In hormone depletion-insensitive prostate cancer, the androgen receptor supports hormone-depletion insensitive growth by non-classical mechanisms of action on target genes.
Abstract
A need for androgen response elements (AREs) for androgen receptor (AR)-dependent growth of hormone depletion-insensitive prostate cancer is generally presumed. In such cells, androgen-independent activation by AR of certain genes has been attributed to selective increases in basal associations of AR with putative enhancers. We examined the importance of AR binding to DNA in prostate cancer cells in which proliferation in the absence of hormone was profoundly (∼90%) dependent on endogenous AR and where the receptor was not up-regulated or mutated but was predominantly nuclear. Here, ARE-mediated promoter activation and the binding of AR to a known ARE in the chromatin remained entirely androgen dependent, and the cells showed an androgen-responsive gene expression profile with an unaltered sensitivity to androgen dose. In the same cells, a different set of genes primarily enriched for cell division functions was activated by AR independently of hormone and significantly overlapped the signature gene overexpression profile of hormone ablation-insensitive clinical tumors. After knockdown of endogenous AR, hormone depletion-insensitive cell proliferation and AR apoprotein-dependent gene expression were rescued by an AR mutant that was unable to bind to ARE but that could transactivate through a well-established AR tethering protein. Hormone depletion-insensitive AR binding sites in the chromatin were functional, binding, and responding to both the wild-type and the mutant AR and lacked enrichment for canonical or noncanonical ARE half-sites. Therefore, a potentially diverse set of ARE-independent mechanisms of AR interactions with target genes must underlie truly hormone depletion-insensitive gene regulation and proliferation in prostate cancer.
The androgen receptor (AR) plays an essential role in the development and physiology of the prostate by mediating the actions of the natural androgens, testosterone, and dihydrotestosterone (1). The major form of AR signaling is transcriptional (2, 3) with a relatively minor contribution from its nongenomic/cytosolic interactions (4–6). Similar to other steroid receptors, the AR apo-protein (protein molecule with no bound ligand) occurs in a cytosolic complex containing heat shock proteins; ligand binding causes the receptor to dissociate from this complex and translocate to the nucleus (7) and to bind as a homodimer to a hormone response element in its target genes (8, 9). The agonist bound AR molecule then recruits coactivators; in contrast, when bound to antagonists, corepressors are preferentially recruited (10, 11). AR shares the typical domain structure of other steroid receptors (12) but also has several distinctive characteristics in its structural and functional organization (2, 13–16), including its ability to bind as a homodimer to both direct and inverted repeat androgen response elements (AREs) (17).
AR is also commonly expressed in malignant prostate, where it is believed to support both androgen-dependent growth and subsequent refractoriness to androgen ablation (18–21). The development of androgen ablation-insensitivity presents a major problem in treating prostate cancer that in its early stages responds well to androgen ablation (22). AR may support androgen-independent growth of prostate tumors through one or more mechanisms, notably up-regulation of AR, AR mutations, an altered AR coregulator complement, and changes in the phosphorylation or acetylation status of AR (22, 23). Dysregulated signaling pathways that support androgen-independent prostate cancer growth, including mitogen activated protein kinase, phosphoinositide 3-kinase/v-akt murine thymoma viral oncogene homolog 1, and protein kinase C, converge on AR (24, 25). Cellular and molecular changes in hormone depletion-insensitive prostate cancer cells apparently enable AR to enter the nucleus and regulate genes independently of androgen.
Although DNA sequence variations of the ARE and its interactions with AR have been well characterized (26), the functionally relevant AREs from which individual androgen-responsive genes are regulated have only been definitively identified for a limited number of genes, because AR appears to commonly regulate its target gene promoters from multiple sites at great distances from the target promoter, generally more than 10 kb (27, 28). Nevertheless, the concept of ARE-mediated gene activation by androgen extends to the prevalent view of transcriptional regulation by AR in all hormone depletion-insensitive cells. For example, it has been demonstrated that overexpression of AR in prostate cancer cells will sensitize the cells to postablation levels of androgen in vivo or up to an 80% lower androgen concentration in vitro and also result in an agonist response to classical androgen antagonists (23). It has also been suggested that AR mutations that alter its ligand specificity may enable its activation by cross-reacting ligands and antiandrogens in hormone depletion-insensitive tumors (29–31). In both these cases (i.e. hypersensitization and altered ligand specificity), the ligand bound AR requires a functional DNA-binding domain (DBD), and therefore, it appears to exert its transcriptional activity through its classical mechanism by binding to response elements in its target genes (23). On the other hand, posttranslational modifications and some mutations of AR associated with androgen-independent growth of prostate cancer have been presumed to alter its conformation, not only allowing hormone depletion-insensitive nuclear localization of the receptor but also its association as a homodimer with target AREs (22, 24, 32, 33).
There is not an adequate amount of direct and unequivocal evidence for a necessary role for AREs in situations in which AR signaling supports the proliferation of prostate cancer cells completely deprived of hormone (i.e. in “truly” hormone depletion-insensitive cells). In a recent study of hormone depletion-insensitive cells (34), specific AREs were assigned to a few selected AR-activated genes based on an increase in androgen-independent association of AR compared with the same regions in hormone-sensitive cells. Nevertheless, androgen further increased the binding of AR at those sites in the hormone depletion-insensitive cells, indicating that the binding of AR to those AREs was suboptimal in the absence of androgen. On the other hand, in hormone depletion-insensitive cells, the well-established AREs of the androgen-responsive prostate-specific antigen (PSA) gene (35–37) are not occupied by AR in the absence of hormone (38), indicating that AR either targets such genes indirectly or that it acts through an ARE-independent mechanism. Identification of hormone depletion-insensitive sites of AR binding in the chromatin of hormone depletion-insensitive cells has been limited by the less extensive and weak signals obtained by chromatin immunoprecipitation (ChIP) methods (34). It was the goal of this study to use a different approach to examine the importance of AREs in the activation of genes critical for the growth of hormone depletion-insensitive prostate cancer cells. This information is of obvious importance for the design of mechanism-based treatment strategies, particularly in view of the fact that many advanced prostate tumors do not have amplified AR or show heterogeneity in AR expression levels.
The AR-positive LNCaP prostate cancer cell line is heterogeneous and is known to become androgen independent after extended growth in vitro or in castrated mice in vivo. Androgen independence of the late passage LNCaP cells presumably reflects selection pressure for a subpopulation of cells identified by profiling of cluster designation cell surface markers (39), especially when androgen is unavailable or is limiting. Hormone depletion-insensitive LNCaP cells developed by serial transplantation of tumor xenografts in castrated mice tend to survive by acquiring hypersensitivity to postablation levels of androgen in association with up-regulation of AR (23). In contrast, we found that a subpopulation of LNCaP cells selected for during prolonged growth in vitro (named LP50 cells) exhibited AR-dependent but truly hormone depletion-insensitive growth, i.e. without an associated increase in AR expression or hypersensitization to androgen. Because advanced prostate tumors are quite heterogeneous with respect to AR expression (40, 41), we chose the LP50 cells as a reasonable model to examine the role of AREs in AR signaling in the context of truly hormone depletion-insensitive cell proliferation.
Results
Proliferation of LP50 cells is AR dependent but hormone depletion insensitive without an associated increase in AR
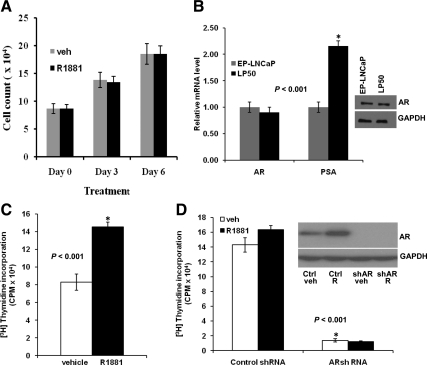
LNCaP is a heterogeneous cell line that was derived from a hormone ablation-insensitive prostate tumor, but the cells in early passage (EP) (EP-LNCaP) are known to exhibit androgen dependence. Under normal culture conditions, LP50 cells grew more rapidly than EP-LNCaP cells, with a doubling time that was half that of EP-LNCaP cells. Addition of the synthetic androgen, R1881, did not influence the growth of LP50 cells in hormone-depleted media (Fig. 1A). The basal level of the AR protein, as well as AR mRNA in LP50 cells, was comparable with that of EP-LNCaP cells (Fig. 1B). Further, the sequencing of reverse-transcribed endogenous AR mRNA in LP50 cells showed an identical sequence to that in EP- LNCaP cells. However, LP50 cells showed a higher level of mRNA for PSA (Fig. 1B).
Fig. 1.
Role of androgen and AR in the proliferation of EP-LNCaP and LP50 cells. A, Hormone-depleted LP50 cells were treated with R1881 (1 nm) or vehicle (veh). The cells were counted in a coulter particle counter. B, EP-LNCaP and LP50 cells were hormone depleted. The endogenous AR protein levels or GAPDH [loading control (ctrl)] were measured by Western blotting (inset). The mRNA for AR and PSA were measured by real-time RT-PCR. C, Hormone-depleted EP-LNCaP cells were treated with R1881 (1 nm) or vehicle. At 18 h of the treatment, [3H]thymidine was added to the media, and 6 h later, the incorporation of the radiolabel into DNA was measured. D, LP50 cells were infected with AR shRNA lentivirus or nontarget control lentivirus; 12 h after infection, the cells were treated to deplete them of hormone and to measure [3H]thymidine incorporation in response to treatment with either vehicle or R1881, all exactly as described for C. D, inset, Western blotting showing the expression of AR or GAPDH (loading control) in LP50 cells infected with AR shRNA lentivirus or nontarget control shRNA lentivirus and treated with vehicle or R1881. CPM, Count per minute; *, P < 0.001.
R1881 stimulated the incorporation of 3H-labeled thymidine in EP-LNCaP cells (Fig. 1C) but did not significantly stimulate [3H]thymidine incorporation in LP50 cells (Fig. 1D), although the uptake and functionality of R1881 in LP50 cells was apparent in consideration of its known ability to stabilize AR (Fig. 1D, inset). Infection of LP50 cells with AR short hairpin RNA (shRNA) lentivirus resulted in a knockdown of AR to an undetectable level (Fig. 1D, inset) and an inhibition of proliferation by more than 90% (Fig. 1D); this demonstrates a profound dependence of LP50 cells on hormone depletion-insensitive AR signaling.
The AR in LP50 cells is localized in the nucleus
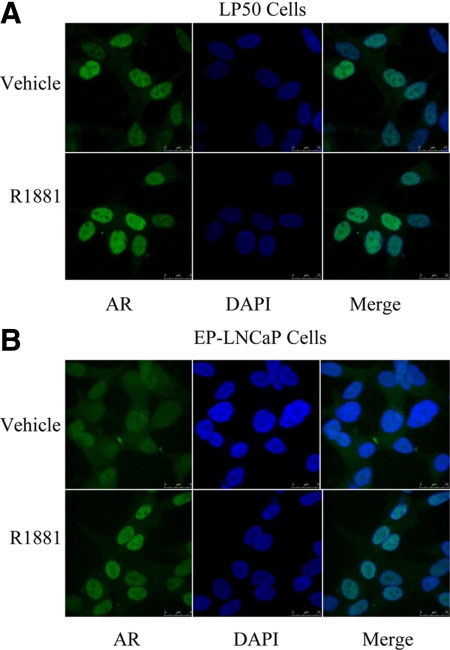
The intracellular localization of AR observed by confocal microscopy was predominantly nuclear in LP50 cells even in the absence of hormone (Fig. 2A); in contrast, the EP-LNCaP cells generally showed a mixed distribution of AR between the nucleus and the cytosol and a dependence on androgen for nuclear localization of AR (Fig. 2B). In LP50 cells, hormone depletion-insensitive nuclear localization of AR is consistent with its ability to support androgen-independent proliferation.
Fig. 2.
Subcellular localization of AR in LP50 cells and in EP-LNCaP cells. LP50 cells (A) or EP-LNCaP cells (B) grown in chamber slides were depleted of hormones and then treated with either vehicle or R1881 for 12 h. Immunofluorescence staining for AR was performed using a primary rabbit antibody to AR and a bovine antirabbit IgG-FITC as the secondary antibody. The nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI), and fluorescence images were captured by confocal microscopy. A, Representative images show predominant nuclear localization of AR (green fluorescence) in LP50 cells both with and without R1881 treatment. B, Representative images show a mixed distribution of AR (green fluorescence) between nuclear and cytosolic compartments in the absence of hormone but a predominantly nuclear distribution after R1881 treatment. Note that the brighter fluorescence staining of AR in both A and B upon R1881 treatment is consistent with the expected stabilization of AR by R1881.
Functional and physical association of AR with the ARE requires androgen in LP50 cells
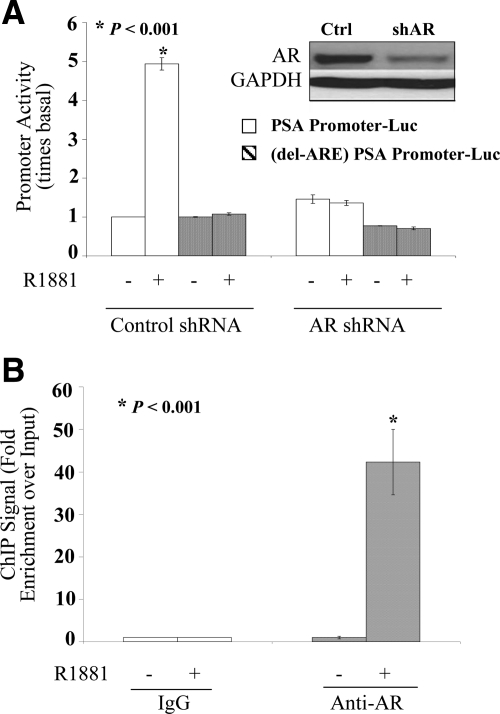
The ability of AR in LP50 cells to activate through the classical PSA enhancer was studied using transfected promoter-luciferase (promoter-Luc) reporter constructs. The PSA promoter plus enhancer region, including 6.1 kb of upstream DNA sequence, is known to be activated by androgen and AR by the binding of the receptor predominantly to a cluster of AREs located at −4366 to −3874 nucleotides (nt). As a negative control for ARE-mediated effects, cells were also transfected with the same promoter construct in which only the AREs were deleted. R1881 stimulated the promoter activity in an ARE-dependent manner (Fig. 3A). Cotransfection of AR shRNA plasmid effectively knocked down AR as evident from both the Western blotting (Fig. 3A, inset) and the inability of R1881 to activate the promoter (Fig. 3A). Knocking down AR did not significantly affect the basal PSA promoter activity (Fig. 3A), indicating that AR could not functionally associate with the ARE in the absence of hormone.
Fig. 3.
Androgen dependence for functional and physical association of AR with classical response elements in LP50 cells. A, Hormone-depleted LP50 cells were transfected by nucleofection with either the PSA promoter (6.1-kb fragment)-Luc reporter construct or with the same construct in which the multiple dispersed AREs were removed by deletion of only the AREs that include −4366 to −3874 nt and an additional internal deletion of −170 to −159 nt (delARE-PSA promoter-Luc) as indicated. The cells were cotransfected with AR shRNA or nontargeting shRNA (negative control). In addition, all wells were cotransfected with the Renilla luciferase control plasmid. R1881 (1 nm) or vehicle was added to the culture media 12 h after transfection; 72 h after transfection, the samples were harvested either to measure luciferase activity or for Western blot analysis. The promoter activity values are plotted as the ratio to the basal activity of the corresponding promoter in the nontargeting shRNA controls. Triplicate samples were included in each experimental set. A, inset, Western blotting showing the expression of AR or GAPDH (loading control). B, Hormone-depleted LP50 cells were treated with R1881 (1 nm) or vehicle for 2 h and subjected to ChIP assay using either an AR-specific rabbit antibody or negative control rabbit IgG. The immunoprecipitated DNA fragments as well as input DNA were amplified. Enrichment for fragments covering the major ARE enhancer region of PSA (−4366 to −3874 nt) within the immunoprecipited DNA compared with input DNA was measured by real-time PCR targeting the enhancer region. Specificity of the immunoprecipitation was also confirmed by the lack of enrichment of an irrelevant target sequence within the open reading frame of the PSA gene.
The ability of AR to associate with AREs in the chromatin context in situ was also tested in LP50 cells by ChIP analysis. The major cluster of AREs within the PSA enhancer region (−4366 to −3874 nt) was chosen as the target region for the ChIP assay. The AR ChIP signal was strikingly enriched in the R1881-treated cells relative to the input genomic DNA, but there was not a significant enrichment in the absence of hormone (Fig. 3B), indicating the requirement for hormone for AR to physically associate with the ARE in situ.
LP50 cells are not hypersensitized to androgen
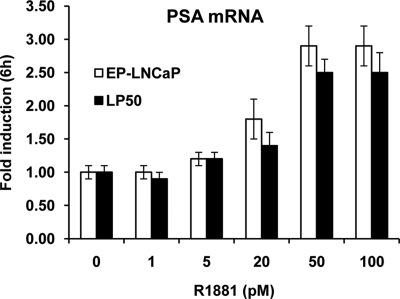
It has been demonstrated (23) that androgen-independent prostate cancer cells could simply be sensitized to lower androgen concentrations rather than being truly androgen independent; hypersensitization to androgen was associated with an increase in AR expression. Although LP50 cells did not have elevated AR, it was nevertheless of significance in this study to test whether they had acquired hypersensitivity to androgen by any other means. Therefore, the R1881 dose dependence for induction of endogenous PSA mRNA was compared between EP-LNCaP cells and LP50 cells. After hormone depletion, both cells showed a similar R1881 dose response, which was optimal at 50 pm (Fig. 4). Therefore, LP50 cells are not hypersensitized to low levels of R1881 compared with their androgen-dependent counterpart and are thus truly androgen insensitive.
Fig. 4.
R1881 dose response of endogenous PSA expression in LP50 cells vs. EP-LNCaP cells. LP50 cells and EP-LNCaP cells were depleted of hormone and then treated with vehicle or R1881 (1–500 pm) in triplicate wells for 6 h. Total RNA was extracted from the cells, and the level of mRNA for PSA was measured by quantitative real-time RT-PCR. The values of the PSA mRNA levels were normalized to the corresponding values for GAPDH mRNA.
In hormone-depleted LP50 cells, the AR-activated gene set is primarily enriched for cell division functions and reflects gene overexpression in advanced prostate tumors
The influence of completely knocking down AR (using shRNA lentivirus; see Fig. 1D, inset) on the mRNA profile of LP50 cells was examined in the absence of hormone by Affymetrix DNA microarray analysis. For this purpose, RNA was obtained from hormone-deprived control and AR knockdown cells. A decrease in the basal expression by more than or equal to 50% was observed for a total of 2015 probe sets upon knocking down AR (annotated gene list provided in Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). The Affymetrix probe set identifiers for the genes down 2-fold or more (1291 IDs) were submitted to the Database for Annotation, Visualization, and Integrated Discovery server (42) for functional classification. Compared with the background set of all human genes, this group was predominantly enriched for gene clusters supporting cell division.
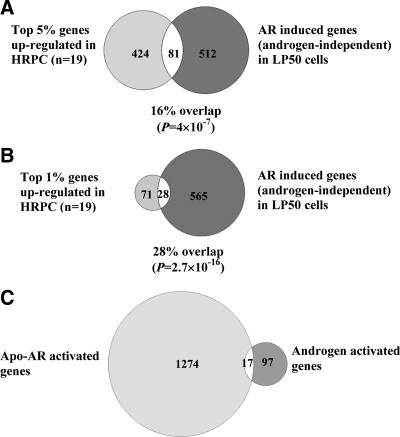
The possible relevance of the genes identified in LP50 cells as targets of hormone depletion-insensitive regulation by AR to clinical prostate cancer progression was examined. Tomlins et al. (43) have recently reported the pattern of mRNA up-regulation associated with the malignant epithelial cells in clinical metastatic (hormone ablation-insensitive) prostate tumors by examining a platform of approximately 10,000 unique genes. The Affymetrix platform used in the present study to identify genes up-regulated in LP50 cells by AR independent of hormone represents approximately 20,000 unique annotated genes. Of the 1291 AR-regulated (independent of hormone) genes identified in this study, 593 were represented in the platform used by Tomlins et al. (43); this subset of genes was therefore used to examine overlap with the genes up-regulated in the Tomlins study. A comparison with the top 5% genes (505 genes) most consistently overexpressed in the metastatic tumors (Fig. 5A) showed an overlap of 81 genes (16%); this overlap is highly significant, with a P of 4 × 10−7, and the average fold-up-regulation of these genes by AR in LP50 cells was 4.21. A comparison with the top 1% most consistently overexpressed genes in the metastatic tumors (99 genes) (Fig. 5B) showed a match of 28 genes (28%). This correlation was highly significant, with a P of 2.7 × 10−16. The average fold-up-regulation of the overlapping gene subset by AR in LP50 cells was 3.31; for comparison, the average fold-change for the 1291 AR-dependent genes was 3.47. The annotated gene lists are provided in Supplemental Fig. 2. Using the Database for Annotation, Visualization, and Integrated Discovery server for gene ontology analysis as above, the set of 81 overlapping genes was also predominantly categorized as gene clusters with cell division functions, with the lowest P of 2.7 × 10−18 for mitotic cell cycle.
Fig. 5.
Comparison of gene subsets up-regulated in clinical advanced prostate tumors with those up-regulated by AR in LP50 cells independently of androgen. LP50 cells were infected with AR shRNA lentivirus or nontarget control lentivirus; 12 h after infection, the cells were grown in hormone-free media to deplete androgen. The cells were then treated for 6 h with either vehicle or R1881 (1 nm) and harvested to obtain total RNA. The mRNA profile was determined using replicate samples by Affymetrix microarray analysis. Complete knockdown of AR due to the AR shRNA lentivirus was confirmed by Western blot analysis. mRNAs that were decreased by more than or equal to 50% were identified as those induced by AR independent of hormone. In the analysis in A and B, genes found to be consistently up-regulated in 19 advanced hormone ablation-insensitive prostate tumors within a platform of approximately 10,000 annotated gene probes [Tomlins et al. (43)] were used. A subset of either the top 5% most consistently up-regulated genes (A) or the top 1% most consistently up-regulated genes (B) was compared with 593 genes contained in the same platform that were found to be up-regulated by AR in an androgen-independent manner in LP50 cells. C, All of the genes up-regulated by Apo-AR (i.e. genes identified by Affymetrix DNA microarray analysis of LP50 cells after AR knockdown) are compared with genes that were up-regulated in LP50 cells by a 6-h treatment with R1881 (determined by Affymetrix DNA microarray analysis). HRPC, Hormone refractory prostate cancer.
The results demonstrate that the pattern of hormone depletion-insensitive gene regulation by AR, observed in LP50 cells, is both functionally and clinically relevant to advanced prostate cancer.
A distinct set of AR-regulated genes in LP50 cells is early androgen responsive with only a few among them showing partial androgen independence
The effect of R1881 treatment on the mRNA profile of LP50 cells was determined by Affymetrix DNA microarray analysis. A total of 114 unique annotated genes showed a more than or equal to 2-fold increase in expression within 6 h of treatment, indicating that the ability of androgen to strongly activate genes was retained despite the progression to hormone depletion insensitivity (annotated gene lists provided in Supplemental Fig. 3). Among the androgen-responsive genes, the basal expression of only 17 genes was also decreased by knocking down AR in LP50 cells. This subset of genes represents less than 15% of the 6-h androgen-responsive genes (Fig. 5C).
ARE-independent gene activation and hormone depletion-insensitive cell proliferation are supported by a DBD mutant form of AR (mutAR)
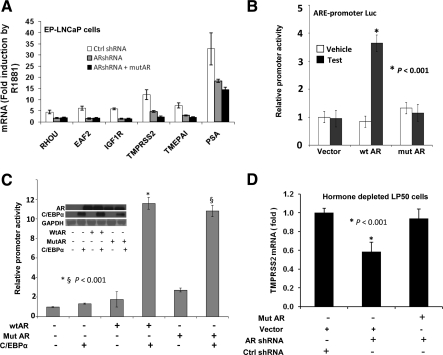
A mutAR that lacked the ability to bind to ARE was used as a tool to directly test whether the ability of AR to support proliferation of LP50 cells was independent of ARE, notwithstanding the limitation that the mutation could also partially or fully disrupt other modes of interaction with target genes. mutAR was identical in amino acid sequence to that of the endogenous AR in LNCaP cells with the exception that it contained a V581F mutation in its DBD; the V581F mutation has been previously demonstrated to disrupt DNA binding (44). mutAR also contained silent mutations to eliminate the target sequence for knockdown by AR shRNA. In EP-LNCaP cells in which endogenous AR was knocked down, ectopic mutAR was unable to rescue androgen activation of known androgen target genes (Fig. 6A). mutAR was also unable to activate an ARE-driven promoter-Luc reporter in transfected HeLa cells in contrast to the wild-type AR (wtAR) (Fig. 6B). Both wtAR and mutAR, however, were able to activate an artificial minimal promoter-Luc reporter construct containing three tandem CCAAT/enhancer-binding protein (C/EBP) elements upstream of a TATA box [(C/EBP)3-TATA-Luc]; the promoter activation was dependent on C/EBPα, a well-established AR tethering protein (45), and the activation was hormone depletion insensitive (Fig. 6C). Further, among the androgen-responsive genes tested in Fig. 6A, the basal level of transmembrane protease, serine 2 (TMPRSS2) was decreased upon knocking down endogenous AR in hormone-depleted LP50 cells; ectopic mutAR recued the basal expression of TMPRSS2 (Fig. 6D).
Fig. 6.
Gene regulation by a DNA-binding mutAR. A, Hormone-depleted EP-LNCaP cells were nucleofected with mutAR or control (Ctrl) vector; 24 h later, the cells were infected with AR shRNA lentivirus or nontarget control lentivirus for 48 h. During the last 12 h of the infection, the cells were treated with R1881 (1 nm) or vehicle. The cells were then harvested to measure mRNA levels for the indicated androgen target genes by real-time RT-PCR. B, HeLa cells were depleted of hormone and transfected with the minimal ARE-driven promoter-Luc reporter plasmid and cotransfected with wtAR or mutAR expression plasmid or with the vector control. Testosterone (10 nm) or vehicle was added to the culture media 24 h after transfection; 24 h later, the cells were harvested to measure luciferase activity. C, Hormone-depleted HeLa cells were transfected with the (C/EBP)3-TATA-Luc construct. The cells were cotransfected with expression plasmid for wtAR, DNA-binding mutAR, or control plasmid as well as C/EBPα expression plasmid or control plasmid. The wtAR and mutAR plasmids were transfected at a dose (200 ng plasmid/1 × 105 cells) at which AR has previously been shown (45) to enter the nucleus in HeLa cells independent of hormone. In addition, all wells were cotransfected with the Renilla luciferase transfection control plasmid; 48 h after transfection, the samples were harvested either to measure luciferase activity or for Western blot analysis. The promoter activity values are plotted as the ratio to the basal activity of the control. Triplicate samples were included in each experimental set. C, inset, Western blotting showing the expression of wtAR, mutAR, C/EBPα, and GAPDH (loading control). D, Hormone-depleted LP50 cells were nucleofected with mutAR or control vector; 24 h later, the cells were infected with AR shRNA lentivirus or nontarget control lentivirus for 48 h. The cells were then harvested to measure mRNA levels for the TMPRSS2 gene by real-time RT-PCR.
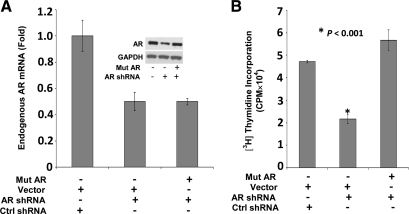
Both mutAR and AR shRNA plasmids were introduced into LP50 cells by electroporation, which yielded a transfection efficiency (determined using a cotransfected green fluorescent protein expression plasmid) of approximately 50%. AR shRNA knocked down the endogenous AR mRNA and protein (Fig. 7A) and decreased cell proliferation (Fig. 7B). Ectopic mutAR expressed at a level that compensated for the loss of endogenous AR in the AR knockdown cells (Fig. 7A, inset) completely rescued cell proliferation (Fig. 7B). The approximately 50% decrease in AR caused by the AR shRNA represents 100% decrease in AR in the transfected subpopulation of cells. This is proven in Fig. 3A, in which AR shRNA was cotransfected with an ARE-driven promoter-Luc and where the response of the promoter to R1881 was completely abrogated, demonstrating that the knockdown of AR was complete in the transfected subpopulation of cells. Therefore, the rescue of proliferation by mutAR observed in Fig. 7B could not be due to heterodimerization of wtAR and mutAR.
Fig. 7.
The ability of a DNA-binding mutAR to support proliferation in LP50 cells. A, LP50 cells were nucleofected with AR shRNA and cotransfected with either mutAR or the vector control (Ctrl). Cells were also cotransfected with the control shRNA plasmid and the vector control. Cells were harvested 72 h after transfection for Western blot analysis or to extract total RNA. The Western blotting was probed for AR and for the GAPDH loading control (A, inset). The RNA was reverse transcribed, and the mRNAs for endogenous AR and GAPDH were measured by real-time PCR. A, The endogenous AR was distinguished from that of mutAR by targeting the TaqMan probe to its 5′UTR. B, In the same experiment as in A, 72 h after transfection, [3H]thymidine incorporation was measured in the cells in separate sets of triplicate wells.
The results demonstrate that in LP50 cells, AR activates genes in the absence of hormone and supports hormone depletion-insensitive cell proliferation in LP50 cells without requiring the binding of AR to ARE.
Chromatin sites of hormone depletion-insensitive AR recruitment are functional and lack AREs
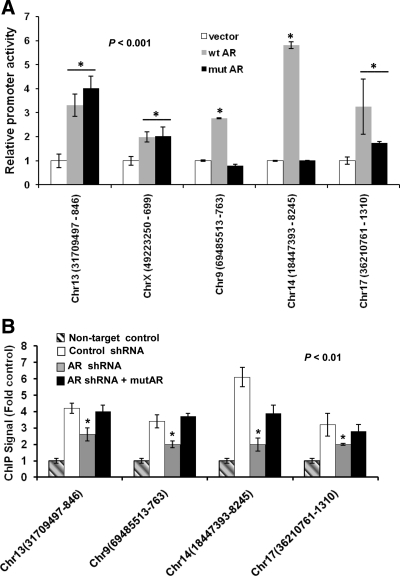
In the chromatin context, androgen commonly appears to regulate target genes by inducing the binding of AR at AREs located at great distances (>10 kb) from the target promoters (27). In the absence of hormone, however, global ChIP analysis showed less extensive and relatively weak signals for AR binding to chromatin even in hormone depletion-insensitive cells (34), but this could possibly reflect a limited efficiency of immunoprecipitation of AR in its hormone depletion-insensitive modes of chromatin binding. The present study sought to use ChIP-chip analysis of LP50 cells to identify a limited set of genomic DNA sequences that gave detectable signals for hormone depletion-insensitive AR binding, to examine whether those sites indeed lacked AREs, and to functionally test the ability of selected sequences to recruit AR in the absence of hormone. Accordingly, the Roche-NimbleGen promoter tiling arrays covering promoter regions −3500 to +1000 nt were chosen for the analysis. The number of androgen-independent peaks of chromatin associations of AR within this limited array was 113 with a false discovery rate (FDR) of less than 0.01 (peak list provided in Supplemental Fig. 4), all of which gave relatively weak ChIP signals (<2-fold enrichment relative to input DNA). The peak sequences were submitted to the Trawler program (43) to identify overrepresented sequences. The analysis yielded several families of motifs but none matching ARE half-sites. Peak DNA sequences were inserted upstream of the minimal promoter within the pG5luc reporter plasmid and tested in HeLa cells for their ability to enhance promoter activity in response to ectopic AR, independent of hormone. HeLa cells were chosen for this experiment because they lack endogenous AR and because in these cells, overexpressed ectopic AR enters the nuclear compartment without the need for ligand binding (45). Among the 15 most intense peaks, five peak sequences were cloned into pG5luc based on the availability of suitable flanking sequences for specific PCR amplification from genomic DNA. All of the five sequences promoted androgen-independent transactivation by wtAR, and three among them supported androgen-independent transactivation by mutAR in the range of 2- to 6-fold (Fig. 8A), indicating that the ChIP-chip peaks include non-ARE sites of relatively strong hormone depletion-insensitive action of AR despite their relatively weak ChIP signal.
Fig. 8.
Validation and functional tests of hormone depletion-insensitive AR recruitment sites in the chromatin. A, Hormone-depleted HeLa cells were transfected with either a minimal promoter-Luc reporter (pG5luc) in which the indicated genomic DNA fragments were inserted upstream of the promoter. The chromosomal locations of the insert sequences are indicated on the x-axis. The cells were cotransfected with either an expression plasmid for wtAR, mutAR, or the vector control; 48 h after transfection, the cells were harvested to measure luciferase activity. The promoter activities are plotted as the ratio of luciferase activities to that of the vector control. Triplicate samples were included in each experimental set. *, P < 0.001. B, Hormone-depleted LP50 cells were transfected with either AR shRNA or control shRNA in combination with mutAR or the vector control; 48 h later, the cells were subjected to ChIP analysis using antibody to AR. TaqMan probes were used to quantify immunoprecipitation of the DNA fragments encompassing the indicated chromatin sites. The TaqMan probe for GAPDH was used for the nontarget control. Chr, Chromosome; *, P < 0.01.
To further validate the chromatin sites selected above, the ability of endogenous wtAR, as well as mutAR to bind to those sites in situ, was tested by ChIP (Fig. 8B). Only four of the five sites were tested because of the inability to develop a suitable TaqMan probe for one of them. In all cases, the endogenous wtAR bound to the chromatin sites, consistent with the ChIP-chip data, and the ChIP signal decreased when the wtAR was knocked down by transfected AR shRNA (transfection efficiency, ∼50%). The ChIP signal was rescued when mutAR was cotransfected with the AR shRNA (Fig. 8B). As discussed in the previous section, the shRNA transfected cells virtually completely lacked wtAR. The mutAR was apparently recruited to all of the sites (Fig. 8B) regardless of its ability to be functional (Fig. 8A), suggesting that the mutation may interfere in some instances with its function.
The above results demonstrate that in LP50 cells, AR may be recruited in a hormone depletion-insensitive and functional manner, to chromatin sites that lack ARE, without the receptor binding directly to DNA.
Discussion
The foregoing studies contradict a common perception that in prostate cancer cells that are truly hormone depletion insensitive, AR supports proliferation through androgen-independent transcriptional signaling by a mechanism that essentially mimics its classical ligand-dependent gene activation at AREs. The LP50 cell model of hormone depletion-insensitive prostate cancer used in this study showed a profound dependence on AR for hormone depletion-insensitive growth. In these cells, the endogenous AR was not up-regulated or mutated and retained the same androgen dose response as its hormone-sensitive counterpart for gene activation by the classical mechanism, indicating that its ability to support cell growth was not associated with hypersensitization to androgen. Further, LP50 cells showed a molecular profile of hormone depletion-insensitive gene regulation by AR that represented advanced clinical tumors. In addition, in these cells the genes most highly up-regulated by AR in a hormone depletion-insensitive manner, including the subset consistently overexpressed in clinical tumors, were primarily genes supporting cell division. Although the AR in these cells still retained its ability to activate genes in an androgen-dependent manner, the majority of the early androgen target genes nevertheless were excluded from the large set of genes directly or indirectly activated by AR in the absence of hormone. The set of genes supported by AR in the absence of hormone in LP50 cells was essentially functionally similar by gene ontology analysis to that previously reported by Wang et al. (34) in LNCaP-abl cells, although the degree of AR dependence for growth as well as gene expression was generally greater in LP50 cells.
Within the LP50 model, in addition to the existence of two distinctive gene regulation profiles, several lines of evidence made a clear distinction between the classical mechanism of androgen-dependent gene regulation and the mechanism of hormone depletion-insensitive gene activation by AR. Hormone depletion-insensitive gene activation by AR supported cell proliferation without direct binding of AR to ARE. The evidence for this conclusion includes studies of physical and functional associations of AR with known AREs both in promoter constructs and in the chromatin. Wider functional chromatin associations of AR were also unrelated to AREs. Further evidence was provided by using a mutAR defective in DNA binding but that could transactivate a promoter through a well-established AR tethering mechanism (i.e. C/EBPα). The mutAR 1) rescued basal expression of TMPRSS2 after its decrease due to knockdown of endogenous AR in LP50 cells, 2) physically and functionally associated with chromatin sites that lacked ARE, and 3) supported hormone depletion-insensitive proliferation of LP50 cells.
AR was localized primarily in the nucleus in LP50 cells even in the absence of hormone. It follows that in the context of transcriptional signaling by AR in the complete absence of hormone, one essential role of cellular changes or changes related to the AR protein is to permit hormone depletion-insensitive entry of AR into the nucleus, although the precise mechanistic basis for the predominantly nuclear localization of AR in LP50 cells was not investigated in this study.
It is formally possible that some unidentified AR agonist is synthesized by LP50 cells, accounting for the nuclear localization of AR in hormone deplete cells and/or gene activation by the receptor. If this were the case, the putative agonist must be readily displaced by R1881, because the androgen dose response for gene activation is the same in LP50 cells vs. EP-LNCaP cells. Further, the putative agonist must not support the classical mechanism of gene activation by AR through association with AREs.
The studies indicate that in LP50 cells, AR must associate in a hormone depletion-insensitive and functional manner with chromatin sites that do not contain classical response elements. Therefore, the functionally significant hormone depletion-insensitive associations of AR must occur through tethering mechanisms. Although AR could conceivably influence gene transcription through different mechanisms that are independent of its ability to bind to DNA, tethering of AR by DNA-bound proteins offers the most likely mechanism for global gene regulation. Tethering mechanisms have been well studied for other steroid receptors, particularly the estrogen receptor. Gene activation by estrogen receptor is known to occur by recruitment of the receptor to its target genes by DNA-bound transcription factors, such as specificity protein family proteins (46) and activator protein 1 (47). AR is known to repress gene activation by activator protein 1 and nuclear factor κB (RelA), but this appears to occur indirectly, principally through sequestration of limiting amounts of the coactivator, CREB binding protein (48, 49). Gene activation through AR tethering, however, has not received adequate attention. It has been reported that in myoblasts, AR activates the skeletal α-actin gene through its recruitment to the target promoter by serum response factor (50). Homeobox B13 can act as an AR tethering transcription factor during prostate development (51). We have recently found that C/EBPα, which is a corepressor of AR, also redirects the transcriptional activity of AR by tethering it to C/EBP elements (45); C/EBPα is a tumor suppressor that is absent in LNCaP cells but is expressed in the nucleus of differentiated prostate epithelial cells (52). Recent studies examining the components of AR interactomes in LNCaP cells have identified a variety of DNA-bound transcription factors that physically associate with AR (53). Many of the candidate AR tethering proteins are substantially up-regulated in advanced prostate tumors (Oncomine database), and studies are underway in this laboratory to systematically examine the potential role of AR as a hormone depletion-insensitive coactivator of each of them.
AR is distinct from other Class I nuclear receptors in that it can form dimeric conformations that can bind to both direct and inverted repeat elements (26). In the absence of hormone, tethering factors may be able to recruit AR to the target promoters as a monomer; this is evident for the recruitment of AR by C/EBPα (45) and is an issue that warrants investigation for other AR tethering proteins as well. Another important characteristic of gene activation by tethered AR evident from our studies of AR-C/EBPα interactions (45) is that it is insensitive to flutamide, a classical androgen antagonist. AR is also distinctive in terms of specific characteristics of its activation functions and coregulator interactions (2, 13–16), which should be reexamined in the context of tethered associations of AR with its target genes.
The frequency and extent of the contribution of known molecular events associated with hormone depletion insensitivity in clinical prostate tumors is unclear; however, AR overexpression is frequently observed in metastatic prostate tumors (please see Oncomine database), and only a few-fold increase in AR expression has been shown to confer sensitization to postablation levels of androgen in prostate cancer cells and to cause a functional switch of classical AR antagonists to agonists (23). Although the tumors hypersensitized to androgen in this manner could conceivably be treated with a new generation of androgen antagonists (54), the tumors are themselves heterogeneous in terms of AR expression (40, 41), and cells within the tumor could circumvent such therapies by acquiring true hormone depletion insensitivity. It is implicit from the present studies of the LP50 model that in the progression of prostate cancer to true hormone depletion-insensitivity, the cells not only acquire the ability to translocate AR into the nucleus independent of hormone but that they also become reliant on nonclassical mechanisms, including tethering to activate a largely different set of target genes. Significantly, our recent studies (45) of one AR tethering protein (C/EBPα) demonstrated insensitivity of this mechanism to a classical androgen antagonist. Future studies should reveal whether progression to androgen independence is accompanied by either overexpression or de novo expression of critical AR tethering proteins and whether the tethering proteins are causally associated with hormone depletion insensitivity or are only critical for supporting it. In any event, the development of peptidomimetic or other agents that target critical AR tethering proteins or their interactions is a conceivable approach to treating such tumors.
Materials and Methods
Chemicals, antibodies, and reagents
DMEM, RPMI 1640, sodium pyruvate, and penicillin/streptomycin/l-glutamine stock mix were purchased from Life Technologies, Inc. (Carlsbad, CA). Fetal bovine serum (FBS) and charcoal stripped FBS (CS-FBS) were from Invitrogen (Carlsbad, CA). Luciferase assay reagents were from Promega (Madison, WI). Affinity-purified rabbit antihuman antibodies to AR (AR-N20 and AR-C19), normal rabbit IgG control (sc-2027), and mouse antihuman antibody to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (sc-47724) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Peroxidase or fluorescein isothiocyanate (FITC)-conjugated secondary antibodies were from Vector Laboratories (Burlingame, CA). Cell Line Nucleofector kits (R) were purchased from Amaxa Biosystems (Basel, Switzerland). Custom oligonucleotide primers were from Life Technologies. Lipofectamine 2000 reagent was from Invitrogen. FuGENE 6 was purchased from Roche Diagnostics (Indianapolis, IN). Vent DNA polymerase was purchased from New England Biolabs (Beverly, MA). Custom oligonucleotide primers were from Integrated DNA Technologies, Inc. (Coralville, IA). The reagents for RT-PCR and real-time PCR, including inventoried TaqMan probes (for Ras homolog gene family, member U, ELL associated factor 2, insulin-like growth factor 1 receptor, prostate transmembrane protein, androgen induced 1, and kallikrein-related peptidase 3), were purchased from Applied Biosystems (Branchburg, NJ). Custom made TaqMan probes (for AR, GAPDH, and selected genomic fragments) were ordered from Integrated DNA Technologies, Inc. Plaque-forming unit DNA polymerase was from Stratagene (La Jolla, CA).
Promoter constructs and expression plasmids
The PSA promoter-Luc reporter construct contains 6.1 kb of DNA sequence upstream from +12 nt. The delARE-PSA promoter-Luc construct is derived from PSA promoter-Luc by deletion of only the AREs that include −4366 to −3874 nt and an additional internal deletion of −170 to −159 nt. (C/EBP)3-TATA-Luc was made by cloning the appropriate annealed oligos with the addition of KpnI(5′)- and NheI(3′)-terminal restriction sites into the large segment of lectin, galactoside-binding, soluble, 4-TATA-Luc digested by KpnI and NheI. (C/EBP)3 is a 3-tandem repeated C/EBP consensus element, (TGCAGATTGCGCCAATCTGCA)3; the sequence underlined is the central binding motif. Genomic sequences corresponding to androgen-independent AR enrichment peaks identified by ChIP-chip analysis were amplified by PCR and cloned upstream of the minimal promoter in the pG5luc plasmid (Promega) by replacing its GAL4 element at KpnI and NheI restriction sites. The Renilla luciferase transfection control in the pRL-null plasmid was from Promega. The expression plasmid for AR (pSG5-hAR) was kindly provided by Lirim Shemshedini (University of Toledo, Toledo, OH). The AR-specific shRNA and nontargeting shRNA control in the lentiviral expression vector, pLKO.1 puro, were purchased from Sigma-Aldrich (St. Louis, MO). The shRNA sequence for AR is: CCGGCACCAATGTCAACT CCAGGATCTCGAGCTCCTGGAGTTGACATTGGTGTTTTT (TRCN0000003718, MISSION TRC shRNA Target Set; Sigma, St. Louis, MO). The control nontargeting shRNA sequence is: CCGGCAACAAGATGAAGAGCACCAACTCGAGTTGGTGCTCTTCA TCTTGTTGTTTTT (MISSION Non-Target shRNA Control Vector; Sigma). mutAR was generated by site directed mutagenesis through a PCR strategy using plaque-forming unit DNA polymerase. The cDNA for mutAR was inserted into the pcDNA3.1 expression plasmid and contains the following mutations: 1) the natural T877A mutation in the endogenous AR in LNCaP cells, 2) a V581F mutation that disrupts DNA binding, and 3) silent mutations within the target site for AR shRNA to disrupt recognition by the shRNA; the AR shRNA target sequence CACCAATGTCAACTCCAGGAT was mutated to CACTAACGTTAATAGTCGAAT.
Cell culture and hormone depletion
EP-LNCaP cells (EP-LNCaP, passage 17) were purchased from American Type Culture Collection (Rockville, MD). Cells were routinely cultured at 37 C and in 5% CO2 in RPMI 1640 medium supplemented with FBS (10%), penicillin (100 U/ml), streptomycin (100 μg/ml), l-glutamine (2 mm), and sodium pyruvate (1 mm). LP50 cells were derived from EP-LNCaP cells by growth selection through 50 serial passages. To obtain hormone depletion, EP-LNCaP or LP50 cells were grown for 72 h in phenol red-free RPMI 1640 medium containing 10% (Dextran CS-FBS) CS-FBS, transferrin (20 μg/ml), penicillin (100 U/ml), streptomycin (100 μg/ml), l-glutamine (2 mm), and sodium pyruvate (1 mm). Androgen-depletion in the cells was confirmed in parallel experiments by observing induction of PSA mRNA by a 6-h treatment with R1881. HeLa cells (American Type Culture Collection) were cultured in DMEM supplemented with FBS (10%), penicillin (100 units/ml), streptomycin (100 mg/ml), and l-glutamine (2 mm). To obtain hormone depletion, HeLa cells were grown in phenol red-free DMEM supplemented with CS-FBS (5% vol/vol), l-glutamine (2 mm), insulin (1 μg/ml), and transferrin (20 μg/ml). 293 FT cells (American Type Culture Collection) were cultured in DMEM supplemented with FBS (10%), penicillin (100 units/ml), streptomycin (100 mg/ml), and l-glutamine (2 mm).
Plasmid transfection
EP-LNCaP and LP50 cells were transfected by nucleofection using the Amaxa reagent kit R (Amaxa, Gaithersburg, MD) following the manufacturer-optimized protocol for LNCaP cells. After nucleofection, the cells were plated in 12-well poly-D-lysine coated plates from Becton Dickinson Labware (Bedford, MA). Typically, each nucleofection was performed using 2 × 106 cells, and a total of 5 μg of DNA. In all cases, the appropriate empty vector plasmids were used to equalize total DNA for transfection. For promoter analysis, 1 μg of each promoter-Luc reporter construct was transfected. In the AR knockdown experiments, 3 μg of AR shRNA or nontargeting control shRNA plasmids were transfected. Uniformity of transfection and promoter specificity was confirmed using the pRL-null plasmid expressing Renilla luciferase and measurement of Renilla luciferase activity in the cell lysates. To measure transfection efficiency (percentage of cells transfected), a green fluorescent protein expression plasmid was cotransfected, and the fluorescent cells were counted by flow cytometry. HeLa cells were transfected with DNA constructs in six-well tissue culture plates (Corning, New York, NY) using FuGENE 6 (Roche Diagnostics), according to the manufacturer's protocol. For transfection in each well (∼3 × 105 cells), 500 ng of each promoter-Luc reporter construct and 300 ng of either the AR expression plasmid or a control plasmid were used. Uniformity of transfection and promoter specificity was confirmed using the pRL-null pasmid expressing Renilla luciferase and measurement of Renilla luciferase activity in the cell lysates.
Lentiviral infection
The AR shRNA vector or the nontargeting shRNA control vector was packaged into lentivirus. The virus particles were generated by transfecting 293 FT cells using Lipofectamine 2000 reagent. Lentivirus was harvested from the supernatant 48 and 72 h after transfection. LP50 cells were plated in 12-well plates the day before infection (1.5 × 105 cells/well); 0.5 ml virus of previously titrated supernatant combined with polybrene (800 μg/ml) was used for the infection of each well together with 0.5 ml media (RPMI 1640 media containing 10% heat inactivated FBS, 2 mm l-glutamine, and 1 mm sodium pyruvate); 4–5 h later, the infection procedure was repeated. About 4–5 h after the second infection, the cells were placed in fresh media.
Cell count proliferation assay
LP50 cells were plated in a six-well tissue culture plate at a density of 3 × 104 cells per well in RPMI medium and placed in a CO2 incubator at 37 degrees for 48 h. The cells were then hormone depleted as described above. The cells were then treated with either R1881 (1 nm) or vehicle for the duration of the growth assay. The cells were counted in a coulter particle counter. The assays were conducted in triplicate wells.
[3H]thymidine incorporation assay
EP-LNCaP cells or LP50 cells in 12-well poly-D-lysine coated plates (1.5 × 105 cells/well), which were depleted of hormones as described above, were treated with either R1881 (0.1 nm) or vehicle; 18 h later, the cells were pulse labled with [3H]thymidine (1 μCi/ml, specific activity 11.3 Ci/mmol; Moravek Biochemicals, Brea, CA) for 6 h. Then, the cells were washed with ice-cold PBS followed by the addition of 1 ml of ice-cold trichloroacetic acid and incubated at 4 C for 30 min. The cells were again washed with cold PBS. [3H]thymidine-labeled DNA was then extracted in 0.5 ml of 0.5 m NaOH/0.5% sodium dodecyl sulfate solution, and the radioactivity was measured in a liquid scintillation counter. The cell numbers were monitored in parallel wells treated identically up to the DNA extraction step. The assays were conducted in sextuplicate wells.
Immunofluorescence and confocal microscopy
EP-LNCaP and LP50 cells (3 × 104), plated in chamber slides and depleted of hormones as described above, were treated with R1881 (1 nm) or vehicle for 12 h. The cells were washed twice with PBS (2 mm KH2PO4, 10 mm Na2HPO4, 2.7 mm KCl, and 137 mm NaCl) and were fixed with freshly prepared 3.7% paraformaldehyde in PBS for 10 min at room temperature. Then, the cells were permeabilized in PBS containing 0.1% BSA and 0.3% Triton X-100 for 5 min at room temperature. The cells were then washed and blocked with PBS containing 5% serum and 0.2% Triton X-100 at room temperature for 1 h. The cells were incubated overnight at 4 C with the antibody to AR at a dilution of 1:50. After washing three times with PBS containing 5% serum and 0.2% Triton X-100, the cells were incubated with bovine antirabbit IgG conjugated to FITC for 1 h in the dark, at room temperature, at a dilution of 1:100. After the final wash, the cells were incubated for 2 min in 4′,6-diamidino-2-phenylindole, washed three times, and mounted using Vectashield mounting medium. Images were acquired using the Leica TCS SP5 Broad band confocal microscope system (Leica, Mannheim, Germany). The acquisition settings were kept constant between specimens, and a negative control sample incubated with normal IgG primary antibody was used to adjust for background. Subcellular localization of AR was examined by acquiring 10 optical sections using ×40 oil immersion with additional ×3.5 optical zoom. The images were compiled as projections of using the Leica LAS software package.
Preparation of cell lysates and luciferase assay
Cells in each well of a 12-well tissue culture plate were washed once with PBS of pH 7.5 (2 mm KH2PO4, 10 mm Na2HPO4, 2.7 mm KCl, and 137 mm NaCl) and lysed in 200 μl of reporter lysis buffer, provided with the luciferase assay system (Promega). The samples were centrifuged at 12,000 × g for 2 min at room temperature. The supernatant was assayed for Firefly or Renilla luciferase activities using the appropriate luciferase substrate from Promega in a luminometer (Lumat LB9501; Berthold, Wildbad, Germany). All luciferase assays were performed at least in triplicate.
Western blottings
Cells were harvested after washing twice with cold (4 C) PBS. The cells were lysed with high salt buffer [400 mm NaCl, 10 mm Tris (pH 8.0), 1 mm EDTA, 1 mm EGTA, 1 mm β-mercaptoethanol, and 0.1% Triton X-100] containing a protease inhibitor cocktail (1 mm phenyl methyl sulfonyl fluoride and 5 μg/ml each of aprotinin, leupeptin, and pepstatin A). Protein samples (20–50 μg) were resolved by electrophoresis on 8% sodium dodecyl sulfate-polyacrylamide gels and electrophoretically transferred to nitrocellulose membranes. The blots were probed with the appropriate primary antibodies followed by goat antirabbit IgG or goat antimouse IgG conjugated to horseradish peroxidase and visualized using the enhanced chemiluminescence method. The same membranes were similarly reprobed with the primary mouse anti-GAPDH antibody and secondary goat antimouse IgG conjugated to horseradish peroxidase.
RNA isolation, RT-PCR, and real-time PCR
Total RNA was prepared using the RNeasy Mini kit (QIAGEN, Valencia, CA). RT-PCR followed by real-time PCR was used to measure mRNAs for PSA and for GAPDH. For the RT reaction, 200 ng of total RNA was reverse transcribed with random primers using the High-Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA) according to the manufacturer's protocol. The resulting cDNA was measured by quantitative real-time PCR using the Real-Time PCR Master Mix (Applied Biosystems) in the StepOne Plus Real Time PCR System (Applied Biosystems). The primers and TaqMan probe for PSA and GAPDH were obtained from Integrated DNA Technologies, Inc. To measure the mRNA for endogenous AR as distinct from transfected mutAR, the TaqMan probe was targeted to a 5′ untranslated region sequence that was absent in mutAR. The sequences of the TaqMan probe and PCR primers for AR are: 5′-FAM-TCCACCTCCTCCTGCCTTCCCC-TAM-3′; forward primer, CCTCTGTTTTCCCCCACTCTCT; reverse primer, GACTGCCTTTTCATCTTTTGATCTC. All samples were assayed in triplicate and normalized to GAPDH values in the same samples.
ChIP and ChIP-chip analyses
LP50 cells were depleted of hormones as described above. Then the cells were treated with either vehicle or R1881 (1 nm) for 2 h, washed with cold PBS, and subjected to ChIP analysis for AR using anti-AR antibody (either AR-N20 or AR-C19) and negative control IgG (sc-2027) following the procedure described previously (55). The recruitment of AR to the major ARE enhancer region of PSA promoter (−4366 to −3874 nt) was measured by real-time PCR. An irrelevant target sequence within the downstream coding region was used as a negative control. The primers and TaqMan probe used to target the PSA ARE enhancer region were: GCCTGGATCTGAGAGAGATATCATC (forward primer); ACACCTTTTTTTTTCTGGATTGTTG (reverse primer); 56FAM-TGCAAGGCCTGCTTTACAAACTTCC-36TAM (probe). The primers and TaqMan probe used to target the irrelevant coding sequence of PSA were: CACACCCGCTCTACGATATGA (forward primer); GAGCTCGGCAGGCTCTGA (reverse primer); 56-FAM-CTCCAGCCACGACCTCATGCTGCT-36TAM (probe); 10 ng of the immunoprecipitated DNA samples that were validated for PSA as well as corresponding input DNA were amplified using the Sigma GenomePlex WGA2 kit (Sigma-Aldrich) to generate at least 4 μg of DNA for chip analysis using the NimbleGen 385K H18 tiling array platform. The labeling and hybridization of DNA samples were performed by NimbleGen Systems, Inc. (Madison, WI). As described in the NimbleGen website, the data were analyzed using the Nimblescan software to identify peaks by searching for four or more probes whose signals were above the specified cutoff values, ranging from 90 to 15%, using a 500-bp sliding window. The cutoff values are a percentage of a hypothetical maximum, which is the mean + 6 (sd). The ratio data was then randomized 20 times to assign a FDR score. An FDR of less than or equal to 0.05 was considered to be highly indicative of binding sites for AR. For validation of ChIP-chip data and also to test the binding of mutAR by regular ChIP assay, selected genomic fragments were targeted for quantitative PCR using custom made TaqMan probes.
mRNA profiling
The Affymetrix DNA microarray analysis was performed as a full service global gene expression study at the transcriptional profiling core facility of The Cancer Institute of New Jersey (New Brunswick, NJ). Total RNA samples were used to generate labeled cRNA, which were hybridized to human U133 Plus 2.0 Affymetrix microarrays. Scanned image files were analyzed using the Gene Chip Operating System version 1.4 software, and standard thresholding and filtering operations were used. The data were normalized using housekeeping genes. Normalization assumes that for a subset of genes (i.e. housekeeping genes), the ratio of measured expression averaged over the set should be one. All data are Minimum Information About a Microarray Experiment (MIAME) compliant, and the raw data have been deposited in a MIAME compliant database (Gene Expression Omnibus), as detailed on the Microarray Gene Expression Data (MGED) Society website available at http://www.mged.org/Workgroups/MIAME/miame.html. The microarray gene expression data have been submitted to Gene Expression Omnibus with the accession no. GSE22483.
Statistical analyses
All experimental values in Figs. 1, 3, 4, and 6–8 are presented as mean ± sd. The statistical significance of differences (P value) between values being compared was determined using ANOVA. In all cases, differences noted in the text are reflected by a P value of less than 0.001.
Acknowledgments
We thank Dr. Lirim Shemshedini for providing plasmid constructs, Dr. David Weaver for assistance in the quantitative analysis of the mRNA expression profiling data, and Dr. Andrea Nestor for assistance with confocal microscopy.
Present address for J.Z.: Crown BioScience, Inc., Beijing, China.
This work was supported by the National Institutes of Health Grant CA103964 and an endowment from the Harold and Helen McMaster Foundation (M.R.).
Disclosure Summary: The authors have nothing to disclose.
NURSA Molecule Pages:
Nuclear Receptors: AR
Ligands: R1881
Footnotes
- AR
- Androgen receptor
- ARE
- androgen response element
- C/EBP
- CCAAT/enhancer-binding protein
- ChIP
- chromatin immunoprecipitation
- CS-FBS
- charcoal stripped FBS
- DBD
- DNA-binding domain
- EP
- early passage
- FBS
- fetal bovine serum
- FDR
- false discovery rate
- FITC
- fluorescein isothiocyanate
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- MIAME
- Minimum Information About a Microarray Experiment
- mutAR
- mutant form of AR
- nt
- nucleotide
- promoter-Luc
- promoter-luciferase
- PSA
- prostate-specific antigen
- shRNA
- short hairpin RNA
- TMPRSS2
- transmembrane protease, serine 2
- wtAR
- wild-type AR.
References
- 1. Roy AK, Lavrovsky Y, Song CS, Chen S, Jung MH, Velu NK, Bi BY, Chatterjee B. 1999. Regulation of androgen action. Vitam Horm 55:309–352 [DOI] [PubMed] [Google Scholar]
- 2. Gelmann EP. 2002. Molecular biology of the androgen receptor. J Clin Oncol 20:3001–3015 [DOI] [PubMed] [Google Scholar]
- 3. Xu Y, Chen SY, Ross KN, Balk SP. 2006. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res 66:7783–7792 [DOI] [PubMed] [Google Scholar]
- 4. Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC. 2001. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104:719–730 [PubMed] [Google Scholar]
- 5. Sun M, Yang L, Feldman RI, Sun XM, Bhalla KN, Jove R, Nicosia SV, Cheng JQ. 2003. Activation of phosphatidylinositol 3-kinase/Akt pathway by androgen through interaction of p85α, androgen receptor, and Src. J Biol Chem 278:42992–43000 [DOI] [PubMed] [Google Scholar]
- 6. Baron S, Manin M, Beaudoin C, Leotoing L, Communal Y, Veyssiere G, Morel L. 2004. Androgen receptor mediates non-genomic activation of phosphatidylinositol 3-OH kinase in androgen-sensitive epithelial cells. J Biol Chem 279:14579–14586 [DOI] [PubMed] [Google Scholar]
- 7. Pratt WB, Toft DO. 1997. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev 18:306–360 [DOI] [PubMed] [Google Scholar]
- 8. Tsai MJ, O'Malley BW. 1994. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 63:451–486 [DOI] [PubMed] [Google Scholar]
- 9. Beato M, Herrlich P, Schütz G. 1995. Steroid hormone receptors: many actors in search of a plot. Cell 83:851–857 [DOI] [PubMed] [Google Scholar]
- 10. Glass CK, Rosenfeld MG. 2000. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev 14:121–141 [PubMed] [Google Scholar]
- 11. McKenna NJ, O'Malley BW. 2002. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108:465–474 [DOI] [PubMed] [Google Scholar]
- 12. Bourguet W, Germain P, Gronemeyer H. 2000. Nuclear receptor ligand-binding domains: three-dimensional structures, molecular interactions and pharmacological implications. Trends Pharmacol Sci 21:381–388 [DOI] [PubMed] [Google Scholar]
- 13. McEwan IJ. 2004. Molecular mechanisms of androgen receptor-mediated gene regulation: structure-function analysis of the AF-1 domain. Endocr Relat Cancer 11:281–293 [DOI] [PubMed] [Google Scholar]
- 14. Berrevoets CA, Doesburg P, Steketee K, Trapman J, Brinkmann AO. 1998. Functional interactions of the AF-2 activation domain core region of the human androgen receptor with the amino-terminal domain and with the transcriptional coactivator TIF2 (transcriptional intermediary factor2). Mol Endocrinol 12:1172–1183 [DOI] [PubMed] [Google Scholar]
- 15. Hong H, Kohli K, Trivedi A, Johnson DL, Stallcup MR. 1996. GRIP1, a novel mouse protein that serves as a transcriptional coactivator in yeast for the hormone binding domains of steroid receptors. Proc Natl Acad Sci USA 93:4948–4952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jenster G, van der Korput HA, Trapman J, Brinkmann AO. 1995. Identification of two transcription activation units in the N-terminal domain of the human androgen receptor. J Biol Chem 270:7341–7346 [DOI] [PubMed] [Google Scholar]
- 17. Shaffer PL, Jivan A, Dollins DE, Claessens F, Gewirth DT. 2004. Structural basis of androgen receptor binding to selective androgen response elements. Proc Natl Acad Sci USA 101:4758–4763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ruizeveld de Winter JA, Janssen PJ, Sleddens HM, Verleun-Mooijman MC, Trapman J, Brinkmann AO, Santerse AB, Schröder FH, van der Kwast TH. 1994. Androgen receptor status in localized and locally progressive hormone refractory human prostate cancer. Am J Pathol 144:735–746 [PMC free article] [PubMed] [Google Scholar]
- 19. Linja MJ, Savinainen KJ, Saramäki OR, Tammela TL, Vessella RL, Visakorpi T. 2001. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res 61:3550–3555 [PubMed] [Google Scholar]
- 20. Zegarra-Moro OL, Schmidt LJ, Huang H, Tindall DJ. 2002. Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res 62:1008–1013 [PubMed] [Google Scholar]
- 21. Li TH, Zhao H, Peng Y, Beliakoff J, Brooks JD, Sun Z. 2007. A promoting role of androgen receptor in androgen-sensitive and -insensitive prostate cancer cells. Nucleic Acids Res 35:2767–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miyamoto H, Messing EM, Chang C. 2004. Androgen deprivation therapy for prostate cancer: current status and future prospects. Prostate 61:332–353 [DOI] [PubMed] [Google Scholar]
- 23. Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. 2004. Molecular determinants of resistance to antiandrogen therapy. Nat Med 10:33–39 [DOI] [PubMed] [Google Scholar]
- 24. Edwards J, Bartlett JM. 2005. The androgen receptor and signal-transduction pathways in hormone-refractory prostate cancer. Part 1: modifications to the androgen receptor. BJU Int 95:1320–1326 [DOI] [PubMed] [Google Scholar]
- 25. Shand RL, Gelmann EP. 2006. Molecular biology of prostate-cancer pathogenesis. Curr Opin Urol 16:123–131 [DOI] [PubMed] [Google Scholar]
- 26. Verrijdt G, Tanner T, Moehren U, Callewaert L, Haelens A, Claessens F. 2006. The androgen receptor DNA-binding domain determines androgen selectivity of transcriptional response. Biochem Soc Trans 34:1089–1094 [DOI] [PubMed] [Google Scholar]
- 27. Wang Q, Li W, Liu XS, Carroll JS, Jänne OA, Keeton EK, Chinnaiyan AM, Pienta KJ, Brown M. 2007. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol Cell 27:380–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bolton EC, So AY, Chaivorapol C, Haqq CM, Li H, Yamamoto KR. 2007. Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev 21:2005–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Veldscholte J, Ris-Stalpers C, Kuiper GG, Jenster G, Berrevoets C, Claassen E, van Rooij HC, Trapman J, Brinkmann AO, Mulder E. 1990. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem Biophys Res Commun 173:534–540 [DOI] [PubMed] [Google Scholar]
- 30. Culig Z, Hobisch A, Cronauer MV, Cato AC, Hittmair A, Radmayr C, Eberle J, Bartsch G, Klocker H. 1993. Mutant androgen receptor detected in an advanced-stage prostatic carcinoma is activated by adrenal androgens and progesterone. Mol Endocrinol 7:1541–1550 [DOI] [PubMed] [Google Scholar]
- 31. Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE, Ogata GK, Keer HN, Balk SP. 1995. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med 332:1393–1398 [DOI] [PubMed] [Google Scholar]
- 32. Grossmann ME, Huang H, Tindall DJ. 2001. Androgen receptor signaling in androgen-refractory prostate cancer. J Natl Cancer Inst 93:1687–1697 [DOI] [PubMed] [Google Scholar]
- 33. Heinlein CA, Chang C. 2004. Androgen receptor in prostate cancer. Endocr Rev 25:276–308 [DOI] [PubMed] [Google Scholar]
- 34. Wang Q, Li W, Zhang Y, Yuan X, Xu K, Yu J, Chen Z, Beroukhim R, Wang H, Lupien M, Wu T, Regan MM, Meyer CA, Carroll JS, Manrai AK, Jänne OA, Balk SP, Mehra R, Han B, Chinnaiyan AM, Rubin MA, True L, Fiorentino M, Fiore C, Loda M, Kantoff PW, Liu XS, Brown M. 2009. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell 138:245–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cleutjens KB, van der Korput HA, van Eekelen CC, van Rooij HC, Faber PW, Trapman J. 1997. An androgen response element in a far upstream enhancer region is essential for high, androgen-regulated activity of the prostate-specific antigen promoter. Mol Endocrinol 11:148–161 [DOI] [PubMed] [Google Scholar]
- 36. Kim J, Coetzee GA. 2004. Prostate specific antigen gene regulation by androgen receptor. J Cell Biochem 93:233–241 [DOI] [PubMed] [Google Scholar]
- 37. Dehm SM, Tindall DJ. 2006. Ligand-independent androgen receptor activity is activation function-2-independent and resistant to antiandrogens in androgen refractory prostate cancer cells. J Biol Chem 281:27882–27893 [DOI] [PubMed] [Google Scholar]
- 38. Jia L, Coetzee GA. 2005. Androgen receptor-dependent PSA expression in androgen-independent prostate cancer cells does not involve androgen receptor occupancy of the PSA locus. Cancer Res 65:8003–8008 [DOI] [PubMed] [Google Scholar]
- 39. Liu AY, Brubaker KD, Goo YA, Quinn JE, Kral S, Sorensen CM, Vessella RL, Belldegrun AS, Hood LE. 2004. Lineage relationship between LNCaP and LNCaP-derived prostate cancer cell lines. Prostate 60:98–108 [DOI] [PubMed] [Google Scholar]
- 40. Chodak GW, Kranc DM, Puy LA, Takeda H, Johnson K, Chang C. 1992. Nuclear localization of androgen receptor in heterogeneous samples of normal, hyperplastic and neoplastic human prostate. J Urol 147:798–803 [DOI] [PubMed] [Google Scholar]
- 41. Tilley WD, Lim-Tio SS, Horsfall DJ, Aspinall JO, Marshall VR, Skinner JM. 1994. Detection of discrete androgen receptor epitopes in prostate cancer by immunostaining: measurement by color video image analysis. Cancer Res 54:4096–4102 [PubMed] [Google Scholar]
- 42. Huang da W, Sherman BT, Tan Q, Collins JR, Alvord WG, Roayaei J, Stephens R, Baseler MW, Lane HC, Lempicki RA. 2007. The DAVID gene functional classification tool: a novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol 8:R183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tomlins SA, Mehra R, Rhodes DR, Cao X, Wang L, Dhanasekaran SM, Kalyana-Sundaram S, Wei JT, Rubin MA, Pienta KJ, Shah RB, Chinnaiyan AM. 2007. Integrative molecular concept modeling of prostate cancer progression. Nat Genet 39:41–51 [DOI] [PubMed] [Google Scholar]
- 44. Lobaccaro JM, Poujol N, Chiche L, Lumbroso S, Brown TR, Sultan C. 1996. Molecular modeling and in vitro investigations of the human androgen receptor DNA-binding domain: application for the study of two mutations. Mol Cell Endocrinol 116:137–147 [DOI] [PubMed] [Google Scholar]
- 45. Zhang J, Gonit M, Salazar MD, Shatnawi A, Shemshedini L, Trumbly R, Ratnam M. 2010. C/EBPα redirects androgen receptor signaling through a unique bimodal interaction. Oncogene 29:723–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Safe S, Kim K. 2004. Nuclear receptor-mediated transactivation through interaction with Sp proteins. Prog Nucleic Acid Res Mol Biol 77:1–36 [DOI] [PubMed] [Google Scholar]
- 47. Jakacka M, Ito M, Weiss J, Chien PY, Gehm BD, Jameson JL. 2001. Estrogen receptor binding to DNA is not required for its activity through the nonclassical AP1 pathway. J Biol Chem 276:13615–13621 [DOI] [PubMed] [Google Scholar]
- 48. Frønsdal K, Engedal N, Slagsvold T, Saatcioglu F. 1998. CREB binding protein is a coactivator for the androgen receptor and mediates cross-talk with AP-1. J Biol Chem 273:31853–31859 [DOI] [PubMed] [Google Scholar]
- 49. Aarnisalo P, Palvimo JJ, Jänne OA. 1998. CREB-binding protein in androgen receptor-mediated signaling. Proc Natl Acad Sci USA 95:2122–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vlahopoulos S, Zimmer WE, Jenster G, Belaguli NS, Balk SP, Brinkmann AO, Lanz RB, Zoumpourlis VC, Schwartz RJ. 2005. Recruitment of the androgen receptor via serum response factor facilitates expression of a myogenic gene. J Biol Chem 280:7786–7792 [DOI] [PubMed] [Google Scholar]
- 51. Norris JD, Chang CY, Wittmann BM, Kunder RS, Cui H, Fan D, Joseph JD, McDonnell DP. 2009. The homeodomain protein HOXB13 regulates the cellular response to androgens. Mol Cell 36:405–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang J, Wilkinson JE, Gonit M, Keck R, Selman S, Ratnam M. 2008. Expression and sub-cellular localization of the CCAAT/enhancer binding protein α in relation to postnatal development and malignancy of the prostate. Prostate 68:1206–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mukhopadhyay NK, Ferdinand AS, Mukhopadhyay L, Cinar B, Lutchman M, Richie JP, Freeman MR, Liu BC. 2006. Unraveling androgen receptor interactomes by an array-based method: discovery of proto-oncoprotein c-Rel as a negative regulator of androgen receptor. Exp Cell Res 312:3782–3795 [DOI] [PubMed] [Google Scholar]
- 54. Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, Wasielewska T, Welsbie D, Chen CD, Higano CS, Beer TM, Hung DT, Scher HI, Jung ME, Sawyers CL. 2009. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 324:787–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shatnawi A, Tran T, Ratnam M. 2007. R5020 and RU486 act as progesterone receptor agonists to enhance Sp1/Sp4-dependent gene transcription by an indirect mechanism. Mol Endocrinol 21:635–650 [DOI] [PubMed] [Google Scholar]