Abstract
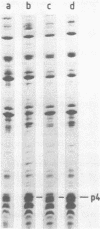
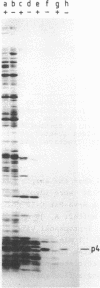
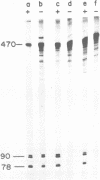
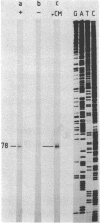
The phage phi 29 protein p4, that controls viral late transcription, was highly purified from Escherichia coli cells harbouring a gene 4-containing plasmid. This protein, representing about 6% of the total cellular protein, was obtained in a highly purified form. The protein was characterized as p4 by amino acid analysis and NH2-terminal sequence determination. The purified protein was active in an in vitro transcription assay, allowing specific initiation of transcription at the phi 29 A3 late promoter in the presence of Bacillus subtilis sigma 43-RNA polymerase holoenzyme.
Full text
PDF


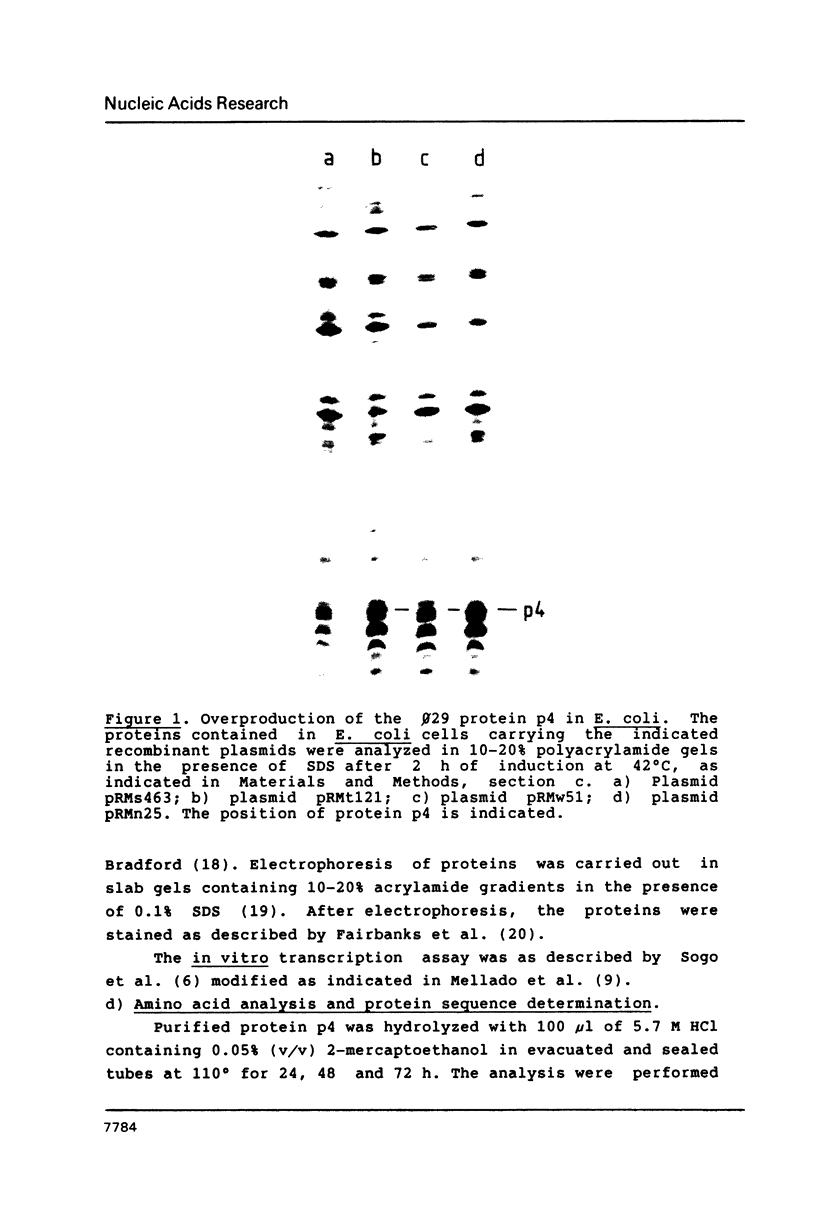


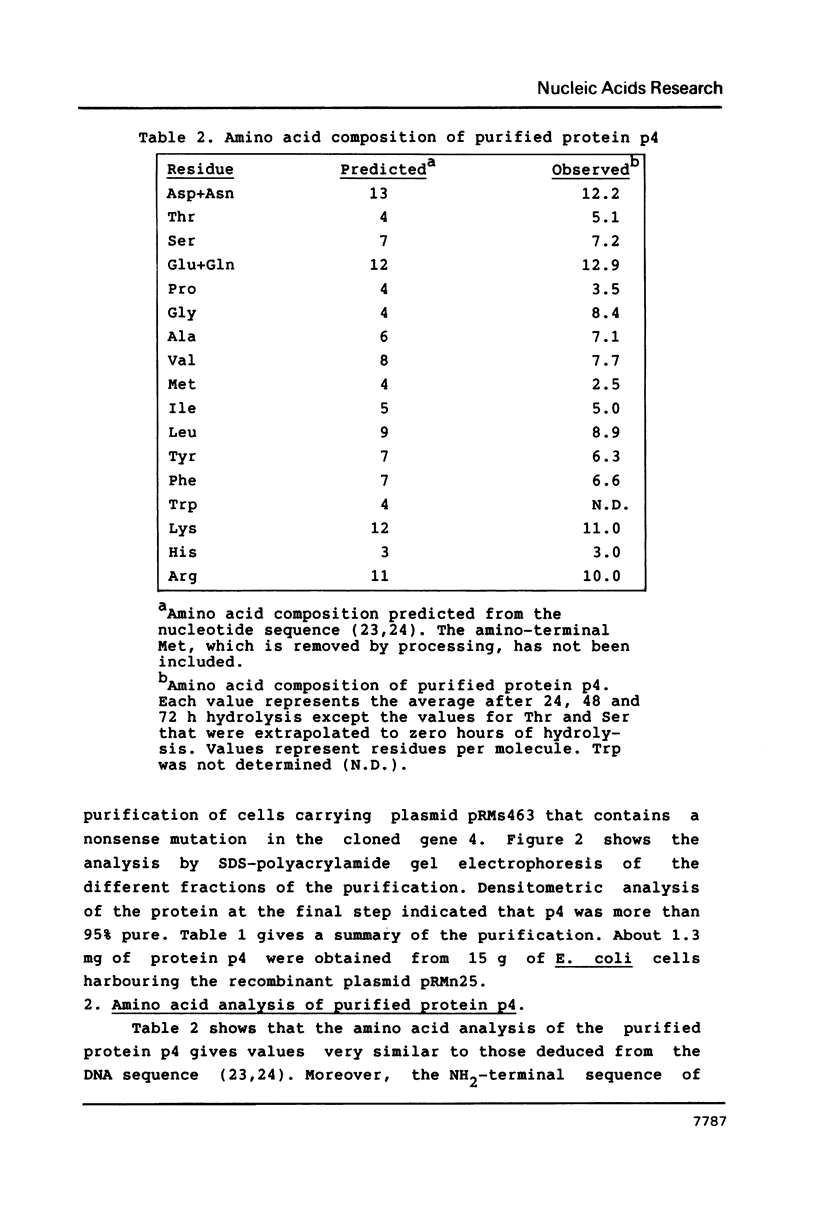
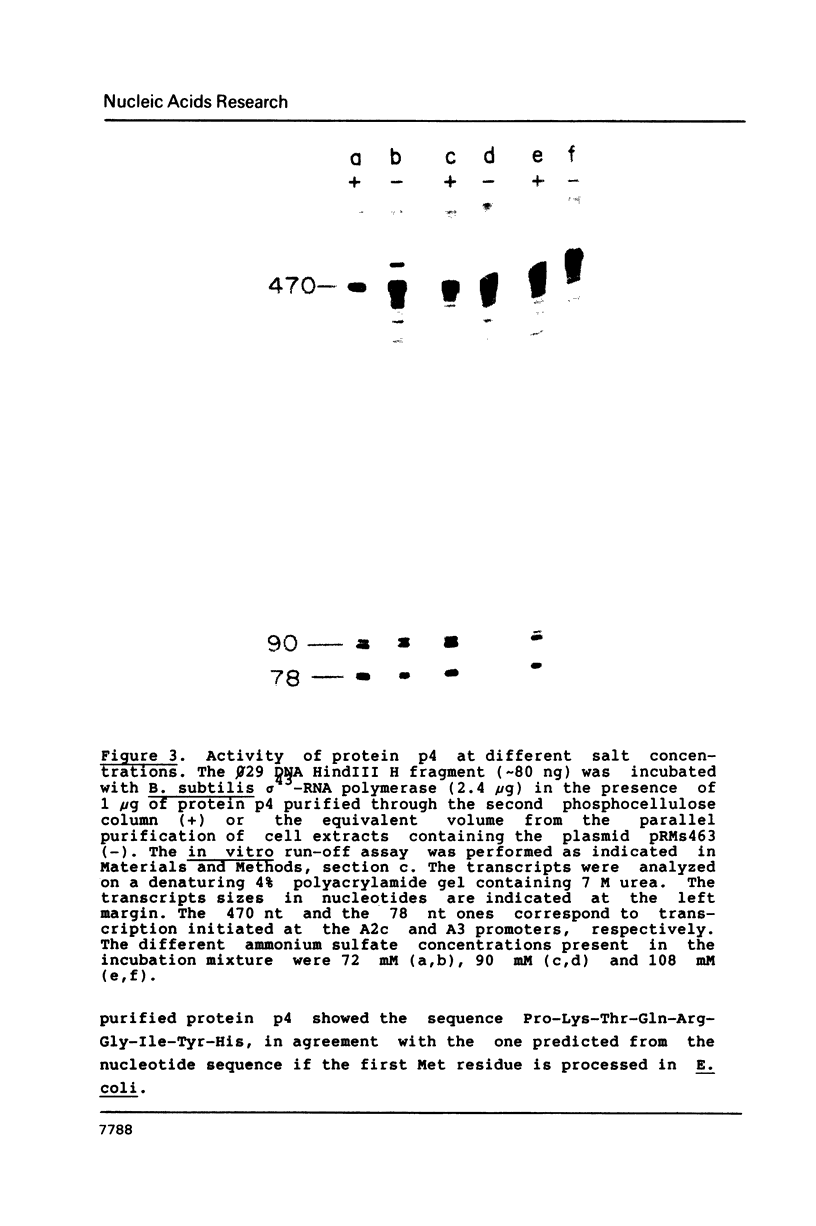
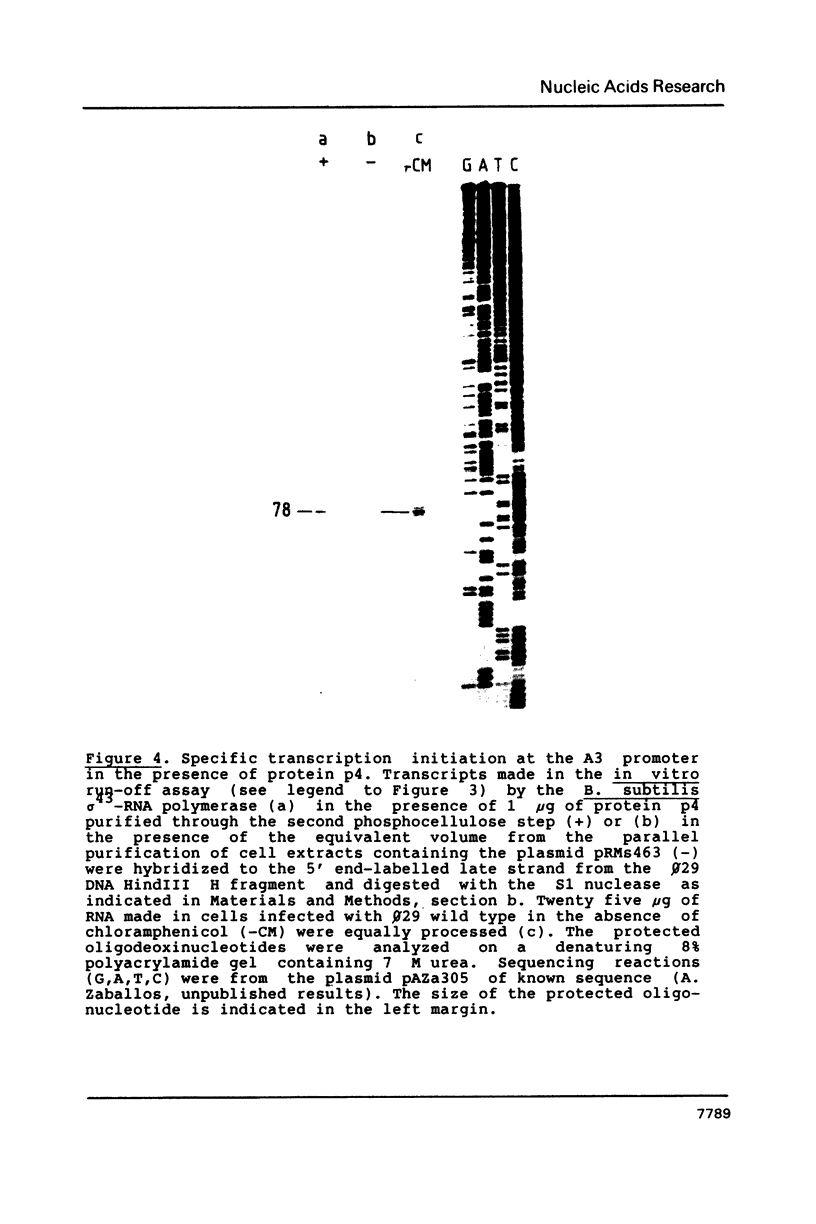
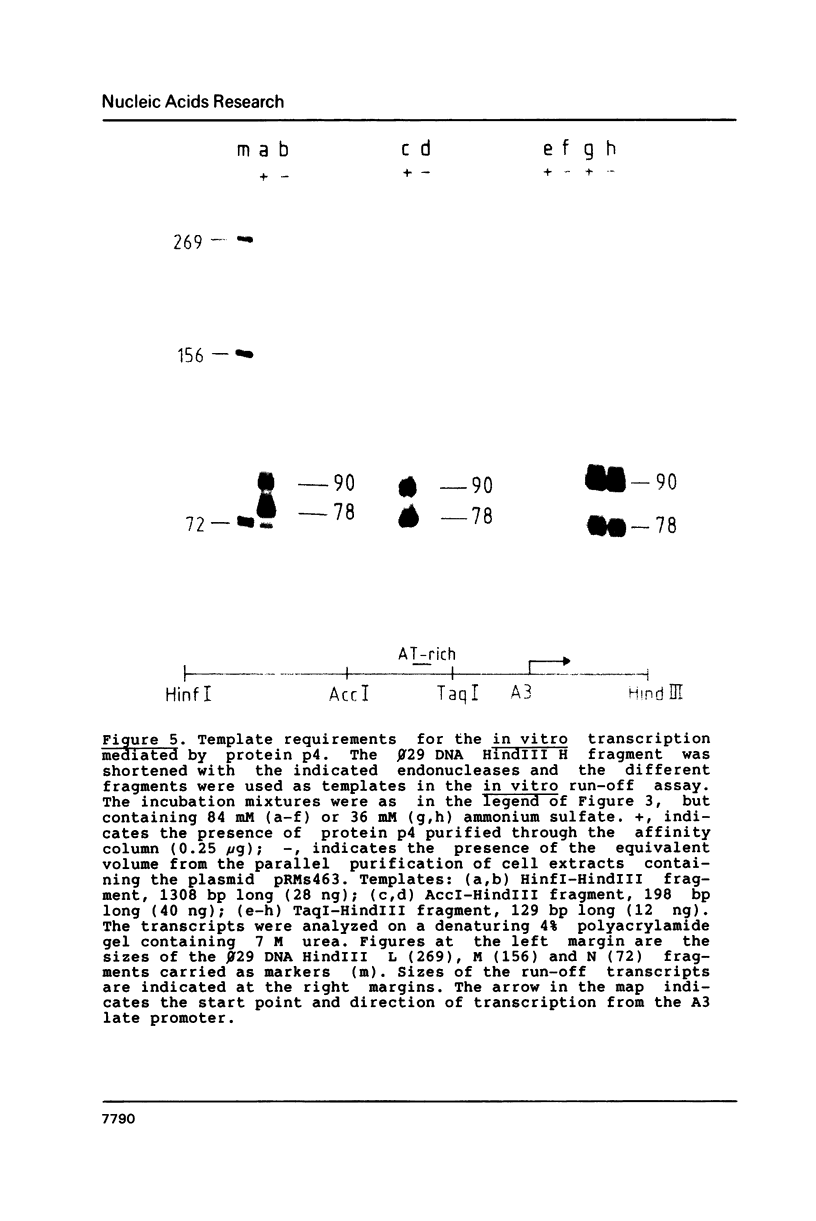



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barthelemy I., Salas M., Mellado R. P. In vivo transcription of bacteriophage phi 29 DNA: transcription initiation sites. J Virol. 1986 Dec;60(3):874–879. doi: 10.1128/jvi.60.3.874-879.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carrascosa J. L., Camacho A., Moreno F., Jiménez F., Mellado R. P., Viñuela E., Salas M. Bacillus subtilis phage phi29. Characterization of gene products and functions. Eur J Biochem. 1976 Jul 1;66(2):229–241. doi: 10.1111/j.1432-1033.1976.tb10512.x. [DOI] [PubMed] [Google Scholar]
- Davison B. L., Murray C. L., Rabinowitz J. C. Specificity of promoter site utilization in vitro by bacterial RNA polymerases on Bacillus phage phi 29 DNA. Transcription mapping with exonuclease III. J Biol Chem. 1980 Sep 25;255(18):8819–8830. [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Escarmís C., Salas M. Nucleotide sequence at the termini of the DNA of Bacillus subtilis phage phi 29. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1446–1450. doi: 10.1073/pnas.78.3.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escarmís C., Salas M. Nucleotide sequence of the early genes 3 and 4 of bacteriophage phi 29. Nucleic Acids Res. 1982 Oct 11;10(19):5785–5798. doi: 10.1093/nar/10.19.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Hjelm H., Hjelm K., Sjöquist J. Protein A from Staphylococcus aureus. Its isolation by affinity chromatography and its use as an immunosorbent for isolation of immunoglobulins. FEBS Lett. 1972 Nov 15;28(1):73–76. doi: 10.1016/0014-5793(72)80680-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mellado R. P., Barthelemy I., Salas M. In vitro transcription of bacteriophage phi 29 DNA. Correlation between in vitro and in vivo promoters. Nucleic Acids Res. 1986 Jun 25;14(12):4731–4741. doi: 10.1093/nar/14.12.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellado R. P., Barthelemy I., Salas M. In vivo transcription of bacteriophage phi 29 DNA early and late promoter sequences. J Mol Biol. 1986 Sep 20;191(2):191–197. doi: 10.1016/0022-2836(86)90256-1. [DOI] [PubMed] [Google Scholar]
- Mellado R. P., Moreno F., Viñuela E., Salas M., Reilly B. E., Anderson D. L. Genetic analysis of bacteriophage phi 29 of Bacillus subtilis: integration and mapping of reference mutants of two collections. J Virol. 1976 Aug;19(2):495–500. doi: 10.1128/jvi.19.2.495-500.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellado R. P., Salas M. High level synthesis in Escherichia coli of the Bacillus subtilis phage phi 29 proteins p3 and p4 under the control of phage lambda PL promoter. Nucleic Acids Res. 1982 Oct 11;10(19):5773–5784. doi: 10.1093/nar/10.19.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellado R. P., Salas M. Initiation of phage phi 29 DNA replication by the terminal protein modified at the carboxyl end. Nucleic Acids Res. 1983 Nov 11;11(21):7397–7407. doi: 10.1093/nar/11.21.7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami Y., Sekiya T., Hirokawa H. Nucleotide sequence of gene F of Bacillus phage Nf. Gene. 1986;42(2):231–235. doi: 10.1016/0378-1119(86)90302-1. [DOI] [PubMed] [Google Scholar]
- Nishi T., Itoh S. Enhancement of transcriptional activity of the Escherichia coli trp promoter by upstream A + T-rich regions. Gene. 1986;44(1):29–36. doi: 10.1016/0378-1119(86)90039-9. [DOI] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- Salas M., Mellado R. P., Viñuela E. Characterization of a protein covalently linked to the 5' termini of the DNA of Bacillus subtilis phage phi29. J Mol Biol. 1978 Feb 25;119(2):269–291. doi: 10.1016/0022-2836(78)90438-2. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., De Sain C. V., Anderson D. L. Transcription during the development of bacteriophage phi29: definition of "early" and "late" phi29 ribonucleic acid. J Virol. 1973 Jan;11(1):9–16. doi: 10.1128/jvi.11.1.9-16.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogo J. M., Inciarte M. R., Corral J., Viñuela E., Salas M. RNA polymerase binding sites and transcription map of the DNA of Bacillus subtilis phage phi29. J Mol Biol. 1979 Feb 5;127(4):411–436. doi: 10.1016/0022-2836(79)90230-4. [DOI] [PubMed] [Google Scholar]
- Sogo J. M., Lozano M., Salas M. In vitro transcription of the Bacillus subtilis phage phi 29 DNA by Bacillus subtilis and Escherichia coli RNA polymerases. Nucleic Acids Res. 1984 Feb 24;12(4):1943–1960. doi: 10.1093/nar/12.4.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr G. E., Beecher J. F., Bell M., McKean D. J. Polyquarternary amines prevent peptide loss from sequenators. Anal Biochem. 1978 Feb;84(2):622–7?0=ENG. doi: 10.1016/0003-2697(78)90086-6. [DOI] [PubMed] [Google Scholar]
- Vlcek C., Paces V. Nucleotide sequence of the late region of Bacillus phage phi 29 completes the 19,285-bp sequence of phi 29 genome. Comparison with the homologous sequence of phage PZA. Gene. 1986;46(2-3):215–225. doi: 10.1016/0378-1119(86)90406-3. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H., Ito J. Nucleotide sequence of the major early region of bacteriophage phi 29. Gene. 1982 Mar;17(3):323–335. doi: 10.1016/0378-1119(82)90149-4. [DOI] [PubMed] [Google Scholar]
- Zaballos A., Salas M., Mellado R. P. Initiation of phage phi 29 DNA replication by mutants with deletions at the carboxyl end of the terminal protein. Gene. 1986;43(1-2):103–110. doi: 10.1016/0378-1119(86)90013-2. [DOI] [PubMed] [Google Scholar]