Using proteomic and genomic analyses, we demonstrate that a large repertoire of DNA-binding transcription factors, including CREB, contribute to estrogen signaling through a tethering pathway.
Abstract
The indirect recruitment (tethering) of estrogen receptors (ERs) to DNA through other DNA-bound transcription factors (e.g. activator protein 1) is an important component of estrogen-signaling pathways, but our understanding of the mechanisms of ligand-dependent activation in this pathway is limited. Using proteomic, genomic, and gene-specific analyses, we demonstrate that a large repertoire of DNA-binding transcription factors contribute to estrogen signaling through the tethering pathway. In addition, we define a set of endogenous genes for which ERα tethering through activator protein 1 (e.g. c-Fos) and cAMP response element-binding protein family members mediates estrogen responsiveness. Finally, we show that functional interplay between c-Fos and cAMP response element-binding protein 1 contributes to estrogen-dependent regulation through the tethering pathway. Based on our results, we conclude that ERα recruitment in the tethering pathway is dependent on the ligand-induced formation of transcription factor complexes that involves interplay between the transcription factors from different protein families.
Estrogen signaling leading to estrogen-dependent transcriptional responses involves multiple distinct molecular mechanisms. One of these mechanisms is the tethering of estrogen receptor (ER) to DNA through protein-protein interactions with other transcription factors, such as activator protein 1 (AP-1) (1). The physiological importance of tethering in vivo was suggested by studies in the NERKI knock-in mouse strain, in which the potential for ERα tethering was maintained, but direct DNA binding by ERα was abolished (2–6). Moreover, several genome-wide ERα localization studies identified sites of ERα binding lacking estrogen response elements (EREs), supporting the indirect association of ERα with DNA through other DNA-bound transcription factors (7–13). For example, a recent study coupling genomics with gene-specific functional analyses identified Runx1 as an ERα-tethering factor (14). Estrogen-dependent regulation of genes harboring AP-1-binding elements (e.g. human collagenase, insulin-like growth protein-1, matrix metalloproteinase-1, and choline acetyltransferase genes; chicken ovalbumin gene; bovine FSH β gene) has also been described in multiple studies (15–20). These genes, together with other recently identified estrogen-regulated genes requiring AP-1 family members (21), highlight the importance of the tethering pathway for ER signaling.
The AP-1 transcription factor consists of members of the Jun, Fos, ATF (activating transcription factor), and MAF (musculoaponeurotic fibrosarcoma) basic region-leucine zipper (bZIP) motif protein families, which dimerize upon activation to regulate gene expression (22). The recognition of distinct sequence enhancer elements by the different possible dimeric combinations of the AP-1 transcription factor is responsible for the activation of specific target genes (23, 24). In vivo, the ultimate outcome of AP-1 effects on gene expression depends on the composition of the dimeric transcription factors, as well as the cellular context (23).
Previous studies have provided evidence of a direct interaction between ERα and c-Jun, as well as ERα and c-Fos (19, 25–27); Jun and Fos represent the two predominant components of the AP-1 transcription factor in mammalian cells (28). Even though the ER/AP-1-tethering pathway is accepted as an important component of estradiol (E2) signaling, our understanding of the ligand-dependent activation of this pathway is limited. In particular, the precise composition of the tethering component is poorly characterized, and the number of genes known to be regulated through the ER/AP-1 pathway is limited.
Using proteomic, genomic, and gene-specific approaches, we provide a more detailed understanding of the estrogen-dependent regulation of gene expression mediated through the tethering mechanism. First, we have isolated and identified an expanded repertoire of enhancer-specific proteins potentially involved in this pathway. Second, we have identified a set of endogenous genes regulated by estrogen signaling in the tethering pathway. Finally, we have shown a functional association between several tethering proteins and the estrogen-mediated regulatory outcome. These observations contribute to a more complete understanding of estrogen action on gene expression.
Results
Ligand-bound ERα stimulates transcription through tetradecanoyl phorbol acetate response elements (TREs) in a biochemical assay
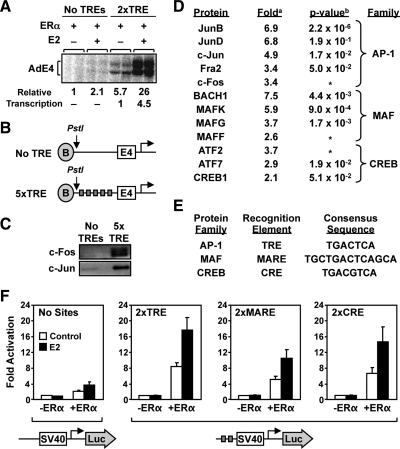
To explore the nature of the tethering component in the ERα-tethering pathway, we developed a set of biochemical assays that can be used to analyze both protein binding and transcriptional outcomes and can be coupled with a proteomic analysis. In an in vitro transcription assay with chromatin templates (29, 30), ERα stimulated transcription in an E2-dependent manner with a template containing TREs, but not with a template lacking TREs (Fig. 1A). In this assay, all of the proteins needed for the transcriptional response, including the TRE-binding proteins, were present and were supplied by a HeLa cell nuclear extract, except for ERα, which was added as a purified recombinant protein. We surmised that a binding assay based on the in vitro transcription assay might be useful for identifying the TRE-binding proteins.
Fig. 1.
Proteomic analysis of TRE-binding proteins and their relationship to ERα-dependent gene regulation. A, ERα stimulates transcription through TREs in the presence of E2 in an in vitro assay with chromatin templates. Plasmid templates containing no TREs or two TRE sites upstream of the adenovirus E4 promoter were assembled into chromatin and transcribed in the presence of ERα and E2, as indicated, using HeLa cell nuclear extract as a source of the RNA polymerase II transcription machinery. B, Schematic diagrams of the immobilized templates used in the proteomic isolation of TRE-associated proteins. C, Western blot showing the presence of c-Fos and c-Jun in the bound fraction used for mass spectrometry analysis. Immobilized templates with or without five TREs were incubated with HeLa cell nuclear extract. Bound material was subjected to Western blotting using antibodies against c-Fos and c-Jun. D, List of bZip proteins identified in the TRE-bound fraction using iTRAQ. The bound proteins from panel C were subjected to iTRAQ analysis as described in Supplemental Materials and Methods. Those proteins with greater than or equal to 2-fold enrichment were included in the list. a, Average iTRAQ117/iTRAQ114 ratio for all unique peptides corresponding to the listed protein. b, P value calculated in ProteinPilot software using the fold ratios for multiple peptides from the same protein. *, P value not determined because the fold ratio was based on single unique/unambiguous peptide sequence. E, Comparison of the consensus response elements for the AP-1, MAF, and CREB families. F, ERα + E2 stimulates gene expression in HeLa cells through TREs, MAREs, and CREs. The results shown are from transient transfection-reporter gene assays with SV40-luciferase reporter constructs lacking or containing two TREs, MAREs, or CREs sites upstream of the promoter (see schematics along bottom). Each reporter was transfected together with or without an expression vector for ERα into HeLa cells, followed by treatment ± E2 (100 nm) for 16 h. The cells were collected and subjected to luciferase assay analyses. The results are presented as fold over the −ERα/−E2 condition. Each bar represents the mean ± sem (n ≥ 3).
Unbiased identification of TRE-binding proteins
Previous studies have shown that ERα can interact with and be recruited by c-Jun and other AP-1 family members at TREs (25, 27, 31). The complete repertoire of TRE-binding proteins that might serve as ERα-tethering proteins, however, has not been determined in an unbiased manner. To explore this in more detail, we used an immobilized template-binding assay with a promoter DNA fragment similar to the one present in the in vitro transcription template described above, but containing five TREs instead of two (Fig. 1B). In this assay, we detected specific binding of both c-Jun and c-Fos from HeLa cell nuclear extract by Western blotting using the TRE template, but not a template lacking TREs (Fig. 1C).
We then coupled this binding assay with an unbiased proteomic identification of TRE-binding proteins. Specifically, we used iTRAQ labeling of peptides generated from the bound material isolated from templates with or without TREs, followed by a determination of the proteins that were enriched in the “5xTRE” condition (Supplemental Fig. 1 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org) (32). Using this approach, we identified a number of proteins that were enriched for binding to the template containing TREs, most notably bZIP proteins in the AP-1, cAMP response element-binding protein-1 (CREB1), and BACH1 families (Fig. 1D). For example, we detected specific enrichments (∼3- to 7-fold) of JunB, JunD, c-Jun, Fra2, and c-Fos in the AP-1 family (Fig. 1D), which were confirmed by Western blotting (Supplemental Fig. 2). We also observed an enrichment of BACH1, CREB1, and related family members, which are known to bind DNA sequences related to the TRE [Fig. 1E; MAF response element (MARE) and cAMP response element (CRE), respectively]. These TRE-binding proteins are candidate ERα-tethering proteins.
ERα transactivates through a TRE, MARE, or CRE
To determine whether ERα might be able to activate transcription through the DNA elements for all three classes of bZIP proteins [i.e. TRE, MARE, and CRE], we used transient transfection reporter gene assays. The reporter plasmids, which contained consensus TRE, MARE, and CRE sequences upstream of an simian virus 40 (SV40) promoter-luciferase reporter, or no binding site as a control, were cotransfected with or without an ERα expression vector into HeLa cells. We then monitored the ERα- and E2-dependent activation of the reporters. The reporters containing the TREs, MAREs, and CREs, but not the reporter lacking protein-binding sites, were activated greater than 10-fold in the presence of ERα, and about 50% of the signal in each case was E2 dependent (Fig. 1F). These results indicate that ERα can transactivate through a variety of bZIP family-binding sites, presumably through a tethering mechanism involving one or more of the HeLa cell proteins that we identified in our proteomic analysis. The remaining analyses described in this study were aimed at exploring this possibility with native genes and proteins in cells.
Identification of potential ERα-tethering sites in cells
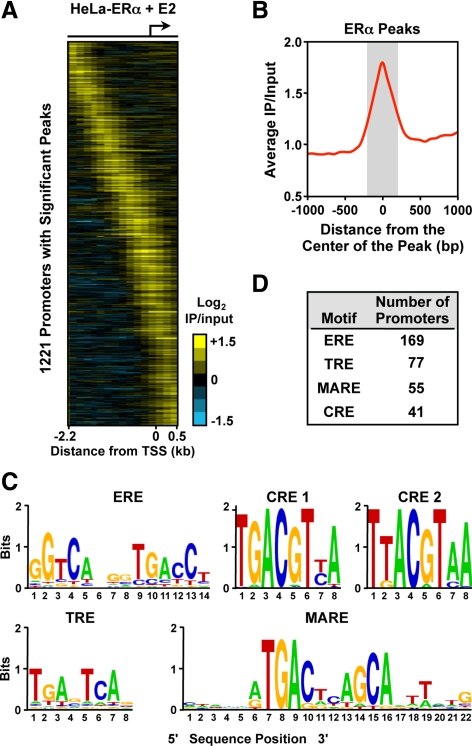
To examine whether the recruitment of ERα through a tethering mechanism to TRE-, CRE-, and MARE-like sequence-containing promoters also applies in vivo, we performed a chromatin immunoprecipitation (ChIP)-chip assay in HeLa cells stably expressing ERα, as has been done previously in breast cancer cells and other cell types (7, 8, 11, 12). We identified 1221 promoters with significant ERα peaks in the presence of E2 using a Nimblegen RefSeq HG18 promoter tiling array (Fig. 2A). We then compared the ERα-binding sites identified in our HeLa-ERα ChIP-chip experiments to ERα-binding sites from MCF-7 cells for the same promoter-proximal genomic locations represented on our array. About 9% of ERα-binding sites from MCF-7 cells overlapped with ERα-binding sites from HeLa-ERα cells (Supplemental Fig. 3), indicating that the genomic binding patterns of ERα vary considerably between cell types, as has been noted previously (33).
Fig. 2.
Analysis of TREs, CREs, MAREs, and EREs in promoter-proximal ERα-binding sites in HeLa-ERα cells. A, Analysis of promoter-proximal ERα-binding sites in HeLa-ERα cells by ChIP-chip using Nimblegen promoter arrays containing approximately 19,000 promoters tiled from 2200 bp upstream to 500 bp downstream of the TSS. The data are shown as a heat map of the log2 ERα + E2 ChIP-chip data for 1221 promoters with significant binding. B, Metagene analysis of the log2 enrichment ratios from regions in panel A showing significant ERα binding. The probe signals from all significant and unique ERα-binding sites were centered on the peak and averaged across the regions. C, Sequences generated from the TRE, CRE, MARE, and ERE position weight matrices used in the bioinformatic analysis of the significant and unique ERα-binding site peaks. The sequences are shown as web logos. The TRE, CRE, and MARE position weight matrices are from TRANSFAC (58). The ERE position weight matrix was generated based on information from O'Lone et al. (60). D, Bioinformatic analysis of TREs, CREs, MAREs, and EREs under the significant and unique ERα-binding site peaks in HeLa-ERα cells. The position weight matrices from panel C were used with MAST (34) to map the location of the binding sites. The number of promoters with a significant peak of ERα and containing a TRE, CRE, MARE, or an ERE are indicated. IP, Immunoprecipitation.
To determine what types of DNA sequence motifs might be present under the ERα-binding sites in HeLa-ERα cells, we performed a bioinformatic analysis in the regions under the ERα peaks (within 250 bp of the center of the peak; Fig. 2B). TRANSFAC matrices representing TRE, CRE, and MARE sequences (Fig. 2C) were used with motif-finding algorithms to map the location of the motifs using Motif Alignment and Search Tool (MAST) (34) within both bound and unbound regions. To avoid ambiguity concerning ERα recruitment, motifs were considered as having a potential association with ERα binding if 1) they were located within 250 bp on either side of the ERα peak center (Fig. 2B) and 2) no estrogen response element (ERE) or any of the other scanned motifs were located in this region. We identified approximately 40–80 ERα-bound TRE-, CRE-, and MARE-containing promoters and 169 ERE-containing promoters (Fig. 2D and Supplemental Fig. 4). Comparisons with MCF-7 cells revealed a similar, but distinct, distribution of EREs, TREs, CREs, and MAREs under ERα-binding sites at the promoter and, to a lesser extent, across the whole genome (Supplemental Table 1). In general, EREs and TRE-related motifs are present under a significant, but minor, fraction of the ERα-binding sites in both cell types. The genes whose promoters contain significant peaks of ERα binding in HeLa-ERα cells are listed in Supplemental Table 2, along with the motifs found under the peaks.
Correlation between receptor occupancy and gene regulation
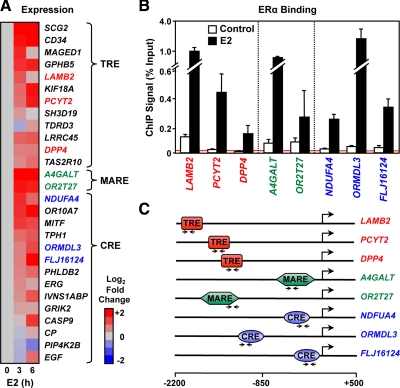
Having identified the promoters bound by ERα in the presence of E2 in our cell system, we next determined whether the presence of ERα at the potential tethering sites facilitates E2-dependent modulation of the expression of those genes. Gene-specific primers were used to examine the potential regulation of 43 genes after 3 or 6 h of E2 treatment. We identified 28 genes as being regulated after E2 treatment (Fig. 3A), including genes from all three enhancer classes, demonstrating that the recruitment of ERα correlates with the transcriptional regulation of these genes. A selection of genes representing all three classes was used to verify receptor recruitment to the response elements in the presence of E2 with ChIP-quantitative real-time PCR (qPCR) (Fig. 3, B and C).
Fig. 3.
E2-dependent regulation of ERα-bound genes lacking EREs. A, Heat map of RT-qPCR expression data for E2-regulated genes from HeLa-ERα cells presented as log2 fold ratio and separated into three classes based on the tethering protein-binding site. The cells were treated ± E2 (100 nm) as indicated. B, ChIP-qPCR analysis of the E2-dependent ERα binding to the promoters of a subset of the genes shown in panel A. Each bar represents the mean ± sem (n ≥ 3). C, Schematics of the promoters analyzed in panel B showing the enhancer element type and its location relative to the TSS. Arrow pairs indicate the location of the primers used for qPCR.
Although we do not have genome-scale gene expression data from HeLa-ERα cells, we can gauge the extent to which ERα binding in the promoter region, as well as the underlying motifs, corresponds to gene-regulatory outcomes based on existing data from MCF-7 cells. We found in MCF-7 cells that promoter-proximal ERα binding after 45 min of E2 treatment corresponds to a significant gene-regulatory outcome after 3 h of E2 treatment for about 7% of the promoters examined (Supplemental Fig. 5). The fraction of regulated genes was higher when the analysis was limited to ERα-binding sites containing EREs (9%), TREs (12%), and CREs (15%) (Supplemental Fig. 5). These results suggest that promoter-proximal ERα binding is more likely to be productive when the binding site contains an ERE, TRE, or CRE. Our more limited gene-specific analysis in HeLa-ERα cells is in agreement with this conclusion.
The DNA binding of proteins identified by proteomic analyses is modulated by E2 signaling in cells
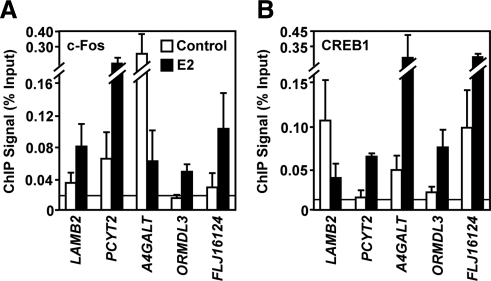
Next, we determined whether the binding of potential tethering proteins at the native E2-regulated genes that we identified might be regulated by E2 and dependent on ERα. Examination of various potential tethering proteins by ChIP-qPCR demonstrated the E2-dependent binding of these proteins at endogenous response elements (Fig. 4). Binding of c-Fos and other AP-1 proteins were enhanced at TRE sites by E2 treatment, suggesting that the presence of ERα at these regions either increases the affinity of AP-1 for DNA or plays a role in the recruitment of these AP-1 proteins. Surprisingly, the CREB family member, CREB1, is also recruited in an E2-dependent manner to some of the sites, including a TRE site. Importantly, we noticed that AP-1 proteins can bind at CREs, possibly due to the high sequence similarity between these motifs or dimerization between members of these families. In fact, CREB1 and c-Fos are both recruited to both types of binding sites (e.g. the TRE in PCYT2 and the CRE in ORMDL3 and FLJ16124) (Fig. 4). Because CREB1 and ATF2 also showed an E2-dependent recruitment, an active role for ERα in the recruitment of these proteins is likely. Our results also suggest that modulation of other proteins belonging to the CREB family (e.g. ATF2), or even the MAF family (e.g. BACH1), may play a key role in conveying E2 responsiveness at these promoters.
Fig. 4.
E2-dependent modulation of c-Fos and CREB1 binding at ERα-bound promoters that lack EREs. ChIP-qPCR analysis of c-Fos (A) and CREB1 (B) binding in HeLa-ERα cells to the promoters of a subset of the genes shown in Fig. 3A. The cells were treated ± E2 (100 nm) for 45 min. Each bar represents the mean ± sem (n ≥ 3).
Inhibition of tethering proteins inhibits E2-dependent reporter activation
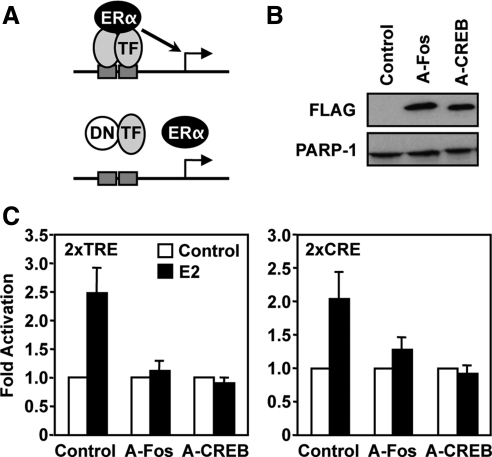
To explore further the role of the potential tethering proteins in E2-dependent gene regulation, we determined their contribution to the ligand-dependent response in transient transfection assays. For this purpose, we used the flag-tagged dominant negative (DN) versions of c-Fos (A-Fos) and CREB1 (A-CREB) described previously (35). These DN proteins prevent the wild-type proteins and their dimerization partners from binding to DNA. The E2-dependent activation of TRE and CRE reporters in transient transfection assays was abolished when c-Fos and its dimerization partners were inhibited using the A-Fos construct (Fig. 5). Interestingly, inhibiting the normal function of CREB1 and its dimerization partners also prevented the E2-dependent activation with both the TRE and CRE reporters, suggesting a critical role for CREB1 in E2 signaling with both types of binding sites.
Fig. 5.
Inhibition of E2-dependent TRE- and CRE-reporter gene activity with DN c-Fos or CREB1. A, Schematic diagram of DN inhibitor effects on c-Fos and CREB1 binding, and ERα recruitment. B, Western blot showing the expression of A-Fos and A-CREB in transfected HeLa cells vs. an empty vector control. Poly(ADP-ribose) polymerase (PARP-1) was used as a loading control. C, Effects of A-Fos and A-CREB on E2-dependent TRE- and CRE-reporter gene activity in HeLa cells. SV40-luciferase reporter constructs lacking or containing two TREs or CREs were transfected with an expression vector for ERα into HeLa cells, followed by treatment ± E2 (100 nm) for 16 h. The cells were collected and subjected to luciferase assay analyses. The results are presented as fold over the control/−E2 condition. Each bar represents the mean ± sem (n ≥ 3). TF, Transcription factor.
Functional interplay between tethering proteins and regulatory outcomes with native genes
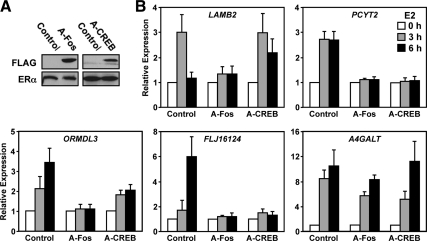
Next, we determined whether the effects of the DNs could also be observed for more complex endogenous promoters. We generated HeLa-ERα cells stably expressing the DN proteins using a retrovirus infection. Expression of the DNs in the cell lines was monitored by Western blotting (Fig. 6A). Effects on gene regulation were tested after 3 or 6 h of E2 treatment and compared with a control matched cell line generated at the same time as the DN lines. Consistent with the reporter assays, we observed that inhibition of c-Fos by A-Fos reduced activation of the TRE-containing genes PCYT2 and LAMB2, and the CRE-containing genes ORMDL3 and FLJ16124 (Fig. 6B). In addition, in the presence of the A-CREB, PCYT2 lost responsiveness to E2, whereas LAMB2 was unaffected (Fig. 6B). The CRE-containing gene FLJ16124 was unresponsive to E2 in the presence of A-CREB, whereas ORMDL3 was less responsive, but was still regulated. A redundant role of CREB family members may explain the remaining regulation, even though the recruitment of ATF2 is also inhibited in the A-CREB cell line (Supplemental Fig. 6A). Activation of A4GALT, on the other hand, remains unaffected by inhibition of c-Fos or CREB1 (Fig. 6B), suggesting the involvement of proteins other than c-Fos and CREB1.
Fig. 6.
Inhibition of E2-dependent expression of native TRE- and CRE-containing genes with DN c-Fos or CREB1. A, Western blot showing the stable expression of A-Fos, A-CREB, and ERα in HeLa-ERα cells vs. an empty vector control. ERα was used as a loading control. B, mRNA expression analyses by RT-qPCR in control, A-Fos, and A-CREB HeLa-ERα cells in response to 0, 3, and 6 h of E2 treatment (100 nm) as indicated. The data presented as fold over the 0 h E2 condition in each cell line. Each bar represents the mean ± sem (n ≥ 3).
c-Fos and CREB1 interdependency affects tethering of ERα
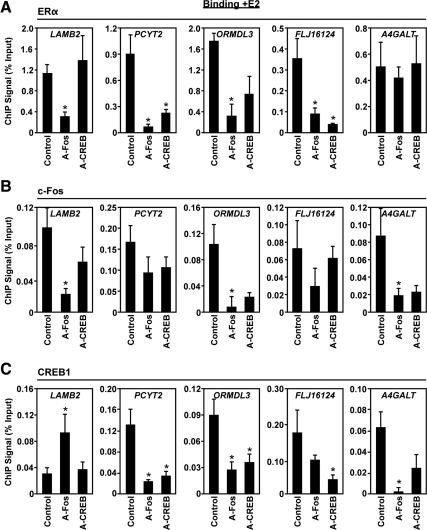
ChIP-qPCR experiments performed in the DN lines to investigate the E2-mediated corecruitment of c-Fos (Fig. 7B) and CREB1 (Fig. 7C) suggest an interdependent recruitment of these proteins to the PCYT2, ORMDL3, and A4GALT promoters. Inhibition of either protein prevents the other from proper recruitment at these promoters, whereas recruitment to LAMB2, where CREB1 is not normally recruited (Fig. 4), shows proper c-Fos recruitment and is also still regulated in the A-CREB line. The recruitment of CREB1 and c-Fos to the FLJ16124 promoter is not affected by each other's inhibition; however, the presence of both proteins is crucial for the E2-dependent regulatory effect (Fig. 6B). Functionally, the lack of response seen for PCYT2, LAMB2, ORMDL3, and FLJ16124 in the case of the A-Fos line can be explained by a loss of ERα recruitment to the binding sites (Fig. 7A). Clearly, tethering of ERα is dependent on c-Fos, but it is also dependent on CREB1 at the promoters where c-Fos and CREB1 both bind (e.g. PCYT2, FLJ16124, and ORMDL3). At A4GALT, however, we observed ligand-dependent ERα recruitment in the presence of A-Fos and A-CREB (Fig. 7A), which fits well with the expression outcome. The results of the expression and ChIP experiments with A-Fos and A-CREB are summarized in Supplemental Table 3. Taken together, our results indicate that ERα recruitment in the tethering pathway is dependent on the ligand-induced formation of transcription factor complexes that involves interplay between the transcription factors from different protein families.
Fig. 7.
Inhibition of ERα, c-Fos, and CREB1 binding to the promoters of native TRE- and CRE-containing genes with DN c-Fos or CREB1. ChIP-qPCR analysis of ERα (A), c-Fos (B), and CREB1 (C) binding in control, A-Fos, and A-CREB HeLa-ERα cells to the promoters of a subset of the genes shown in Fig. 3A. The cells were treated ± E2 (100 nm) for 45 min. Each bar represents the mean ± sem (n ≥ 3). Bars marked with an asterisk are significantly different from the control (P < 0.05).
Discussion
Our study demonstrates that a broader repertoire of DNA-binding transcription factors than previously identified is involved in mediating estrogen signaling through the tethering pathway. As is true for estrogen signaling in general, regulation of the tethering pathways is likely to be highly dependent on cell context, ER subtype, and ligand structure (16, 19). Kushner et al. (36) proposed a dual mechanism, including activation function-mediated or activation function-independent ER activation of AP-1 that emphasizes the role of coregulators as regulatory determinants. Interestingly, our data showing that E2 modulates the binding of the core tethering component at endogenous binding sites (e.g. Fig. 4) suggest that ERα-mediated activities at the binding elements are not limited to the previously described coregulator-dependent mechanisms. This observation supports an active role of ERα in the formation of the DNA-binding protein complex, rather than simply a passive tethering of ERα by the DNA-bound proteins or shared coregulators.
The fact that members of the CREB and MARE protein families also associate with TREs suggests more complexity and flexibility than has been previously appreciated for gene activation in the tethering pathway. Whether this association results from a direct ER/DNA-binding protein interaction or from binding through an intermediate coregulator is unclear. Evidence supporting the first hypothesis for a direct interaction between ERα and CREB1 under certain conditions has been previously reported (37). Our data demonstrate that reporter constructs and, more importantly, endogenous genes harboring CREs and MAREs are activated by E2 likely via a tethering mechanism similar to that involving TREs. Indeed, we see ERα recruited not only to the endogenous TRE, but also to CREs and MAREs, consistent with a tethering mechanism.
Using DN inhibitors to block DNA binding by c-Fos and CREB1, we observed cross talk between these two proteins in the estrogen-signaling pathway. The fact that both CREB1 and c-Fos are recruited at the endogenous TRE-containing promoter PCYT2 and that E2-dependent activation is inhibited by both A-CREB and A-Fos suggest an important functional cross talk between these representatives of two distinct bZIP families. This is evident at several promoters where inhibition of either CREB or c-Fos prevents the other protein from being recruited in an E2-dependent manner to regulate gene regulation. Also, in many cases, tethering of ERα is dependent on the binding of both c-Fos and CREB1.
The CREB1 result fits well with the literature, especially because CREs and CREB1 have been implicated previously as part of the estrogen-signaling pathway (37, 38). A recent global analysis of ERα and ERK2 binding across the genome showed that CREs are enriched at ERα/ERK2-binding sites, and re-ChIP experiments demonstrated that ERα, ERK2, and CREB1 all colocalize to the same site (39). Interestingly, CREB1 expression is significantly increased in patients with poor prognosis breast cancers, as well as in tumor-bearing breast adipose tissue compared with normal breast adipose tissue (40, 41). Moreover, CREs are closely linked to estrogen signaling and breast cancer via regulation of BRCA1 and aromatase gene expression (41–43), both of which play a role in the etiology and progression of breast cancers. Additional CRE-dependent estrogen-regulated genes include those encoding the IGF-1 receptor, somatostatin receptor SST2, and cyclin D1 (44–46). The estrogen-dependent up-regulation of IGF-1 receptor in prostate cancer is mediated through nongenotropic signaling involving ERK pathways (44), whereas the estrogen-dependent up-regulation of SST2 requires an ERE half-site in combination with an ATF/CRE (45). In contrast, the induction of cyclin D1 as part of the mitogenic action of estrogen is dependent on both intact ERα and the binding of c-Jun/ATF2 heterodimers to a CRE site in the promoter, which is consistent with a tethering mechanism, although the recruitment of ERα to the site was not shown (46). Our results indicate that, in addition to activating CREB1/ATF2 signaling via kinase-dependent mechanisms to regulate target genes, ERα may play a more direct role through recruitment to the promoters of estrogen-dependent genes that lack an ERE-like sequence.
In summary, we conclude that ERα recruitment in the tethering pathway is dependent on the ligand-induced formation of transcription factor complexes that involves interplay between the proteins from different transcription factor families. A fascinating feature of the ERα-tethering pathway is that, under certain conditions, selective ER modulators, which are traditionally thought to function as ERα antagonists, can function as agonists (16, 19, 36). This observation raises an interesting question of whether different ligands and, thereby receptor conformations, may induce differences in the tethering factor composition and response element preferences, which could play a role in determining selective ligand pharmacology. This hypothesis will be addressed in future studies.
Materials and Methods
Plasmids
The adenovirus E4 promoter- and TRE-containing plasmid templates used in the in vitro transcription assays and elsewhere in these studies (i.e. p2xTRE-E4 and p5xTRE-E4) were constructed from pIE0 (47) by the addition of double-stranded oligonucleotides with the TRE sequence. The unique PstI site in both plasmids was moved to the same location relative to the transcription start. The pCMV-based expression plasmids for A-Fos and A-CREB (48) were kindly provided by Dr. Charles Vinson (Laboratory of Metabolism, National Institutes of Health). Retrovirus vectors for expressing FLAG-tagged A-Fos or FLAG-tagged A-CREB were made by PCR amplifying the inserts with the tags from the pCMV-based vectors and cloning into pQCXIP (BD Biosciences, Palo Alto, CA) using NotI and EcoRI sites. The TRE, CRE, and MARE luciferase reporter vectors were constructed by cloning double-stranded oligonucleotides with the binding sites 5′ of the promoter in pGL3 (Promega Corp., Madison, WI). The pCMV5-based ERα expression vector has been described previously (49). The β-galactosidase expression vector (pCMVβ) used an internal control in the transfection assays was from CLONTECH Laboratories, Inc. (Palo Alto, CA).
Antibodies
Custom rabbit polyclonal antibodies for ERα and c-Fos have been described previously (29, 30). Rabbit polyclonal antibodies for Fra-2 (sc-604), CREB1 (SC-186), JunB (sc-73), JunD (sc-74), and ATF-2 (sc-187) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). A custom rabbit polyclonal antibody for Bach-1 was kindly provided by Dr. Kazuhiko Igarashi (School of Medicine, Tohoku University) (50).
Primers
Detailed sequence information about the oligonucleotide primers used for RT-qPCR and ChIP-qPCR can be found in Supplemental Tables 4 and 5.
Cell lines
HeLa S3 cells were purchased from the National Cell Culture Center and maintained in suspension culture in MEM Eagle medium (Sigma M0518; Sigma Chemical Co., St. Louis, MO) supplemented with 5% calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and MEM nonessential amino acids (Sigma M7145). Adherent HeLa cells were purchased from American Type Culture Collection (Manassas, VA). HeLa-ERα cells were kindly provided by Dr. David Shapiro (University of Illinois, Urbana-Champaign,) (51). Both cell types were maintained in DMEM/F12 (Sigma, D2906) supplemented with 10% charcoal-dextran-stripped calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 25 μg/ml gentamicin. DN Fos- and CREB1-overexpressing cell lines were generated by retroviral-mediated gene transfer of a pQCXIP vector containing FLAG-tagged A-Fos or A-CREB1 followed by selection using 1 μg/ml puromycin. Control cells infected with empty pQCXIP vector were generated in parallel.
In vitro chromatin assembly and transcription assays
In vitro chromatin assembly and transcription reactions were carried out as described using the p2xTRE-E4 plasmid as a template (29, 30). Briefly, purified wild-type ERα (40 nm) and E2 (400 nm) were added during the chromatin assembly reaction. In vitro transcription was performed using HeLa cell nuclear extract as a source of AP-1 proteins and the RNA polymerase II transcription machinery. Because of dilution during reaction setup, the final concentrations of ERα and E2 in the transcription assays were 12 nm and 120 nm, respectively. RNA products from the transcription reactions were analyzed by primer extension. The assays were quantified by PhosphorImager analysis with Imagequant software (Molecular Dynamics, Sunnyvale, CA). All transcription reactions were carried out in duplicate, and each experiment was performed three or more times to ensure reproducibility.
Immobilized DNA template-binding assays coupled to peptide iTRAQ labeling and mass spectrometry identification and quantification
Detailed information about protein isolation and identification can be found in the Supplemental Materials and Methods. The nuclear extract preparation protocol is based on the method of Dignam et al. (52), and the quantitative proteomics is based on the methods from Ranish et al. (32). The proteomics data have been deposited and may be downloaded from ProteomeCommons.org Tranche using the following five hashes:
guk27GD3bLhwVvJ/JvsXrwogIKH5Fhr/RQ9ms+EbjzPhMjH6j6AQU5WsTnDy6XxXVnvZZcs1+KA7+Tbecq99ffN1BDcAAAAAAAADHw==
LEVnvUl/z4H3jqAKklSUTkPIqDbpaKT3Afqi7+nHJAVVi9J3W7UsC1ZkoAOZVYfkmvQwqwmL2NdY7ZLiIYs0aK7zNgIAAAAAAAAh2 g==
8O+4rHnoxwU5FDIEVA5VZxklzkNTnwmu1jx7MmXZeky6pC6cpKW914dYL6PzYN6uEVE4ygmIxib18jzzaCgGjYo+tVEAAAAAAAAM4w==
phXPf40CDJF+PyWrpCg5EW8qX8m/aENsjhNHi0C0vJwuz58bRiiQIHGuzYBoYXsbEvlHMq4fHXeXyRMlF1uwiqprwWEAAAAAAAArgg==
te3GsncHgbcVjleKHu8zEQYAb45AXzr/hyDITnfKYRBZQnIu0OtN0kmNom3lQTsN4PtAjJdSC+iBKixUUB1x8MInOGwAAAAAAAAVQw==
ChIP assays
ChIP assays were performed essentially as described previously (53). Briefly, HeLa-ERα cells were plated and grown for 3 d to approximately 80% confluence. The cells were cross-linked with 10 mm dimethyl superimidate (Pierce Chemical Co., Rockford, IL) in PBS for 10 min at 37 C and 1% paraformaldehyde in PBS for 10 min at 37 C, followed by quenching in 125 mm glycine in PBS for 5 min at 4 C. The cells were collected by centrifugation and sonicated in lysis buffer (50 mm Tris·HCl, pH 7.9; 10 mm EDTA; 1% sodium dodecyl sulfate; 1 mm dithiothreitol + 1× protease inhibitor cocktail; Roche) to generate chromatin fragments of approximately 300–700 bp in length. The containing lysate was clarified by centrifugation, diluted 10-fold in dilution buffer (20 mm Tris·HCl, pH 7.9; 150 mm NaCl; 2 mm EDTA; 0.5% Triton X-100; 1 mm dithiothreitol + 1× protease inhibitor cocktail), and precleared with protein A-agarose beads (Millipore Corp., Bedford, MA; 16–125). The precleared, chromatin-containing supernatant was used in immunoprecipitation reactions with antibodies against ERα, CREB1, c-Fos, Bach 1, Fra2, ATF-2, JunB, and JunD; a no-antibody condition was used as a control. The immunoprecipitated genomic DNA was cleared of protein and residual RNA by digestion with proteinase K and ribonuclease H, respectively. The DNA was then extracted with phenol-chloroform-isoamyl alcohol and precipitated with ethanol. For gene-specific ChIP analyses, qPCR was used to determine the enrichment of immunoprecipitated material relative to the input material using primer sets directed to the specified regions. Each ChIP experiment was conducted a minimum of three times with independent chromatin isolates to ensure reproducibility.
ChIP-chip
For the ERα ChIP-chip analyses, immunoprecipitated genomic DNA prepared from E2-treated HeLa-ERα cells was blunted, amplified by ligation-mediated PCR, and used to probe a Nimblegen promoter array (HG18 RefSeq promoter array; C4226-00-01). The array includes approximately 19,000 promoters tiled from 2200 bp upstream to 500 bp downstream of the transcription start site (TSS) with 50- to 75-mer probes. The ERα ChIP-chip analyses were performed in duplicate from two independent preparations of ChIP material to ensure reproducibility. The data were processed as described previously (54) with modifications.
Briefly, the log2 signal ratios for each probe on the arrays were subjected to lowess normalization (55), and the normalized data were scaled to equivalent sum of squares. A single-array error model was generated using a 600-bp moving window with 150-bp steps in which both the mean probe log2 ratio and P values were calculated for each window. The P values are from a nonparametric Wilcoxon signed-rank test. Significant windows on each array were defined as those with 1) six of six positive probes per window, 2) a Wilcoxon P value <0.01, and 3) a window mean greater than 0. Peaks were defined as individual, adjacent, or consecutive windows that were significant in both replicates. The relative distance of all windows to the closest TSS was determined using RefSeq gene annotations from the UCSC genome browser. Window means were averaged for graphing purposes, not for peak analysis. The TSS anchored heat map used to visualize the ChIP-chip data (Fig. 2) was generated with Java Treeview (56). For genes with multiple TSS the most 5′-TSS was used. The ChIP data have been deposited in National Center for Biotechnology Information's Gene Expression Omnibus and are accessible through GEO Series accession no. GSE22099 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE22099).
Motif analyses
The promoter regions (∼ −2 kb to +0.5 kb) were scanned for the presence of transcription factor motifs with MAST (34, 57) using a sixth order Markov model and the default match threshold (P = 0.0001). Motif sequences found within 250 bp (or more stringently within 75 bp) of the peak center were determined. Promoters with one unique motif sequence within 250 bp of the center of the ERα peak were selected for further investigation. Transcription factor searches were conducted using weight matrices obtained from the TRANSFAC database (58, 59). The TRANSFAC transcription factor motifs used for mapping were as follows: AP-1 (TRE) (M00199), CRE (M00040, M00041), MARE (M00284). The ERE motif was a consensus defined by O'Lone et al. (60).
Transient transfection reporter gene assays
HeLa cells were seeded at 1 × 105 cells per well in 12-well plates 24 h before transfection in DMEM/F12 supplemented with 2% charcoal-dextran-stripped calf serum. Cells were transfected at about 65% confluence with 300 ng of luciferase reporter, 10 ng of pCMV5-ERα, 50 ng of pCMVβ, and 50 ng pCMV-A-Fos or pCMV-A-CREB per well using Gene Juice as instructed by the manufacturer (Novagen, Madison, WI). The cells were treated 6 h after transfection with or without E2 (100 nm) for an additional 16 h before they were collected for determination of luciferase and ß-galactosidase activity. Luciferase activity and ß-galactosidase were measured using a 96-well plate reader (LD400; Beckman Coulter, Fullerton, CA); luciferase activity was normalized to ß-galactosidase. To ensure reproducibility, each assay was run in duplicate, and each experiment was performed at least three times.
mRNA analyses by RT-qPCR
HeLa-ERα, HeLa-ERα/A-Fos, HeLa-ERα/A-CREB, and HeLa-ERα/control cells were seeded at approximately 1.5 × 105 cells per well in six-well plates in DMEM/F12 medium supplemented with 2% charcoal-dextran-stripped calf serum. After growth to about 80% confluence, the cells were treated with E2 (100 nm) for 0, 3, or 6 h, and total RNA was isolated using Trizol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Total RNA was reverse transcribed and subjected to real-time PCR with gene-specific primers. All target gene transcripts were normalized to the β-actin transcript. A fold change of 1.5 was set as a cutoff for the genes to be considered regulated. Each experiment was conducted a minimum of three times with independent isolates of total RNA to ensure reproducibility.
Acknowledgments
We thank members of the Kraus laboratory for technical advice, helpful discussions, and critical comments on this study and manuscript. We thank Dr. David Shapiro for the HeLa-ERα cells, Dr. Charles Vinson for providing the DN A-Fos construct, and Dr. Kazuhiko Igarashi for providing the BACH1 antibody. We thank Tim Quill for assistance with editing this manuscript.
This work was supported by a Marie Curie Outgoing International Fellowship (to N.H.); a predoctoral fellowship from the US Department of Defense Breast Cancer Research Program (to G.D.I.); and Grant DK058110 from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (to W.L.K.).
Present address for N.H.: Department of Neuroscience, Karolinska Institutet, 17177 Stockholm, Sweden.
Present address for G.D.I.: Liberty University, Department of Chemistry and Biology, Lynchburg, Virginia 24502.
Disclosure Summary: The authors have nothing to disclose.
NURSA Molecule Pages:
Nuclear Receptors: ER-α
Ligands: 17β-estradiol
Footnotes
- AP-1
- Activator protein 1
- ATF
- activating transcription factor
- bZIP
- basic region-leucine zipper
- CRE
- cAMP response element
- CREB1
- cAMP-response element-binding protein-1
- DN
- dominant negative
- E2
- estradiol
- ER
- estrogen receptor
- ERE
- estrogen response element
- MAF
- musculoaponeurotic fibrosarcoma
- MARE
- MAF response element
- MAST
- Motif Alignment and Search Tool
- qPCR
- quantitative real-time PCR
- SV40
- simian virus 40
- TRE
- tetradecanoyl phorbol acetate response element
- TSS
- transcription start site.
References
- 1. Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M, Gustafsson JA. 2007. Estrogen receptors: how do they signal and what are their targets. Physiol Rev 87:905–931 [DOI] [PubMed] [Google Scholar]
- 2. McDevitt MA, Glidewell-Kenney C, Jimenez MA, Ahearn PC, Weiss J, Jameson JL, Levine JE. 2008. New insights into the classical and non-classical actions of estrogen: evidence from estrogen receptor knock-out and knock-in mice. Mol Cell Endocrinol 290:24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jakacka M, Ito M, Martinson F, Ishikawa T, Lee EJ, Jameson JL. 2002. An estrogen receptor (ER)α deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Mol Endocrinol 16:2188–2201 [DOI] [PubMed] [Google Scholar]
- 4. O'Brien JE, Peterson TJ, Tong MH, Lee EJ, Pfaff LE, Hewitt SC, Korach KS, Weiss J, Jameson JL. 2006. Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor α binding to classical estrogen response elements. J Biol Chem 281:26683–26692 [DOI] [PubMed] [Google Scholar]
- 5. Syed FA, Mödder UI, Fraser DG, Spelsberg TC, Rosen CJ, Krust A, Chambon P, Jameson JL, Khosla S. 2005. Skeletal effects of estrogen are mediated by opposing actions of classical and nonclassical estrogen receptor pathways. J Bone Miner Res 20:1992–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weiss J, Bernhardt ML, Laronda MM, Hurley LA, Glidewell-Kenney C, Pillai S, Tong M, Korach KS, Jameson JL. 2008. Estrogen actions in the male reproductive system involve estrogen response element-independent pathways. Endocrinology 149:6198–6206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, Fox EA, Silver PA, Brown M. 2005. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122:33–43 [DOI] [PubMed] [Google Scholar]
- 8. Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M. 2006. Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38:1289–1297 [DOI] [PubMed] [Google Scholar]
- 9. Gao H, Fält S, Sandelin A, Gustafsson JA, Dahlman-Wright K. 2008. Genome-wide identification of estrogen receptor α-binding sites in mouse liver. Mol Endocrinol 22:10–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kininis M, Chen BS, Diehl AG, Isaacs GD, Zhang T, Siepel AC, Clark AG, Kraus WL. 2007. Genomic analyses of transcription factor binding, histone acetylation, and gene expression reveal mechanistically distinct classes of estrogen-regulated promoters. Mol Cell Biol 27:5090–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin CY, Vega VB, Thomsen JS, Zhang T, Kong SL, Xie M, Chiu KP, Lipovich L, Barnett DH, Stossi F, Yeo A, George J, Kuznetsov VA, Lee YK, Charn TH, Palanisamy N, Miller LD, Cheung E, Katzenellenbogen BS, Ruan Y, Bourque G, Wei CL, Liu ET. 2007. Whole-genome cartography of estrogen receptor α binding sites. PLoS Genet 3:e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Welboren WJ, van Driel MA, Janssen-Megens EM, van Heeringen SJ, Sweep FC, Span PN, Stunnenberg HG. 2009. ChIP-Seq of ERα and RNA polymerase II defines genes differentially responding to ligands. EMBO J 28:1418–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vega VB, Lin CY, Lai KS, Kong SL, Xie M, Su X, Teh HF, Thomsen JS, Yeo AL, Sung WK, Bourque G, Liu ET. 2006. Multiplatform genome-wide identification and modeling of functional human estrogen receptor binding sites. Genome Biol 7:R82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stender JD, Kim K, Charn TH, Komm B, Chang KC, Kraus WL, Benner C, Glass CK, Katzenellenbogen BS. 2010. Genome-wide analysis of estrogen receptor α DNA binding and tethering mechanisms identifies Runx1 as a novel tethering factor in receptor-mediated transcriptional activation. Mol Cell Biol 30:3943–3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gaub MP, Bellard M, Scheuer I, Chambon P, Sassone-Corsi P. 1990. Activation of the ovalbumin gene by the estrogen receptor involves the fos-jun complex. Cell 63:1267–1276 [DOI] [PubMed] [Google Scholar]
- 16. Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. 1997. Differential ligand activation of estrogen receptors ERα and ERβ at AP1 sites. Science 277:1508–1510 [DOI] [PubMed] [Google Scholar]
- 17. Schmitt FC. 1995. Multistep progression from an oestrogen-dependent growth towards an autonomous growth in breast carcinogenesis. Eur J Cancer 31A:2049–2052 [DOI] [PubMed] [Google Scholar]
- 18. Umayahara Y, Kawamori R, Watada H, Imano E, Iwama N, Morishima T, Yamasaki Y, Kajimoto Y, Kamada T. 1994. Estrogen regulation of the insulin-like growth factor I gene transcription involves an AP-1 enhancer. J Biol Chem 269:16433–16442 [PubMed] [Google Scholar]
- 19. Webb P, Lopez GN, Uht RM, Kushner PJ. 1995. Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cell-specific estrogen-like effects of antiestrogens. Mol Endocrinol 9:443–456 [DOI] [PubMed] [Google Scholar]
- 20. Webb P, Nguyen P, Valentine C, Lopez GN, Kwok GR, McInerney E, Katzenellenbogen BS, Enmark E, Gustafsson JA, Nilsson S, Kushner PJ. 1999. The estrogen receptor enhances AP-1 activity by two distinct mechanisms with different requirements for receptor transactivation functions. Mol Endocrinol 13:1672–1685 [DOI] [PubMed] [Google Scholar]
- 21. DeNardo DG, Kim HT, Hilsenbeck S, Cuba V, Tsimelzon A, Brown PH. 2005. Global gene expression analysis of estrogen receptor transcription factor cross talk in breast cancer: identification of estrogen-induced/activator protein-1-dependent genes. Mol Endocrinol 19:362–378 [DOI] [PubMed] [Google Scholar]
- 22. Newman JR, Keating AE. 2003. Comprehensive identification of human bZIP interactions with coiled-coil arrays. Science 300:2097–2101 [DOI] [PubMed] [Google Scholar]
- 23. Eferl R, Wagner EF. 2003. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer 3:859–868 [DOI] [PubMed] [Google Scholar]
- 24. van Dam H, Castellazzi M. 2001. Distinct roles of Jun:Fos and Jun:ATF dimers in oncogenesis. Oncogene 20:2453–2464 [DOI] [PubMed] [Google Scholar]
- 25. Lambertini E, Tavanti E, Torreggiani E, Penolazzi L, Gambari R, Piva R. 2008. ERα and AP-1 interact in vivo with a specific sequence of the F promoter of the human ERα gene in osteoblasts. J Cell Physiol 216:101–110 [DOI] [PubMed] [Google Scholar]
- 26. Qi X, Borowicz S, Pramanik R, Schultz RM, Han J, Chen G. 2004. Estrogen receptor inhibits c-Jun-dependent stress-induced cell death by binding and modifying c-Jun activity in human breast cancer cells. J Biol Chem 279:6769–6777 [DOI] [PubMed] [Google Scholar]
- 27. Teyssier C, Belguise K, Galtier F, Chalbos D. 2001. Characterization of the physical interaction between estrogen receptor α and JUN proteins. J Biol Chem 276:36361–36369 [DOI] [PubMed] [Google Scholar]
- 28. Hess J, Angel P, Schorpp-Kistner M. 2004. AP-1 subunits: quarrel and harmony among siblings. J Cell Sci 117:5965–5973 [DOI] [PubMed] [Google Scholar]
- 29. Kraus WL, Kadonaga JT. 1998. p300 and estrogen receptor cooperatively activate transcription via differential enhancement of initiation and reinitiation. Genes Dev 12:331–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cheung E, Acevedo ML, Cole PA, Kraus WL. 2005. Altered pharmacology and distinct coactivator usage for estrogen receptor-dependent transcription through activating protein-1. Proc Natl Acad Sci USA 102:559–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Siewit CL, Gengler B, Vegas E, Puckett R, Louie MC. 2010. Cadmium promotes breast cancer cell proliferation by potentiating the interaction between ERα and c-Jun. Mol Endocrinol 24:981–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ranish JA, Yi EC, Leslie DM, Purvine SO, Goodlett DR, Eng J, Aebersold R. 2003. The study of macromolecular complexes by quantitative proteomics. Nat Genet 33:349–355 [DOI] [PubMed] [Google Scholar]
- 33. Krum SA, Miranda-Carboni GA, Lupien M, Eeckhoute J, Carroll JS, Brown M. 2008. Unique ERα cistromes control cell type-specific gene regulation. Mol Endocrinol 22:2393–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bailey TL, Williams N, Misleh C, Li WW. 2006. MEME: discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res 34:W369–W373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Olive M, Krylov D, Echlin DR, Gardner K, Taparowsky E, Vinson C. 1997. A dominant negative to activation protein-1 (AP1) that abolishes DNA binding and inhibits oncogenesis. J Biol Chem 272:18586–18594 [DOI] [PubMed] [Google Scholar]
- 36. Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P. 2000. Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol 74:311–317 [DOI] [PubMed] [Google Scholar]
- 37. Lazennec G, Thomas JA, Katzenellenbogen BS. 2001. Involvement of cyclic AMP response element binding protein (CREB) and estrogen receptor phosphorylation in the synergistic activation of the estrogen receptor by estradiol and protein kinase activators. J Steroid Biochem Mol Biol 77:193–203 [DOI] [PubMed] [Google Scholar]
- 38. Aronica SM, Kraus WL, Katzenellenbogen BS. 1994. Estrogen action via the cAMP signaling pathway: stimulation of adenylate cyclase and cAMP-regulated gene transcription. Proc Natl Acad Sci USA 91:8517–8521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Madak-Erdogan Z, Lupien M, Stossi F, Brown M, Katzenellenbogen BS. 2011. Genomic collaboration of estrogen receptor α and extracellular signal-regulated kinase 2 in regulating gene and proliferation programs. Mol Cell Biol 31:226–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chhabra A, Fernando H, Watkins G, Mansel RE, Jiang WG. 2007. Expression of transcription factor CREB1 in human breast cancer and its correlation with prognosis. Oncol Rep 18:953–958 [PubMed] [Google Scholar]
- 41. Sofi M, Young MJ, Papamakarios T, Simpson ER, Clyne CD. 2003. Role of CRE-binding protein (CREB) in aromatase expression in breast adipose. Breast Cancer Res Treat 79:399–407 [DOI] [PubMed] [Google Scholar]
- 42. Bulun SE, Lin Z, Zhao H, Lu M, Amin S, Reierstad S, Chen D. 2009. Regulation of aromatase expression in breast cancer tissue. Ann NY Acad Sci 1155:121–131 [DOI] [PubMed] [Google Scholar]
- 43. Ghosh S, Lu Y, Hu Y. 2008. A role of CREB in BRCA1 constitutive promoter activity and aromatase basal expression. Int J Biomed Sci 4:260–265 [PMC free article] [PubMed] [Google Scholar]
- 44. Genua M, Pandini G, Sisci D, Castoria G, Maggiolini M, Vigneri R, Belfiore A. 2009. Role of cyclic AMP response element-binding protein in insulin-like growth factor-i receptor up-regulation by sex steroids in prostate cancer cells. Cancer Res 69:7270–7277 [DOI] [PubMed] [Google Scholar]
- 45. Kimura N, Takamatsu N, Yaoita Y, Osamura RY, Kimura N. 2008. Identification of transcriptional regulatory elements in the human somatostatin receptor sst2 promoter and regions including estrogen response element half-site for estrogen activation. J Mol Endocrinol 40:75–91 [DOI] [PubMed] [Google Scholar]
- 46. Sabbah M, Courilleau D, Mester J, Redeuilh G. 1999. Estrogen induction of the cyclin D1 promoter: involvement of a cAMP response-like element. Proc Natl Acad Sci USA 96:11217–11222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pazin MJ, Kamakaka RT, Kadonaga JT. 1994. ATP-dependent nucleosome reconfiguration and transcriptional activation from preassembled chromatin templates. Science 266:2007–2011 [DOI] [PubMed] [Google Scholar]
- 48. Rishi V, Gal J, Krylov D, Fridriksson J, Boysen MS, Mandrup S, Vinson C. 2004. SREBP-1 dimerization specificity maps to both the helix-loop-helix and leucine zipper domains: use of a dominant negative. J Biol Chem 279:11863–11874 [DOI] [PubMed] [Google Scholar]
- 49. Kim MY, Woo EM, Chong YT, Homenko DR, Kraus WL. 2006. Acetylation of estrogen receptor α by p300 at lysines 266 and 268 enhances the deoxyribonucleic acid binding and transactivation activities of the receptor. Mol Endocrinol 20:1479–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Suzuki H, Tashiro S, Hira S, Sun J, Yamazaki C, Zenke Y, Ikeda-Saito M, Yoshida M, Igarashi K. 2004. Heme regulates gene expression by triggering Crm1-dependent nuclear export of Bach1. EMBO J 23:2544–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang CC, Krieg S, Shapiro DJ. 1999. HMG-1 stimulates estrogen response element binding by estrogen receptor from stably transfected HeLa cells. Mol Endocrinol 13:632–643 [DOI] [PubMed] [Google Scholar]
- 52. Dignam JD, Lebovitz RM, Roeder RG. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res 11:1475–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kininis M, Isaacs GD, Core LJ, Hah N, Kraus WL. 2009. Postrecruitment regulation of RNA polymerase II directs rapid signaling responses at the promoters of estrogen target genes. Mol Cell Biol 29:1123–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Krishnakumar R, Gamble MJ, Frizzell KM, Berrocal JG, Kininis M, Kraus WL. 2008. Reciprocal binding of PARP-1 and histone H1 at promoters specifies transcriptional outcomes. Science 319:819–821 [DOI] [PubMed] [Google Scholar]
- 55. Smyth GK, Speed T. 2003. Normalization of cDNA microarray data. Methods 31:265–273 [DOI] [PubMed] [Google Scholar]
- 56. Saldanha AJ. 2004. Java Treeview–extensible visualization of microarray data. Bioinformatics 20:3246–3248 [DOI] [PubMed] [Google Scholar]
- 57. Bailey TL, Gribskov M. 1998. Methods and statistics for combining motif match scores. J Comput Biol 5:211–221 [DOI] [PubMed] [Google Scholar]
- 58. Wingender E, Chen X, Fricke E, Geffers R, Hehl R, Liebich I, Krull M, Matys V, Michael H, Ohnhäuser R, Prüss M, Schacherer F, Thiele S, Urbach S. 2001. The TRANSFAC system on gene expression regulation. Nucleic Acids Res 29:281–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wingender E, Chen X, Hehl R, Karas H, Liebich I, Matys V, Meinhardt T, Prüss M, Reuter I, Schacherer F. 2000. TRANSFAC: an integrated system for gene expression regulation. Nucleic Acids Res 28:316–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. O'Lone R, Frith MC, Karlsson EK, Hansen U. 2004. Genomic targets of nuclear estrogen receptors. Mol Endocrinol 18:1859–1875 [DOI] [PubMed] [Google Scholar]