Abstract
Objective
Caregivers of patients with advanced cancer experience physical and emotional strain that can raise their own risk for morbidity and mortality. This analysis was performed to determine whether ENABLE II, a patient-focused palliative care intervention that increased patients’ quality of life, reduced symptom intensity, and lowered depressed mood compared to usual care, would affect caregiver burden.
Methods
Caregivers of patients with advanced cancer from the parent study completed a caregiver burden scale and patients completed quality of life, symptom intensity, and depressed mood measures. Data were collected at baseline, 1 month, and every 3 months thereafter until patient death or the study ended. Decedents’ caregivers were asked to complete an after death interview regarding the quality of care that the patient received.
Results
There were no significant differences in caregiver burden between intervention and usual care conditions. Follow-up analyses showed that higher caregiver objective burden and stress burden were related to lower patient quality of life, higher symptom intensity, and higher depressed mood. Caregivers who perceived that patients had unmet needs at end-of-life reported higher objective burden, and those who perceived that patients were not treated with respect reported higher demand burden.
Significance of results
The results indicate that a successful patient-focused intervention did not have a similar beneficial effect on caregiver burden. Future interventions should focus on caregivers as well as patients, with particular attention to caregivers’ perceptions of patient care, and seek to change both negative and positive effects of informal caregiving.
Keywords: caregiver burden, intervention, palliative care, cancer
The American Cancer Society estimates that 11.1 million Americans were living with cancer in 2005, and that 1.5 million new cases of cancer were diagnosed in 2009 (American Cancer Society, 2009). Because of the debilitating nature of advanced cancers and their treatment, many persons with advanced cancer require the assistance of an informal caregiver, defined as an unpaid individual who assists someone else who has functional impairment with activities of their daily living (Scott, 2006). Over 22 million Americans (with estimates running as high as 52 million) are involved in informal caregiving (Scott, 2006), and a nationally representative survey of older Americans revealed that end-of-life caregivers provide an average of 43 hours of assistance per week (Wolff, Dy, Frick, & Kasper, 2007). Unfortunately, there is substantial evidence documenting deleterious caregiver physical and mental health effects, often referred to as caregiver burden. As people live longer with cancer, the negative effects of caregiver burden will only continue to grow.
A meta-analysis of 84 caregiver burden studies across all types of chronic disease showed that caregivers exhibit higher levels of stress and depression, lower subjective well-being, and worse physical health compared to non-caregiver controls (Pinquart & Sörenson, 2003a). Caregiver burden has also been identified as an independent risk factor for mortality: controlling for demographics and co-morbid disease, caregivers were 63% more likely to die within a 4-year span than non-caregiver controls (Schulz & Beach, 1999). Although the negative effects of caregiving are most pronounced in caregivers of patients with dementia, informal caregivers of patients with cancer also report worse mental health (Ringdal et al., 2004; Braun, Mikulincer, Rydall, Walsh, & Rodin, 2007; Janda et al., 2007; Rhee et al., 2008). In addition, caring for someone with a slow-developing cancer, such as colon or lung cancer, has been shown to increase 9-year mortality rates versus caregivers of patients with ‘quick’ cancers (Elwert & Christakis, 2008). Even hospitalization for cancer may increase the risk of spouse mortality within 1 year (Christakis & Allison, 2006). The evidence seems clear that informal caregiving can exact a heavy toll.
Because of the significant effect of caregiving on health outcomes, various interventions to alleviate caregiver burden have been tested, but these have produced equivocal results (Harding & Higginson, 2003). A meta-analysis of 78 caregiver interventions across a wide array of chronic ailments found only marginal effects (0.14 < d < 0.41) (Sörenson, Pinquart, & Duberstein, 2002). A review of end-of-life palliative care studies concluded that the evidence for interventions improving outcomes for caregivers of patients with cancer was weak (Lorenz et al., 2008).
Psychoeducational interventions that teach caregivers about symptom management, self-care, and coordination of resources have been shown to improve caregivers’ physical health, distress, and depression relative to controls, but these effects appeared short-lived as group differences disappeared shortly after the interventions ceased (McCorkle, Robinson, Nuamah, Lev, & Benoliel, 1998; Jepson, McCorkle, Adler, Nuamah, & Lusk, 1999). Caregiver interventions designed to improve problem-solving and coping skills have been shown to improve physical and social functioning (Toseland, Blanchard, & McCallion, 1995). The benefits of both types of intervention, however, were most evident for those caregivers that entered the study with the highest impairment (Toseland et al., 1995; Jepson et al., 1999). One exception was a problem-solving and coping skills intervention that successfully improved caregiver quality of life and reduced caregiver burden at 30 days post-intervention compared to both a usual care condition and an emotional support intervention (McMillan et al., 2005). Unfortunately, this intervention failed to change the proposed mediators (e.g. problem-focused coping), leaving the mechanism behind this success unknown. In a disheartening statement, a review of interventions for caregivers of patients with cancer concluded that “no intervention can be recommended for nursing practice as an evidence-based strategy to reduce strain and burden in caregivers” (Honea et al., 2008)
One explanation for the ineffectiveness of earlier caregiver interventions is that these programs neglected the deteriorating health of caregivers’ loved ones (Hebert, Arnold, & Schulz, 2007). Cancer patients’ physical health (e.g. functional impairment, experience of pain) and quality of life have been found to predict caregiver burden, distress, depression, and quality of life (Miaskowski, Kragness, Dibble, & Wallhagen, 1997; Beach, Schulz, Yee, & Jackson, 2000; Fang, Manne, & Pape, 2001; Harding, Higginson, & Donaldson, 2003; Given et al., 2004). A meta-analysis showed that among couples in which one partner had cancer, each partner’s level of distress was significantly correlated (r = .29) and distress was not significantly different between partners (Hagedoorn, Sanderman, Bolks, Tuinstra, & Coyne, 2008). Additionally, a randomized controlled trial (RCT) of a caregiver intervention found that only a reduction in patient symptoms predicted decreases in caregiver depression (Kozachik et al., 2001). It was argued, therefore, that caregiver interventions will not be effective if the patient is suffering, and that patient-directed interventions might help caregivers indirectly by improving patient outcomes (Hebert et al., 2007). However, previous palliative care interventions for patients with cancer have failed to affect caregiver outcomes despite demonstrating improvements in patients’ quality of life (Clark et al., 2006). For example, a RCT of a psychoeducational intervention produced marked improvements in patient depression and symptom severity in the intervention condition but a non-significant trend that caregivers in the intervention showed higher depression scores than controls (Kurtz, Kurtz, Given, & Given, 2005). This finding indicates that certain interventions may inadvertently result in increased caregiver burden. Even a nursing intervention that increased longevity among patients with cancer only resulted in short-term benefits for caregiver stress and depression (Giarelli, Pisano, & McCorkle, 2000).
The ENABLE II (Educate, Nurture, Advise Before Life Ends) palliative care intervention provided a prime opportunity to determine if an effective patient-directed intervention could alleviate caregiver burden. ENABLE II was designed to improve problem-solving skills, symptom management, and communication skills, as well as promote advance care planning (e.g., advanced directives and “do not resuscitate” orders) among patients with advanced cancer. Importantly, participants were recently diagnosed with cancer and the intervention occurred concurrently with cancer treatment, such as chemotherapy and radiation, in an effort to provide elements of palliative care to patients before death was imminent (Bakitas et al., 2009a). In this RCT, the intervention proved effective in improving patients’ quality of life, reducing symptom intensity, and lowering depressed mood compared to a usual care control condition in the 7 months following study enrollment (Bakitas et al., 2009b). Although the intervention did not directly involve caregivers, we hypothesized that the intervention would improve caregiver outcomes indirectly through improvements in patient outcomes (Hebert et al., 2007).
Method
Sample
All procedures were approved by the Institutional Review Board of Dartmouth College. Patients in the parent study were recruited from an oncology clinic at a comprehensive cancer center in northern New England, affiliated outreach clinics, and an academically-affiliated Veterans Affairs Medical Center. Recruitment extended from November 2003 to May 2007. Eligible patients were at least 18 years old, had received a diagnosis of lung, breast, gastrointestinal, or genitourinary cancer within the past 12 weeks, and had a life expectancy of approximately 1 year. Patients were excluded if they were diagnosed with dementia/severe confusion, schizophrenia, bipolar disorder, or an active substance use disorder. Upon enrollment, patients were invited to identify someone close to them who was involved with their care (the caregiver) who could participate in the study. Patients were not excluded from the study if they failed to provide the name of a caregiver. Both patients and caregivers signed separate informed consents. Caregivers were asked to complete questionnaires on the same schedule as the patients, and caregivers of decedents were asked to participate in an interview to evaluate the quality of care patients received near the end of life.
Instruments
Caregiver burden
Caregivers completed the Montgomery Borgatta Caregiver Burden Scale (Montgomery, Borgatta, & Borgatta, 2000). The 14-item measure contained 3 subscales: objective burden (α = .81, n = 192), or perceived infringement on tangible aspects of life (e.g., time available for recreational activities); stress burden (α = .76, n = 191), or the emotional impact of caregiving (e.g., perceived anxiety); and demand burden (α = .90, n = 184), or the caregiver’s perceptions that the caregiving responsibilities are too demanding (e.g., unreasonable requests by the patient).
Quality of Care
The quality of care that patients received at end-of-life from their medical team was assessed using a revised version of the After Death Bereaved Family Member Interview (ADI) (Teno, Clarridge, Casey, Edgman-Levitan, & Fowler, 2001). Caregivers were asked to complete this 67-item measure approximately 4-to-6 months following death of the patient. The scale measured perceptions of the quality of care that the patient received in 8 domains: number of problems in unmet needs, coordination of care, emotional and spiritual support, shared decision making, match between care and preferences, problems with symptoms, support self-efficacy, and respectful treatment. Previous research has shown that close others’ reports of patients’ well-being and quality of care are reliable (McPherson & Addington-Hall, 2003).
Patient quality of life
Patients reported their quality of life by completing the Functional Assessment of Chronic Illness Therapy – Palliative Care (FACIT-Pal) (Brady & Cella, 1999). This 46-item scale assessed the patient’s physical, social, and emotional well-being at the end of life. Previous research demonstrated that the FACIT-Pal is reliable (α = .80, n = 189 in our sample) and has strong construct validity, being significantly related to symptom severity, depression, and longevity (Lyons et al., 2009).
Physical symptoms
Patients completed the Edmonton Symptom Assessment Scale (ESAS), a reliable (α = .80, n = 191 in our sample) and validated scale often used in palliative care research to measure severity of symptoms (Bruera, Kuehn, Miller, Selsmer, & Macmillan, 1991; Bruera, 1996). This 10-item measure asked respondents to rate their intensity of pain, activity, nausea, depression, anxiety, drowsiness, appetite, shortness of breath, and sensation of well-being.
Depressed mood
Patients completed the Center for Epidemiological Studies – Depression (CES-D) scale (Radloff, 1977). This 20-item scale asked respondents to rate the frequency of experiencing depressive symptoms. This scale is the most widely used measure of depressed mood in epidemiological studies, and extensive research has supported its reliability (α = .84, n = 185 in our sample) and validity (Plutchick & Conte, 1989).
Procedure
Upon enrollment, patients were randomly assigned to either the intervention or usual care condition. In the intervention, specialized nurse educators conducted 4 weekly telephone sessions on topics of problem-solving skills, communicating with health care providers, managing symptoms, and advance care planning. Caregivers were invited to participate in these educational sessions but were not required to do so. After these sessions ended, nurses called patients at least monthly to provide support and further information. Patients in the usual care condition received standard care (oncology and/or palliative) provided at the cancer center. Caregivers and patients were asked to complete questionnaires at baseline (T0), 1 month after baseline (T1), and follow-up questionnaires were mailed every 3 months until the study ended (December 2007) or the patient died. If the patient died during the study caregivers were also asked to complete the ADI. A detailed description of the intervention procedure is published elsewhere (Bakitas et al., 2009a; 2009b).
Statistical analysis
Longitudinal caregiver burden data were analyzed using mixed effects modeling for repeated measures. For these analyses, we adopted a factorial design of Time (T0, T1, T2, T3), Condition (Intervention vs. Control), and Patient Gender (Male, Female) with an unstructured covariance matrix. The contribution of each independent variable was tested as a main effect and in interaction with the other independent variables for each of the 3 burden subscales. Following these analyses, we conducted a series of correlation analyses that related measures of caregiver burden to measures of patient well-being (FACIT-Pal, ESAS, and CES-D) and after death reports by the caregiver of the quality of patient care. All analyses were conducted using SPSS version 17.0 (2008).
Results
Demographics
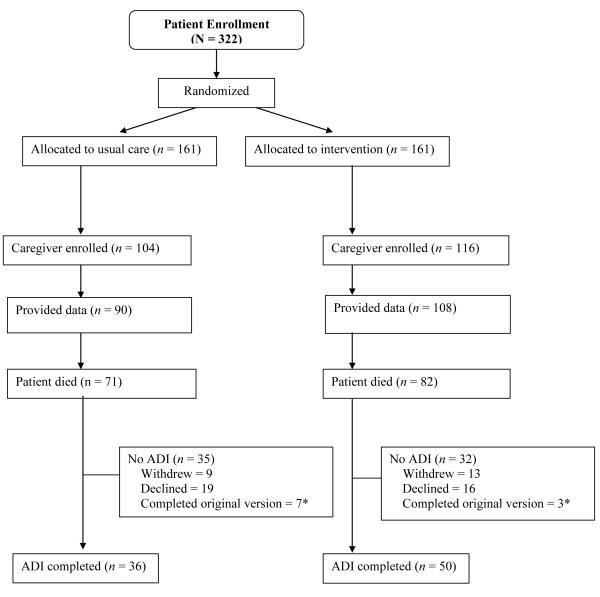
Two-hundred twenty participants from the parent study nominated a caregiver (68%). No caregiver declined enrollment, but only 198 (90%) provided any data (see Figure 1). The demographic characteristics of caregivers in the intervention and control groups (N = 198) appear in Table 1. The majority of caregivers in the study were Caucasian (96%), female (77%), and the spouse or partner of the patient (71%). Caregivers of patients assigned to the intervention condition were more highly educated than caregivers of patients assigned to the usual care condition (p < .05). The demographic characteristics of the subset of caregivers who completed the ADI also appear in Table 1 (N = 86).
Figure 1.
Caregiver enrollment diagram for caregiver burden measures and ADI.
*A revised ADI was instituted early in the study and data from these two versions could not be combined for analysis.
Table 1.
Demographic Characteristics of Caregiver Participants
| Mean ± SD or n (%) | ||||||
|---|---|---|---|---|---|---|
| Provided data | Completed ADI | |||||
| Usual Care (N = 90) |
Intervention (N = 108) |
P-value* | Usual Care (N = 36) |
Intervention (N = 50) |
P-value* | |
| Age | 59.9 ± 13.0 | 58.0 ± 11.9 | 0.28 | 62.7 ± 12.7 | 60.8 ± 10.6 | 0.48 |
| Gender | ||||||
| Male | 20 (22.2) | 25 (23.2) | 1.00 | 4 (11.1) | 11 (22.0) | 0.26 |
| Marital status | ||||||
| Never married | 7 (7.8) | 5 (4.6) | 0.53 | 2 (5.6) | 2 (4.0) | 1.00 |
| Married or living with partner | 72 (80.0) | 93 (86.1) | 28 (77.8) | 41 (82.0) | ||
| Divorced or Separated | 6 (6.7) | 4 (3.7) | 2 (5.6) | 2 (4.0) | ||
| Widowed | 2 (2.2) | 4 (3.7) | 1 (2.8) | 3 (6.0) | ||
| Missing | 3 (3.3) | 2 (1.9) | 3 (8.3) | 2 (4.0) | ||
| Education | ||||||
| Less than high school graduate | 10 (11.1) | 2 (1.9) | 0.02 | 4 (11.1) | 0 (0.0) | 0.052 |
| High school graduate | 52 (57.8) | 64 (59.3) | 15 (41.7) | 26 (52.0) | ||
| College graduate | 24 (26.7) | 38 (35.2) | 14 (38.9) | 22 (44.0) | ||
| Missing | 4 (4.4) | 4 (3.7) | 3 (8.3) | 2 (4.0) | ||
| Ethnicity | ||||||
| White | 86 (95.8) | 105 (97.2) | 1.00 | 32 (88.9) | 47 (94.0) | 1.00 |
| Other | 1 (1.1) | 1 (0.9) | 1 (2.8) | 1 (2.0) | ||
| Missing | 3 (3.3) | 2 (1.9) | 3 (8.3) | 2 (4.0) | ||
| Employment status | ||||||
| Employed | 39 (43.3) | 46 (42.6) | 0.38 | 13 (36.1) | 22 (44.0) | 0.84 |
| Retired | 31 (34.4) | 31 (28.7) | 14 (38.9) | 17 (34.0) | ||
| Not employed | 15 (16.7) | 26 (24.1) | 6 (16.7) | 9 (18.0) | ||
| Missing | 5 (5.6) | 5 (4.6) | 3 (8.3) | 2 (4.0) | ||
| Relationship to Patient | ||||||
| Spouse/partner | 67 (74.4) | 73 (67.6) | 0.56 | 26 (72.2) | 35 (70.0) | 1.00 |
| Friend | 4 (4.4) | 8 (7.4) | 2 (5.6) | 3 (6.0) | ||
| Child | 12 (13.3) | 20 (18.5) | 5 (13.9) | 8 (16.0) | ||
| Parent | 1 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Grandchild | 1 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Niece/nephew | 0 (0.0) | 1 (0.9) | 0 (0.0) | 1 (2.0) | ||
| Other | 4 (4.4) | 4 (3.7) | 3 (8.3) | 3 (6.0) | ||
| Missing | 1 (1.1) | 2 (1.9) | 0 (0.0) | 0 (0.0) | ||
| Primary disease site of Patient | ||||||
| Gastrointestinal | 39 (43.3) | 45 (41.7) | 0.50 | 14 (38.9) | 19 (38.0) | 1.00 |
| Lung | 36 (40.0) | 36 (33.3) | 14 (38.9) | 18 (36.0) | ||
| Genitourinary | 10 (11.1) | 16 (14.8) | 6 (16.7) | 9 (18.0) | ||
| Breast | 5 (5.6) | 11 (10.2) | 2 (5.6) | 4 (8.0) | ||
Note. P-values from Fisher exact test for categorical variables and t-test for continuous variables.
Longitudinal analyses of caregiver burden
There were no significant main effects or interactions for Time, Condition, or Patient Gender for any of the measures of caregiver burden (all ps > .05).
Caregiver burden and patient well-being
To further explore the relationship between patient status and caregiver burden, correlation analyses were conducted relating caregiver burden to patient’s self-reported well-being. Listwise deletion of missing data was adopted so that all correlations would be based on the same participants (n = 118). Because follow-up measures at T2 and T3 suffered considerable attrition, results for these waves are not reported.
As seen in Table 2, measures of patient well-being were highly correlated both within and across measurement periods: quality of life (FACIT-Pal) was negatively correlated with symptom intensity (ESAS) and depressed mood (CES-D), whereas the latter 2 variables were positively correlated. In contrast, caregiver burden measures were less consistently and less highly correlated. Although objective burden was positively correlated with stress burden it was not related to demand burden. In essence, caregivers who perceived objective sources of burden reported stress, but did not feel that the patient was inappropriately demanding. On the other hand, demand burden was positively related to stress burden: caregivers who perceived the patient was unduly demanding reported higher stress.
Table 2.
Correlation Matrix of Caregiver Burden and Patient Quality of Life, Symptom Intensity, and Depressed Mood
| OB0 | OB1 | SB0 | SB1 | DB0 | DB1 | FP0 | FP1 | ES0 | ES1 | CD0 | CD1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OB0 | 1.00 | |||||||||||
| OB1 | .79** | 1.00 | ||||||||||
| SB0 | .23* | .16 | 1.00 | |||||||||
| SB1 | .49** | .51** | .46** | 1.00 | ||||||||
| DB0 | .08 | .05 | .37** | .22* | 1.00 | |||||||
| DB1 | .07 | .10 | .09 | .33** | .28** | 1.00 | ||||||
| FP0 | −.24** | −.28** | −.22* | −.23* | −.10 | −.08 | 1.00 | |||||
| FP1 | −.15 | −.22* | −.11 | −.19* | .07 | .04 | .78** | 1.00 | ||||
| ES0 | .19* | .30** | .24** | .22* | −.04 | .04 | −.67** | −.54** | 1.00 | |||
| ES1 | .09 | .24** | .07 | .15 | −.13 | −.06 | −.48** | −.73** | .56** | 1.00 | ||
| CD0 | .11 | .17 | .14 | .12 | .01 | .02 | −.72** | −.65** | .63** | .46** | 1.00 | |
| CD1 | .16 | .24** | .03 | .14 | −.06 | .08 | −.61** | −.81** | .50** | .76** | .65**1.00 | 1.00 |
Note. Listwise N = 118.
p < .05 (two-tailed);
p < .01 (two-tailed).
OB = Objective Burden, SB = Stress Burden, DB = Demand Burden, FP = FACIT-Pal, ES = ESAS, CD = CES-D. 0 = Time 0 (baseline); 1 = Time 1 (one month follow-up).
With respect to relations between caregiver reports and patient reports, Table 2 shows that lower patient quality of life (FACIT-Pal) was related to higher caregiver reports of both objective burden and stress burden. Similarly, higher patient symptom intensity (ESAS) and depressed mood (CES-D) were related to higher caregiver reports of objective burden and stress burden, although in the case of depressed mood this relation was true only at T1. Demand burden was unrelated to patient reports of well-being. In general, then, caregivers reported more infringement on their lives and higher stress when the patient reported lower well-being, more intense symptoms, and greater depressed mood.
The same correlation analyses were conducted separately for male and female patients. These analyses showed similar patterns as those observed in Table 2, although because of the smaller number of observations in each category, statistical significance was diminished. Analyses that separated patients into those in the intervention and control conditions showed similar, albeit less statistically significant patterns.
Caregiver burden, patient well-being, and quality of care
Each of the 3 measures of patient well-being (FACIT-Pal, ESAS, CES-D) and 3 subscales of caregiver burden at both T0 and T1 were correlated with 8 measures of the quality of patients’ end-of-life care, as reported by the caregiver after the patient’s death. Due to missing data, pairwise deletion was adopted for all analyses. Out of 96 correlations, 5 achieved statistical significance. At T0, more problems in patient emotional and spiritual support were associated with decreased patient well-being, r(N = 37) = −.49, p < .01, and decreased caregiver stress burden, r(N = 41) = −.33, p < .05. In addition, more problems with symptoms at T0 were associated with decreased patient well-being, r(N = 35) = −.45, p < .01. At T1, more problems with unmet patient needs were associated with increased stress burden, r(N = 74) = .24, p < .05, and more problems with respectful treatment of the patient were associated with increased demand burden, r(N = 73) = .23, p < .05.
Discussion
Despite implementing an effective palliative care intervention for patients with advanced cancer (ENABLE II) (Bakitas et al., 2009b), we found no evidence of improvements in caregiver burden in the intervention condition compared to usual care. To further understand how patient status was related to caregiver burden, we found that patient quality of life (FACIT-PAL), symptom intensity (ESAS), and, to a lesser extent, depressed mood (CES-D), were positively related to objective burden and stress burden. As expected, caregivers expressed more tangible limitations and higher stress associated with caregiving when patients reported lower quality of life and more severe symptoms (Miaskowski et al., 1997; Beach et al., 2000; Fang et al., 2001; Harding et al., 2003; Given et al., 2004). Despite ENABLE II improving patient quality of life, symptom intensity, and depressed mood, these benefits did not appear to produce commensurate changes in any facet of caregiver burden. These findings are similar to other effective palliative care interventions for patients with cancer that failed to show improvements in caregiver outcomes (Giarelli et al., 2000; Kurtz et al., 2005; Clark et al., 2006).
Given the dearth of significant findings in earlier caregiver interventions, these null results are not surprising, albeit disappointing. Such findings challenge the notion that interventions focused primarily on reducing patient suffering can improve caregiver outcomes (Hebert et al., 2007). These results suggest that the relation between patient status and caregiver burden is more complex than previously considered. First, it has been suggested that the association between patient status and caregiver burden is bidirectional (Nijboer et al., 1998; Fang et al., 2001). Because many patients with cancer require daily assistance (Wolff et al., 2007) an overburdened caregiver will likely provide insufficient help to the patient due to their own limitations, leading to further decrements in patient quality of life. This decline may, in turn, increase caregiver burden (Nijboer et al., 1998). In addition, the majority of caregivers are patients’ partners (Wolff et al., 2007), many of whom are experiencing their own physical limitations and medical problems prior to their partner’s cancer diagnosis, which can exacerbate the ill effects of caregiving (Jepson et al., 1999; Giarelli et al., 2000). Second, because caregivers are often caring for end-of-life patients, they may fail to perceive the benefits of a patient-centered intervention. What researchers define as patient ‘improvements’ are often compared to a control group, and at an individual level may actually be stabilization or a slower decline in health. Although interventions that produce these effects are important for patients, these differences may be lost on caregivers who cannot see patient status at the group level.
Further analyses involving caregivers’ after death reports of patients’ care help explain factors in palliative care associated with caregiver burden. Patient’s unmet needs at end-of-life were associated with higher caregiver stress burden and problems with respectful treatment toward the patient were associated with higher caregiver demand burden. These results suggest that practitioners’ conduct toward patients may influence caregiver burden, and both results highlight areas that future palliative care interventions should address. Improvements in patient care may better serve both patients with cancer and their caregivers.
Another facet of caregiving that is often ignored in research is the perceived benefits of caregiving (Nijboer et al., 1998). In a national survey, end-of-life caregivers reported significant physical, emotional, and financial strains associated with caregiving, but over 60% also indicated that caregiving is a rewarding experience (Wolff et al., 2007). In fact, increased help provided to one’s spouse has been related to decreases in anxiety and depression (Beach et al., 2000). Additional research has shown that caregiver interventions can increase ‘uplifts’ associated with caregiving (d = .15) (Sörenson et al., 2002), and a meta-analysis of 228 studies showed that these ‘uplifts’ are negatively associated with caregiver burden and depression (rs = −.16 & −.17, respectively) (Pinquart & Sörenson, 2003b). These results suggest that by conceptualizing the health effects of caregiving as a bi-dimensional construct, rather than a unipolar scale ranging from ‘bad’ to ‘worse,’ researchers may better understand why multiple well-designed interventions have failed to affect caregiver burden and how to improve those processes in the future. Future research should measure both the negative and positive effects of caregiving, and interventions should simultaneously attempt to alleviate the bad while accentuating the good (Hudson, Aranda, & Hayman-White, 2005).
Several limitations to our study must be noted. First, patients in the study were not required to provide the name of a caregiver. This recruitment procedure may have created a selection bias in which caregivers in the study were somehow different from those caregivers potentially omitted. Second, caregivers were provided the opportunity to be involved with patients’ intervention sessions, but were not required to do so; therefore, the experience of caregivers in the intervention condition likely varied by their level of involvement. Third, the majority of caregivers were White females from rural New England, which calls into question the generalizability of these findings. Fourth, participant attrition limited our ability to study the effects of the intervention on caregiver outcomes past 4 months. Finally, caregivers’ after death reports in the current study focused solely on patients’ quality of care, not on their own experiences. Although no significant effect of the intervention on caregiver burden was found, these caregivers may have shown faster improvements in mental health in the bereavement phase (Braun et al., 2007).
The current study provides evidence that reducing caregiver burden requires more than just improving patient outcomes. Our results suggest that caregivers, along with patients, require specialized interventions to ease the physical and emotional strains that come with caregiving. In particular, future interventions should investigate treating caregivers and patients as a dyad whose health outcomes are inextricably linked. Such interventions will require well-designed RCTs to determine their feasibility and effectiveness in a palliative care setting. Future work should consider the pre-existing limitations of older caregivers (Jepson et al., 1999) and measure caregiver outcomes for longer durations in order to determine whether the intervention results in delayed positive effects (Kurtz et al., 2005). In addition, aside from the obvious patient benefits that result when the patient’s needs are met and the patient is treated with respect, such care may mitigate undue burden for caregivers. Such steps should help develop evidence-based programs to improve the health of those helping others cope with cancer.
Acknowledgments
This study was supported by National Cancer Institute grant R01 CA101704. We thank the study participants and oncology clinicians from the Section of Hematology/Oncology, outreach clinics, and VA Medical Center for their cooperation with the conduct of this study (all without payment). We also thank Ira Byock, MD, Director and the Palliative Care Team, Section of Palliative Medicine; Daphne Ellis, AS, Julie Wolf, RN, Luann E. Graves, BS, MT, CCRP, and Linda Eickhoff, MS, who provided diligent recruitment, data collection, and data management as funded research coordinators; Kathleen Barnett, M.A., APRN and Elizabeth McKinstry, M.S., R.N. who delivered the intervention; and Tor Tosteson, ScD, for consultation with statistical analyses.
References
- American Cancer Society [Retrieved January 4, 2010];Cancer Facts & Figures. 2009 from http://www.cancer.org/downloads/STT/500809web.pdf.
- Bakitas M, Lyons KD, Hegel MT, Balan S, Barnett K, Brokaw FC, Byock I, Hull J, Li Z, McKinstry E, Seville J, Ahles TA. The project ENABLE II randomized controlled trial to improve palliative care for rural patients with advanced cancer: Baseline findings, methodological challenges, and solutions. Palliative and Supportive Care. 2009a;7:75–86. doi: 10.1017/S1478951509000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakitas M, Lyons KD, Hegel MT, Balan S, Brokaw FC, Seville J, Hull JG, Li Z, Tosteson T, Byock IR, Ahles TA. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer. Journal of the American Medical Association. 2009b;302:741–749. doi: 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach SR, Schulz R, Yee JL, Jackson S. Negative and positive health effects of caring for a disabled spouse: Longitudinal findings from the caregiver health effects study. Psychology and Aging. 2000;15:259–271. doi: 10.1037//0882-7974.15.2.259. [DOI] [PubMed] [Google Scholar]
- Brady MJ, Cella D. Assessing quality of life in palliative care. Cancer Treatment and Research. 1999;100:203–216. doi: 10.1007/978-1-4615-5003-7_11. [DOI] [PubMed] [Google Scholar]
- Braun M, Mikulincer M, Rydall A, Walsh A, Rodin G. Hidden morbidity in cancer: Spouse caregivers. Journal of Clinical Oncology. 2007;25:4829–4834. doi: 10.1200/JCO.2006.10.0909. [DOI] [PubMed] [Google Scholar]
- Bruera E. Patient assessment in palliative cancer care. Cancer Treatment Reviews. 1996;22(Supplement A):3–12. doi: 10.1016/s0305-7372(96)90058-4. [DOI] [PubMed] [Google Scholar]
- Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. Journal of Palliative Care. 1991;7:6–9. [PubMed] [Google Scholar]
- Christakis NA, Allison PD. Mortality after the hospitalization of a spouse. New England Journal of Medicine. 2006;354:719–730. doi: 10.1056/NEJMsa050196. [DOI] [PubMed] [Google Scholar]
- Clark MM, Rummans TA, Sloan JA, Jensen A, Atherton PJ, Frost MH, Richardson JW, Bostwick JM, Johnson ME, Hanson JM, Brown PD. Quality of life of caregivers of patients with advanced-stage cancer. American Journal of Hospice and Palliative Medicine. 2006;23:185–191. doi: 10.1177/1049909106289074. [DOI] [PubMed] [Google Scholar]
- Elwert F, Christakis NA. The effect of widowhood on mortality by the causes of death of both spouses. American Journal of Public Health. 2008;98:2092–2098. doi: 10.2105/AJPH.2007.114348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang CY, Manne SL, Pape SJ. Functional impairment, marital quality, and patient psychological distress as predictors of psychological distress among cancer patients’ spouses. Health Psychology. 2001;20:452–457. [PubMed] [Google Scholar]
- Giarelli E, Pisano R, McCorkle R. Stable & able. American Journal of Nursing. 2000;100:26–32. [PubMed] [Google Scholar]
- Given B, Wyatt G, Given C, Sherwood P, Gift A, DeVoss D, Rahbar M. Burden and depression among caregivers of patients with cancer at the end of life. Oncology Nursing Forum. 2004;31:1105–1115. doi: 10.1188/04.ONF.1105-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedoorn M, Sanderman R, Bolks HN, Tuinstra J, Coyne JC. Distress in couples coping with cancer: A meta-analysis and critical review of role and gender effects. Psychological Bulletin. 2008;134:1–30. doi: 10.1037/0033-2909.134.1.1. [DOI] [PubMed] [Google Scholar]
- Harding R, Higginson IJ. What is the best way to help caregivers in cancer and palliative care? A systematic literature review of interventions and their effectiveness. Palliative Medicine. 2003;17:63–74. doi: 10.1191/0269216303pm667oa. [DOI] [PubMed] [Google Scholar]
- Harding R, Higginson IJ, Donaldson N. The relationship between patient characteristics and carer psychological status in home palliative cancer care. Supportive Care in Cancer. 2003;11:638–643. doi: 10.1007/s00520-003-0500-6. [DOI] [PubMed] [Google Scholar]
- Hebert RS, Arnold RM, Schulz R. Improving well-being in caregivers of terminally ill patients: Making the case for patient suffering as a focus of intervention research. Journal of Pain and Symptom Management. 2007;34:539–546. doi: 10.1016/j.jpainsymman.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea NJ, Brintnall R, Given B, Sherwood P, Colao DB, Somers SC, Northouse LL. Putting evidence into practice: Nursing assessment and interventions to reduce family caregiver strain and burden. Clinical Journal of Oncology Nursing. 2008;12:507–516. doi: 10.1188/08.CJON.507-516. [DOI] [PubMed] [Google Scholar]
- Hudson PL, Aranda S, Hayman-White K. A psycho-educational intervention for family caregivers of patients receiving palliative care: A randomized control trial. Journal of Pain and Symptom Management. 2005;30:329–341. doi: 10.1016/j.jpainsymman.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Janda M, Seginga S, Langbecker D, Dunn J, Walker D, Eakin E. Quality of life among patients with a brain tumor and their carers. Journal of Psychosomatic Research. 2007;63:617–623. doi: 10.1016/j.jpsychores.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Jepson C, McCorkle R, Adler D, Nuamah I, Lusk E. Effects of home care on caregivers’ psychosocial status. Journal of Nursing Scholarship. 1999;31:115–120. doi: 10.1111/j.1547-5069.1999.tb00444.x. [DOI] [PubMed] [Google Scholar]
- Kozachik SL, Given CW, Given BA, Pierce SJ, Azzouz F, Rawl SM, Champion VL. Improving depressive symptoms among caregivers of patients with cancer: Results of a randomized clinical trial. Oncology Nursing Forum. 2001;28:1149–1157. [PubMed] [Google Scholar]
- Kurtz ME, Kurtz JC, Given CW, Given B. A randomized, controlled trial of a patient/caregiver symptom control intervention: Effects of depressive symptomatology of caregivers of cancer patients. Journal of Pain and Symptom Management. 2005;30:112–122. doi: 10.1016/j.jpainsymman.2005.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz KA, Lynn J, Dy SM, Shugarman LR, Wilkinson A, Mularski RA, Morton SC, Hughes RG, Hilton LK, Maglione M, Rhodes SL, Rolon C, Sun VC, Shekelle PG. Evidence for improving palliative care at the end of life: A systematic review. Annals of Internal Medicine. 2008;148:147–161. doi: 10.7326/0003-4819-148-2-200801150-00010. [DOI] [PubMed] [Google Scholar]
- Lyons KD, Bakitas M, Hegel MT, Hanscom B, Hull J, Ahles TA. Reliability and validity of the Functional Assessment of Chronic Illness Therapy – Palliative Care (FACIT-Pal) scale. Journal of Pain and Symptom Management. 2009;37:23–32. doi: 10.1016/j.jpainsymman.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCorkle R, Robinson L, Nuamah I, Lev E, Benoliel JQ. The effects of home nursing care for patients during terminal illness on the bereaved’s psychological distress. Nursing Research. 1998;47:2–10. doi: 10.1097/00006199-199801000-00002. [DOI] [PubMed] [Google Scholar]
- McMillan SC, Small BJ, Weitzner M, Schonwetter R, Tittle M, Moody L, Haley WE. Impact of coping skills intervention with family caregivers of hospice patients with cancer. Cancer. 2005;106:214–222. doi: 10.1002/cncr.21567. [DOI] [PubMed] [Google Scholar]
- McPherson CJ, Addington-Hall JM. Judging the quality of care at the end of life: Can proxies provide reliable information? Social Science & Medicine. 2003;56:95–109. doi: 10.1016/s0277-9536(02)00011-4. [DOI] [PubMed] [Google Scholar]
- Miaskowski C, Kragness L, Dibble S, Wallhagen M. Differences in mood states, health status, and caregiver strain between family caregivers of oncology outpatients with and without cancer-related pain. Journal of Pain and Symptom Management. 1997;13:138–147. doi: 10.1016/s0885-3924(96)00297-7. [DOI] [PubMed] [Google Scholar]
- Montgomery RJV, Borgatta EF, Borgatta ML. Societal and family change in the burden of care. In: Liu WT, Kendig H, editors. Who Should Care for the Elderly? Singapore University Press; Singapore: 2000. pp. 27–54. [Google Scholar]
- Nijboer C, Tempelaar R, Sanderman R, Triemstra M, Spruijt RJ, van den Bos GAM. Cancer and caregiving: The impact on the caregiver’s health. Psycho-Oncology. 1998;7:3–13. doi: 10.1002/(SICI)1099-1611(199801/02)7:1<3::AID-PON320>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Pinquart M, Sörenson S. Differences between caregivers and noncaregivers in psychological health and physical health: A meta-analysis. Psychology and Aging. 2003a;18:250–267. doi: 10.1037/0882-7974.18.2.250. [DOI] [PubMed] [Google Scholar]
- Pinquart M, Sörenson S. Associations of stressors and uplifts of caregiving with caregiver burden and depressive mood: A meta-analysis. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2003b;58:112–128. doi: 10.1093/geronb/58.2.p112. [DOI] [PubMed] [Google Scholar]
- Plutchick R, Conte HR. Self-report scales for the measurement of depression. Psychiatric Annals. 1989;19:367–371. [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–392. [Google Scholar]
- Rhee YS, Yun YH, Park S, Shin DO, Lee KM, Yoo HJ, Kim JH, Kim SO, Lee R, Lee YO, Kim NS. Depression in family caregivers of cancer patients: The feeling of burden as a predictor of depression. Journal of Clinical Oncology. 2008;26:5890–5895. doi: 10.1200/JCO.2007.15.3957. [DOI] [PubMed] [Google Scholar]
- Ringdal GI, Ringdal K, Jordhøy MS, Ahlner-Elmqvist M, Jannert M, Kaasa S. Health-related quality of life (HRQOL) in family members of cancer victims: Results from a longitudinal intervention study in Norway and Sweden. Palliative Medicine. 2004;18:108–120. doi: 10.1191/0269216304pm878oa. [DOI] [PubMed] [Google Scholar]
- Schulz R, Beach SR. Caregiving as a risk factor for mortality: The caregiver health effects study. Journal of the American Medical Association. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- Scott JA. Informal caregiving. Paper presented at the Blaine House Conference on Aging; September, 2006; [Retrieved January 4, 2009]. 2006. from http://www.umaine.edu/mainecenteronaging/documents/issuebriefinformalcaregiving.pdf. [Google Scholar]
- Sörenson S, Pinquart M, Duberstein P. How effective are interventions with caregivers? An updated meta-analysis. Gerontologist. 2002;42:356–372. doi: 10.1093/geront/42.3.356. [DOI] [PubMed] [Google Scholar]
- SPSS Statistics (Version 17.0) SPSS Inc.; Chicago: 2008. [Google Scholar]
- Teno JM, Clarridge B, Casey V, Edgman-Levitan S, Fowler J. Validation of toolkit after-death bereaved family member interview. Journal of Pain and Symptom Management. 2001;22:752–758. doi: 10.1016/s0885-3924(01)00331-1. [DOI] [PubMed] [Google Scholar]
- Toseland RW, Blanchard CG, McCallion P. A problem solving intervention for caregivers of cancer patients. Social Science & Medicine. 1995;40:517–528. doi: 10.1016/0277-9536(94)e0093-8. [DOI] [PubMed] [Google Scholar]
- Wolff JL, Dy SM, Frick KD, Kasper JD. End-of-life care: Findings from a national survey of informal caregivers. Archives of Internal Medicine. 2007;167:40–46. doi: 10.1001/archinte.167.1.40. [DOI] [PubMed] [Google Scholar]