Abstract
In female rats, vaginal-cervical stimulation (VCS) received during mating induces bicircadian prolactin surges that are required for the maintenance of pregnancy or pseudopregnancy (PSP). The neural circuits that transmit VCS inputs to the brain have not been fully described, although mating stimulation is known to activate medullary noradrenergic cell groups that project to the forebrain. In response to VCS, these neurones release noradrenaline within the ventrolateral division of the ventromedial hypothalamus (VMHvl) and the posterodorsal medial amygdala (MePD), two forebrain sites that are implicated in the initiation of PSP. Noradrenaline receptor activation within the VMHvl is both necessary and sufficient for PSP induction, suggesting that noradrenaline acting within the VMHvl is particularly important in mediating the effects of VCS towards the establishment of PSP. We therefore investigated whether or not endogenous, VCS-induced noradrenaline release within the VMHvl is involved in PSP induction in the rat. Before the receipt of sufficient mating stimulation to induce PSP, a retrograde neurotoxin, dopamine-β-hydroxylase-saporin (DBH-SAP), was infused bilaterally into the either the VMHvl or the MePD to selectively destroy afferent noradrenergic nuclei in the brainstem. DBH-SAP infusions into the VMHvl lesioned mating-responsive noradrenergic neurones in A1 and A2 medullary nuclei and reduced the incidence of PSP by 50%. Infusions of DBH-SAP into the MePD had no effect on the subsequent induction of PSP. These results suggest that VCS is conveyed to mating-responsive forebrain areas by brainstem noradrenergic neurones, and that the activity of noradrenergic cells projecting to the VMHvl is involved in the induction of PSP.
Keywords: noradrenaline, hypothalamus, medial amygdala, vaginocervical stimulation, dopamine-β-hydroxylase, saporin
In rodents and several other mammalian species, vaginal cervical stimulation (VCS) received during mating is required to initiate critical neuroendocrine changes that allow pregnancy to occur. One important component of this process involves the release of noradrenaline in forebrain regions after receipt of VCS. In female rats, noradrenaline is involved in multiple aspects of reproduction, including sexual behaviour (1,2) and the secretion of luteinising hormone and prolactin (3–5) during pro-oestrus. Noradrenaline actions have also been implicated in the induction of pregnancy or pseudopregnancy (PSP) in response to VCS (6,7).
A threshold amount of VCS is required by the female rat to induce the twice-daily prolactin surges needed to establish pregnancy. These prolactin surges are triggered even after a sterile mating, resulting in a 12–14-day acyclic period of PSP (8–11). For VCS to induce prolactin secretion, information received by the vagina and cervix must be conveyed to the central nervous system by the hypogastric and pelvic nerves (12–14). Mating stimulation is then relayed through ascending spinal pathways to activate medullary noradrenergic cell groups that project to the hypothalamus and thalamus (15–17).
Hypothalamic noradrenaline derives mainly from A1 and A2 medullary nuclei (17). These neurones send projections through the ventral noradrenergic bundle (VNAB), with terminal fields innervating the ventromedial nucleus of the hypothalamus (18) and other hypothalamic targets (17). Receipt of VCS causes a rapid increase in noradrenaline concentration in the ventrolateral division of the ventromedial hypothalamic nucleus (VMHvl) (19) and in the posterodorsal division of the medial amygdala (MePD) (7), two brain areas that are implicated in PSP induction. Electrolytic lesioning of the VNAB reduces PSP by 80% (20,21), suggesting that inputs to one or both of these areas from A1 and A2 noradrenergic cell groups are important for PSP induction. This premise is further confirmed by studies examining the expression of Fos, a marker for neuronal activation, in which VCS specifically activated neurones in the A1 and A2 nuclei, but not in A5 or A6 (locus coeruleus; LC) noradrenergic nuclei (22,23).
Fos expression has also been used to demonstrate that multiple forebrain nuclei, including the VMHvl, the bed nucleus of the stria terminalis (BNST) and the MePD respond to VCS (24–28). Although additional brain regions, including the dorsomedial–ventromedial hypothalamic region, the medial preoptic area, and the paraventricular nucleus of the hypothalamus (PVN), are involved in VCS-induced prolactin release (29–31), the MePD and VMHvl have been shown to be essential for the initial transduction of the VCS signal. The MePD appears to summate individual VCS inputs through rapid activation of NMDA glutamate receptors, and to transmit this information downstream to the BNST and VMHvl (32,33). By contrast, VMHvl contributions to the establishment of VCS-induced PSP appear to involve activation of noradrenaline and oxytocin receptors (6,34). Thus, the VMHvl is a likely site where noradrenaline released from A1 and A2 neurones may act to influence PSP induction. The present study directly tested the hypothesis that mating-responsive noradrenaline neurones that project to the VMHvl are involved in PSP induction. A noradrenaline-specific retrograde toxin, dopamine-β-hydroxylase-saporin (DBH-SAP), was infused before mating into either the VMHvl or the MePD to selectively kill afferent noradrenergic neurones projecting to each site. The results obtained demonstrate that mating-induced activation of noradrenergic cell groups projecting to the VMHvl, but not the MePD, contributes to PSP induction.
Materials and methods
Two separate experiments were conducted. In Experiment 1, drug infusions targeted the VMHvl and in Experiment 2, infusions targeted the MePD. Both experiments were performed using the same protocol unless specified.
Animals
Experimental animals were virgin Long-Evans female rats (200–225 g) purchased from Charles River Laboratories (Wilmington, MA, USA). Animals were housed individually in suspended metal cages under a reversed 12 : 12 h light/dark cycle (lights on 21.00 h). Access to food and water was provided ad lib. Throughout the experiments, the ovarian cyclicity of all females was monitored by daily examination (08.00 h) of vaginal cytology using the lavage method (35). Only females that exhibited two complete oestrous cycles (4–5 days) before drug infusions were used for experimentation. For Experiment 1, 50 animals were infused into the VMHvl, as described below. Infusions into the MePD were without effect in any animals, and so Experiment 2 was discontinued after four to eight animals in each group had been tested (16 animals total) to minimise the use of experimental animals.
Drug treatments
All animals were infused on the day of metoestrus to minimise the possible effects of infusion on subsequent ovarian cyclicity. Overall, drug infusions had no effect on normal oestrous cyclicity. In cases where the length of the first postoperative oestrous cycle was extended as a result of the stress of surgery or drug infusion, animals were not mated until they resumed normal cyclicity. Individual animals that failed to show normal oestrous cycles after drug infusion were excluded from the experiment.
DBH-SAP was used to selectively lesion noradrenergic neurones that project to the VMHvl or MePD. DBH is the rate-limiting enzyme in the conversion of dopamine to noradrenaline and is commonly used as a specific marker for noradrenaline neurones. DBH-SAP contains a mouse anti-DBH antibody conjugated to saporin, a toxin that inactivates ribosome function (Advanced Targeting Systems, San Diego, CA, USA). During noradrenaline neurotransmitter exocytosis, when vesicles are exposed to the synaptic cleft, DBH-SAP binds to vesicular DBH (36). The DBH-SAP complex is then internalised by noradrenaline terminals during vesicular endocytosis and is transported retrogradely to the cell body. Upon reaching the cell bodies of noradrenaline neurones, saporin inactivates ribosome function, preventing protein synthesis and causing cell death (37).
Experiment 1: VMHvl infusions
All infusion drugs were diluted in 0.05 mol/l phosphate-buffered saline (PBS). Animals were divided into six experimental groups. Two groups received VMHvl infusions of high or low doses of DBH-SAP (60 ng/0.6 μl, n = 14; 2 ng/0.6 μl, n = 6). As a control for the effects of DBH-SAP, matching high and low doses of nonspecific mouse immunoglobulin (IgG) bound to saporin (IgG-SAP) were administered to two additional groups (60 ng/0.6 μl, n = 10; 2 ng/0.6 μl, n = 10). The IgG portion of IgG-SAP has no antigen specificity and thus does not target specific cells (Advanced Targeting Systems). A fifth group of females received control infusions of artificial cerebral spinal fluid (aCSF) (n = 5) and, to control for the possibility that DBH-SAP infusion could itself induce PSP, a sixth group (n = 5) was infused with the high dose of DBH-SAP but subsequently received no mating stimulation (‘home cage’ animals; HC). For all infusions, animals were anaesthetised with isoflurane gas (2.5% in 100% oxygen) and positioned in a Kopf stereotaxic instrument. The skull of each animal was exposed and cleared of connective tissue to reveal the anatomical sutures. Bilateral holes were drilled in the skull to target the VMHvl at the following positions relative to Bregma: anteroposterior −3.14 mm; mediolateral ±1.0 mm (38). A 1.0 μl Hamilton syringe (Model #7003; Hamilton Co., Reno, NV, USA) was filled with DBH-SAP or control solutions and was lowered with the bevel of the needle pointed medially, towards the lateral edge of the VMHvl, until the tip resided −9.5 mm ventral to the dural membrane. Bilateral infusions were administered sequentially at a rate of 0.6 μl/2 min per side. Needles were left in place for an additional 7 min after infusion to allow for diffusion of the drug away from the needle tip. After infusions, incisions were sutured, and animals were placed on a heating pad for postoperative recovery before being returned to their home cages.
Experiment 2: MePD infusions
Drug infusions targeting the MePD were administered to separate groups of female rats as described above, with slight modifications. Drug concentrations were selected based on the results from Experiment 1. A 60-ng dose of DBH-SAP was selected because it was found to most consistently destroy medullary noradrenaline cells, and 2 ng of IgG-SAP was chosen as a control treatment to avoid nonspecific effects that had been observed with the higher 60-ng dose. To target the MePD, bilateral holes were drilled at the following positions relative to Bregma: anteroposterior −2.8 mm; mediolateral ±3.5 mm (38). A 1.0-μl Hamilton syringe was filled with DBH-SAP (60 ng in 0.6 μl, n = 8), IgG-SAP (2 ng in 0.6 μl, n = 4) or aCSF (n = 4) and lowered −8.1 mm from the dural membrane such that the tip of the needle was just ventral to the dorsolateral edge of the optic tract. The bevel of the needle was pointed medially, directing the infusate from the site of penetration into the MePD, as described previously (33). Infusions were administered sequentially to the right and left MePD at 0.6 μl/2 min per side, and needles were left in place for an additional 7 min to allow the drug to diffuse away from the needle tip. As described above, head wounds were sutured and after recovery, animals were returned to their home cages until behavioural testing.
Mating tests
Mating tests occurred 10–15 days after drug infusions. Ten days previously has been shown to be an effective length of time for the DBH-SAP toxin to fully lesion noradrenaline cells before behavioural testing (7,36). Mating tests were conducted in a dimly illuminated room (25 W yellow light) between 11.00 h and 13.00 h on the day of pro-oestrus. Mating stimulation was provided by sexually experienced, vasectomised males that had been previously exposed to the testing chamber. On the day of testing, each experimental female was placed in the testing chamber with an individual male. Females received 15 intromissions (15I), some including ejaculations, in addition to unlimited mounts without intromissions. Behavioural observations were recorded to determine if drug infusions had any effect on sexual readiness. As measures of sexual receptivity, the intensity of each lordosis (lordosis rating) and lordosis quotient (percent of lordotic responses) were scored as previously reported (6). HC animals that had been infused with 60 ng of DBH-SAP remained in their home cages on the day of testing, receiving no exposure to males.
After mating, PSP was considered to have been induced if 8–13 successive days of dioestrous vaginal cytology, characterised by a prevalence of leukocytes and round enucleate cells, were observed (11,35). After the initial mating session, females were allowed to resume oestrous cyclicity (at least one full oestrous cycle). Pro-oestrous females were then paired with males a second time. All females except HC animals were allowed to receive 15I, and were euthanised for immunohistochemical experiments 90 min after the first intromission, when induction of Fos by VCS is optimal (28). HC animals, which had remained in their cages and were not paired with males, were euthanised along with the mated females.
Tissue collection and processing
After the second mating session (90 min after first intromission), all animals from both experiments were injected i.p. with a lethal dose of sodium pentobarbital. Animals were then perfused transcardially with ice-cold 0.9% PBS for 30 s followed by ice-cold 4% paraformaldehyde in 0.1 mol/l phosphate buffer for 10 min. Brains were removed, post-fixed in 4% paraformaldehyde for 24 h, and stored at 4 °C in a 25% sucrose-PBS cryoprotectant solution until sectioning. Separate blocks of tissue containing forebrains and brainstems were isolated and frozen. Tissue was then sectioned coronally using a freezing microtome (section thickness = 30 μm). For each animal tested, four series of forebrain sections and four series of brainstem sections were collected. Sections were stored at −20 °C in an antifreeze solution consisting of 50% PBS (pH 7.4), 30% ethylene glycol and 20% glycerol until histological processing. For all histological procedures, tissue was rinsed well with PBS (3 × 10 min) upon removal from antifreeze and between all incubation steps.
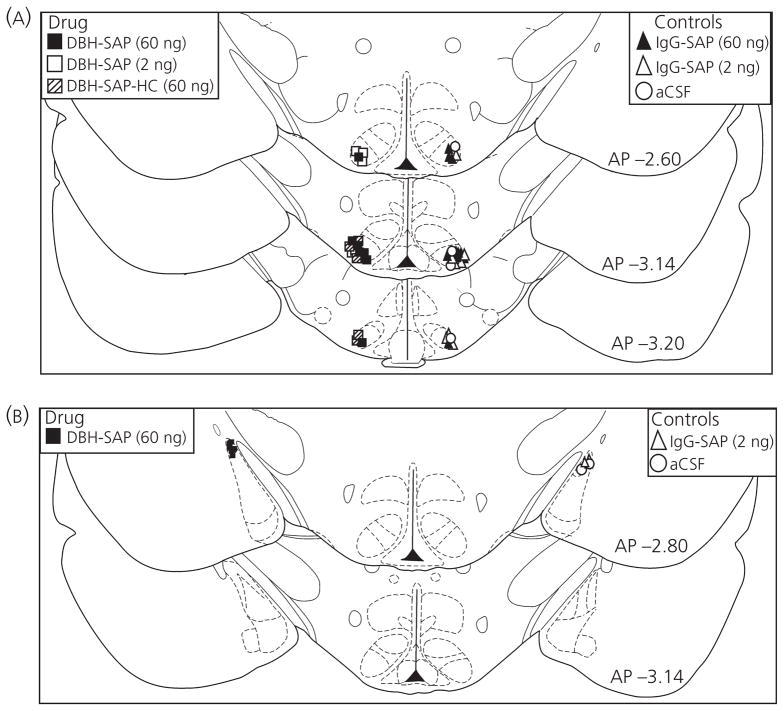
For histological verification of infusion sites in the VMHvl and MePD, one series of forebrain sections from each animal was mounted onto gelatin-coated slides for Nissl staining. Sections were dehydrated with ethanol, stained with cresyl violet, and overlaid with cover slips using Permount mounting medium (6). Infusion sites were identified for each animal by observing the location of damage produced by the bevelled tip (1 mm) of the infusion needle, according to the atlas of Paxinos and Watson (38). As shown in Fig. 1, placements were accepted if the tip of the needle targeted the lateral edge of the VMHvl or the dorsolateral aspect of the MePD. Animals were excluded from the experiments if the infusion sites were not bilaterally localised to the appropriate target nuclei.
Fig. 1.
Infusion sites in the ventromedial hypothalamus (VMHvl) (A) and posterodorsal medial amygdala (MePD) (B) for animals in Experiment 1 (n = 50) and Experiment 2 (n = 16), respectively. Animals received bilateral infusions of either dopamine-β-hydroxylase-saporin (DBH-SAP) or control treatments. For clarity of presentation, a single, unilateral infusion site is depicted for each experimental animal. aCSF, artificial cerebral spinal fluid; AP, anteroposterior; HC, home cage; IgG, immunoglobulin.
Nissl stained sections were also examined to confirm that significant neuronal death proximal to the infusion sites was not a confounding factor in these experiments. Targeted areas (VMHvl and MePD) were observed using light microscopy for the absence of stained neurones or excessive gliosis. In all animals, minimal neuronal loss was evident along the needle tract and damage to target nuclei was not observed with any of the experimental treatments (Fig. 2).
Fig. 2.
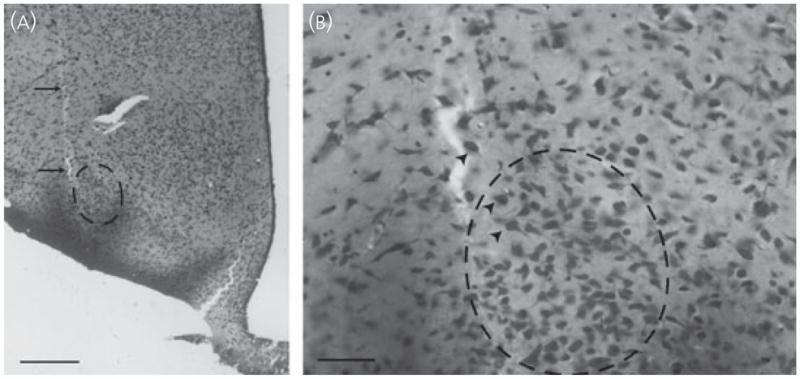
(A) Photomicrograph of a Nissl-stained section depicting the infusion tract (arrows) from a female infused with 60 ng of dopamine-β-hydroxylase-saporin into the ventrolateral division of the ventromedial hypothalamus. Scale bar = 500 μm. (B) Infusions caused minimal damage, and neurones at the infusion site (arrowheads) exhibited a normal distribution and morphology. Scale bar = 100 μm.
DBH-Fos double-immunolabelling
Free-floating brainstem sections from animals infused into the VMHvl were stained for DBH and Fos immunoreactivity (IR), as described previously (7), with slight modifications. Briefly, one series of brainstem sections from each animal was incubated at 4 °C for 15 h in a rabbit anti-Fos primary antiserum (SC-52; Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted 1 : 1000 in PBS containing 0.4% Triton X-100. To reduce nonspecific labelling, sections were then incubated in 3% normal goat serum (NGS) and 1% hydrogen peroxide in 0.4% Triton X-100/PBS for 1.5 h. Fos-IR was visualised by sequential incubation with a biotinylated goat anti-rabbit IgG secondary antibody (dilution 1 : 200 in 0.4% Triton X-100 in PBS; Vector Laboratories, Burlingame, CA, USA), Vectastain Elite avidin-biotin complex (Vector Laboratories), and nickel-enhanced 3,3′-diaminobenzidine (DAB) peroxidase substrate (Vector Laboratories). Fos-labelled sections were then placed in a mouse anti-DBH primary antibody (MAB308; Chemicon, Temecula, CA, USA) diluted 1 : 2000 in 0.4% Triton X-100 in PBS for 16 h at room temperature. Sections were then sequentially incubated with 1% hydrogen peroxide and 3% NGS in PBS as above, followed by biotinylated goat anti-mouse IgG (dilution 1 : 200 in 0.4% Triton X-100 in PBS; Vector Laboratories), Vectastain Elite avidin-biotin complex, and DAB peroxidase substrate without nickel enhancement. Using these procedures, Fos-containing nuclei were labelled with a dark blue–black precipitate and DBH-containing soma were stained a light brown color (Fig. 3). Sections were then mounted onto gelatin-coated slides, dehydrated in ethanol, cleared in xylenes, and sealed under cover slips using Permount mountant.
Fig. 3.
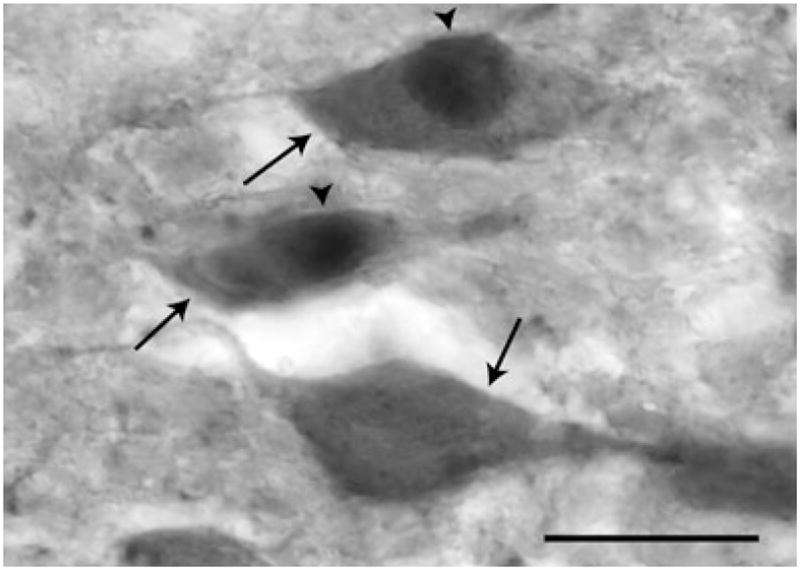
Photomicrograph of dopamine-β-hydroxylase-immunoreactive (DBH-IR) and DBH/Fos-IR cells in the rostral A2 nucleus. DBH-IR cells (arrows) were identified by their brown cytoplasmic staining. Dark purple-black nuclear label was observed in Fos-IR cells (arrowheads). Single- and double-labelled cells were readily identifiable using a 20× objective. Scale bar = 20 μm.
Validation of drug specificity
A second series of forebrain sections was selected for immunolabelling of oxytocin neurones in the PVN to assess if cell loss had occurred in non-noradrenergic afferent cell groups. The PVN contains a population of mating-responsive oxytocin cells that is considered to provide afferent projections to multiple limbic and hypothalamic targets, including the VMHvl (39–43). Free-floating sections from the medial (anteroposterior −1.88 mm) and posterior (anteroposterior −2.12 mm) PVN of VMHvl-infused females were incubated at room temperature for 24 h with a rabbit anti-oxytocin primary antibody (AB911; Chemicon) diluted 1 : 10 000 in 0.4% Triton-X in PBS. The next day, sections were incubated with 1% H2O2 and 3% NGS in 0.4% Triton-X in PBS to decrease nonspecific labelling. Sections were then sequentially incubated in a biotinylated goat anti-rabbit IgG secondary antibody (dilution 1 : 400 in 0.4% Triton-X in PBS), Vectastain Elite avidin-biotin complex, and DAB chromagen for visualisation of oxytocin-IR neurones. Sections were mounted and cover slipped as above for quantification.
Quantification of immunoreactive cells
DBH- and DBH/Fos-IR neurones
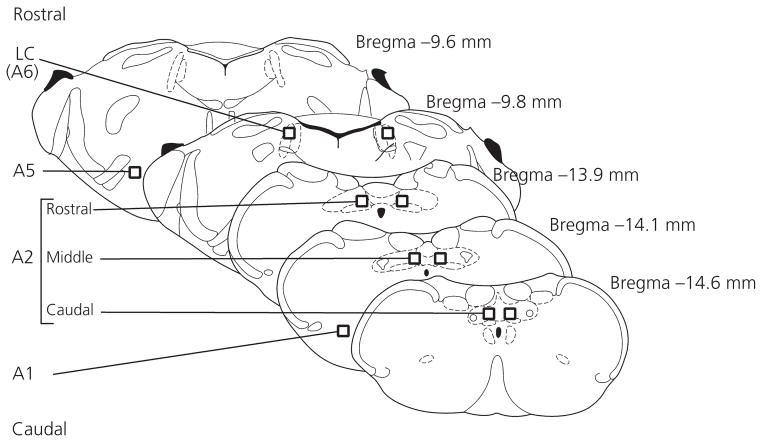
As shown in Fig. 4, brainstem sections from VMHvl-infused females containing A1, A2, A5 and A6 noradrenergic brainstem nuclei were selected for quantification of DBH- and DBH/Fos-IR. The A2 nucleus was subdivided into rostral, middle and caudal regions for further quantification, as described previously (7). For each area, DBH-IR cells and cells double-labelled for DBH and Fos were counted bilaterally within a single section, and mean bilateral cell counts were generated. Cell counts from rostral, middle and caudal A2 sections were summed to generate total A2 values.
Fig. 4.
Areas in which dopamine-β-hydroxylase (DBH)- and DBH/Fos-immunoreactivity were quantified. Rectangles indicate position and relative size of templates (for details, see text). Cells were counted bilaterally in each section. LC, locus coeruleus.
Cells were counted by an experimenter blind to the treatment condition for each animal. Identification and analysis of DBH- and DBH/Fos-IR were performed as described previously (7). Cells were determined to be Fos-IR if they had dark blue–black nuclear staining with distinct nuclear boundaries, and cells with brown cytoplasmic staining were identified as DBH-positive (Fig. 3). Using a camera lucida, labelled cells were manually transcribed into quadrilateral templates (350 × 350 μm) that were superimposed over each region.
Oxytocin-IR neurones
The identification and analysis of oxytocin-IR parvocellular cells in the PVN was performed as described previously (39). Using a camera lucida and standard templates, neuronal cell bodies labelled for oxytocin were quantified bilaterally in single sections representing the medial (anteroposterior −1.88 mm) and posterior (anteroposterior −2.12 mm) levels of the PVN. The mean bilateral cell counts from each of the two sections were combined to generate values used for group analyses.
Statistical analysis
Cell counts
The effects of drug treatments on the numbers of DBH-IR and DBH/Fos-IR cell counts in brainstem noradrenergic cell groups and on the number of oxytocin-IR cells in the PVN were determined by one-way ANOVA followed by a two-tailed Fisher’s exact probability post-hoc analysis, where appropriate. P ≤ 0.05 was considered statistically significant.
To determine the effects of drug treatments on VCS-induced Fos expression within DBH-IR neurones, the percentage of DBH-IR cells that expressed Fos was calculated for each animal. Group comparisons were performed using one-way ANOVA followed by a two-tailed Fisher’s exact probability test. P ≤ 0.05 was considered statistically significant.
PSP induction by mating
PSP induction was defined as a period of extended dioestrus (8–12 days) in females after being paired with a male (11,35). The percentage of females that became PSP (% PSP) was calculated for each treatment group, and a nonparametric Fisher’s exact probability test was used to compare % PSP between each mated group and the unmated HC group. P ≤ 0.05 was considered statistically significant.
Behavioural measures
The effects of drug treatments on behavioral parameters recorded during mating sessions were compared by one-way ANOVA. P ≤ 0.05 was considered statistically significant. A lack of significant effects precluded post-hoc analysis.
Results
Effect of DBH-SAP infusions into the VMHvl on mating responsive DBH-IR cells
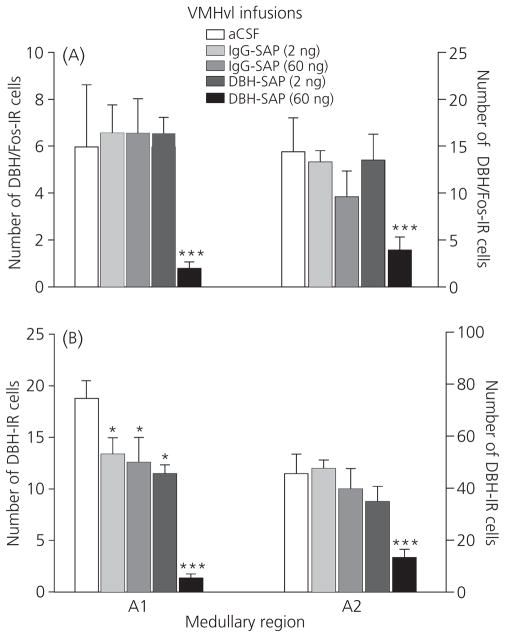
The effects of VMHvl infusions on the numbers of noradrenergic neurones activated by mating are shown in Fig. 5. Overall, bilateral infusions of 60 ng of DBH-SAP into the VMHvl reduced the numbers of cells double-labelled for DBH and Fos in both the A1 (F4,44 = 5.66, P ≤ 0.001) and A2 (F4,44 = 4.83, P ≤ 0.003) medullary nuclei (Fig. 5A). In both regions, a 60 ng infusion of DBH-SAP significantly decreased DBH/Fos-IR cell number compared to all other treatments (P ≤ 0.05). Low dose DBH-SAP and IgG-SAP infusions were without effect. Within the nucleus of the solitary tract (NTS), similar patterns of cell loss were observed throughout, with analysis of variance revealing significant group differences in rostral (F4,44 = 3.13, P ≤ 0.03), middle (F4,44 = 2.77, P ≤ 0.05) and caudal (F4,44 = 3.42, P ≤ 0.02) levels (not depicted). As shown in Fig. 5(B), drug treatments also influenced total DBH-IR cell number in A1 (F4,44 = 19.01, P ≤ 0.001) and A2 (F4,44 = 8.46, P ≤ 0.001) cell groups, with a 60 ng infusion of DBH-SAP into the VMHvl reducing cell counts compared to all other treatments (P ≤ 0.05). In A1, total numbers of DBH-IR cells were also reduced in animals infused with the 2-ng dose of DBH-SAP or either dose of IgG compared to those infused with aCSF (P ≤ 0.05). The effects of 2 ng of DBH-SAP and IgG-SAP on DBH-IR cell number were not observed in the NTS. The percentage of DBH-IR cells that were double-labelled for Fos did not differ among the treatment groups in any area. DBH/Fos-IR was also quantified in the A5 and A6 (locus coeruleus) nuclei, brainstem noradrenergic nuclei that project to dorsal and medial hypothalamic cell groups but provide only sparse projections to the VMHvl (44–46). Consistent with previous studies (23), numbers of Fos-expressing cells were negligible in these areas (0–3 Fos-IR cells per animal), and there was no effect of drug treatments on the numbers of DBH/Fos-IR cells (not shown).
Fig. 5.
Mean ± SEM number of dopamine-β-hydroxylase (DBH)/Fos-immunoreactive (IR) (A) and total DBH-IR (B) cells observed in the A1 and A2 (nucleus of the solitary tract) noradrenergic areas after ventromedial hypothalamus (VMHvl) infusion of DBH-saporin (SAP), immunoglobulin (IgG)-SAP or artificial cerebral spinal fluid (aCSF). ***Significantly lower number of cells compared to all other treatments (P ≤ 0.05). *Significantly lower number of cells compared to aCSF infused animals (P ≤ 0.05).
Effects of DBH-SAP treatment on the induction of PSP
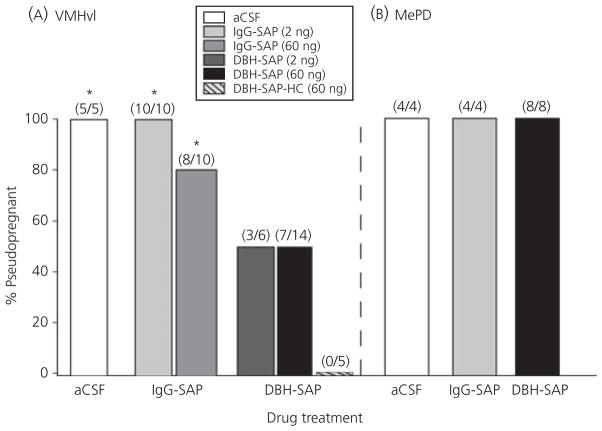
The incidence of PSP (% PSP) in animals that had received infusions into the VMHvl at least 10 days before mating is presented in Fig. 6(A). As shown, none of the unmated HC animals infused with 60 ng of DBH-SAP became PSP, and all mated females that had been infused with either aCSF or 2 ng of IgG-SAP became PSP. The incidence of PSP was slightly reduced (20%) in animals that had received VMHvl infusions of 60 ng of IgG-SAP but remained significantly elevated compared to the unmated HC group (P ≤ 0.007). In mated females that had been infused into the VMHvl with either the 60-ng or 2-ng dose of DBH-SAP, the incidence of PSP was reduced by 50%, to levels that were not statistically different from unmated HC animals (P = 0.11 and 0.18, respectively). In the MePD, there was no effect of DBH-SAP, IgG-SAP or aCSF infusion on the incidence of PSP in mated animals (Fig. 6B).
Fig. 6.
The effect of dopamine-β-hydroxylase-saporin (DBH-SAP) infusions into the ventromedial hypothalamus (VMHvl) (A) and posterodorsal medial amygdala (MePD) (B) on the incidence of pseudopregnancy (PSP). Numbers in parentheses indicate the number of PSP animals/total number of animals in each group. *Significantly greater incidence of PSP than home cage (HC) controls (P ≤ 0.05). IgG, immunoglobulin.
Specificity of DBH-SAP toxicity
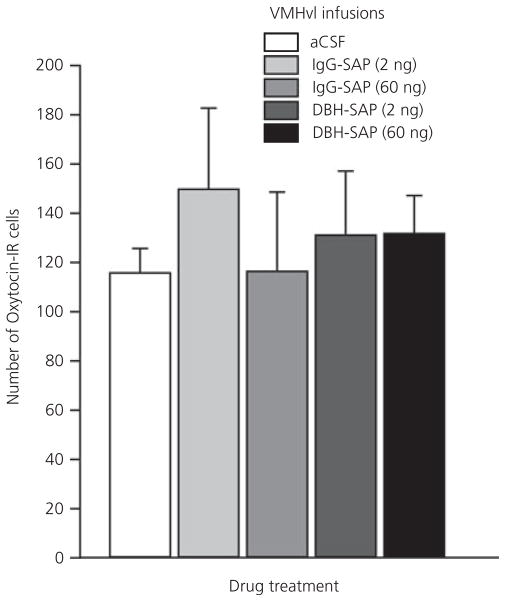
To confirm that the neurotoxic effects of DBH-SAP were specific for noradrenergic afferent cell groups, parvocellular oxytocin neurones of the PVN, a non-noradrenergic cell population that is considered to send direct projections to the VMHvl (40–42), were also quantified. There was no difference in the number of oxytocin-IR neurones after any of the drug treatments (Fig. 7).
Fig. 7.
Numbers of oxytocin-immunoreactive (IR) neurones in the paraventricular nucleus of females infused with dopamine-β-hydroxylase-saporin (DBH-SAP), immunoglobulin (IgG)-SAP, or artificial cerebral spinal fluid (aCSF) into the ventromedial hypothalamus (VMHvl).
DBH-SAP effects on sexual behaviour
Behavioural measures are presented in Table 1. Infusion of DBH-SAP into the VMHvl or the MePD before mating did not influence mating behaviour in either the first or second round of mating. Drug treatment more than 10 days before mating had no significant effect on lordosis rating or lordosis quotient, nor was there an effect on the numbers of mounts, intromissions and ejaculations received from the male.
Table 1.
Effect of Dopamine-β-Hydroxylase-Saporin (DBH-SAP) Infusions into the Ventromedial Hypothalamus (VMHvl) or Posterodorsal Medial Amygdala (MePD) on Mating Behaviour.
| Brain region | Drug | N value | Received from vasectomised male |
LR | LQ | ||
|---|---|---|---|---|---|---|---|
| Intromissions | Mounts | EJ | |||||
| VMHvl | Test 1 | ||||||
| aCSF | 5 | 15.0 ± 0.00 | 5.50 ± 1.91 | 1.25 ± 0.50 | 2.90 ± 0.12 | 96.83 ± 4.14 | |
| IgG-SAP (2 ng) | 10 | 15.0 ± 0.00 | 7.33 ± 2.16 | 0.83 ± 0.75 | 2.97 ± 0.48 | 99.33 ± 1.63 | |
| IgG-SAP (60 ng) | 11 | 15.0 ± 0.00 | 7.90 ± 2.33 | 1.17 ± 0.66 | 2.92 ± 0.13 | 99.22 ± 2.77 | |
| DBH-SAP (2 ng) | 6 | 15.0 ± 0.00 | 5.50 ± 7.29 | 1.50 ± 0.55 | 2.97 ± 0.07 | 99.01 ± 2.40 | |
| DBH-SAP (60 ng) | 15 | 15.0 ± 0.00 | 7.93 ± 5.35 | 1.13 ± 0.35 | 2.89 ± 0.22 | 100.00 ± 0.98 | |
| VMHvl | Test 2 | ||||||
| aCSF | 5 | 15.0 ± 0.00 | 9.75 ± 5.12 | 1.80 ± 0.82 | 2.99 ± 0.14 | 99.8 ± 0.44 | |
| IgG-SAP (2 ng) | 10 | 15.0 ± 0.00 | 9.33 ± 4.68 | 1.83 ± 0.75 | 2.98 ± 0.08 | 99.82 ± 2.54 | |
| IgG-SAP (60 ng) | 11 | 15.0 ± 0.00 | 8.70 ± 5.62 | 1.10 ± 0.57 | 2.92 ± 0.13 | 99.94 ± 1.56 | |
| DBH-SAP (2 ng) | 6 | 15.0 ± 0.00 | 6.67 ± 7.84 | 1.20 ± 0.45 | 2.83 ± 0.21 | 98.03 ± 3.65 | |
| DBH-SAP (60 ng) | 15 | 15.0 ± 0.00 | 6.94 ± 7.67 | 1.13 ± 0.50 | 2.91 ± 0.21 | 98.21 ± 5.38 | |
| MePD | Test 1 | ||||||
| aCSF | 4 | 15.0 ± 0.00 | 4.75 ± 4.50 | 1.00 ± 0.00 | 2.96 ± 0.06 | 99.04 ± 1.90 | |
| IgG-SAP | 4 | 15.0 ± 0.00 | 4.75 ± 2.50 | 1.75 ± 0.96 | 3.00 ± 0.08 | 100.0 ± 2.94 | |
| DBH-SAP | 8 | 15.0 ± 0.00 | 3.63 ± 1.30 | 1.50 ± 0.75 | 2.94 ± 0.21 | 100.0 ± 5.81 | |
| MePD | Test 2 | ||||||
| aCSF | 4 | 15.0 ± 0.00 | 7.00 ± 3.56 | 1.00 ± 0.00 | 2.97 ± 0.06 | 97.07 ± 1.85 | |
| IgG-SAP | 4 | 15.0 ± 0.00 | 5.25 ± 5.31 | 1.00 ± 0.82 | 2.93 ± 0.08 | 97.72 ± 2.76 | |
| DBH-SAP | 8 | 15.0 ± 0.00 | 6.50 ± 3.21 | 1.38 ± 0.74 | 2.89 ± 0.22 | 97.92 ± 6.62 | |
Data are shown as the mean ± SD. EJ, ejaculations; Ig, immunoglobulin; LR, lordosis rating; LQ, lordosis quotient.
Discussion
The results obtained in the present study indicate that noradrenergic (noradrenaline) neurones that project to the VMHvl contribute to the induction of PSP by mating. Bilateral infusions of 60 ng DBH-SAP into the VMHvl that caused a marked decrease in the numbers of mating-responsive DBH-IR cells in A1 and A2 medullary nuclei significantly depressed the incidence of PSP in mated females. This effect appears specifically to be a result of the neurotoxic effects of DBH-SAP in afferent noradrenaline cell groups. Examination of infusion sites verified that there was minimal damage to cells in the VMHvl, and no loss of oxytocin neurones in the PVN was observed. The results of the present study suggest that by reducing noradrenaline release in the VMHvl after mating, DBH-SAP treatments interfered with the transduction of the VCS stimulus and impeded PSP induction. This hypothesis is in accordance with previous studies demonstrating that VCS causes rapid noradrenaline release within the VMHvl, and that VMHvl noradrenaline receptor activation is required for PSP induction (6,19).
The results of the present study also suggest that noradrenergic projections from A1 and A2 to the VMHvl, specifically, are required for the processing of VCS towards PSP induction. PSP block was associated with reduced numbers of mating-activated noradrenaline cells in A1 and A2, but not in A5 or A6 (LC). Previous studies in which VCS induced Fos expression in noradrenergic neurones in A1 and A2, but not in A6, are in accordance with this finding (22,23). Anatomical reports also support this interpretation, demonstrating that noradrenergic inputs to the VMHvl are derived primarily from lateral tegmental and dorsal medullary cell groups, such as A1 and A2, which send ascending projections through the VNAB (17,47). By contrast, the LC sends dense projections throughout the thalamus and to dorsal and medial hypothalamic structures (44,48,49), but projects only sparsely to the VMHvl (45,46,50). The findings of the present study do not, however, preclude the possibility that LC neurones may contribute, in part, to transduction of the VCS stimulus; the LC is a heterogeneous structure, and LC neurones other than those we quantified may have been affected by DBH-SAP infusions into the VMHvl. Thus, although we did not observe significant neuronal loss in LC after DBH-SAP infusions into the VMHvl, the possibility that inputs from the LC may also contribute to PSP induction warrants further investigation.
DBH-SAP infusions failed to block PSP in all animals. Characterisations of DBH-SAP toxicity show that the drug does not kill 100% of noradrenaline neurones (51), and it is likely that limited noradrenaline activity in the VMHvl was retained in the present study. The use of intact, oestrous females may also have contributed to this variability because differences in circulating oestradiol levels could have influenced neuronal sensitivity to VCS in brainstem noradrenergic nuclei (52) or in the VMHvl (53). Thus, a subset of females may have exhibited heightened sensitivity to peri-threshold levels of mating-induced noradrenaline activity, such that the reduced populations of noradrenaline neurones in A1 and A2 were capable of adequately transducing the VCS stimulus. It is also possible that the varying effectiveness of DBH-SAP infusions in preventing PSP may reflect a role for compensatory pathways in transducing the VCS stimulus, as has been suggested for the MePD (54).
Heightened neuronal sensitivity in the VMHvl may also explain the lack of treatment effects on measures of sexual receptivity. Although noradrenaline plays an important role in regulating female sexual behaviour (2), the results of the present study suggest that high levels of mating-induced noradrenergic activity in the VMHvl are not required for the display of sexual receptivity. This finding is consistent with previous pharmacological experiments, in which noradrenaline receptor agonists and antagonists were administered into the VMHvl before mating (2,55). These studies indicated that, although lordosis quotients were significantly influenced by noradrenergic activity in the VMHvl, complete blockade of noradrenaline receptors reduced lordosis quotients by only 50%.
Low dose (2 ng) DBH-SAP infusions into the VMHvl did not cause significant loss of mating-activated noradrenergic neurones but were effective at blocking PSP. This suggests that low doses of DBH-SAP may have exerted sub-lethal toxic effects on noradrenaline neurones projecting to the VMHvl. The immunohistochemical approaches employed in these studies would not have detected loss of function in surviving cells, such as decreased levels of neurotransmitter synthesis or release. However, both free saporin and DBH-SAP are reported to have dose-dependent effects on protein synthesis and cytotoxicity (51,56), supporting the possibility that infusions of 2 ng of DBH-SAP may have impaired cellular function without causing detectable neuronal death. To a lesser degree, 60 ng of IgG-SAP also prevented PSP induction. IgG-SAP does not mimic the ability of DBH-SAP to target noradrenergic systems but, when administered at a supra-threshold concentration, it may be taken up nonspecifically by neurones through bulk endocytosis (Advanced Targeting Systems). Thus, the 60-ng dose of IgG-SAP may have marginally compromised the ability of mating-activated noradrenergic neurones to transduce the VCS stimulus, blocking PSP in a small percentage of females. It also remains possible that VMHvl neurones proximal to the infusion sites, although appearing normal in Nissl-stained sections, were compromised by the treatments, interfering with the transduction of VCS towards PSP induction.
DBH-SAP infusions into the MePD were without effect in the present experiments. Similar to the VMHvl, the MePD receives noradrenaline inputs from A1 and A2 cell groups, and VCS sufficient for PSP induction causes an increase in noradrenaline release in the MePD (7). The results of the present study suggest that the noradrenaline activity observed in the MePD after mating is not related to PSP induction. Rather, given the prominent roles played by both the amygdala and the noradrenaline neurotransmitter system in regulating stress responses, this response may reflect the increased stress associated with receipt of supra-threshold mating stimulation. The finding that DBH-SAP infusions into the VMHvl, but not the MePD, influenced PSP is consistent with the proposed roles that these two nuclei play in PSP induction. A preponderance of evidence points to the MePD as a site where information from individual VCS events is immediately summated towards a threshold for PSP induction through activation of NMDA glutamate receptors (57). It is postulated that this information is then conveyed by the MePD to downstream brain regions, including the VMHvl. The VMHvl, along with other forebrain nuclei, appears to be involved in the subsequent establishment of a long-term ‘mnemonic’, whereby the single mating episode causes repeated prolactin surges for 10–12 days (57–59). The findings of the present study support this model. Furthermore, they suggest that under physiological mating conditions, VMHvl inputs from both the MePD and noradrenergic medullary nuclei may contribute to the induction of PSP.
The neuronal pathways that transduce the VCS signal to initiate the prolactin surges of pregnancy/PSP have not been completely defined. VCS is detected by visceral sensory neurones in the vagina and cervix, including the pelvic and hypogastric nerves (14). The pelvic nerve appears to be essential for communicating VCS to the central nervous system for PSP induction; transection of the pelvic nerve before mating or VCS prevents Fos induction in mating-responsive brain sites and effectively blocks PSP induction (24,60,61). Pelvic nerve afferents enter the spinal cord at the lumbar– sacral level (L6–S1), and previous anatomical studies suggest that VCS is processed primarily by dorsal horn neurones in laminae I and II at these levels (62–64). VCS is then conveyed through ascending spinal tracts to activate neurones in medullary nuclei, including noradrenergic neurones in the A1 and A2 cell groups (22,23). These cell groups send their principal projections through the VNAB to provide widespread noradrenaline inputs to forebrain nuclei, including the VMHvl and the MePD (17,18). Characterisation of Fos responses to mating demonstrate that VCS activates neurones in numerous limbic and hypothalamic structures, including the dorsomedial hypothalamus, the MePD, the bed nuclei of the stria terminalis and the medial preoptic area (24–28), as well as oxytocin neurones in the parvocellular PVN (39,43). All of these nuclei, along with tuberoinfundibular dopamine neurones in the arcuate nucleus (65), have also been shown to be involved in VCS-induced prolactin release. A rich anatomical literature links these areas into an intricate neuroendocrine network (66–69), although little is known about the neurotransmitters that act within and between these areas to process VCS towards the expression of prolactin surges and PSP. The present study provides new insight by demonstrating a direct role for brainstem noradrenergic projections to the VMHvl in the induction of PSP in rats.
Acknowledgments
This work was performed in the laboratory of Dr Mary S. Erskine, who was the Principal Investigator for these experiments. The manuscript is being published posthumously in her name. The project was supported by National Institutes of Health Grants MH64187 and MHO1435 to M.S.E. Dr E. K. Polston’s efforts were partially supported by an award through the Specialized Neuroscience Research Program at Howard University College of Medicine (U54NS39407-09). We would like to thank Dr Angela Seliga for her assistance with the microscopic calibrations, as well as Drs Michael Baum and Thomas Gilmore for their comments on the manuscript.
References
- 1.Vathy I, Etgen AM. Hormonal activation of female sexual behaviour is accompanied by hypothalamic norepinephrine release. J Neuroendocrinol. 1989;1:383–388. doi: 10.1111/j.1365-2826.1989.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 2.Etgen AM. Intrahypothalamic implants of noradrenergic antagonists disrupt lordosis behaviour in female rats. Physiol Behav. 1990;48:31–36. doi: 10.1016/0031-9384(90)90256-4. [DOI] [PubMed] [Google Scholar]
- 3.Le W-W, Berghorn KA, Smith MS, Hoffman GE. Alpha-1 adrenergic receptor blockade blocks LH secretion but not LHRH cFos activation. Brain Res. 1997;747:236–245. doi: 10.1016/s0006-8993(96)01269-3. [DOI] [PubMed] [Google Scholar]
- 4.Mohankumar PS, Thyagarajan S, Quadri SK. Correlations of catecholamine release in the medial preoptic area with proestrous surges of luteinising hormone and prolactin: effects of aging. Endocrinology. 1994;135:119–126. doi: 10.1210/endo.135.1.8013343. [DOI] [PubMed] [Google Scholar]
- 5.Rance N, Wise PM, Selmanoff MK, Barraclough C. Catecholamine turnover rates in discrete hypothalamic areas and associated changes in median eminence luteininsing hormone-releasing hormone and serum gonadotropins on proestrus and dioestrus day 1. Endocrinology. 1981;108:1795–1802. doi: 10.1210/endo-108-5-1795. [DOI] [PubMed] [Google Scholar]
- 6.Northrop LE, Shadrach JL, Erskine MS. Noradrenergic innervation of the ventromedial hypothalamus is involved in mating-induced pseudopregnancy in the female rat. J Neuroendocrinol. 2006;18:577–583. doi: 10.1111/j.1365-2826.2006.01453.x. [DOI] [PubMed] [Google Scholar]
- 7.Cameron NM, Carey P, Erskine MS. Medullary noradrenergic neurones release norepinephrine in the medial amydgala in females in response to mating stimulation sufficient for pseudopregnancy. Brain Res. 2004;1022:137–147. doi: 10.1016/j.brainres.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Wilson JR, Adler NT, Le Boeuf B. The effects of intromission frequency on successful pregnancy in the rat. Proc Natl Acad Sci USA. 1965;53:1392–1395. doi: 10.1073/pnas.53.6.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adler NT. Effects of male’s copulatory behaviour on successful pregnancy of the female rat. J Comp Physiol Psychol. 1969;69:613–622. doi: 10.1037/h0028244. [DOI] [PubMed] [Google Scholar]
- 10.Erskine MS. Solicitation behaviour in the oestrous female rat: a review. Horm Behav. 1989;23:473–502. doi: 10.1016/0018-506x(89)90037-8. [DOI] [PubMed] [Google Scholar]
- 11.Gunnett JW, Freeman ME. The mating-induced release of prolactin: a unique neuroendocrine response. Endocr Rev. 1983;4:44–61. doi: 10.1210/edrv-4-1-44. [DOI] [PubMed] [Google Scholar]
- 12.Komisaruk BR, Bianca R, Sansone G, Gomez LE, Cueva-Rolon R, Beyer C, Whipple B. Brain-mediated responses to vaginocervical stimulation in spinal cord-transected rats: role of the vagus nerves. Brain Res. 1996;708:128–134. doi: 10.1016/0006-8993(95)01312-1. [DOI] [PubMed] [Google Scholar]
- 13.Peters LD, Kristal MB, Komisaruk BR. Sensory innervation of the external and internal genitalia of the female rat. Brain Res. 1987;408:199–204. doi: 10.1016/0006-8993(87)90372-6. [DOI] [PubMed] [Google Scholar]
- 14.Steinman JL, Carlton SM, Willis WD. The segmental distribution of afferent fibers from the vaginal cervix and hypogastric nerve in rats. Brain Res. 1992;575:25–31. doi: 10.1016/0006-8993(92)90418-9. [DOI] [PubMed] [Google Scholar]
- 15.Lee JW, Erskine MS. Vaginocervical stimulation suppresses the expression of c-fos induced by mating in thoracic, lumbar and sacral segments of the female rat. Neuroscience. 1996;74:237–249. doi: 10.1016/0306-4522(96)00103-0. [DOI] [PubMed] [Google Scholar]
- 16.Hubscher CH, Berkley KJ. Spinal and vagal influences on the responses of rat solitary nucleus neurones to stimulation of uterus, cervix and vagina. Brain Res. 1995;702:251–254. doi: 10.1016/0006-8993(95)01121-8. [DOI] [PubMed] [Google Scholar]
- 17.Moore RY, Bloom FE. Central catecholamine neurone systems: anatomy and physiology of the norepinephrine and epinephrine systems. Ann Rev Neurosci. 1979;2:13–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- 18.Palkovits M, Zaborszky L, Feminger A, Mezey E, Fekete MI, Herman JP, Kanyicska B, Szabo D. Noradrenergic innervation of the rat hypothalamus: experimental biochemical and electron microscopic studies. Brain Res. 1980;191:161–171. doi: 10.1016/0006-8993(80)90320-0. [DOI] [PubMed] [Google Scholar]
- 19.Etgen AM, Morales JC. Somatosensory stimuli evoke norepinephrine release in the anterior ventromedial hypothalamus of sexually receptive female rats. J Neuroendocrinol. 2002;14:213–218. doi: 10.1046/j.0007-1331.2001.00764.x. [DOI] [PubMed] [Google Scholar]
- 20.Hansen S, Stanfield EJ, Everitt BJ. The role of ventral bundle noradrenergic neurones in sensory components of sexual behaviour and coitusinduced pseudopregnancy. Nature. 1980;286:152–154. doi: 10.1038/286152a0. [DOI] [PubMed] [Google Scholar]
- 21.Hansen S, Stanfield EJ, Everitt BJ. The effects of lesions of lateral tegmental noradrenergic neurones on components of sexual behaviour and pseudopregnancy in female rats. Neuroscience. 1981;6:1105–1117. doi: 10.1016/0306-4522(81)90075-0. [DOI] [PubMed] [Google Scholar]
- 22.Cameron NM, Ha GK, Erskine MS. Fos expression after mating in the noradrenergic cells of the A1 and A2 areas of the medulla is altered by adrenalectomy. J Neuroendocrinol. 2004;16:750–757. doi: 10.1111/j.1365-2826.2004.01231.x. [DOI] [PubMed] [Google Scholar]
- 23.Yang S, Voogt J. Mating-activated brainstem catecholaminergic neurones in the female rat. Brain Res. 2001;894:159–166. doi: 10.1016/s0006-8993(01)01990-4. [DOI] [PubMed] [Google Scholar]
- 24.Rowe DW, Erskine MS. c-Fos proto-oncogene activity induced by mating in the preoptic area, hypothalamus and amygdala in the female rat: role of afferent input via the pelvic nerve. Brain Res. 1993;621:25–34. doi: 10.1016/0006-8993(93)90294-w. [DOI] [PubMed] [Google Scholar]
- 25.Pfaus JG, Kleopoulos SP, Mobbs CV, Gibbs RR, Pfaff DW. Sexual stimulation activates c-fos within oestrogen-concentrating regions of the female rat forebrain. Brain Res. 1993;624:253–267. doi: 10.1016/0006-8993(93)90085-2. [DOI] [PubMed] [Google Scholar]
- 26.Tetel MJ, Getzinger MJ, Blaustein JD. Fos expression in the rat brain following vaginal-cervical stimulation by mating and manual probing. J Neuroendocrinol. 1993;5:397–404. doi: 10.1111/j.1365-2826.1993.tb00500.x. [DOI] [PubMed] [Google Scholar]
- 27.Cameron NM, Erskine MS. c-fos expression in the forebrain after mating in the female rat is altered by adrenalectomy. Neuroendocrinology. 2003;77:305–313. doi: 10.1159/000070283. [DOI] [PubMed] [Google Scholar]
- 28.Polston EK, Erskine MS. Patterns of induction of the immediate-early genes c-fos and egr-1 in the female rat brain following differential amounts of mating stimulation. Neuroendocrinology. 1995;62:370–384. doi: 10.1159/000127027. [DOI] [PubMed] [Google Scholar]
- 29.Gunnett JW, Mick C, Freeman ME. The role of dorsomedial-ventromedial area of the hypothalamus in the control of prolactin secretion induced by cervical stimulation. Endocrinology. 1981;109:1846–1850. doi: 10.1210/endo-109-6-1846. [DOI] [PubMed] [Google Scholar]
- 30.Arey BJ, Freeman ME. Hypothalamic factors involved in the endogenous stimulatory rhythm regulating prolactin secretion. Endocrinology. 1989;124:878–883. doi: 10.1210/endo-124-2-878. [DOI] [PubMed] [Google Scholar]
- 31.Gunnett JW, Freeman ME. The interaction of the medial preoptic area and the dorsomedial-ventromedial nuclei of the hypothalamus in the regulation of the mating-induced release of prolactin. Neuroendocrinology. 1985;40:232–237. doi: 10.1159/000124079. [DOI] [PubMed] [Google Scholar]
- 32.Polston EK, Heitz M, Barnes W, Cardamone K, Erskine MS. NMDA-mediated activation of the medial amygdala initiates a downstream neuroendocrine memory responsible for pseudopregnancy in the female rat. J Neurosci. 2001;21:4104–4110. doi: 10.1523/JNEUROSCI.21-11-04104.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehmann ML, Mckellar H, Erskine MS. Coding for the initiation of pseudopregnancy by temporally patterned activation of amygdalar NMDA receptors. J Neurosci. 2005;25:8696–8703. doi: 10.1523/JNEUROSCI.1893-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Northrop LE, Erskine MS. Selective oxytocin receptor activation in the ventrolateral portion of the ventromedial hypothalamus is required for mating-induced pseudopregnancy in the female rat. Endocrinology. 2008;149:836–842. doi: 10.1210/en.2007-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper RL, Goldman JM, Vandenbergh JG. Monitoring of the oestrous cycle in the laboratory rodent by vaginal lavage. In: Heindel JCR, editor. Methods in Toxicology. Orlando, FL: Academic Press; 1992. pp. 45–56. [Google Scholar]
- 36.Wrenn CC, Picklo MJ, Lappi DA, Roberston D, Wiley RG. Central noradrenergic lesioning using anti-DBH-saporin: anatomical findings. Brain Res. 1996;740:175–184. doi: 10.1016/s0006-8993(96)00855-4. [DOI] [PubMed] [Google Scholar]
- 37.Ippoliti R, Lendaro E, Bellelli A, Brunori M. A ribosomal protein is specifically recognised by saporin, a plant toxin which inhibits protein synthesis. FEBS Lett. 1992;298:145–148. doi: 10.1016/0014-5793(92)80042-f. [DOI] [PubMed] [Google Scholar]
- 38.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic; 1998. [Google Scholar]
- 39.Polston EK, Centorino KM, Erskine MS. Diurnal fluctuations in mating-induced oxytocinergic activity within the paraventricular and supraoptic nuclei do not influence prolactin secretion. Endocrinology. 1998;139:4849–4859. doi: 10.1210/endo.139.12.6341. [DOI] [PubMed] [Google Scholar]
- 40.Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 41.Daniels D, Flanagan-Cato LM. Functionally-defined compartments of the lordosis neural circuit in the ventromedial hypothalamus in female rats. J Neurobiol. 2000;45:1–13. [PubMed] [Google Scholar]
- 42.Insel T, Young L, Wang Z. Central oxytocin and reproductive behaviours. Rev Reproduction. 1997;2:28–37. doi: 10.1530/ror.0.0020028. [DOI] [PubMed] [Google Scholar]
- 43.Flanagan LM, Pfaus JG, Mcewen BS. Induction of FOS immunoreactivity in oxytocin neurones after sexual activity in female rats. Neuroendocrinology. 1993;58:352–358. doi: 10.1159/000126562. [DOI] [PubMed] [Google Scholar]
- 44.Foote SL, Bloom FE, Aston-Jones G. Nucleus locus coeruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- 45.Jones BE, Moore RY. Ascending projections of the locus coeruleus in the rat. II. Autoradiographic study. Brain Res. 1977;127:25–53. [PubMed] [Google Scholar]
- 46.Kobayshi R, Palkovits M, Kopin I, Jacobowitz D. Biochemical mapping of noradrenergic nerves arising from the rat locus coeruleus. Brain Res. 1974;77:269–279. doi: 10.1016/0006-8993(74)90790-2. [DOI] [PubMed] [Google Scholar]
- 47.Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand Suppl. 1971;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- 48.Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res. 1982;257:275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- 49.Dahlstrom A, Fuxe K. Evidence for the existence of monoamines-containing neurones in the central nervous system I. Demonstration of monoamines in the cell bodies of brainstem neurones. Acta Physiol Scand Suppl. 1964;232:1–55. [PubMed] [Google Scholar]
- 50.Lindvall O, Bjorklund A. The organization of the ascending catecholamine neurone systems in the rat brain as revealed by the glyoxylic acid fluorescence method. Acta Physiol Scand Suppl. 1974;412:1–48. [PubMed] [Google Scholar]
- 51.Picklo MJ, Wiley RG, Lappi DA, Robertson D. Noradrenergic lesioning with an anti-dopamine beta-hydroxylase immunotoxin. Brain Res. 1994;666:195–200. doi: 10.1016/0006-8993(94)90772-2. [DOI] [PubMed] [Google Scholar]
- 52.Jennes L, Jennes ME, Purvis C, Nees M. c-fos expression in noradrenergic A2 neurones of the rat during the oestrous cycle and after steroid hormone treatments. Brain Res. 1992;586:171–175. doi: 10.1016/0006-8993(92)91391-q. [DOI] [PubMed] [Google Scholar]
- 53.Lee AW, Kyrozis A, Chevaleyre V, Kow LM, Devidze N, Zhang Q, Etgen AM, Pfaff DW. Estradiol modulation of phenylephrine-induced excitatory responses in ventromedial hypothalamic neurones of female rats. Proc Natl Acad Sci USA. 2008;105:7333–7338. doi: 10.1073/pnas.0802760105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coopersmith CB, Gans S, Rowe DW, Erskine MS. Infusions of lidocaine into the amygdala, but not the preoptic area, block pseudopregnancy in the rat. J Neuroendocrinol. 1996;8:259–266. doi: 10.1046/j.1365-2826.1996.04432.x. [DOI] [PubMed] [Google Scholar]
- 55.Kow L-M, Weesner GD, Pfaff DW. Alpha-1 adrenergic agonists act on the ventromedial hypothalamus to cause neuronal excitation and lordosis facilitation: electrophysiological and behavioural evidence. Brain Res. 1992;588:237–245. doi: 10.1016/0006-8993(92)91581-x. [DOI] [PubMed] [Google Scholar]
- 56.Bagga S, Hosur MV, Batra JK. Cytotoxicity of ribosome-inactivating protein saporin is not mediated through alpha2-macroglobulin receptor. FEBS Lett. 2003;541:16–20. doi: 10.1016/s0014-5793(03)00280-1. [DOI] [PubMed] [Google Scholar]
- 57.Erskine MS, Lehmann ML, Cameron NM, Polston EK. Co-regulation of female sexual behaviour and pregnancy induction: an exploratory synthesis. Brain Behav Res. 2004;153:295–315. doi: 10.1016/j.bbr.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 58.Terkel J, Witcher JA, Adler NT. Evidence for ‘memory’ of cervical stimulation for the promotion of pregnancy in rats. Horm Behav. 1990;24:40–49. doi: 10.1016/0018-506x(90)90025-s. [DOI] [PubMed] [Google Scholar]
- 59.Freeman ME, Smith M, Nazian SJ, Neill JD. Ovarian and hypothalamic control of the daily surges of prolactin secretion during pseudopregnancy in the rat. Endocrinology. 1974;94:875–882. doi: 10.1210/endo-94-3-875. [DOI] [PubMed] [Google Scholar]
- 60.Pfaus JG, Manitt C, Coopersmith CB. Effects of pelvic, pudendal, or hypogastric nerve cuts on Fos induction in the rat brain following vaginocervical stimulation. Physiol Behav. 2006;89:627–636. doi: 10.1016/j.physbeh.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 61.Kollar EJ. Reproduction in the female rat after pelvic nerve neurectomy. Anat Rec. 1953;115:641–658. doi: 10.1002/ar.1091150406. [DOI] [PubMed] [Google Scholar]
- 62.Lee JW, Erskine MS. Pseudorabies virus tracing of neural pathways between the uterine cervix and CNS: effects of survival time, estrogen treatment, rhizotomy, and pelvic nerve transection. J Comp Neurol. 2000;418:484–503. [PubMed] [Google Scholar]
- 63.Lee JW, Erskine MS. Changes in pain threshold and lumbar spinal cord immediate-early gene expression induced by paced and nonpaced mating in female rats. Brain Res. 2000;861:26–36. doi: 10.1016/s0006-8993(00)01957-0. [DOI] [PubMed] [Google Scholar]
- 64.Chinapen S, Swann JM, Steinman JL, Komisaruk BR. Expression of c-fos protein in lumbosacral spinal cord in response to vaginocervical stimulation in rats. Neurosci Lett. 1992;145:93–96. doi: 10.1016/0304-3940(92)90211-o. [DOI] [PubMed] [Google Scholar]
- 65.Lerant A, Herman ME, Freeman ME. Dopaminergic neurones of periventricular and arcuate nuclei of pseudopregnant rats: semicircadian rhythm in Fos-related antigens immunoreactivities and in dopamine concentration. Endocrinology. 1996;137:3621–3628. doi: 10.1210/endo.137.9.8756525. [DOI] [PubMed] [Google Scholar]
- 66.Coolen L, Wood R. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. J Comp Neurol. 1999;399:189–209. doi: 10.1002/(sici)1096-9861(19980921)399:2<189::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 67.Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- 68.Polston EK, Simerly RB. Sex-specific patterns of galanin, cholecystokinin, and substance P expression in neurones of the principal bed nucleus of the stria terminalis are differentially reflected within three efferent preoptic pathways in the juvenile rat. J Comp Neurol. 2003;465:551–9. doi: 10.1002/cne.10841. [DOI] [PubMed] [Google Scholar]
- 69.Polston EK, Gu G, Simerly RB. Neurons in the principal nucleus of the bed nuclei of the stria terminalis provide a sexually dimorphic GABAergic input to the anteroventral periventricular nucleus of the hypothalamus. Neuroscience. 2004;123:793–803. doi: 10.1016/j.neuroscience.2003.09.034. [DOI] [PubMed] [Google Scholar]