Abstract
Purpose
A range of impulse control disorders (ICDs) are reported to occur in Parkinson’s disease (PD). However, alterations in brain activity at rest and during risk taking occurring with ICDs in PD are not well understood.
Methods
We used both arterial spin labeling (ASL) perfusion fMRI to directly quantify resting cerebral blood flow (CBF) and blood oxygenation level dependent (BOLD) fMRI to measure neural responses to risk taking during performance on the Balloon Analogue Risk Task (BART).
Results
18 PD patients, either with a diagnosis of one or more ICDs (N=9) or no lifetime ICD history (N=9), participated. BOLD fMRI data demonstrated that PD patients without an ICD activate the mesocorticolimbic pathway during risk taking. Compared with non-ICD patients, ICD patients demonstrated significantly diminished BOLD activity in the right ventral striatum during risk taking and significantly reduced resting CBF in the right ventral striatum.
Conclusion
ICDs in PD are associated with reduced right ventral striatal activity at rest and diminished striatal activation during risk taking, suggesting that a common neural mechanism may underlie ICDs in individuals with PD and those without PD. Thus, treatments for ICDs in non-PD patients warrant consideration in PD patients with ICDs.
INTRODUCTION
Individuals with Parkinson’s disease (PD) may experience impulse control disorders (ICDs), including compulsive gambling, buying, sexual behavior, and eating.1–4 As defined in the current Diagnostic and Statistical Manual (DSM-IV-TR),5 ICDs constitute a broad range of psychiatric disorders, and an essential feature of ICDs is a failure to resist an impulse, drive, or temptation to perform an act that is harmful to the person or to others. ICDs, sometimes called “behavioral addictions”,6 occur in the context of PD, but also in other disorders (e.g., restless legs syndrome (RLS)7 and fibromyalgia) for which dopamine replacement therapies are used.8
Human brain imaging and lesions studies have demonstrated an important role for the mesocorticolimbic network in the processing of risk-reward decision-making and impulse control.9;10 Recent functional magnetic resonance imaging (fMRI) studies reported diminished ventral striatum and ventromedial prefrontal activation in non-PD pathological gamblers during simulated gambling11, a task involving response inhibition,12 and following gambling cue exposure.13
Although ICDs are observed in PD,1;2 functional brain differences in PD patients with and without ICDs in this population remain poorly understood. It is unclear if ICDs in PD, a disease characterized by degeneration of dopaminergic substantia nigra-striatum pathways, demonstrate similar neural deficits in the mesolimbic “reward” system as reported for ICDs in the general population. To investigate ICDs in PD, we used conventional blood oxygenation level dependent (BOLD) fMRI and measured neural responses underlying risk taking in PD patients with and without ICDs during the performance of a modified Balloon Analogue Risk Task (BART).14;15 Behavioral studies using the BART support it as an ecologically valid model for the assessment of risk-taking propensity and behavior,14;16;17 and a brain imaging study has demonstrated that risk taking during the BART induced robust activation in the mesocorticolimbic “reward” network in healthy controls.15 In addition, a recent study involving PD patients used the BART to assess risk-taking behavior outside of the scanner and found that dopamine agonists (DAs) significantly strengthened the correlations between risk-taking performance on the BART and regional brain activation in the orbitofrontal cortex during administration of a probabilistic reward task.18
Since BOLD fMRI measures relative task-induced signal changes and lacks absolute quantification of neural activity, we also used arterial spin labeling (ASL) perfusion fMRI19;20 in order to investigate resting neural activity. Using magnetically labeled water in arterial blood as a diffusible tracer, ASL perfusion fMRI provides a non-invasive imaging method of quantifying cerebral blood flow (CBF), a biomarker of regional brain function.21 ASL fMRI has been used successfully to characterize resting perfusion changes in neurodegenerative disease disorders, including Alzheimer’s disease.22
Based on previous findings from fMRI studies of pathological gamblers in the general population,11;12 we hypothesized that PD patients with ICDs, compared with PD patients without an ICD, would demonstrate diminished neural activity in mesolimbic-prefrontal cortex brain regions, particularly in the ventral striatum. We also hypothesized that ICD patients would demonstrate increased risk-taking behavior, as measured by an increased average adjusted number of inflations per balloon on the BART, compared with non-ICD patients.
SUBJECTS and METHODS
Subjects
Eighteen PD patients (age range 40–75 years, male/female =15/3) participated in the study. Nine patients were diagnosed as having one or more active ICDs at the time of assessment and imaging (ICD group), and 9 had no lifetime ICD history (non-ICD group). The specific ICDs are presented in Table 1. Based on clinical interview, all ICD patients had no lifetime history of an ICD prior to initiating DA treatment in the context of PD. All subjects in the ICD group and 8 subjects in the non-ICD group were taking a DA at the time of imaging, and 8 of the subjects in each group were taking levodopa. ICD status was ascertained by the Massachusetts Gambling Screen23 for problem or pathological gambling, the Minnesota Impulsive Disorder Interview24 for compulsive sexual behavior and buying, and DSM-IV-TR research criteria for binge-eating disorder,5 and dopamine dysregulation syndrome (DDS) was ruled out in all subjects on the basis of proposed criteria.25 The mean (SD) duration of ICD behaviors was 4.5 (3.2) years.
Table 1.
Demographic and clinical characteristics of subjects
| Variablesa | ICD Group (N=9) | Non-ICD Group (N=9) | P value |
|---|---|---|---|
| ICD Type | Eating only (N=2) Gambling only (N=1) Sexual behavior only (N=1) Buying only (N=1) Gambling and sexual behavior (N=1) Buying and eating (N=1) Eating, sexual behavior, and gambling (N=1) All four ICDs (N=1) |
- | - |
| Sex (% male) | 77.8 | 88.9 | 0.53 |
| Age (years) | 56.2 (10.7) | 54.4 (9.6) | 0.72 |
| Education (years) | 17.2 (2.9) | 16.0 (2.6) | 0.36 |
| PD Duration (years) | 7.2 (4.4) | 7.6 (6.6) | 0.87 |
| Hoehn & Yahr stage | 2.1 (0.3) | 2.1 (0.2) | >0.99 |
| Dopamine replacement therapy | |||
| - Levodopa LEDDb | 418 (306) | 309 (171) | .36 |
| - DA LEDD | 278 (116) | 319 (187) | .58 |
| - Total LEDD | 696 (399) | 628 (313) | .70 |
| GDS-15 score | 5.4 (3.2) | 2.9 (3.3) | 0.12 |
| MoCA score | 27.1 (1.8) | 27.0 (2.5)c | 0.78 |
Percentage, mean (SD), or median values
LEDD = levodopa equivalent daily dosage
N=8
Mean (SD) values for demographic and clinical variables for the entire sample were: age =55.3 (9.9) years, formal education =16.6 (2.7) years, PD duration =7.4 (5.4) years, Hoehn & Yahr stage26 of PD severity =2.1 (0.3), DA levodopa equivalent daily dosage (LEDD)27 =299 (152) mg, levodopa LEDD =363 (247) mg, 15-item Geriatric Depression Scale (GDS-15)28 score =4.2 (3.4), and Montreal Cognitive Assessment (MoCA)29 score =27.1 (2.1). There were no differences between the ICD group and the non-ICD group on any of these variables (Table 1).
The study was approved by the Institutional Review Board of the University of Pennsylvania, and subjects provided written informed consent prior to study participation. Each participant was compensated $35 for study participation.
Experimental protocol and procedures
The fMRI BART paradigm has been described elsewhere.15 In summary, participants completed a modified version of the original BART.14 Participants pressed a button to sequentially inflate (i.e., “pump”) a virtual balloon presented on a laptop computer screen, which either grows larger or explodes with each inflation. The maximum number of inflations prior to explosion per balloon was 12. Larger balloons were associated with greater risk of explosion, as well as higher virtual monetary reward/loss (i.e., exponential increases). The probability of explosion was set to increase nonlinearly from 0 to 89.6%, and the wager nonlinearly increased from $0 to $5.15. Participants were told that they should try to maximize their virtual monetary reward during completion of the task. After electively terminating a trial (“win outcome”) or explosion of a balloon (“loss outcome”), the next balloon presented itself at the smallest balloon size and lowest wager amount.
The timing of inflation during the BART was controlled by a cue, with a jittered time interval of 1.5–2.5 seconds between inflations within a trial, and 2–4 seconds between trials. The number of balloons participants completed during the scan was not pre-determined, but depended on the response speed of subjects.
Imaging data acquisition and analysis
Functional imaging was conducted on a Siemens 3.0T Trio whole-body scanner (Siemens AG, Erlangen, Germany), using a product 8-channel array coil. Before the scan, participants had the opportunity to practice the task to ensure that they could perform it correctly in the scanner.
First, a modified pseudo-continuous ASL perfusion sequence30 was used for the resting state scan. Participants were instructed that there was no specific task and to be in a relaxed awake state during this portion of the scanning process. Interleaved images with and without labeling were acquired using a gradient echo-planar imaging (EPI) sequence. The tagging/control duration was 2s. A delay of 1s was inserted between the end of the labeling pulse and image acquisition to reduce transit artifact. Acquisition parameters consisted of the following: FOV =22cm, matrix =64×64, TR =4s, TE =17ms, flip angle =90°. Sixteen axial slices (6mm thickness with 2mm gap) were acquired from inferior to superior in sequential order. The resting perfusion scanning protocol lasted 4 minutes and consisted of 60 acquisitions. Three patients (1 ICD and 2 non-ICD) did not finish the perfusion scan due to MR technical problems; hence the perfusion data consisted of 15 patients (ICD group =8, non-ICD group =7).
After the perfusion scan, BOLD imaging data were acquired using a standard EPI sequence (TR =3000ms, TE =24ms, flip angle =90°, 50 interleaved axial slices with 3mm thickness, in-plane resolution =3.44mm × 3.44mm) while subjects completed the modified BART. Each participant completed an 8-minute BOLD scan. After the functional scans were completed, high-resolution T1-weighted anatomic images were obtained using 3D MPRAGE (TR =1620ms, TI =950ms, TE =3ms, flip angle =15°, 160 contiguous slices of 1.0mm thickness, in-plane resolution =1mm × 1mm).
Imaging Data Analysis
Functional imaging data processing and analyses were conducted using Statistical Parametric Mapping software (SPM2, Wellcome Department of Cognitive Neurology, UK, implemented in Matlab 6.5, Math Works, Natick, MA), with some additional modifications for perfusion analysis (http://cfn.upenn.edu/perfusion/software.htm).
For the perfusion data, images of each subject were realigned to correct head motion, co-registered with the anatomical image, and smoothed in space with a three-dimensional, 10mm FWHM (Full Width at Half Maximum) Gaussian kernel. Perfusion weighted image series were then generated by pair-wise subtraction of the label and control images, followed by conversion to absolute CBF image series based on a single compartment continuous arterial spin labeling (CASL) perfusion model.31 For each subject, one mean resting CBF image was generated, normalized to the Montreal Neurological Institute (MNI) template, and then entered into the whole brain voxel-wise analysis using a general linear model (GLM). The GLM analysis used a two-sample t-test to compare the resting CBF in the ICD and non-ICD groups. The threshold was set as uncorrected P<0.001 at voxel, and whole brain corrected P<0.05 for cluster. This threshold identified a cluster in the right ventral striatum, and regional of interest (ROI) analysis were carried out in this cluster by calculating quantitative regional CBF values in this region and comparing the CBF values between the two groups after adjusting for global CBF differences.
For BOLD data, EPI images of each subject were realigned to correct for head motion, corrected for slice acquisition time differences, co-registered with the anatomical image, smoothed in space with a three-dimensional, 10mm FWHM Gaussian kernel, and entered into a voxel-wise analysis using the GLM. A high-pass filter with a cut-off at 128s was used to remove low frequency fluctuations. An event-related design was used and the BOLD time series data were modeled using a standard hemodynamic response function (HRF) with time derivative. The GLM included three regressors representing a balloon inflation, a win outcome, and a loss outcome, respectively. The risk level associated with each balloon (i.e., the probability of explosion, orthogonalized by mean central correction) was entered into the model as a linear parametric modulation of the balloon inflation regressor. For each subject, three contrast images (beta maps) from the risk level, loss outcome, and win outcome regressors were calculated from individual-level GLM analysis, respectively. These beta maps were normalized to the standard MNI template, and then entered into one-sample t-tests for the group-level random-effect analyses. A threshold of whole brain false discovery rate (FDR)32 corrected for P<0.05 and cluster size larger than 30 voxels was used to identify significant activation areas associated with risk (from the parametric regression with risk levels) in the non-ICD group. For other contrasts which showed no activation cluster surviving this corrected threshold, a more liberal threshold of uncorrected P<0.001 was used.
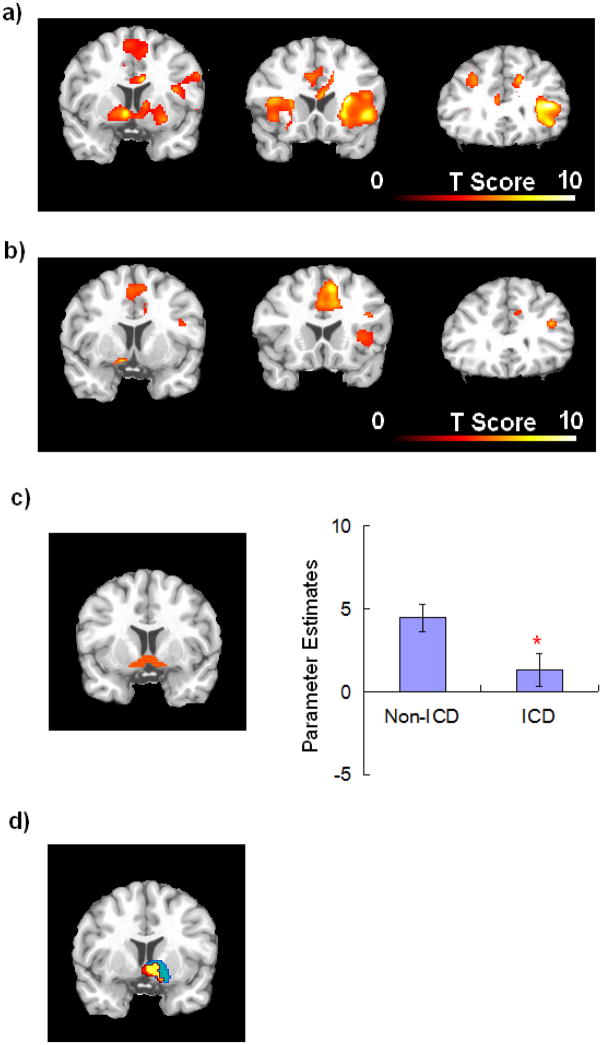
A group-level two sample t-test analysis was also applied to the individual contrast images to compare the risk taking-related activation in the ICD and non-ICD groups. Since our hypothesis focused on the mesolimbic reward system, specifically the ventral striatum, small volume correction (SVC) was applied using bilateral ventral striatum (nucleus accumbens) as the a priori defined region of interest (ROI) from manual structure segmentation (Figure 2c). Bold activation levels (parameter estimates) were also calculated and a two-sample t-test was conducted to examine the difference between the non-ICD and ICD patients in this ROI.
Figure 2.
BOLD imaging data showing (a) increased neural activation for the non-ICD group in bilateral mesolimbic-prefrontal cortex regions during risk taking; (b) increased neural activation for the ICD group in visual regions, the anterior cingulate cortex/medial frontal cortex, left striatum, and right prefrontal cortex, with no activation in the right striatum during risk taking; (c) the ventral striatum region of interest (ROI), a priori defined from manual structure segmentation, and parametric estimates in this ROI showed significantly lower BOLD activation levels for ICD group compared with the non-ICD group (error bar represents standard error; *P<0.05); (d) BOLD activation differences in the right ventral striatum overlapped with resting CBF differences (red=BOLD, blue=CBF, yellow=both).
RESULTS
Behavioral performance
The primary outcome measure reported for the BART is the average adjusted number of pumps (for the unexploded balloons only), with a higher score indicating increased risk taking14. There were no differences between the ICD and non-ICD groups on either of these measures, nor on any of the other exploratory outcome measures (Table 2).
Table 2.
Behavioral performance for the BART
| Variablesa | ICD (N=9) | Non-ICD (N=9) | P value |
|---|---|---|---|
| Average adjusted pumps (unexploded balloons only) | 5.6 (1.0) | 5.8 (0.9) | 0.63 |
| Average adjusted pumps (all balloons) | 5.4 (1.1) | 5.6 (0.8) | 0.77 |
| Balloons completed without explosion | 23.4 (5.2) | 22.4 (3.7) | 0.64 |
| Number of explosions (“loss outcomes”) | 5.3 (1.4) | 5.1 (1.7) | 0.57 |
| Number of completed balloons (“win outcomes”) | 17.3 (5.5) | 16.9 (4.8) | 0.85 |
Mean (SD) values
Resting perfusion
Resting perfusion data showed no between-group differences in global CBF (ICD vs. non-ICD groups, 57 vs. 56 ml/100g/min, P>0.8). However, voxel-wise comparisons on regional CBF revealed significantly lower perfusion in the ventral striatum in the ICD group (mainly in the right hemisphere, peak MNI coordinates =[14, 24, 6], uncorrected P<0.001, cluster corrected P<0.05, small volume corrected P<0.01; Figure 1a), and a region-of-interest (ROI) analysis demonstrated a 39% reduction in resting right ventral striatum CBF for the ICD group vs. the non-ICD group (39 vs. 64 ml/100g/min, respectively, P<0.001, Figure 1b).
Figure 1.
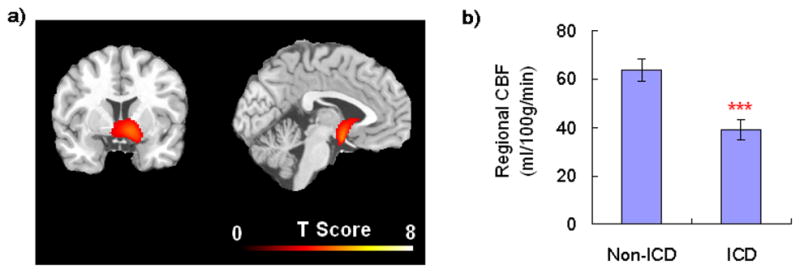
(a) Resting perfusion imaging data showing significant CBF differences in the ventral striatum between ICD and non-ICD PD patients. Threshold was set as cluster corrected for P<0.05; (b) Quantitative analysis showing regional resting CBF in the right ventral striatum significantly decreased for the ICD group compared with the non-ICD group (error bar represents standard error, ***P<0.001).
BOLD fMRI
BOLD data for the BART task demonstrated significant between-group differences in neural activation during risk taking. Using a whole brain-corrected threshold, the non-ICD group demonstrated increased activation that co-varied with risk (i.e., the contrasts of risk regressor) in bilateral visual regions and mesolimbic-frontal regions, including the midbrain, ventral striatum, anterior insula, dorsal lateral prefrontal cortex, and anterior cingulate cortex/medial frontal cortex (Table 3 and Figure 2a), duplicating activation patterns observed in younger healthy subjects15.
Table 3.
Activation during risk taking on the BART
| Brain Regions | Peak MNI Coordinates | Z Scores | P Values (corrected) | Cluster size | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Non-ICD groupa | ||||||
| R. Insula/PFC | 40 | 28 | 10 | 5.05 | 0.014 | 1893 |
| R. Parietal | 36 | −32 | 44 | 4.58 | 0.014 | 983 |
| L. Parietal/Occipital | −14 | −80 | 50 | 4.27 | 0.016 | 110 |
| L. Insula | −26 | 26 | 12 | 4.21 | 0.016 | 747 |
| L. Striatum | −8 | 8 | −4 | 4.13 | 0.017 | |
| R. Striatum | 8 | 4 | 4 | 3.98 | 0.020 | |
| R. ACC | 8 | 8 | 28 | 4.13 | 0.018 | 298 |
| R. Occipital | 42 | −78 | 22 | 4.11 | 0.018 | 153 |
| R. Midbrain/Thalamus | 6 | −24 | −8 | 3.94 | 0.020 | 91 |
| R. Parietal | 22 | −58 | 48 | 3.74 | 0.025 | 234 |
| L. PFC | −30 | 40 | 34 | 3.73 | 0.025 | 164 |
| L. ACC | −8 | 30 | 14 | 3.62 | 0.029 | 39 |
| R. MFC | 10 | 2 | 56 | 3.51 | 0.034 | 41 |
| L. ACC | −6 | 22 | 32 | 3.51 | 0.034 | 63 |
| R. Parietal | 58 | −36 | 22 | 3.37 | 0.041 | 47 |
| ICD groupb | ||||||
| L. Fusiform/Occipital | −24 | −74 | −12 | 5.04 | 0.055 | 848 |
| L. Occipital | −34 | −82 | 20 | 4.81 | 0.055 | 466 |
| R. ACC/MFC | 8 | 18 | 42 | 4.37 | 0.055 | 1274 |
| R. Occipital | 32 | −84 | 16 | 4.35 | 0.055 | 456 |
| L. Striatum | −12 | 8 | −12 | 4.26 | 0.061 | 54 |
| R. PFC | 26 | 50 | 18 | 3.87 | 0.065 | 36 |
| R. PFC | 42 | 12 | 20 | 3.42 | 0.087 | 35 |
Threshold set as whole brain FDR corrected P<.05 and cluster size larger than 30 voxels.
Threshold set as the uncorrected P<.001 and cluster size larger than 30 voxels
The ICD group showed no significant activation in any brain region at this level of stringency. Using a more liberal threshold (uncorrected P<0.001), the ICD group demonstrated increased activation that co-varied with risk in visual regions, the anterior cingulate cortex/medial frontal cortex, left striatum, and right prefrontal cortex (Table 3 and Figure 2b), while no activation was found in the right striatum. Direct voxel-wise comparisons showed that the ICD group had reduced activation during risk taking compared with the non-ICD group in the right ventral striatum (peak MNI coordinates =[2, 8, −6], uncorrected P =0.001, small volume corrected P <0.05). Independent ROI analysis confirmed reduced ventral striatal activation for the ICD group compared with the non-ICD group (P = 0.03; Figure 2c). The brain regions demonstrating between-group differences in BART task activation overlapped substantially with those showing between-group differences in resting CBF (Figure 2d).
Neither group showed significant regional activations relating to loss or win outcomes, even when employing a more liberal threshold of uncorrected P < 0.005.
DISCUSSION
Consistent with our first hypothesis, we found that PD patients with an ICD compared with those without an ICD demonstrated relatively diminished activation in the ventral striatum during risk taking. The between-group difference involved predominantly the right ventral striatum, and this region was also identified as demonstrating between-group differences in resting blood flow as assessed by ASL. In contrast to both the imaging findings and our second hypothesis, no between-group differences were observed in performance on the risk-taking task. Implications are discussed below.
Neural activity during risk taking
In healthy subjects, increasing reward exposure using a gambling task is associated with increased brain activity in mesocorticolimbic regions.15;33 In non-PD populations, reduced activation of the mescorticolimbic “reward” system has been reported in pathological gambling, substance use disorders, and eating disorders.6;11–13;34 Thus, our finding of reduced ventral striatum activation during risk taking in PD patients with a range of ICDs suggests that ICDs in PD share a common mechanism with those in the general population. Our finding of differences in the right striatum specifically is similar to that reported in a fMRI study of pathological gamblers in the general population,11 and the ability to detect differences in brain activity in spite of normal task performance was similar to that reported in an fMRI Stroop study of pathological gambling subjects.12
Resting cerebral blood flow
The finding of reduced resting CBF in the ventral striatum of the ICD group compared with the non-ICD group, which overlapped with the between-group differences in neural activity in response to risk taking, suggests several possibilities. For example, diminished right ventral striatal function may represent a trait effect of ICDs in PD, and diminished ventral striatal activation might thus be observed across multiple tasks, including ones not directly related to risk taking. However, the specificity of ventral striatal findings to the modeling of the hemodynamic response function to risk-taking and not to win or loss outcomes on the BART does not provide support for this interpretation.
The current ASL findings complement those from a PET study of PD patients with pathological gambling in which diminished ventral striatal dopamine D2/3 receptor binding potential was observed.35 However, as the current study did not investigate dopaminergic influences, the extent to which the present findings might relate to dopamine function in individuals with PD and ICDs warrants direct investigation.
A recent SPECT study36 in PD patients with pathological gambling showed increased brain perfusion in multiple right hemisphere regions linked with impulse control, including the orbitofrontalcortex, hippocampus, amygdala, insula, and ventralpallidum. Multiple factors, including small sample sizes in both studies, differences in patient populations (e.g., 11 subjects with pathological gambling in the Cilia et al. study versus 4 patients in our study; average ICD duration of 1.7 years in Cilia et al. study versus 4.5 years in our study), resolution of imaging techniques, and analysis methods may have contributed to apparent differences in results from the two studies. Future, larger studies are needed to examine these possibilities.
The ventral striatum and ICD behaviors
Abnormalities in risk-reward processing and decision-making have been reported in PD.37–39 Unmedicated PD patients demonstrate an impaired mesolimbic reward prediction response40 and diminished functional connectivity of the ventral striatum with other regions of the limbic cortex,41;42 suggesting a neural substrate that may predispose to ICD development.
An alternate possibility involves drugs, such as DAs and levodopa, that influence ventral striatal function and have been associated with ICDs in PD. A recent PET neuroimaging study in PD patients with pathological gambling using 11C raclopride, a radioligand that binds to dopamine D2/D3 receptors, demonstrated decreased D2/D3 binding potential at baseline and a relatively greater decrease in binding potential during performance of a gambling task.35 Via action on ventral striatal dopamine function, dopamine replacement therapies in PD patients could potentially alter reward responsivity43;44 and abilities to learn from negative decision outcomes.45;46 Given the cross-sectional nature of the present study and the study’s use of brain imaging techniques that do not measure dopamine function, additional research is needed to examine these and other hypotheses.
The current findings of relatively diminished ventral striatal activation in individuals with ICDs are similar to findings in non-PD populations. Specifically, individuals with pathological gambling have shown relatively diminished ventral striatal activation during simulated gambling and following exposure to gambling cues.11;13 As such, the present findings suggest that this pattern may extend to PD samples and be particularly relevant to risk taking. Additionally, as many of the subjects with ICDs in the present study did not have pathological gambling, the findings suggest that diminished ventral striatal function may be relevant to a broader range of ICDs.
Clinical relevance
Although discontinuation of or a reduction in DA treatment may be associated with improvements in or resolution of ICD symptoms,47 PD patients are often reluctant to make changes in their pharmacotherapy due to motor or psychological benefits derived from the medications. In addition, reductions or changes in DA dosage may not uniformly lead to resolution of ICD symptoms, so other interventions are needed. If there is an association between ventral striatum activity and ICDs in PD, non-dopaminergic treatments that target this brain region have the potential to improve ICD symptoms without worsening the motor symptoms of PD. For instance, nalmefene and naltrexone, two opioid antagonists, have been shown to be efficacious in the treatment of non-PD pathological gambling48;49 and are hypothesized to generate clinical effects through indirect modulation of dopamine function in the mesolimbic system.50 Investigation of the efficacy and tolerability of opioid antagonists in the treatment of ICDs in PD is warranted, as are studies of the biological mechanisms of action.
Study limitations
There are several study limitations. First, with 18 patients for the BOLD study and 15 patients for the ASL perfusion study, the sample sizes may have been too small to detect more subtle between-group differences in other regional neural activity (e.g., the prefrontal cortex) during risk taking, or for activations related to loss and win outcomes. In addition, inclusion and grouping of data for four different ICDs may have contributed to less robust differences between the ICD and non-ICD groups, due to possible differences in the neural substrates for these four ICDs. Second, due to time limitations inherent in the fMRI experimental design, the maximum number of possible balloon inflations in our modified BART task was reduced to 12, and most subjects completed only 20–30 balloon trials during the 8 minutes of BOLD scanning. Thus, the experimental design may have decreased the sensitivity to detect between-group differences in behavioral performance. However, BART performance in PD patients on a DA has previously been shown to correlate with fMRI brain activity in the orbitofrontal cortex despite lack of a direct DA effect on BART behavioral performance,18 and a recent fMRI study using a probabilistic learning task in PD patients with and without an ICD reported between-group differences in orbitofrontal cortex and dorsolateral putamen activity associated with task prediction error, in spite of no between-group differences in task behavioral performance.51 The use of a virtual reward may have also decreased our sensitivity to detect between-group differences associated with risk taking. While some neuroimaging studies have identified regional brain activations in “reward system” circuitry when processing virtual non-money rewards,52 others have detected greater changes in metabolism when using actual monetary rewards versus non-monetary rewards.53 As the study was cross-sectional in nature, conclusions regarding causality cannot be drawn. Additionally, since the study did not include brain measures evaluating dopamine function, statements relating the findings to dopaminergic function in PD and dopamine replacement therapies must be considered cautiously. Finally, the finding of relatively diminished BOLD activity and CBF in the same region in ICD patients raises the possibility that the BOLD findings may not be specific to risk-taking behaviors.
Conclusions
In summary, ICDs in PD that occur in the context of DA treatment are associated with diminished right ventral striatum activity in resting state as well as in response to risk-taking. Our results provide converging evidence linking alterations in the mesolimbic reward system to a range of behavioral and drug addictions.6;13
Acknowledgments
This research was supported by a Project Development (ProDev) Grant from the Center for Functional Neuroimaging at the University of Pennsylvania, the NSF grant BCS-0224007, the NIH Grants P30 NS045839, K23 MH067894, R01 DA019039, and R01 AA017539, VA VISN1 MIRECC, Sun Yat-Sen University 985 Project, and a Center of Excellence in Gambling Research grant from the National Center for Responsible Gaming and its affiliated Institute for Research on Gambling Disorders. The contents of the manuscript are solely the responsibility of the authors and do not necessarily represent the official views of any of the funding agencies.
Footnotes
CONFLICT OF INTEREST: The authors report no conflicts of interest.
AUTHORS’ ROLES: HR, JAD, and DW participated in the conception and design of the study, recommended statistical analyses, drafted and revised the report, and saw and approved the final version of the manuscript. EM, MNP, ADS, and MBS participated in the conception and design of the study, revised the report, and saw and approved the final version of the manuscript.
FULL FINANCIAL DISCLOSURES OF ALL AUTHORS FOR THE PAST YEAR:
HR: Grant support: National Natural Science Foundation of China, Project Development (ProDev) Grant from the Center for Functional Neuroimaging at the University of Pennsylvania
EM: None
JD: Grant support: NIH, NSF, Wyeth, Penn-AstraZeneca Alliance; Consultancy: Pfizer, NIH, Dana Foundation, St. Jude Medical; Royalties: Inventor on Penn’s ASL Patent; Advisory Board: Pittsburg NMR Center; Editorial Board: Journal of Neuroimaging
AS: Grant support: NINDS; Honoraria: Teva, Solvay; Consultancy: Novartis, Supernus Pharmaceuticals
MS: Consultancy: Teva, Novartis, Ipsen, Schering-Plough, Adamas Pharmaceuticals, and Boeringher-Ingelheim
MP: Consults for and is an advisor to Boehringer Ingelheim; has financial interests in Somaxon; has received research support from the National Institutes of Health, Veteran’s Administration, Mohegan Sun Casino, the National Center for Responsible Gaming and its affiliated Institute for Research on Gambling Disorders, and Forest Laboratories pharmaceuticals; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse control disorders or other health topics; has consulted for law offices on issues related to addictions or impulse control disorders; has provided clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews for the National Institutes of Health and other agencies; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts.
DW: Grant support: NIH, Michael J Fox Foundation for Parkinson Research, Boehinger Ingelheim; Honoraria: Boehringer Ingelheim, Pfizer, Consultancy: Acadia, Merck Serono, Sanofi Aventis; Intellectual property rights: Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease licensed to University of Pennsylvania
References
- 1.Weintraub D, Siderowf AD, Potenza MN, et al. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol. 2006;63:969–973. doi: 10.1001/archneur.63.7.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voon V, Hassan K, Zurowski M, et al. Prevalence of repetitive and reward-seeking behaviors in Parkinson disease. Neurology. 2006;67:1254–1257. doi: 10.1212/01.wnl.0000238503.20816.13. [DOI] [PubMed] [Google Scholar]
- 3.Nirenberg MJ, Waters C. Compulsive eating and weight gain related to dopamine agonist use. Mov Disord. 2006;21:524–529. doi: 10.1002/mds.20757. [DOI] [PubMed] [Google Scholar]
- 4.Weintraub D, Koester J, Potenza MN, et al. Dopaminergic therapy and impulse control disorders in Parkinson’s disease: A cross-sectional study of dopaminergic therapy and other clinical features in 3,090 patients. Arch Neurol. In press. [Google Scholar]
- 5.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. Text Revision. [Google Scholar]
- 6.Holden C. ‘Behavioral’ addictions: do they exist? Science. 2001;294:980–982. doi: 10.1126/science.294.5544.980. [DOI] [PubMed] [Google Scholar]
- 7.Ondo W, Lai D. Predictors of impulsivity and reward seeking behavior with dopamine agonists. Parkinsonism and Related Disorders. 2008;14:28–32. doi: 10.1016/j.parkreldis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Holman AJ. Impulse control disorder behaviors associated with pramipexole used to treat fibromyalgia. Journal of Gambling Studies. 2009 doi: 10.1007/s10899-009-9123-2. [DOI] [PubMed] [Google Scholar]
- 9.Hollander E, Evers M. New developments in impulsivity. The Lancet. 2001;358:949–950. doi: 10.1016/S0140-6736(01)06114-1. [DOI] [PubMed] [Google Scholar]
- 10.Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;56 (Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reuter J, Raedler T, Rose M, Hand I, Gläscher J, Büchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nat Neurosci. 2005;8:147–148. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- 12.Potenza MN, Leung H-C, Blumberg HP, et al. An fMRI Stroop task study in ventromedial prefrontal cortical function in pathological gamblers. Am J Psychiatry. 2003;160:1990–1994. doi: 10.1176/appi.ajp.160.11.1990. [DOI] [PubMed] [Google Scholar]
- 13.Potenza MN. The neurobiology of pathological gambling and drug addiction: an overview and new findings. Philos Trans R Soc Lond B Biol Sci. 2008;363:3181–3189. doi: 10.1098/rstb.2008.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lejuez CW, Read JP, Kahler CW, et al. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) Journal of Experimental Psychology: Applied. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- 15.Rao H, Korczykowski M, Pluta J, Hoang A, Detre JA. Neural correlates of voluntary and involuntary risk taking in the human brain: An fMRI Study of the Balloon Analog Risk Task (BART) NeuroImage. 2008;42:902–910. doi: 10.1016/j.neuroimage.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lejuez CW, Aklin WM, Jones HA, et al. The Balloon Analogue Risk Task (BART) differentiates smokers and nonsmokers. Experimental and Clinical Psychopharmacology. 2003;11:26–33. doi: 10.1037//1064-1297.11.1.26. [DOI] [PubMed] [Google Scholar]
- 17.Hunt MK, Hopko DR, Bare R, Lejuez CW, Robinson EV. Construct validity of the Balloon Analog Risk Task (BART): associations with psychopathy and impulsivity. Assessment. 2005;12:416–428. doi: 10.1177/1073191105278740. [DOI] [PubMed] [Google Scholar]
- 18.Van Eimeren T, Ballanger B, Pellecchia G, Miyasaki JM, Lang AE, Strafella AP. Dopamine agonists diminish value sensitivity of the orbitofrontal cortex: A trigger for pathological gambling in Parkinson’s disease? Neuropsychopharmacology. 2009;34:2758–2766. doi: 10.1038/sj.npp.npp2009124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23:37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- 20.Detre JA, Wang J. Technical aspects and utility of fMRI using BOLD and ASL. Clin Neurophysiol. 2002;113:621–634. doi: 10.1016/s1388-2457(02)00038-x. [DOI] [PubMed] [Google Scholar]
- 21.Raichle ME. Behind the scenes of functional brain imaging: a historical and physiological perspective. Proc Natl Acad Sci U S A. 1998;95:765–772. doi: 10.1073/pnas.95.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alsop DC, Detre JA, Grossman M. Assessment of cerebral blood flow in Alzheimer’s disease by spin-labeled magnetic resonance imaging. Ann Neurol. 2000;47:93–100. [PubMed] [Google Scholar]
- 23.Shaffer HJ, LaBrie R, Scanlan KM, Cummings TN. Pathological gambling among adolescents: Massachusetts gambling screen (MAGS) Journal of Gambling Studies. 1994;10:339–362. doi: 10.1007/BF02104901. [DOI] [PubMed] [Google Scholar]
- 24.Christenson GA, Faber RJ, deZwaan M. Compulsive buying: descriptive characteristics and psychiatric comorbidity. J Clin Psychiatry. 1994;55:5–11. [PubMed] [Google Scholar]
- 25.Giovannoni G, O’Sullivan JD, Turner K, Manson AJ, Lees AJL. Hedonistic homeostatic dysregulation in patients with Parkinson’s disease on dopamine replacement therapies. J Neurol Neurosurg Psychiatry. 2000;68:423–428. doi: 10.1136/jnnp.68.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fahn S, Elton RL . the UPDRS Development Committee. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Calne D, Goldstein M, editors. Recent developments in Parkinson’s disease. Florham Park, NJ: Macmillan Health Care Information; 1987. pp. 153–163. [Google Scholar]
- 27.Hobson P, Gallacher J, Meara J. Cross-sectional survey of Parkinson’s disease and parkinsonism in a rural area of the United Kingdom. Mov Disord. 2005;20:995–998. doi: 10.1002/mds.20489. [DOI] [PubMed] [Google Scholar]
- 28.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. In: Brink TL, editor. Clinical Gerontology: A Guide to Assessment and Intervention. New York: The Haworth Press; 1986. pp. 165–173. [Google Scholar]
- 29.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez-Seara MA, Edlow BL, Hoang A, Wang J, Feinberg DA, Detre JA. Minimizing acquisition time of arterial spin labeling at 3T. Magn Reson Med. 2008;59:1467–1471. doi: 10.1002/mrm.21633. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Zhang Y, Wolf RL, Roc AC, Alsop DC, Detre JA. Amplitude modulated continuous arterial spin labeling perfusion MR imaging with single coil at 3.0 Tesla. Radiology. 2005;235:218–228. doi: 10.1148/radiol.2351031663. [DOI] [PubMed] [Google Scholar]
- 32.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 33.Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315:515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- 34.Brewer JA, Potenza MN. The neurobiology and genetics of impulse control disorders: relationships to drug addictions. Biochem Pharmacol. 2008;75:63–75. doi: 10.1016/j.bcp.2007.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steeves TDL, Miyasaki J, Zurowski M, et al. Increased striatal dopamine release in parkinsonian patients with pathological gambling: a 11C raclopride PET study. Brain. 2009;132:1376–1385. doi: 10.1093/brain/awp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cilia R, Siri C, Marotta G, et al. Functional abnormalities underlying pathological gambling in Parkinson disease. Arch Neurol. 2008;65:1604–1611. doi: 10.1001/archneur.65.12.1604. [DOI] [PubMed] [Google Scholar]
- 37.Brand M, Labudda K, Kalbe E, et al. Decision-making impairments in patients with Parkinson’s disease. Behavioural Neurology. 2004;15:77–85. doi: 10.1155/2004/578354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pagonabarraga J, García-Sánchez C, Llebaria G, Pascual-Sedano B, Gironell A, Kulisevsky J. Controlled study of decision-making and cognitive impairment in Parkinson’s disease. Mov Disord. 2007;22:1430–1435. doi: 10.1002/mds.21457. [DOI] [PubMed] [Google Scholar]
- 39.Kobayakawa M, Koyama S, Mimura M, Kawamura M. Decision making in Parkinson’s disease: analysis of behavioral and physiological patterns in the Iowa gambling task. Mov Disord. 2008;23:547–552. doi: 10.1002/mds.21865. [DOI] [PubMed] [Google Scholar]
- 40.Künig G, Leenders KL, Martin-Sölch C, Missimer J, Magyar S, Schultz W. Reduced reward processing in the brains of Parkinsonian patients. Neuroreport. 2000;11:3681–3687. doi: 10.1097/00001756-200011270-00019. [DOI] [PubMed] [Google Scholar]
- 41.Thiel A, Hilker R, Kessler J, Habedank B, Herholz K, Heiss W-D. Activation of basal ganglia loops in idiopathic Parkinson’s disease: a PET study. J Neural Transm. 2003;110:1289–1301. doi: 10.1007/s00702-003-0041-7. [DOI] [PubMed] [Google Scholar]
- 42.Schott BH, Niehaus L, Wittmann BC, et al. Ageing and early-stage Parkinson’s disease affect separable neural mechanisms of mesolimbic reward processing. Brain. 2007;130:2412–2424. doi: 10.1093/brain/awm147. [DOI] [PubMed] [Google Scholar]
- 43.Evans AH, Lawrence AD, Lees AJ. Changes in psychomotor effects of L-dopa and methyphenidate after sustained dopaminergic therapy in Parkinson disease. J Neurol Neurosurg Psychiatry. 2009;80:267–272. doi: 10.1136/jnnp.2006.108993. [DOI] [PubMed] [Google Scholar]
- 44.Bódi N, Kéri S, Nagy H, et al. Reward-learning and the novelty seeking personality: a between- and within-subjects study of the effects of dopamine agonists on young Parkinson’s patients. Brain. 2009;132:2385–2395. doi: 10.1093/brain/awp094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;318:1309–1312. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- 46.Cools R. Dopaminergic modulation of cognitive function - implications for L-DOPA treatment in Parkinson’s disease. Neurosci Biobehav Rev. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 47.Mamikonyan E, Siderowf AD, Duda JE, et al. Long-term follow-up of impulse control disorders in Parkinson’s disease. Mov Disord. 2008;23:75–80. doi: 10.1002/mds.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grant JE, Potenza MN, Hollander E, et al. Multicenter investigation of the opioid antagonist nalmefene in the treatment of pathological gambling. Am J Psychiatry. 2006;163:303–312. doi: 10.1176/appi.ajp.163.2.303. [DOI] [PubMed] [Google Scholar]
- 49.Grant JE, Kim SW, Hartman BK. A double-blind, placebo-controlled study of the opiate antagonist naltrexone in the treatment of pathological gambling urges. J Clin Psychiatry. 2008;69:783–789. doi: 10.4088/jcp.v69n0511. [DOI] [PubMed] [Google Scholar]
- 50.Kim SW. Opioid antagonists in the treatment of impulse-control disorders. J Clin Psychiatry. 1998;59:159–164. [PubMed] [Google Scholar]
- 51.Voon V, Pessiglione M, Brezing C, et al. Mechanisms underlying dopamine-mediated reward bias in compulsive behaviors. Neuron. 2010;65:135–142. doi: 10.1016/j.neuron.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Delgado MR, Schotter A, Ozbay EY, Phelps EA. Understanding overbidding: using the neural circuitry of reward to design economic auctions. Science. 2010;321:1849–1852. doi: 10.1126/science.1158860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hollander E, Pallanti S, Baldini Rossi N, Sood E, Baker BR, Buchsbaum MS. Imaging monetary reward in pathological gamblers. World Journal of Biological Psychiatry. 2005;6:113–120. doi: 10.1080/15622970510029768. [DOI] [PubMed] [Google Scholar]