Abstract
Background and Purpose
Receptors for calcitonin gene-related peptide (CGRP) are composed of the calcitonin-like receptor in association with receptor activity-modifying protein-1 (RAMP1). CGRP is an extremely potent vasodilator and may protect against vascular disease through other mechanisms.
Methods
We tested the hypothesis that overexpression of RAMP1 enhances vascular effects of CGRP using transgenic mice with ubiquitous expression of human RAMP1 (hRAMP1). Because angiotensin II (Ang II) is a key mediator of vascular disease, we also tested the hypothesis that RAMP1 protects against Ang II-induced vascular dysfunction.
Results
Responses to CGRP in carotid and basilar arteries in vitro as well as cerebral arterioles in vivo were selectively enhanced in hRAMP1 transgenic mice compared to littermate controls (P<0.05), and this effect was prevented by a CGRP receptor antagonist (P<0.05). Thus, vascular responses to CGRP are normally RAMP1-limited. Responses of carotid arteries were examined in vitro following overnight incubation with vehicle or Ang II. In arteries from control mice, Ang II selectively impaired responses to the endothelium-dependent agonist acetylcholine by ∼50% (P<0.05) via a superoxide-mediated mechanism. In contrast, Ang II did not impair responses to acetylcholine in hRAMP1 transgenic mice.
Conclusions
RAMP1 overexpression increases CGRP-induced vasodilation and protects against Ang II-induced endothelial dysfunction. These findings suggest that RAMP1 may be a new therapeutic target to regulate CGRP-mediated effects during disease including pathophysiological states where Ang II plays a major role.
Keywords: cerebral circulation, adrenomedullin, basilar artery, microcirculation, carotid artery, oxidative stress, calcitonin gene-related peptide, receptor activity-modifying protein
Introduction
Calcitonin gene-related peptide (CGRP) is one of the most potent vasodilators known.1 Effects of CGRP are particularly prominent in the cerebral circulation where the peptide regulates vascular tone and other aspects of vascular function.2-6 For example, CGRP mediates vasodilation following activation of trigeminal sensory fibers and inhibits vasospasm following subarachnoid hemorrhage.5,7 Thus, modifying CGRP-mediated signaling may be an important therapeutic target to affect cerebral blood flow and for treatment of vascular disease.
Receptors for CGRP consist of the calcitonin-like receptor (CLR) associated with receptor activity-modifying protein-1 (RAMP1).8 Of the known RAMP proteins, RAMP1 confers ligand specificity for CGRP, while association of CLR with RAMP2 or RAMP3 results in a receptor with high affinity for adrenomedullin.8 Relaxation of the aorta to CGRP is dependent on expression of RAMP1 and responses to CGRP are augmented following increased expression of RAMP1 in vascular muscle in culture.9,10 However, it is unknown if effects of CGRP are RAMP1-limited in intact blood vessels. We tested the hypothesis that overexpression of RAMP1 enhances cerebrovascular effects of CGRP. To achieve this goal, we studied mice expressing human RAMP1 (hRAMP1) in all tissues, including blood vessels.11
Angiotensin II (Ang II) is a key effector of the renin-angiotensin system and is thought to underlie many of the vascular changes that occur during aging and diseases states including hypertension and atherosclerosis.12,13 One of the major detrimental effects of Ang II is to promote oxidative stress.14,15 In this regard, members of the calcitonin/CGRP peptide family (CGRP, adrenomedullin, and intermedin) exert antioxidant effects on vascular cells in culture.16-18 In a model of chronic hypertension, pressor effects of Ang II are greatly attenuated in hRAMP1 transgenic mice11 through mechanisms that may include vascular changes. We hypothesized that overexpression of RAMP1 protects against vascular dysfunction produced by Ang II. To address this question, we examined direct effects on Ang II on the vessel wall using a model of Ang II-induced oxidative stress and vascular dysfunction.14
Methods
All protocols and procedures conform to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Iowa.
Generation of hRAMP1 transgenic mice
The strategy for generating ubiquitous hRAMP1 transgenic mice using conditional activation by the lox P-cre system has been described.11 Briefly, the hRAMP1 cDNA was inserted downstream of GFP and a polyadenylation site flanked by lox P sites under control of the CX1 promoter (a hybrid of β-actin and cytomegalovirus sequences). As a consequence, GFP (but not hRAMP1), was ubiquitously expressed in all tissues until removal of the GFP stop sequences by cre recombinase under control of the adenoviral Ella promoter in double transgenic mice. Transgenic mice were identified using the primers listed in the Supplemental Table. All PCR products were confirmed by sequencing.
Measurement of RAMP1 expression levels
To evaluate expression of hRAMP1, total RNA was isolated from tissues from double transgenic mice and control littermates using the Trizol method followed by DNAse I (Invitrogen, CA) to remove DNA contamination. RNA integrity was checked by gel electrophoresis. Total RNA (1.5-2 μg) was reverse transcribed using random hexamers and a Taqman RT-PCR mix.19 Primers are listed in the Supplemental Table. As controls, GAPDH and β-actin were detected in all tissues and hRAMP1 was not detected from the double transgenic mice when amplified in the absence of reverse transcriptase.
Real time PCR was performed as described.11,19 Reactions were performed in duplicate and analyzed using a Bio-Rad (Hercules, CA) MY-IQ thermocycler. Samples that did not yield a homogenous curve were excluded. The β-actin, mRAMP1, and hRAMP1 Ct values were converted to absolute copy numbers using standard curves generated with 1:10 serial dilutions of pGEM-Qbeta-actin, pGEM-QmRAMP1 and pbsCX1-LEL-hRAMP1 plasmids in 10 ng/ml yeast tRNA.
Studies of carotid and cerebral arteries in vitro
Briefly, mice were euthanized with pentobarbital (∼150 mg/kg, ip) followed by removal of brain and both carotid arteries. Methods to measure responses of carotid arteries in mice have been described in detail.14 Relaxation responses were measured following submaximal precontraction (∼50-60% of maximum) using U46619. In some experiments, the effect of CGRP(8-37) (10-6 mol/L, a CGRP receptor antagonist) was tested.
To test whether overexpression of RAMP1 protects against vascular dysfunction in response to Ang II, segments of arteries were placed in cell culture dishes as described.14 Vessels were then incubated with either vehicle (double-distilled H2O) or Ang II (10 nmol/L, a concentration known to increase superoxide and produce endothelial dysfunction in this model) for 22 hours at 37°C.14 After incubation, vascular rings were connected to force transducers and changes in isometric tension were measured as described above.
Basilar arteries were isolated, cannulated, and pressurized to 60 mmHg so lumen diameter could be measured as described.12 After an equilibration period of at least 30 min, we examined changes in diameter of the artery in response to KCl (50 mM). To examine dilator responses, arteries were constricted submaximally (∼40% of the response to KCl) with U46619.
Studies of cerebral arterioles in vivo
Mice were anesthetized with pentobarbital sodium (75-90 mg/kg ip), supplemented at ∼20 mg/kg/h. Animals were ventilated mechanically and a cranial window was prepared and arterial blood pressure and blood gases were monitored as described.20 Arteriolar diameter was measured under control conditions and during topical application of drugs. Mean arterial pressure and arterial blood gases were similar in both groups and similar to previous studies (data not shown).20
Statistical analysis
All data are expressed as mean±SE. Responses to KCl in the basilar artery and responses of cerebral arterioles in vivo were calculated as percent change in vessel diameter from the baseline control level. For vasodilator responses, results are expressed as percent dilation (% of induced tone), with 100% representing the difference between the resting value under basal conditions and the constricted value with U-46619. Data was evaluated using analysis of variance followed by Student-Neumann Kuells post-hoc test, or Students unpaired t-test, as appropriate. Statistical significance was accepted at P<0.05.
Results
Measurement of hRAMP1 and total RAMP1 levels in transgenic mice
GFP-hRAMP1 mice were bred with EIIa-cre mice to obtain double transgenic mice expressing the hRAMP1 gene (Figure 1A). About 25% of the progeny were double transgenic offspring with an approximately equal sex distribution. Double transgenic mice appeared to develop normally and were fertile. The Ella promoter had widespread activity. Visually using fluorescent microscopy, the GFP signal was qualitatively reduced to background levels in all tissues examined from double transgenic EIIa/hRAMP1 mice (Figure 1A). Ubiquitous expression of hRAMP1 was detected by reverse transcriptase PCR amplification of RNA from tissue from double transgenic mice (including arteries), but not from control GFP-hRAMP1 and Ella-cre mice. The levels of hRAMP1 and endogenous mouse RAMP1 RNAs were determined by quantitative PCR using hRAMP1 and mouse RAMP1 specific primers (Figure 1B). Endogenous mouse RAMP1 was detected in all tissues and the levels were similar between double transgenic mice and their littermates, suggesting that transgene expression did not affect expression of the endogenous RAMP1 gene. In double transgenic mice, the total RAMP1 RNA levels (combined hRAMP1 and mouse RAMP1) in the vasculature were 4-8 fold greater than in controls (Figure 1C).
Figure 1. EIIa/hRAMP1 transgenic mice.
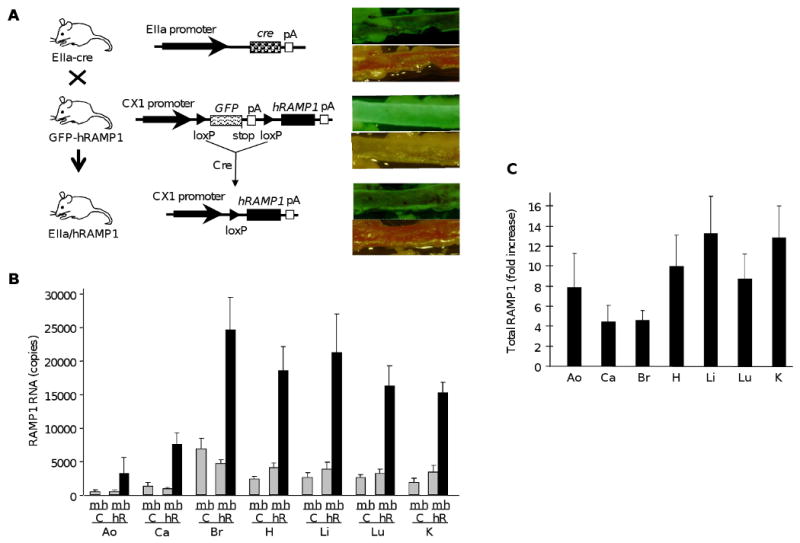
A. Strategy for producing hRAMP1 transgenic mice. Transgenic mice with the CX1 promoter and GFP–stop–hRAMP1 cassette containing a termination codon (stop) and polyadenylation signal (pA) (GFP–hRAMP1) were mated with transgenic mice with EIIa promoter driving cre recombinase (EIIa–cre). Double transgenic offspring (EIIa/hRAMP1) have cre-mediated excision of the GFP–stop sequence at the flanking loxP sites with hRAMP1 transgene expression from the CX1 promoter. Loss of GFP is shown by comparison brightfield and fluorescent images of postmortem aorta from each genotype. B. Quantitative PCR measurement of RAMP1 levels in EIIa/hRAMP1 (hR) mice and control (C) littermates. The levels of hRAMP1 (h), mRAMP1 (m) and β-actin RNA were determined by real-time PCR from aorta (Ao), carotid (Ca), brain (Br), heart (H), liver (Li), lung (Lu), and kidney (K). The copy numbers were calculated from standard curves and normalized to 50,000 copies of β-actin mRNA. Data are the mean from 3 mice in each group, with the SE from 3 independent experiments. C. Fold increase in total RAMP1 RNA levels in EIIa/hRAMP1 mice relative to control mice calculated from panel B.
Responses in carotid arteries
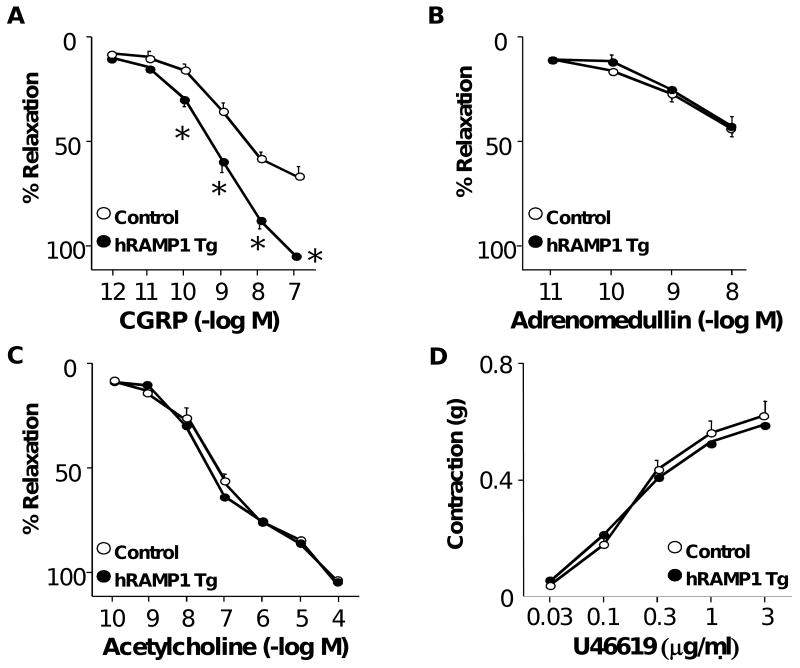
Maximal relaxation of carotid arteries to CGRP was approximately doubled in mice expressing hRAMP1 compared to controls (Figure 2A). Responses to adrenomedullin, acetylcholine (an endothelium-dependent agonist), and U46619 were similar in both groups (Figure 2B-2D), suggesting that effects of expression of hRAMP1 were relatively selective. This increase in vasodilation to CGRP in hRAMP1 transgenic mice was inhibited by CGRP(8-37) (see Supplemental Figure).
Figure 2.
Cumulative relaxation responses of carotid arteries in response to CGRP (A), adrenomedullin (B), and acetylcholine (C) in control mice (n=9) and mice expressing hRAMP1 (n=10). (D) Constrictor responses of the carotid artery to U46619. All data are mean±SE. * P<0.05 vs control
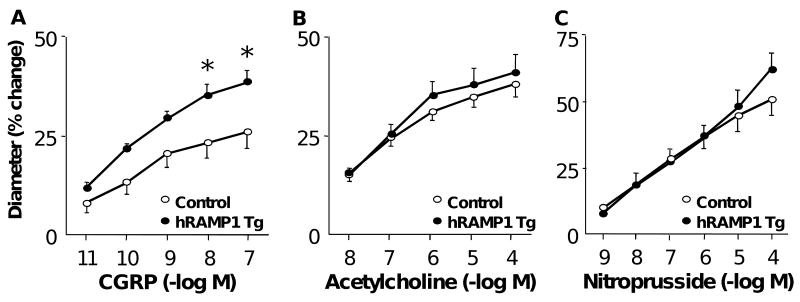
Expression of hRAMP1 augments responses of the cerebral circulation to CGRP
Baseline diameters of the basilar artery in control and hRAMP1 transgenic mice were similar [142±5 μm (n=10) and 133±5 μm (n=10), respectively (P>0.05)]. Vasodilation to CGRP was augmented in mice expressing hRAMP1 compared to controls (Figure 3A). In contrast, there was no effect of hRAMP1 expression on responses to acetylcholine (Figure 3B), nitroprusside (Figure 3C), papaverine, or KCl (data not shown).
Figure 3.
Dilation of the basilar artery in response to CGRP (A, n=10), acetylcholine (B, n=10), and nitroprusside (C, n=6) in control mice and mice expressing hRAMP1. All data are mean±SE. * P<0.05 vs control.
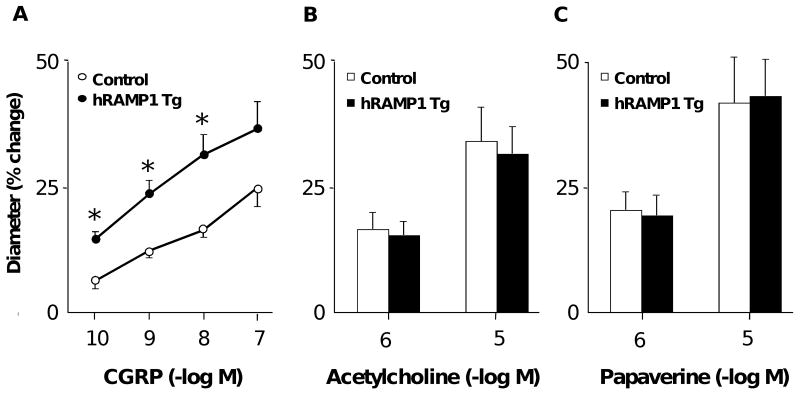
In vivo, baseline diameters of cerebral arterioles in control and hRAMP1 transgenic mice were similar [∼30 μm (P>0.05)]. Vasodilation to CGRP was increased approximately 2-fold in mice expressing hRAMP1 compared to controls (Figure 4A). In contrast, there was no effect of hRAMP1 expression on responses to acetylcholine or papaverine (Figures 4B & C).
Figure 4.
Changes in diameter of cerebral arterioles in response to CGRP (A, n=7-8), acetylcholine (B, n=7-8) and papaverine (C, n=7-8) in control mice and mice expressing hRAMP1. All data are mean±SE. * P<0.05 vs control.
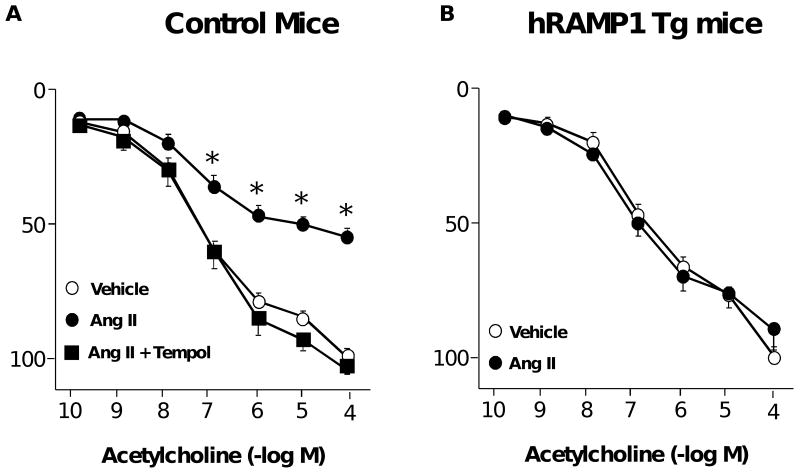
Expression of hRAMP1 protects against Ang II-induced vascular dysfunction
Similar to previous studies,14 Ang II produced marked impairment of acetylcholine-induced relaxation in carotid arteries from control mice (Figure 5A). This effect of Ang II was mediated by superoxide as it was prevented in vessels treated with Tempol (a scavenger of superoxide) (Figure 5A). Expression of hRAMP1 prevented Ang II-induced endothelial dysfunction (Figure 5B). Ang II had no effect on responses to nitroprusside or U46619 (P>0.05, data not shown) in either group of mice.
Figure 5.
Cumulative relaxation responses to acetylcholine in carotid arteries from control mice (A, n=6-15) and mice expressing hRAMP1 (B, n=8) treated with vehicle or 10 nmol/L Ang II or Ang II plus tempol (1 mM). All data are mean±SE. * P<0.05 vs control.
Discussion
This study had several major new findings. First, increasing expression of RAMP1 augments vasodilator responses to CGRP, supporting the new concept that vascular responses to CGRP are normally RAMP1-limited. In mice with ubiquitous expression of hRAMP1, we found that dilation to CGRP was enhanced in resistance vessels supplying the brain (basilar artery and cerebral arterioles), as well as in a large muscular artery (carotid artery). This effect was evident in vivo in microvessels with myogenic tone. RAMP1 confers specificity of CLR for CGRP, whereas RAMP2 and RAMP3 confer specificity of CLR for adrenomedullin.1,8 The finding that responses to CGRP, and not adrenomedullin, were enhanced indicates the selectivity and usefulness of this model for the study of CGRP and RAMP1 in vascular biology. Second, we obtained the first evidence that Ang II-induced oxidative stress and endothelial dysfunction is prevented following expression of hRAMP1. These results support the concept that RAMP1 may be an important mediator of vascular protection and thus a new therapeutic target in vascular disease.
CGRP and regulation of vascular tone
CGRP is known to make important contributions to regulation of vasomotor tone and effects on vascular structure.1,4,5,21 Local sources of CGRP include plasma, sensory nerves, as well as autocrine or paracrine production within the vessel wall.1,4,22 CGRP is an extremely potent vasodilator in many species including humans (present study).1-4,23
Functional CGRP receptors consist of CLR associated with RAMP1.8 Previous studies have shown that increased expression of RAMP1 in vascular muscle in culture increases formation of cAMP in response to CGRP.10 Deletion of RAMP1 in mice markedly reduced responses of the aorta to CGRP in vitro.9 Such findings indicate that responses of vascular cells to CGRP are dependent on expression of RAMP1. The findings that vascular responses to CGRP, and not adrenomedullin, were enhanced following overexpression of RAMP1 is consistent with this concept but also provides the first evidence that responses to CGRP in intact vessels and in vivo are RAMP1-limited.
Reponses to CGRP can be mediated by CGRP receptors but also receptors for related peptides including amylin.25 CGRP receptors are particularly sensitive to CGRP8-37.1,25 Thus, the ability of CGRP8-37 to inhibit increases in responses to CGRP in hRAMP1 mice suggests these responses were mediated (at least in part) by CGRP receptors. Interestingly, CGRP8-37 has been reported to have little effect on CGRP-induced relaxation in normal mouse aorta.24 Thus, the presence of residual effects of CGRP in arteries from transgenic mice treated with CGRP8-37 may indicate that responses in this model are mediated by both CGRP and other related receptors.25
Our finding that increasing expression of RAMP1 augments vasodilator responses to CGRP are complementary to studies in which overexpression of RAMP2 increased vascular responses to adrenomedullin but not CGRP.26 Together this work supports the concept that vascular responses to members of the calcitonin/CGRP peptide family are normally limited by the expression of the appropriate RAMP protein.
Role of CGRP in vascular disease
CGRP plays an important role in regulation of vascular tone and other vascular endpoints.1,4-7,21 Blood vessels of the intracranial circulation are innervated by CGRP-containing sensory fibers from the trigeminal ganglion.3,4,27 CGRP mediates cerebral vasodilation in response to a variety of stimuli including hypotension, cortical spreading depression, and inflammation.5,6,27,28 CGRP inhibit vasoconstrictor responses including vasospasm following experimental subarachnoid hemorrhage.7 In addition, CGRP is thought to play a major role in the pathogenesis of migraine via effects on blood vessels, neurons, and mast cells.27 CGRP receptor antagonists have emerged as useful anti-migraine agents.27
Expression of components of receptors for CGRP, including CLR and RAMP1, in various tissues including the cardiovascular system may change substantially in response to physiological stimuli and disease including hypertension and preeclampsia.3,11,29-32 The level of total RAMP1 expression present in the vasculature of hRAMP1 transgenic mice is well within the range of changes in RAMP1 expression described in models of disease.29-32 Our data showing increased vasodilator responses to CGRP following RAMP1 overexpression suggests that specific targeting of RAMP1 and the CGRP receptor complex may be a novel molecular target in the design of approaches to affect vascular resistance and blood flow in disease, including headache, subarachnoid hemorrhage and other forms of stroke.
Overexpression of RAMP1 protects against Ang II-induced vascular dysfunction
Ang II is known to contribute to vascular dysfunction in multiple disease states and aging12,13 by producing oxidative stress, endothelial dysfunction, and abnormal vascular growth.13,14,33 Ang II increases formation of reactive oxygen species and causes reactive oxygen species-dependent endothelial dysfunction.13,14,34 Oxidative stress and proliferation of vascular muscle in culture in response to Ang II is inhibited by CGRP and adrenomedullin.17,18,35 Thus, there is evidence suggesting that activation of CGRP receptors may counteract detrimental effects of Ang II. Until this study, the functional importance of RAMP1 during Ang II-induced vascular dysfunction was unknown.
There is substantial evidence that impairment of endothelial function in response to Ang II is mediated by NADPH oxidase-derived superoxide.34 In the current study, we obtained evidence consistent with this concept in that Ang II-induced vascular dysfunction in control mice was prevented by a scavenger of superoxide. Our finding that increased expression of RAMP1 protects the vasculature is consistent with findings suggesting that members of the calcitonin/CGRP peptide family exert antioxidant effects on vascular cells, including inhibitory effects on NADPH oxidase.16-18 Although the current studies do not establish a role for such a mechanism, we speculate that increased expression of RAMP1 may protect against Ang II-induced endothelial dysfunction via inhibitory effects on NADPH oxidase.
Recent work from our group has shown that increases in blood pressure in a model of Ang II-induced hypertension are greatly attenuated in hRAMP1 mice.11 The basis for this phenotype has not been defined but may involve vascular mechanisms. We hypothesized that expression of hRAMP1 in vessels may protect against vascular dysfunction produced by Ang II. The present results indicate that Ang II produced superoxide-mediated vascular dysfunction in control mice and show for the first time that this effect was prevented in hRAMP1 transgenic mice. Related to these findings, overexpression of RAMP2 in another transgenic mouse protects against vascular inflammation and hypertrophy in a model of Ang II-dependent hypertension.36 Collectively, these findings support the emerging concept that members of the RAMP protein family may protect the vasculature from Ang II.
In summary
CGRP-induced vasodilation is selectively enhanced following overexpression of RAMP1 suggesting that vascular responses to CGRP are normally RAMP1-limited. These data provide insight into a specific molecular target of the CGRP receptor complex that may be useful in the treatment of vascular disease. Our findings suggest a key role for RAMP1 in the vasculature and demonstrate the utility of the hRAMP1 transgenic mouse for studies in vascular biology and cerebral circulation.
Acknowledgments
Sources of Funding: This work was supported by National Institutes of Health grants HL-38901, NS-24621, DE-016511, and HL-62984 as well as American Heart Association grants 0575092N and 0855944G. S. Chrissobolis was supported by a CJ Martin Fellowship from the National Health and Medical Research Council of Australia (359282), and a postdoctoral fellowship from the American Heart Association (0725643Z).
Footnotes
Disclosures: None.
References
- 1.Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. 2004;84:903–934. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- 2.Kitazono T, Heistad DD, Faraci FM. Role of ATP-sensitive K+ channels in CGRP-induced dilatation of basilar artery in vivo. Am J Physiol. 1993;265:H581–H585. doi: 10.1152/ajpheart.1993.265.2.H581. [DOI] [PubMed] [Google Scholar]
- 3.Sams A, Knyihar-Csillik E, Engberg J, Szok D, Tajti J, Bodi I, Edvinsson L, Vecsei L, Jansen-Olesen I. CGRP and adrenomedullin receptor populations in human cerebral arteries: in vitro pharmacology and molecular investigations in different artery sizes. Eur J Pharmacol. 2000;408:183–193. doi: 10.1016/s0014-2999(00)00781-0. [DOI] [PubMed] [Google Scholar]
- 4.Jansen I, Alafaci C, Uddman R, Edvinsson L. Evidence that calcitonin gene-related peptide contributes to the capsaicin-induced relaxation of guinea pig cerebral arteries. Regulatory Peptides. 1990;31:167–178. doi: 10.1016/0167-0115(90)90003-f. [DOI] [PubMed] [Google Scholar]
- 5.Busija DW, Bari F, Domoki F, Horiguchi T, Shimizu K. Mechanisms involved in the cerebrovascular dilator effects of cortical spreading depression. Progress Neurobiol. 2008;86:379–395. doi: 10.1016/j.pneurobio.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin HK, Hong KW. Importance of calcitonin gene-related peptide, adenosine and reactive oxygen species in cerebral autoregulation under normal and diseased conditions. Clinical Experimental Pharmacol Physiol. 2004;31:1–7. doi: 10.1111/j.1440-1681.2004.03943.x. [DOI] [PubMed] [Google Scholar]
- 7.Toyoda K, Faraci FM, Watanabe Y, Ueda T, Andresen JJ, Chu Y, Otake S, Heistad DD. Gene transfer of calcitonin gene-related peptide prevents vasoconstriction after subarachnoid hemorrhage. Circulation Res. 2000;87:818–824. doi: 10.1161/01.res.87.9.818. [DOI] [PubMed] [Google Scholar]
- 8.Poyner DR, Sexton PM, Marshall I, Smith DM, Quirion R, Born W, Muff R, Fisher JA, Foord SM. International union of pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacological Rev. 2002;54:233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- 9.Tsujikawa K, Yayama K, Hayashi T, Matsushita H, Yamaguchi T, Shigeno T, Ogitani Y, Hirayama M, Kato T, Fukada S, Takatori S, Kawasaki H, Okamoto H, Ikawa M, Okabe M, Yamamoto H. Hypertension and dysregulated proinflammatory cytokine production in receptor activity-modifying protein 1-deficient mice. Proc Natl Acad Sci. 2007;104:16702–16707. doi: 10.1073/pnas.0705974104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z, Dickerson IM, Russo AF. Calcitonin gene-related peptide receptor activation by receptor activity-modifying protein-1 gene transfer to vascular smooth muscle cells. Endocrinology. 2006;147:1932–1940. doi: 10.1210/en.2005-0918. [DOI] [PubMed] [Google Scholar]
- 11.Sabharwal R, Zhang Z, Lu Y, Abboud FM, Russo AF, Chapleau MW. Receptor activity-modifying protein-1 increases baroreflex sensitivity and attenuates angiotensin-induced hypertension. Hypertension. 2010;55:627–35. doi: 10.1161/HYPERTENSIONAHA.109.148171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Modrick ML, Didion SP, Sigmund CD, Faraci FM. Role of oxidative stress and AT1 receptors in cerebral vascular dysfunction with aging. Am J Physiol. 2009;296:H1914–H1919. doi: 10.1152/ajpheart.00300.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reckelhoff JF, Romero JC. Role of oxidative stress in angiotensin-induced hypertension. Am J Physiol. 2003;284:R893–R912. doi: 10.1152/ajpregu.00491.2002. [DOI] [PubMed] [Google Scholar]
- 14.Chrissobolis S, Didion SP, Kinzenbaw DA, Schrader LI, Dayal S, Lentz SR, Faraci FM. Glutathione peroxidase-1 plays a major role in protecting against angiotensin II-induced vascular dysfunction. Hypertension. 2008;51:872–877. doi: 10.1161/HYPERTENSIONAHA.107.103572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kazama K, Anrather J, Zhou P, Girouard H, Frys K, Milner TA, Iadecola C. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circulation Res. 2004;95:1019–26. doi: 10.1161/01.RES.0000148637.85595.c5. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Shimosawa T, Matsui H, Meng F, Supowit SC, DiPette DJ, Ando K, Fujita T. Adrenomedullin inhibits angiotensin II-induced oxidative stress via Csk-mediated inhibition of Src activity. Am J Physiol. 2007;292:H1714–21. doi: 10.1152/ajpheart.00486.2006. [DOI] [PubMed] [Google Scholar]
- 17.Schaeffer C, Vandroux D, Thomassin L, Athias P, Rochette L, Connat JL. Calcitonin gene-related peptide partly protects cultured smooth muscle cells from apoptosis induced by an oxidative stress via activation of ERK1/2 MAPK. Biochim Biophys Acta. 2003;1643:65–73. doi: 10.1016/j.bbamcr.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimoto T, Gochou N, Fukai N, Sugiyama T, Shichiri M, Hirata Y. Adrenomedullin inhibits angiotensin II-induced oxidative stress and gene expression in rat endothelial cells. Hypertension Res. 2005;28:165–172. doi: 10.1291/hypres.28.165. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z, Winborn CS, Marquez de Prado B, Russo AF. Sensitization of calcitonin gene-related peptide receptors by receptor activity-modifying protein-1 in the trigeminal ganglion. J Neurosci. 2007;27:2693–2703. doi: 10.1523/JNEUROSCI.4542-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sobey CG, Faraci FM. Effects of a novel inhibitor of guanylyl cyclase on dilator responses of mouse cerebral arterioles. Stroke. 1997;28:837–842. doi: 10.1161/01.str.28.4.837. [DOI] [PubMed] [Google Scholar]
- 21.Wang W, Sun W, Wang X. Intramuscular gene transfer of CGRP inhibits neointimal hyperplasia after balloon injury in the rat abdominal aorta. Am J Physiol. 2004;287:H1582–H1589. doi: 10.1152/ajpheart.00168.2004. [DOI] [PubMed] [Google Scholar]
- 22.Ye F, Deng PY, Li D, Luo D, Li NS, Deng S, Deng HW, Li YJ. Involvement of endothelial cell-derived CGRP in heat stress-induced protection of endothelial function. Vascular Pharmacol. 2007;46:238–246. doi: 10.1016/j.vph.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Aukes AM, Bishop N, Godfrey J, Cipolla MJ. The influence of pregnancy and gender on perivascular innervation of rat posterior cerebral arteries. Reprod Sci. 2008;15:411–419. doi: 10.1177/1933719107314067. [DOI] [PubMed] [Google Scholar]
- 24.Ashton D, Hieble P, Gout B, Aiyar N. Vasodilatory effect of adrenomedullin in mouse aorta. Pharmacology. 2000;61:101–105. doi: 10.1159/000028388. [DOI] [PubMed] [Google Scholar]
- 25.Hay DL, Poyner DR, Quirion R. International union of pharmacology. LXIX. Status of the calcitonin gene-related peptide subtype 2 receptor. Pharmacol Rev. 2008;60:143–145. doi: 10.1124/pr.108.00372. [DOI] [PubMed] [Google Scholar]
- 26.Tam CW, Husmann K, Clark NC, Clark JE, Lazar Z, Ittner LM, Gotz J, Douglas G, Grant AD, Sudgen D, Poston L, Poston R, McFadzean I, Marber MS, Fisher JA, Born W, Brain SD. Enhanced vascular responses to adrenomedullin in mice overexpressing receptor-activity-modifying protein 2. Circulation Res. 2009;98:262–270. doi: 10.1161/01.RES.0000200737.63865.58. [DOI] [PubMed] [Google Scholar]
- 27.Villalon CM, Olesen J. The role of CGRP in the pathophysiology of migraine and efficacy of CGRP receptor antagonists as acute antimigraine drugs. Pharmacol Ther. 2009;124:309–323. doi: 10.1016/j.pharmthera.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Brian JE, Jr, Heistad DD, Faraci FM. Mechanisms of endotoxin-induced dilatation of cerebral arterioles. Am J Physiol. 1995;269:H783–H788. doi: 10.1152/ajpheart.1995.269.3.H783. [DOI] [PubMed] [Google Scholar]
- 29.Hay DL, Poyner DR, Sexton PM. GPCR modulation by RAMPs. Pharmacol Ther. 2006;109:173–197. doi: 10.1016/j.pharmthera.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 30.Dong YL, Green KE, Vegiragu S, Hankins GD, Martin E, Chauhan M, Thota C, Yallampalli C. Evidence for decreased calcitonin gene-related peptide (CGRP) receptors and compromised resonsiveness to CGRP of fetoplacental vessels in preeclamptic prenancies. J Clin Endocrin Metabol. 2005;90:2336–2343. doi: 10.1210/jc.2004-1481. [DOI] [PubMed] [Google Scholar]
- 31.Cuielle C, Birot O, Bigard X, Hagner S, Garel JM. Post-transcriptional regulation of CRLR expression during hypoxia. Biochem Biophys Res Comm. 2004;326:23–29. doi: 10.1016/j.bbrc.2004.10.205. [DOI] [PubMed] [Google Scholar]
- 32.Nagae T, Mukoyama N, Sugawara A, Mori K, Yahata K, Kasahara M, Suganami T, Makino H, Fujinaga Y, Yoshioka T, Tanaka I, Nakao K. Rat receptor-activity-modifying proteins (RAMPs) for adrenomedullin/CGRP receptor: Cloning and upregulation in obstructive nephropathy. Biochem Biophys Res Comm. 2000;270:89–93. doi: 10.1006/bbrc.2000.2390. [DOI] [PubMed] [Google Scholar]
- 33.Baumbach GL, Sigmund CD, Faraci FM. Cerebral arteriolar structure in mice overexpressing human renin and angiotensinogen. Hypertension. 2003;41:50–55. doi: 10.1161/01.hyp.0000042427.05390.5c. [DOI] [PubMed] [Google Scholar]
- 34.Chrissobolis S, Faraci FM. Role of oxidative stress and NADPH oxidase in cerebrovascular disease. Trends Molecular Med. 2008;14:495–502. doi: 10.1016/j.molmed.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qin XP, Ye F, Hu CP, Liao DF, Deng HW, Li YJ. Effect of calcitonin gene-related peptide on angiotensin II-induced proliferation of rat vascular smooth muscle cells. Eur J Pharmacol. 2004;488:45–49. doi: 10.1016/j.ejphar.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 36.Liang L, Tam CW, Pozsgai G, Siow R, Clark N, Keeble J, Husmann K, Born W, Fischer JA, Poston R, Shah A, Brain SD. Protection of angiotensin II-induced vascular hypertrophy in vascular smooth muscle-targeted receptor activity-modifying protein 2 transgenic mice. Hypertension. 2009;54:1254–1261. doi: 10.1161/HYPERTENSIONAHA.109.129783. [DOI] [PubMed] [Google Scholar]