at a dose of 5.0×106 CFU. The intranasally immunized groups received anesthesia (0.1 ml i.p. containing 80 mg/kg ketamine and 15 mg/kg xylazine) followed by a dose of bacteria diluted in saline (20μL of bacteria total or 10μL/nare) and administered with a pipette into the nares of the mice. The i.p. groups were immunized with bacterial suspension at a volume of 0.25 mL delivered with a 25 gauge, 5/8 inch needle. Each group received the same vaccine dose 3 times at 2 week intervals. Lateral tail vein bleeds (0.150 mL) were performed on the mice on day 14 after the final vaccine dose. Three weeks after the last immunization, mice were challenged i.p. with fully virulent Salmonella enterica serovar Typhi strain Ty2. The IP challenge dose consisted of 4×105 CFU (i.e. 20×LD50) of Ty2 in 500uL of 5% hog gastric mucin. Survival was monitored for 14 days.
2.11. Statistics
Statistical analyses were performed by means of an unpaired t test using GraphPad Prism version 5. A p-value of ≤0.05 (two-tailed) was considered to be statistically significant.
3. Results
3.1 Process comparison
There are several ways in which biological macromolecules can be desiccated. The processing methods can be varied in temperature, pressure, and other physical stresses imposed on the samples as well as in processing time. Freeze drying and spray drying represent two desiccation methods that have been used in vaccine manufacture. Initial efforts to develop a stable lyophilized formulation of Ty21a in a variety of formulations resulted in similar storage stability to that previously reported [15, 32, 33]. Ty21a formulated in 28% (w/v) sucrose and 1% (w/v) gelatin in 25mM potassium phosphate at pH7 resulted in 0.8log10 process-associated loss and titer loss of 0.5log10 following 1 week of storage at 25°C (3.20±0.33% residual water content) (Table 1). Ty21a spray dried following the same strategy as used by Wong et al. [34], i.e., in the absence of buffer salts, resulted in high process loss and poor storage stability; the process loss for Ty21a formulated in un-buffered solution containing 7% (w/v) sucrose was 0.7log10 and demonstrated loss in titer of 1.7log10 CFU following 1 week of storage at 25°C (3.14±0.16% residual water content) (Table 1). In comparison, the process loss and storage stability of the foam dried Ty21a, in one of the formulations that will be described in the manuscript, demonstrated 0.3log10 process loss and no loss in titer following 4 weeks of storage at 25°C (3.75±0.78% residual water content) (Table 1). A photograph of a typical foam dried sample is shown in Figure 1, which is clearly different in physical appearance to a freeze dried cake. Although the foam dried material demonstrated improved process recovery and storage stability, a firm conclusion of its superiority over freeze dried and spray dried material cannot be made, as rigorous formulation and process comparison was not attempted. The superiority of foam drying process for other biological systems, however, has been reported elsewhere [35, 36].
Table 1.
Process-associated loss and storage stability at 25°C for freeze dried, spray dried, and foam dried Ty21a.
| Process | Process Loss (log10 CFU) | Stability Loss after 1 week at 25°C (log10 CFU) |
|---|---|---|
| Freeze drying1 | 0.8 ± 0.1 | 0.5 ± 0.0 |
| Spray drying2 | 0.7 ± 0.1 | 1.7 ± 0.1 |
| Foam drying3 | 0.3 ± 0.2 | No loss4 |
Formulation contained 28% (w/v) sucrose, 1% (w/v) gelatin in 25mM KPO4, pH7
Formulation contained 7% (w/v) sucrose
Formulation contained 25% (w/v) sucrose, 0.5% (w/v) methionine, 2.4wt% DMSO, 25mM KPO4, pH8
Storage stability following 4 weeks at 25°C
All data represent the average (+/− SEM) of duplicate experiments.
Figure 1.

Typical appearance of a foam dried sample (A), in this case Ty21a in 25% sucrose, 25mM potassium phosphate. The key attribute of the foam dried sample is the translucent, foamy structure that occupies a large volume. A vial of freeze dried sample of the same composition (B) is shown for comparison.
3.2 Bacterial growth optimization
3.2.1 Effect of Ty21a growth phase on foam drying process recovery
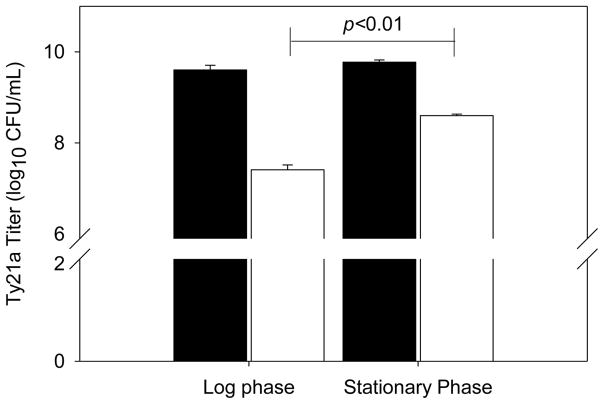
It has been reported that bacteria recovered at different phases of fermentation growth (i.e. lag, logarithmic, stationary phases) may exhibit different phenotypic profiles [37], and possibly different degrees of resistance to dehydration stresses. To this end, we examined the recovery of Ty21a obtained from different growth phases following foam drying. Ty21a was grown in BHI broth overnight and was harvested in both the log phase (1.1 OD600nm) and the stationary phase (1.7 OD600nm). 0.5mL aliquot of each bacteria, formulated in 1M trehalose, was placed into individual vials and then foam dried. The samples were reconstituted with sterile water, diluted and plated out on TSA plates to determine process recovery (Figure 2). The recovery of bacteria harvested in the stationary phase was higher than those collected in the log phase (i.e., 1.18log10 decrease in titer compared to 2.19log10).
Figure 2.
The effect of growth phase on Ty21a stability against process-related stress from foam drying; Ty21a titer prior to (■) and post (□) foam drying. The data represent the average (+/− SEM) of duplicate experiments. A p value of less than 0.05 is considered statistically significant.
3.2.2 Effect of growth media on the growth kinetics of Ty21a
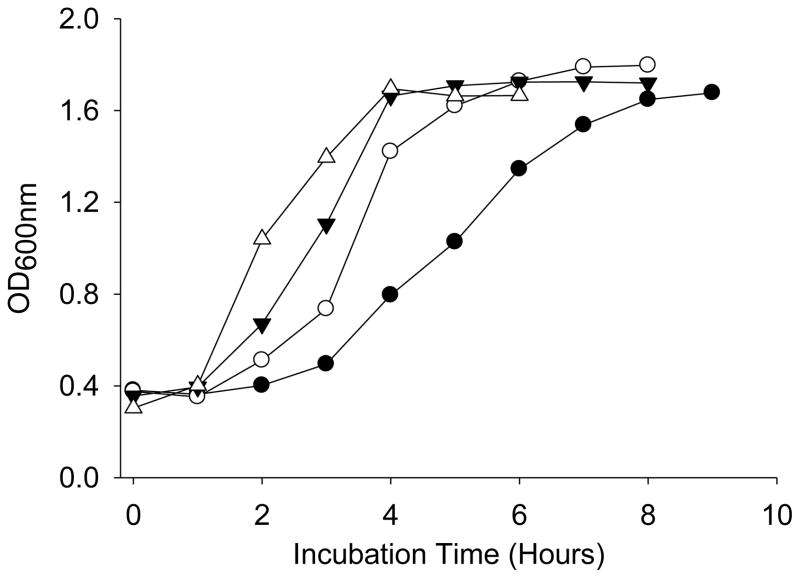
It has been suggested that bacteria grown under osmotic stress may be better conditioned to resist osmotic stresses such as those associated with dehydration [37–39]. To examine the effects of ‘osmotic conditioning’, Ty21a was grown in BHI broth with and without NaCl to examine the effects of osmotic stress on its growth kinetics and recovery following foam drying. NaCl was present at concentrations ranging from 200 to 400mM. The kinetics of bacterial growth was monitored at OD600nm (Figure 3). The addition of NaCl delayed the growth kinetics of Ty21a monotonically with increasing salt concentration. The stationary phase (OD600nm ~ 1.6) was reached in approximately 4 hours in BHI broth, whereas upon the addition of 400mM NaCl, the time required to reach the same OD600nm value was delayed to 8 hours.
Figure 3.
The growth curve of Ty21a, as measured by OD600nm, grown at 37°C with aeration in BHI broth containing NaCl at various concentrations; 0mM (△), 200mM (▼), 300mM (○), and 400mM (●) NaCl.
3.2.3 Effect of Ty21a growth media on foam drying process recovery
Ty21a was grown in the presence of varying amounts of NaCl, harvested from both the log phase and the stationary growth phase, formulated in 1M trehalose, and then foam dried. Following storage at 25°C for up to 16 weeks, the data showed that Ty21a harvested in the stationary phase demonstrated improved process recovery compared to those harvested in the log phase at all NaCl concentrations examined (Figure 4). Bacteria that were harvested in the stationary phase and grown in the presence of 300mM NaCl demonstrated the highest survival following foam drying (<0.1log10 loss in titer). The effect of salt concentration on process recovery for Ty21a harvested in the log phase was insignificant.
Figure 4.
The process recovery of Ty21a upon foam drying. Bacteria were grown in BHI media containing varying amounts of NaCl, ranging from 0–300mM NaCl, and were harvested either in the log phase (Log) or the early stationary phase (Stat), as indicated.
3.3 Foam Drying Cycle Development
The foam drying cycle was optimized to minimize process loss and enhance foaming. An example of a foam drying cycle is shown in Table 2. In most cases, a primary drying pressure of 100mTorr was reached within 3 hours and processing was conducted above 10°C to avoid freezing. There were process conditions in which foam freezing was observed, however. Typically, freezing was avoided as it may introduce additional stresses (i.e., ice crystal formulation, surface denaturation, etc.). To understand the correlation between the amount of water removal to loss in Ty21a titer, the foam drying process (Table 2) was arrested at various time points to recover samples for analysis of water content and bacterial titer (Figure 5). Within the first 100 minutes of processing, the majority of activity loss as well as water removal was complete, suggesting that the process-related stresses, and hence the critical phase of processing, occurs early in the foam drying process. The inverse relationship observed between evaporation and activity loss suggests that dehydration and/or rate of dehydration is a major mechanism of activity loss. By decreasing the systemic pressure stepwise, while allowing for short equilibration time, the process-associated loss was lowered from 0.8 to 0.3log10. More specifically, the pressure was decreased gradually from 10 Torr to <100mTorr, while maintaining the shelf temperature above 10°C, allowing sufficient time for equilibration at 3 to 6 different pressures during its decrease. All data on formulation development (to be described below) were developed using this optimized cycle.
Table 2.
Example of a foam drying cycle, in which the system pressure is decreased in a step-wise manner from atmospheric to 100mTorr, while maintaining the temperature above freezing.
| Temperature | Pressure | Time |
|---|---|---|
| 10°C | atmospheric | 15 min |
| 10°C | 5 Torr | 20 min |
| 22°C | 1 Torr | 2 hr |
| 15°C | 100 mTorr | 45 hr |
Figure 5.
Correlation between activity loss (○) and amount of water removed (●) at various time points during foam drying according to the process described in Table 2 utilizing Ty21a formulated in 25% (w/v) trehalose, 1% (w/v) methionine, and 2.1wt% glycerol.
3.4 Formulation Development
3.4.1 Optimization of pH
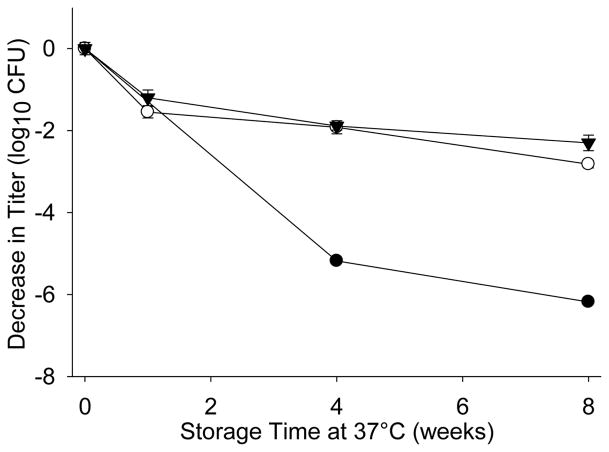
Previously, we showed that the pH range in which Ty21a appeared most stable is pH 6–8 [40]. Therefore, a pH screen ranging from pH 6 to 8 was conducted using a base formulation containing 30% (w/v) trehalose in 25mM potassium phosphate buffer. The storage stability of the foam dried Ty21a was optimal under neutral-to-alkaline condition (pH8), demonstrating 2.1log10 decrease in titer following 8 weeks of storage at 37°C (−0.81log10/wk1/2), whereas the titer of Ty21a foam dried at pH6 decreased by >6log10 (−2.26log10/wk1/2) (Figure 6). Furthermore, the process-associated loss was higher under acidic condition compared to that prepared at pH8, resulting in titer loss of 0.93 and 0.40log10, respectively.
Figure 6.
Storage stability at 37°C of foam dried Ty21a prepared at solution pH 6 (●), pH 7 (○), and pH 8 (▼) in the presence of 30% (w/v) trehalose and 25mM potassium phosphate buffer.
3.4.2 Effect of methionine
Methionine has been considered as a natural, amino acid based stabilizer which imparts its stabilization effect as a mild anti-oxidant [41]. To this end, methionine was added to the trehalose-potassium phosphate formulation at pH8, ranging in concentration from 0.5–2% (w/v). Incorporation of 0.5% (w/v) methionine marginally improved storage stability, as the titer loss upon storage was reduced from 2.1log10 to 1.8log10, following 8 weeks of storage at 37°C (−0.81 and −0.68log10/wk1/2, respectively). At higher methionine concentrations (i.e., 2%), however, the stability worsened, resulting in titer loss greater than 3log10 (−1.26log10/wk1/2) (Figure 7). The foam drying process-associated decrease in titer was also higher for formulations containing higher methionine concentrations, 0.92log10 compared to 0.45log10 loss for formulations containing 2 and 0.5% (w/v) methionine, respectively (data not shown). Thus, optimal storage stability was observed for foam-dried Ty21a in the presence of 0.5% methionine.
Figure 7.
Storage stability at 37°C of foam dried Ty21a containing varying amounts of methionine, ranging from 0–2% (w/v); 0 % (●), 0.5 % (○), 1 % (▼), and 2 % (△).
3.4.3 Effect of plasticizers
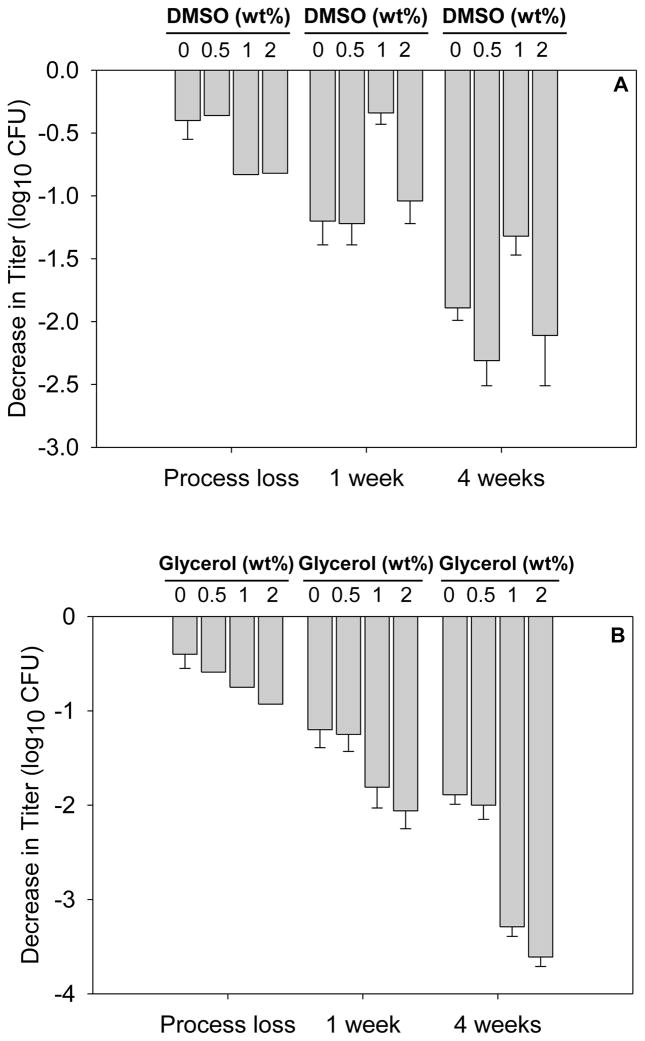
The addition of plasticizers has been demonstrated to improve the storage stability of various proteins and enzymes when incorporated to a sugar solution upon dehydration [42, 43]. DMSO and glycerol, ranging in concentration from 0.5 to 2 wt%, were incorporated into the trehalose-potassium phosphate buffered formulation at pH8 and then foam dried. The foam drying process loss ranged from 0.3 to 1.0log10, with the higher loss associated with higher plasticizer concentrations (Figure 8). The storage stability of the vaccine was significantly improved with the inclusion of DMSO. Formulation containing DMSO at 1wt% reduced the stability loss to 1.3log10 following 4 weeks of storage at 37°C (−0.66log10/wk1/2), while all other compositions resulted in >2log10 decrease (>−1.0log10/wk1/2) (Figure 8A). In the absence of plasticizers, the stability loss was 1.9log10 (−0.95log10/wk1/2). In comparison to DMSO, glycerol was not as effective a stabilizer; Ty21a containing 0.5wt% glycerol demonstrated similar stability to the formulation without any plasticizers, while the inclusion of higher concentrations of glycerol resulted in decreased stability (Figure 8B). In fact, upon the addition of increasing amounts of glycerol, the rate constant of degradation increased monotonically from −1.00 to −1.81log10/wk1/2, at 0.5 and 2% glycerol, respectively. The addition of DMSO typically resulted in improved storage stability compared to foam dried Ty21a formulated with equal concentrations of glycerol (see Figure 8 for comparison at 1wt% inclusion). Optimal storage stability was obtained with foam-dried Ty21a containing 1wt% DMSO.
Figure 8.
Storage stability at 37°C of foam dried Ty21a containing varying amounts of either (A) DMSO or (B) glycerol, both ranging in concentration from 0.5–2 wt%.
The addition of DMSO and methionine, individually, was shown to have a stabilizing effect on foam dried Ty21a (Figures 7 and 8). The effect of both components on the storage stability of Ty21a was evaluated next. While maintaining the methionine composition at 0.5% (w/v), the DMSO concentration was varied from 0 to 2wt% (Figure 9). All formulations, with the exception of that prepared with 0.5wt% DMSO, demonstrated less than 0.5log10 loss upon foam drying. The optimal amount of DMSO, 1wt%, was the same as that observed previously in the absence of methionine (Figure 8A). The rate constant of degradation was the lowest at −0.51log10/wk1/2, while at other DMSO concentrations within the range examined, the degradation rate was greater than −0.65log10/wk1/2. An additive effect on the storage stability of Ty21a was observed upon the inclusion of both components to the trehalose formulation. While Ty21a formulated in 0.5% (w/v) methionine decreased in titer by 1.9log10 (Figure 7) and that in 1wt% DMSO by 1.3log10 (Figure 8A), the decrease in titer for Ty21a containing both methionine and DMSO was minimized to 1log10 (Figure 9). Similar additive effect was also observed for the formulation prepared at pH7 (data not shown).
Figure 9.
Storage stability at 37°C of foam dried Ty21a containing varying amounts of DMSO, ranging from 0–2wt%. The base formulation contained 25% (w/v) trehalose, 0.5% (w/v) methionine, and 25mM potassium phosphate buffer at pH8.
3.4.4 Effect of gelatin
Gelatin, a frequently used stabilizer in several commercial vaccines, was incorporated into the formulation to not only enhance the vaccine stability but also the solution viscosity, which is thought to minimize the rate of cavitation associated with the foaming process. The effect of gelatin addition on the storage stability of foam dried Ty21a was examined on formulations containing trehalose along with another excipient selected from methionine, glycerol, or DMSO. The storage stability data for the same samples lacking gelatin were presented earlier and their slopes of titer decrease are shown in Table 3. Upon the inclusion of gelatin, the storage stability of foam dried Ty21a was improved, irrespective of the formulation composition; for the formulation containing methionine and trehalose, gelatin addition reduced the slope of titer decrease from −1.06 to −0.27log10/wk1/2, for glycerol and trehalose formulation from −1.65 to −0.50log10/wk1/2, and for DMSO and trehalose formulation from −0.66 to −0.50log10/wk1/2 (Table 3). From these combinations, a formulation containing methionine with gelatin and trehalose was observed to be the most stable formulation for foam dried Ty21a, demonstrating ~12 weeks of storage stability at 37°C (time to 1log10 loss).
Table 3.
The effects of gelatin incorporation on the stability of foam dried Ty21a in various formulations stored at 37°C.
| Slope (log10/wk1/2) at 37°C |
|||
|---|---|---|---|
| Methionine3 | Glycerol4 | DMSO5 | |
| Without gelatin1 | −1.06±0.16 | −0.66±0.01 | −1.65±0.02 |
| With gelatin2 | −0.27±0.07 | −0.50±0.09 | −0.50±0.06 |
Formulation further contained 30% (w/v) trehalose and 25mM KPO4 at pH8
Formulation further contained 25% (w/v) trehalose, 5% (w/v) gelatin, and 25mM KPO4 at pH8
Methionine present at 1% (w/v)
Glycerol present at 1 wt%
DMSO present at 1 wt%
The storage stability of the lead formulation was also evaluated at 25 and 4°C. After 12 weeks of storage at either of those temperatures, the foam dried Ty21a demonstrated negligible loss in titer (Figure 10).
Figure 10.
Storage stability at 4°C (▼), 25°C (○), and 37°C (●) of foam dried Ty21a containing methionine, trehalose, and gelatin in potassium phosphate buffer at pH8.
In summary, stabilization of the Ty21a vaccine was made possible through the development of a foam drying process. Furthermore, through the screening of various combinations of stabilizers, including plasticizers (glycerol and DMSO), methionine, and trehalose, improved storage stability of Ty21a vaccine was obtained. The addition of the aforementioned stabilizers, at appropriate concentrations, to the trehalose formulation resulted in improved storage stability at 37°C, which was further enhanced upon the addition of gelatin. Optimal storage stability was obtained by combining gelatin and methionine with trehalose; room temperature stabilization was demonstrated for up to four months with no activity loss, while stabilization at 37°C was demonstrated for over two months (time to 1log10 loss of activity).
3.5 Immunogenicity and Protection in Mice
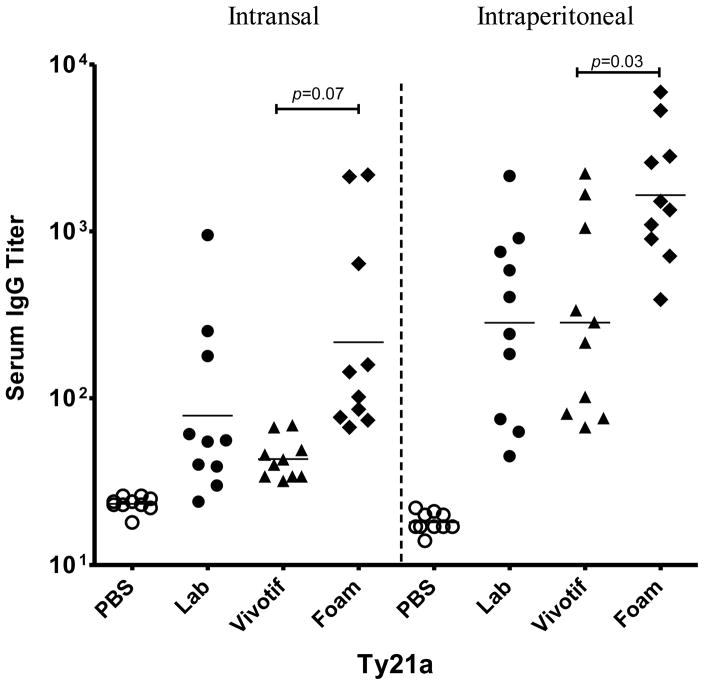
The immunogenic response to foam dried Ty21a was assessed to determine whether subjecting Ty21a vaccine to the formulation and foam drying process altered the immunogenicity of the vaccine. As shown in Figure 11, mice vaccinated with formulated, foam dried Ty21a vaccine elicited slightly higher anti-LPS IgG titers (although not statistically significant for the intranasal route) to that of the unformulated vaccine bulk. Furthermore, mice from the group vaccinated with foam dried Ty21a were better protected (60%) against 20x LD50 (4x 105 CFU) challenge dose, compared to mice immunized with the control lab strain and Vivotif™ commercial strain (both around 20%) (data not shown), whereas all of the PBS control mice died by day 14 post challenge. These data demonstrated that the foam-dried vaccine had enhanced immunogenicity after formulation and processing.
Figure 11.
Immunogenicity of foam dried Ty21a. Anti-LPS serum IgG titers of mice immunized with freshly grown Ty21a (●), Vivotif bulk liquid formulation (▲) or stabilized, foam dried Ty21a (◆). A p value of less than 0.05 is considered statistically significant.
4. Discussion
Several processing methods were evaluated to stabilize Ty21a, including freeze drying and spray drying, prior to developing the foam drying process. A commercial scale freeze drying process for Ty21a was developed by Clarke et al. in which a shelf-life of 18–24 months at 2–8°C was reported [32, 33]. However, at elevated temperatures, the vaccine potency of such freeze dried product decreased dramatically; e.g. the shelf-life decreased to several months or to several days, for storage at room temperature or 37°C respectively [15, 33]. Initial efforts to develop a stable lyophilized preparation of Ty21a from a variety of formulations resulted in similar storage stability; 0.8log10 process-associated loss and titer loss of 0.5log10 following 1 week of storage at 25°C (Table 1). Although the exact mechanism of Ty21a inactivation is unknown, the various processing methods were expected to result in differing process recovery, as the stresses involved in desiccation vary widely. For freeze drying, low temperature stress, ice crystal formation, and phase separation have been suggested to be significant contributors to inactivation of a wide range of biomolecules [44–46], while for spray drying, high temperature stress and shear stress (i.e., atomization of liquid feed into droplets) are two of the most significant contributing factors leading to inactivation [47–49]. Furthermore, the time scale involved for dehydration is significantly different for these processes; freeze drying requires several days whereas spray drying is completed on a millisecond time scale [50–53]. Thus, both the rate of water removal and the thermal history of the biomolecule during processing are quite different and are expected to lead to differing results.
Research has led to a spray drying method that effectively stabilizes Mycobacterium smegmatis [34]. Specifically, salts and other traditional cryoprotectants, such as glycerol, were removed from the formulation which was then air dried. Contrary to expectations, the activity of the recovered bacteria was improved by up to 2log10. The same strategy was utilized with Ty21a, however, neither the process loss nor the storage stability was as high as those reported by Wong et al. [34]; the process loss for Ty21a formulated in un-buffered solution containing 7% (w/v) sucrose was 0.7log10 and demonstrated loss in titer of 1.7log10 CFU following 1 week of storage at 25°C (Table 1).
Compared to the two processes described above, foam drying is conducted at a moderate rate of water removal (process complete in hours to days). Furthermore, the entire process is conducted at a mild temperature, generally in the range of 15–25°C. The foaming process reported here is modestly different from that originally developed in the 1960s by Annear for use in the preservation of vaccines and bacteria [54]. The overall method involves boiling, or foaming, of the solution under lowered vapor pressure followed by rapid evaporation, leaving a structure resembling solidified foam (Figure 1). The temperature is carefully controlled so as to avoid freezing due to evaporative cooling. More recently, the foam drying method has been adapted to be conducted under both non-freezing [21] and freezing [24] conditions followed by rapid evaporation and/or sublimation of water. Foam drying is conducted using a conventional freeze dryer and the process can be thought of as a freeze drying method conducted under a cake collapse condition. It should be noted, however, that it is difficult to directly compare various processes because different formulation components may be required for the processes being compared and optimization can be conducted to varying degrees. For the purpose of this study, a rigorous process comparison was not conducted as the focus has been on foam drying process and formulation development, based on publications which reported superiority of this dehydration technique over freeze drying and spray drying [35, 36].
The foam drying cycle was first optimized to minimize process loss. For processing methods in which the system pressure is decreased too quickly, the solution has a greater tendency to freeze and will not foam effectively. Conversely, foaming will be inhibited by decreasing the pressure too slowly, with the sample solution resembling a slurry. Examination of activity loss during processing and the water content of the samples suggest an inverse relationship between the residual water content and the remaining activity of Ty21a (Figure 5). It is evident that the majority of activity loss as well as water removal was completed within the first 100min of processing. A similar study using other process cycles suggested that the rate of systemic pressure decrease, particularly during the early stages of foam drying, has a significant effect on the viability of Ty21a. By decreasing the systemic pressure in a stepwise manner, the process-associated loss was decreased from 0.8 to 0.3log10. This suggests that the rate of water removal during the initial stages of the foam drying cycle (i.e., the rate at which the physical structure of the sample is set prior to desiccation), is crucial in determining the activity Ty21a. This may be related to the increase in solute concentration encountered during dehydration and the associated changes in osmotic stress across the bacterial membrane. The rate of water desorption is expected to be slower for foam dried material in relation to a similar formulation processed by freeze drying, due to the lower specific surface area of the former [35]. Thus, longer secondary drying process may be required to reduce the residual water content to similar levels as achieved by freeze drying.
Several groups have suggested that growth and harvesting conditions also have a significant effect on the physical stability of bacteria [55–58]. Sodium chloride at various concentrations was incorporated in BHI broth to determine its effect on both the growth kinetics of Ty21a as well as its development of resistance to osmotic stress. In order to reach the same optical density (OD600nm ~ 1.6, corresponding to the stationary phase), Ty21a required incubation for 8 hours at 37°C in the presence of 400mM NaCl, compared to 4 hours in its absence. Thus, there appears to be no benefit of including osmotic stress-inducing agents in the growth media, at least from the perspective of growth kinetics. However, an improvement in desiccation tolerance was observed for Ty21a grown in the presence of salts (Figure 4). Ty21a grown in the presence of 300mM NaCl demonstrated much improved process recovery compared to those grown in the absence of salt, <0.1log10 and 0.8log10 loss in titer, respectively. However, this effect was observed only for bacteria harvested in the stationary phase. For Ty21a harvested in the log phase, there was little-to-no effect of salt addition on the process recovery of bacteria following foam drying. Similar improvement in process recovery of Ty21a from the change in harvesting time to stationary phase was also noted in the absence of salt (Figure 2). Thus, both the presence of osmolytes and the growth stage of bacteria appeared to play a role in determining the desiccation tolerance of bacteria. The increased osmolarity of the growth media may allow Ty21a to develop tolerance to osmotic stress, which would be encountered during desiccation. The higher titer of bacteria at stationary phase may benefit from a similar self-protective mechanism to desiccation encountered by proteins at high concentrations [59].
Zeng et al. [40] reported that Ty21a stability was improved at pH6 – 7 compared to that prepared at pH8. This enhanced stability was attributed to the difference in membrane fluidity. In the current study, both the process-associated loss and storage stability was poorer for Ty21a samples foam dried at pH6 compared to that prepared at pH8 (Figure 6). Zeng presented stability conditions for Ty21a in the liquid phase, whereas the data presented here relates to stability in the dried state. The maintenance of membrane fluidity and distribution has been attributed to have an effect on the stability of a model membrane system, i.e., liposomes (i.e., leakage of encapsulated compounds) [60–62]. Similarly, the avoidance of phase transition and phase separation of various membrane components may have an effect on Ty21a stability during desiccation. The inclusion of trehalose, as presented in this work, would be expected to lower the phase transition temperature of the bacterial membrane, as was reported for a variety of phospholipidic components [63–65], and allow the membrane to remain in the fluid phase throughout the foam drying process as well as during reconstitution (i.e., thus avoiding the occurrence of phase transition). The formation of an amorphous glass during desiccation has also been demonstrated to resist the phase separation of membrane components, which would have occurred in the absence of sugars, perhaps contributing to the enhanced stability of Ty21a [66, 67]. Furthermore, the pH of the external media may affect the stability of Ty21a through the alteration of charge distribution on the bacterial membrane, as has been reported for liposomes [60]. In addition to stabilizing the lipid components of the bacterial membrane, the membrane proteins also require stabilization, as has been demonstrated by the addition of trehalose to E. coli and B. thuringiensis [68].
Several amino acids, such as arginine, phenylalanine, and glycine, have been successfully used to stabilize a wide variety of complex biomolecules [69–73]. In this study, methionine was demonstrated to be an effective stabilizer. The amino acid was incorporated into a trehalose-potassium phosphate formulation at pH8 in a concentration range of 0.5–2% (w/v). Optimum stability was observed with 0.5% methionine, and decreased at higher concentrations (Figure 7). Typically, methionine has been employed as an antioxidant, as is the case for NovoSeven® RT, recombinant human coagulation factor VIIa, that demonstrates room temperature stability for up to 2 years [74]. Similarly, in the present case, methionine may be acting as an antioxidant, but, there may be other contributing factors.
Plasticizers, such as glycerol and DMSO, have been demonstrated to improve the storage stability of various proteins and enzymes even further than that possible by sugars alone [42]. Typically, the use of plasticizers has been avoided due to its effect on lowering the glass transition temperature (Tg) of the dried composition. On a global scale (i.e., translational motion), this may appear to be the overriding factor determining the stability of the biomolecule. However, locally (i.e., vibrational and rotational motion), plasticizers have been demonstrated to reduce molecular mobility by dampening the high frequency motion [43]. It is this effect on the local motion, to which the authors attribute the enhanced storage stability. Both DMSO and glycerol were incorporated into the foam dried Ty21a formulation, but their effects on storage stability were quite different. While increasing glycerol composition resulted in decreased storage stability, DMSO demonstrated an optimal concentration at 1wt% (Figure 8A). Furthermore, the storage stability of foam dried Ty21a was better in formulations containing DMSO in comparison to glycerol at equal composition. For both plasticizers, the process loss associated with foam drying increased with higher plasticizer concentrations. Thus, the amount of plasticizer needs to be optimized to balance the titer loss associated with processing and subsequent storage.
The solids content of the formulation is an important parameter that requires optimization for efficient foaming. One key component is the viscosity of the solution, which can be enhanced through the incorporation of proteins or polymers. Furthermore, these large molecules can enhance the stability of labile biomolecules through their ability to interact, reduce aggregation, and to elevate the Tg of the dried composition [75, 76]. Addition of 5% gelatin to methionine-trehalose formulation resulted in improved storage stability of foam dried Ty21a, reducing the rate of titer decrease from −1.06 to −0.27log10/wk1/2 (Table 3). Similarly, the storage stability was improved for plasticizer-containing formulations. Stabilization conferred to foam dried Ty21a by the individual components (i.e., methionine, glycerol, and DMSO) appears to be enhanced upon the addition of gelatin, perhaps through a complementary stabilization mechanism. The formulation that stabilized Ty21a the most in the foam dried format contained gelatin, methionine, and trehalose, demonstrating ~12 weeks of storage stability at 37°C. At lower storage temperatures, the foam dried sample demonstrated no loss in titer following 4 months of storage at 25 and 4°C. Other formulations of foam-dried Ty21a (not reported here) have been studied over 12–18 months real time for stability and have been found to have a projected shelf-life of >5 years at 4°C, stability at 25°C for 12 months and allowable temperature excursions to 37°C for 8 weeks. Another aspect of stability relates to the maintenance of vaccine titer during processing of the foam dried material, e.g. by jet milling, into powders. In fact, very insignificant loss in titer (< 0.2log10) has been observed (data not shown).
Though not reported here, several other compounds were screened for their ability to stabilize Ty21a during foam drying and subsequent storage as well as to enhance the foam drying process. One class of compounds that was examined in detail was surfactants. Surfactants are very effective in enhancing the foaming process as well as in minimizing inactivation at the air-liquid interface. However, care must be taken to optimize their amount since surfactants can solubilize lipidic components, thus breaching the integrity of bacterial membrane, leading to decreased titer. Pluronic F68, a relatively mild non-ionic surfactant, was incorporated at concentrations ranging from 0.02–0.2wt% to the methionine-gelatin-trehalose formulation. For all cases, the storage stability was compromised, but their inclusion did facilitate the foaming process (i.e., foaming occurred at higher systemic pressure).
Finally, the ability of the foam dried Ty21a to elicit an immunogenic response was examined in mice. As illustrated in Figure 11, mice vaccinated with the foam dried Ty21a (by both intranasal and intraperitoneal administration) elicited an enhanced level of anti-LPS IgG titers when compared to those for the unformulated vaccine as well as for Vivotif™, the commercial vaccine product. This demonstrates that not only does foam drying preserve the physical stability of Ty21a, but may enhance its immunogenic activity (i.e., potency).
5. Conclusion
Foam drying processing methods, growth conditions, and formulation components were developed that produced a heat-stable live attenuated typhoid vaccine. The optimized Ty21a vaccine, formulated with trehalose, methionine, and gelatin, demonstrated stability for longer than 4 and 42 weeks at 37°C and 25°C, respectively, in comparison to 12 hours and 2 weeks, respectively for Vivotif™ (i.e., stability here is indicated as time required for the vaccine to decrease in potency by 0.5log10 CFU) [14, 15, 77, 78]. The foam drying process was optimized to reduce both the residual water content to <4% while minimizing process-associated loss in vaccine titer to <0.3log10 CFU. The initial stages during the foam drying process were identified to be the most critical. Furthermore, desiccation tolerance of the Ty21a vaccine was enhanced through the incorporation of osmotic reagents in the growth media. Collectively, these data suggest that foam-drying methods greatly increase the temperature-stability of live Ty21a vaccine and also enhance product immumogenicity. Application of this technology should increase Ty21a shelf-life and allow for vaccine distribution without the need for refrigeration, a key advantage for developing country use.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Germanier R, Fuer E. Isolation and characterization of Gal E mutant Ty 21a of Salmonella typhi: a candidate strain for a live, oral typhoid vaccine. J Infect Dis. 1975;131(5):553–8. doi: 10.1093/infdis/131.5.553. [DOI] [PubMed] [Google Scholar]
- 2.Levine MM. Typhoid fever vaccines. In: Plotkin S, Orenstein W, Offit P, editors. Vaccines. 5. PA, USA: Saunders-Elsevier; 2008. pp. 887–914. [Google Scholar]
- 3.Levine MM, Ferreccio C, Black RE, Lagos R, San Martin O, Blackwelder WC. Ty21a live oral typhoid vaccine and prevention of paratyphoid fever caused by Salmonella enterica Serovar Paratyphi B. Clin Infect Dis. 2007;45 (Suppl 1):S24–8. doi: 10.1086/518141. [DOI] [PubMed] [Google Scholar]
- 4.Technical information. Centers for Disease Control and Prevention; 2005. Typhoid Fever. [Google Scholar]
- 5.Formal SB, Baron LS, Kopecko DJ, Washington O, Powell C, Life CA. Construction of a potential bivalent vaccine strain: introduction of Shigella sonnei form I antigen genes into the galE Salmonella typhi Ty21a typhoid vaccine strain. Infect Immun. 1981;34(3):746–50. doi: 10.1128/iai.34.3.746-750.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black RE, Levine MM, Clements ML, Losonsky G, Herrington D, Berman S, et al. Prevention of shigellosis by a Salmonella typhi-Shigella sonnei bivalent vaccine. J Infect Dis. 1987;155(6):1260–5. doi: 10.1093/infdis/155.6.1260. [DOI] [PubMed] [Google Scholar]
- 7.Osorio M, Wu Y, Singh S, Merkel TJ, Bhattacharyya S, Blake MS, et al. Anthrax protective antigen delivered by Salmonella enterica serovar Typhi Ty21a protects mice from a lethal anthrax spore challenge. Infect Immun. 2009;77(4):1475–82. doi: 10.1128/IAI.00828-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu DQ, Zhang L, Kopecko DJ, Gao L, Shao Y, Guo B, et al. Bacterial delivery of siRNAs: a new approach to solid tumor therapy. Methods Mol Biol. 2009;487:161–87. doi: 10.1007/978-1-60327-547-7_8. [DOI] [PubMed] [Google Scholar]
- 9.Black R, Levine MM, Young C, Rooney J, Levine S, Clements ML, et al. Immunogenicity of Ty21a attenuated “Salmonella typhi” given with sodium bicarbonate or in enteric-coated capsules. Dev Biol Stand. 1983;53:9–14. [PubMed] [Google Scholar]
- 10.Wahdan MH, Serie C, Germanier R, Lackany A, Cerisier Y, Guerin N, et al. A controlled field trial of liver oral typhoid vaccine Ty21a. Bull World Health Organ. 1980;58(3):469–74. [PMC free article] [PubMed] [Google Scholar]
- 11.Levine MM, Ferreccio C, Black RE, Germanier R. Large-scale field trial of Ty21a live oral typhoid vaccine in enteric-coated capsule formulation. Lancet. 1987;1(8541):1049–52. doi: 10.1016/s0140-6736(87)90480-6. [DOI] [PubMed] [Google Scholar]
- 12.Levine MM, Ferreccio C, Abrego P, Martin OS, Ortiz E, Cryz S. Duration of efficacy of Ty21a, attenuated Salmonella typhi live oral vaccine. Vaccine. 1999;17 (Suppl 2):S22–7. doi: 10.1016/s0264-410x(99)00231-5. [DOI] [PubMed] [Google Scholar]
- 13.Levine MM, Ferreccio C, Cryz S, Ortiz E. Comparison of enteric-coated capsules and liquid formulation of Ty21a typhoid vaccine in randomised controlled field trial. Lancet. 1990;336(8720):891–4. doi: 10.1016/0140-6736(90)92266-k. [DOI] [PubMed] [Google Scholar]
- 14.Corbel MJ. Reasons for instability of bacterial vaccines. Dev Biol Stand. 1996;87:113–24. [PubMed] [Google Scholar]
- 15.Cryz SJ, Jr, Pasteris O, Varallyay SJ, Furer E. Factors influencing the stability of live oral attenuated bacterial vaccines. Dev Biol Stand. 1996;87:277–81. [PubMed] [Google Scholar]
- 16.Annear DI. Observations on drying bacteria from the frozen and from the liquid state. Aust J Exp Biol Med Sci. 1958;36(3):211–21. doi: 10.1038/icb.1958.23. [DOI] [PubMed] [Google Scholar]
- 17.Annear DI. Recoveries of bacteria after drying and heating in glutamate foams. J Hyg (Lond) 1970;68(3):457–9. doi: 10.1017/s0022172400042352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stamp L. The preservation of bacteria by drying. J Gen Microbiol. 1947;1(2):251–65. doi: 10.1099/00221287-1-2-251. [DOI] [PubMed] [Google Scholar]
- 19.Chang BS, Kendrick BS, Carpenter JF. Surface-induced denaturation of proteins during freezing and its inhibition by surfactants. J Pharm Sci. 1996;85(12):1325–30. doi: 10.1021/js960080y. [DOI] [PubMed] [Google Scholar]
- 20.Bhatnagar BS, Pikal MJ, Bogner RH. Study of the individual contributions of ice formation and freeze-concentration on isothermal stability of lactate dehydrogenase during freezing. J Pharm Sci. 2008;97(2):798–814. doi: 10.1002/jps.21017. [DOI] [PubMed] [Google Scholar]
- 21.Brohnstein V. 5766520. Preservation by foam formation. 1997
- 22.Roser BJ. 4891319. Protection of proteins and the like. 1987
- 23.Annear DI. Recovery of Strigomonas oncopelti after drying from the liquid state. Aust J Exp Biol Med Sci. 1961;39:295–303. doi: 10.1038/icb.1961.29. [DOI] [PubMed] [Google Scholar]
- 24.Truong VL. 7381425. Preservation of bioactive materials by freeze dried foam. 2006
- 25.Salton MRJ. Studies of the bacterial cell wall: IV. The composition of the cell walls of some gram-positive and gram-negative bacteria. Biochimica et Biophysica Acta. 1953;10:512–23. doi: 10.1016/0006-3002(53)90296-0. [DOI] [PubMed] [Google Scholar]
- 26.Kita H, Nikaido H. Structure of cell wall lipopolysaccharide from Salmonella typhimurium. IV. Anomeric configuration of L-rhamnose residues and its taxonomic implications. J Bacteriol. 1973;113(2):672–9. doi: 10.1128/jb.113.2.672-679.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quintela JC, de Pedro MA, Zollner P, Allmaier G, Garcia-del Portillo F. Peptidoglycan structure of Salmonella typhimurium growing within cultured mammalian cells. Mol Microbiol. 1997;23(4):693–704. doi: 10.1046/j.1365-2958.1997.2561621.x. [DOI] [PubMed] [Google Scholar]
- 28.Ritz M, Freulet M, Orange N, Federighi M. Effects of high hydrostatic pressure on membrane proteins of Salmonella typhimurium. Int J Food Microbiol. 2000;55(1–3):115–9. doi: 10.1016/s0168-1605(00)00165-3. [DOI] [PubMed] [Google Scholar]
- 29.Crowe JH, Crowe LM, Chapman D. Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science. 1984;223(4637):701–3. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- 30.Crowe LM, Mouradian R, Crowe JH, Jackson SA, Womersley C. Effects of carbohydrates on membrane stability at low water activities. Biochim Biophys Acta. 1984;769(1):141–50. doi: 10.1016/0005-2736(84)90017-8. [DOI] [PubMed] [Google Scholar]
- 31.Crowe JH, Crowe LM, Mouradian R. Stabilization of biological membranes at low water activities. Cryobiology. 1983;20(3):346–56. doi: 10.1016/0011-2240(83)90023-8. [DOI] [PubMed] [Google Scholar]
- 32.Burke CJ, Hsu TA, Volkin DB. Formulation, stability, and delivery of live attenuated vaccines for human use. Crit Rev Ther Drug Carrier Syst. 1999;16(1):1–83. [PubMed] [Google Scholar]
- 33.Clarke PD, Forrest BD. WO/1993/011220. Medical Preparations. 1993
- 34.Wong YL, Sampson S, Germishuizen WA, Goonesekera S, Caponetti G, Sadoff J, et al. Drying a tuberculosis vaccine without freezing. Proc Natl Acad Sci U S A. 2007;104(8):2591–5. doi: 10.1073/pnas.0611430104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdul-Fattah AM, Truong-Le V, Yee L, Pan E, Ao Y, Kalonia DS, et al. Drying-induced variations in physico-chemical properties of amorphous pharmaceuticals and their impact on Stability II: stability of a vaccine. Pharm Res. 2007;24(4):715–27. doi: 10.1007/s11095-006-9191-2. [DOI] [PubMed] [Google Scholar]
- 36.Abdul-Fattah AM, Truong-Le V, Yee L, Nguyen L, Kalonia DS, Cicerone MT, et al. Drying-induced variations in physico-chemical properties of amorphous pharmaceuticals and their impact on stability (I): stability of a monoclonal antibody. J Pharm Sci. 2007;96(8):1983–2008. doi: 10.1002/jps.20859. [DOI] [PubMed] [Google Scholar]
- 37.Prasad J, McJarrow P, Gopal P. Heat and osmotic stress responses of probiotic Lactobacillus rhamnosus HN001 (DR20) in relation to viability after drying. Appl Environ Microbiol. 2003;69(2):917–25. doi: 10.1128/AEM.69.2.917-925.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahdy HM, el-Sheikh HH, Ahmed MS, Refaat BM. Physiological and biochemical changes induced by osmolality in halotolerant aspergilli. Acta Microbiol Pol. 1996;45(1):55–65. [PubMed] [Google Scholar]
- 39.Fonseca F, Beal C, Corrieu G. Operating conditions that affect the resistance of lactic acid bacteria to freezing and frozen storage. Cryobiology. 2001;43(3):189–98. doi: 10.1006/cryo.2001.2343. [DOI] [PubMed] [Google Scholar]
- 40.Zeng Y, Fan H, Chiueh G, Pham B, Martin R, Lechuga-Ballesteros D, et al. Towards development of stable formulations of a live attenuated bacterial vaccine: a preformulation study facilitated by a biophysical approach. Hum Vaccin. 2009;5(5):322–31. doi: 10.4161/hv.5.5.7559. [DOI] [PubMed] [Google Scholar]
- 41.Patel S. Stabilization of protein formulations. 5358708. US. 1993
- 42.Cicerone MT, Soles CL. Fast dynamics and stabilization of proteins: binary glasses of trehalose and glycerol. Biophys J. 2004;86(6):3836–45. doi: 10.1529/biophysj.103.035519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Curtis JE, Dirama TE, Carri GA, Tobias DJ. Inertial suppression of protein dynamics in a binary glycerol-trehalose glass. J Phys Chem B. 2006;110(46):22953–6. doi: 10.1021/jp0615499. [DOI] [PubMed] [Google Scholar]
- 44.Armstrong TK, Anchordoquy TJ. Immobilization of nonviral vectors during the freezing step of lyophilization. J Pharm Sci. 2004;93(11):2698–709. doi: 10.1002/jps.20177. [DOI] [PubMed] [Google Scholar]
- 45.Allison SD, Molina MC, Anchordoquy TJ. Stabilization of lipid/DNA complexes during the freezing step of the lyophilization process: the particle isolation hypothesis. Biochim Biophys Acta. 2000;1468(1–2):127–38. doi: 10.1016/s0005-2736(00)00251-0. [DOI] [PubMed] [Google Scholar]
- 46.Bhatnagar BS, Bogner RH, Pikal MJ. Protein stability during freezing: separation of stresses and mechanisms of protein stabilization. Pharm Dev Technol. 2007;12(5):505–23. doi: 10.1080/10837450701481157. [DOI] [PubMed] [Google Scholar]
- 47.Liao YH, Brown MB, Nazir T, Quader A, Martin GP. Effects of sucrose and trehalose on the preservation of the native structure of spray-dried lysozyme. Pharm Res. 2002;19(12):1847–53. doi: 10.1023/a:1021445608807. [DOI] [PubMed] [Google Scholar]
- 48.Vehring R. Pharmaceutical particle engineering via spray drying. Pharm Res. 2008;25(5):999–1022. doi: 10.1007/s11095-007-9475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walter E, Moelling K, Pavlovic J, Merkle HP. Microencapsulation of DNA using poly(DL-lactide-co-glycolide): stability issues and release characteristics. J Control Release. 1999;61(3):361–74. doi: 10.1016/s0168-3659(99)00151-0. [DOI] [PubMed] [Google Scholar]
- 50.Kawakami K, Sumitani C, Yoshihashi Y, Yonemochi E, Terada K. Investigation of the dynamic process during spray-drying to improve aerodynamic performance of inhalation particles. Int J Pharm. 2010;390(2):250–9. doi: 10.1016/j.ijpharm.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 51.Tang X, Pikal MJ. Design of freeze-drying processes for pharmaceuticals: practical advice. Pharm Res. 2004;21(2):191–200. doi: 10.1023/b:pham.0000016234.73023.75. [DOI] [PubMed] [Google Scholar]
- 52.Roy ML, Pikal MJ. Process control in freeze drying: determination of the end point of sublimation drying by an electronic moisture sensor. J Parenter Sci Technol. 1989;43(2):60–6. [PubMed] [Google Scholar]
- 53.Zbicinski I, Strumillo C, Delag A. Drying Kinetics and Particle Residence Time in Spray Drying. Drying Technology. 2002;20(9):1751–68. [Google Scholar]
- 54.Annear DI. Recoveries of bacteria after drying in vacuo at a bath temperature of 100 degrees C. Nature. 1966;211(5050):761. doi: 10.1038/211761a0. [DOI] [PubMed] [Google Scholar]
- 55.Silva J, Carvalho AS, Ferreira R, Vitorino R, Amado F, Domingues P, et al. Effect of the pH of growth on the survival of Lactobacillus delbrueckii subsp. bulgaricus to stress conditions during spray-drying. J Appl Microbiol. 2005;98(3):775–82. doi: 10.1111/j.1365-2672.2004.02516.x. [DOI] [PubMed] [Google Scholar]
- 56.Koch S, Oberson G, Eugster-Meier E, Meile L, Lacroix C. Osmotic stress induced by salt increases cell yield, autolytic activity, and survival of lyophilization of Lactobacillus delbrueckii subsp. lactis. Int J Food Microbiol. 2007;117(1):36–42. doi: 10.1016/j.ijfoodmicro.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 57.Sheehan VM, Sleator RD, Fitzgerald GF, Hill C. Heterologous expression of BetL, a betaine uptake system, enhances the stress tolerance of Lactobacillus salivarius UCC118. Appl Environ Microbiol. 2006;72(3):2170–7. doi: 10.1128/AEM.72.3.2170-2177.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonaterra A, Cabrefiga J, Camps J, Montesinos E. Increasing survival and efficacy of a bacterial biocontrol agent of fire blight of rosaceous plants by means of osmoadaptation. FEMS Microbiol Ecol. 2007;61(1):185–95. doi: 10.1111/j.1574-6941.2007.00313.x. [DOI] [PubMed] [Google Scholar]
- 59.Jameel F, Tchessalov S, Bjornson E, Lu X, Besman M, Pikal M. Development of freeze-dried biosynthetic Factor VIII: I. A case study in the optimization of formulation. Pharm Dev Technol. 2009;14(6):687–97. doi: 10.3109/10837450902882344. [DOI] [PubMed] [Google Scholar]
- 60.Hays LM, Crowe JH, Wolkers W, Rudenko S. Factors affecting leakage of trapped solutes from phospholipid vesicles during thermotropic phase transitions. Cryobiology. 2001;42(2):88–102. doi: 10.1006/cryo.2001.2307. [DOI] [PubMed] [Google Scholar]
- 61.Leidy C, Wolkers WF, Jorgensen K, Mouritsen OG, Crowe JH. Lateral organization and domain formation in a two-component lipid membrane system. Biophys J. 2001;80(4):1819–28. doi: 10.1016/S0006-3495(01)76152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun WQ, Leopold AC, Crowe LM, Crowe JH. Stability of dry liposomes in sugar glasses. Biophys J. 1996;70(4):1769–76. doi: 10.1016/S0006-3495(96)79740-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leslie SB, Teter SA, Crowe LM, Crowe JH. Trehalose lowers membrane phase transitions in dry yeast cells. Biochim Biophys Acta. 1994;1192(1):7–13. doi: 10.1016/0005-2736(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 64.Crowe LM, Crowe JH, Rudolph A, Womersley C, Appel L. Preservation of freeze-dried liposomes by trehalose. Arch Biochem Biophys. 1985;242(1):240–7. doi: 10.1016/0003-9861(85)90498-9. [DOI] [PubMed] [Google Scholar]
- 65.Crowe LM, Crowe JH, Chapman D. Interaction of carbohydrates with dry dipalmitoylphosphatidylcholine. Arch Biochem Biophys. 1985;236(1):289–96. doi: 10.1016/0003-9861(85)90628-9. [DOI] [PubMed] [Google Scholar]
- 66.Ohtake S, Schebor C, Palecek SP, de Pablo JJ. Phase behavior of freeze-dried phospholipid-cholesterol mixtures stabilized with trehalose. Biochim Biophys Acta. 2005;1713(1):57–64. doi: 10.1016/j.bbamem.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 67.Ricker JV, Tsvetkova NM, Wolkers WF, Leidy C, Tablin F, Longo M, et al. Trehalose maintains phase separation in an air-dried binary lipid mixture. Biophys J. 2003;84(5):3045–51. doi: 10.1016/S0006-3495(03)70030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leslie SB, Israeli E, Lighthart B, Crowe JH, Crowe LM. Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl Environ Microbiol. 1995;61(10):3592–7. doi: 10.1128/aem.61.10.3592-3597.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ohtake S, Martin RA, Yee L, Chen D, Kristensen DD, Lechuga-Ballesteros D, et al. Heat-stable measles vaccine produced by spray drying. Vaccine. 2010;28(5):1275–84. doi: 10.1016/j.vaccine.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 70.Mattern M, Winter G, Kohnert U, Lee G. Formulation of proteins in vacuum-dried glasses. II. Process and storage stability in sugar-free amino acid systems. Pharm Dev Technol. 1999;4(2):199–208. doi: 10.1081/pdt-100101354. [DOI] [PubMed] [Google Scholar]
- 71.Izutsu K, Kadoya S, Yomota C, Kawanishi T, Yonemochi E, Terada K. Freeze-drying of proteins in glass solids formed by basic amino acids and dicarboxylic acids. Chem Pharm Bull (Tokyo) 2009;57(1):43–8. doi: 10.1248/cpb.57.43. [DOI] [PubMed] [Google Scholar]
- 72.Izutsu K, Fujimaki Y, Kuwabara A, Aoyagi N. Effect of counterions on the physical properties of l-arginine in frozen solutions and freeze-dried solids. Int J Pharm. 2005;301(1–2):161–9. doi: 10.1016/j.ijpharm.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 73.Pikal MJ, Dellerman KM, Roy ML, Riggin RM. The effects of formulation variables on the stability of freeze-dried human growth hormone. Pharm Res. 1991;8(4):427–36. doi: 10.1023/a:1015834724528. [DOI] [PubMed] [Google Scholar]
- 74.Product insert for NovoSeven(R) RT from Novo Nordisk.
- 75.Duddu SP, Dal Monte PR. Effect of glass transition temperature on the stability of lyophilized formulations containing a chimeric therapeutic monoclonal antibody. Pharm Res. 1997;14(5):591–5. doi: 10.1023/a:1012144810067. [DOI] [PubMed] [Google Scholar]
- 76.Sarciaux JM, Hageman MJ. Effects of bovine somatotropin (rbSt) concentration at different moisture levels on the physical stability of sucrose in freeze-dried rbSt/sucrose mixtures. J Pharm Sci. 1997;86(3):365–71. doi: 10.1021/js960217k. [DOI] [PubMed] [Google Scholar]
- 77.Cryz SJ., Jr Post-marketing experience with live oral Ty21a vaccine. Lancet. 1993;341(8836):49–50. doi: 10.1016/0140-6736(93)92522-u. [DOI] [PubMed] [Google Scholar]
- 78.World Health Organization. Temperature Sensitivity of Biologicals. 2006. [Google Scholar]