Induction of c-Fos by GnRH in the gonadotrope precedes that of FSHβ, requires the same signaling pathways and occurs primarily through CamKII activation of SRF.
Abstract
Despite extensive studies on GnRH regulation of the gonadotropin subunit genes, very little is known about mechanism of induction of intermediary immediate early genes, such as c-Fos, that are direct targets of GnRH signaling and that upon induction, activate transcription of gonadotropin genes. Although c-Fos is induced by a variety of stimuli in other cell types, in the gonadotropes, only GnRH induces c-Fos and through it FSHβ. Thus, understanding the specificity of c-Fos induction by GnRH will provide insight into GnRH regulation of FSHβ gene expression. GnRH induction of c-Fos in LβT2 cells requires the serum response factor (SRF)-binding site, but not the Ets/ELK1 site. This is in contrast to c-Fos induction by growth factors in other cells, which activate c-Fos transcription via phosphorylation of ELK1 and require the ELK1-binding site. The SRF site alone is sufficient for induction by GnRH, whereas induction by 12-tetradecanoylphorbol-13-acetate (TPA) requires both the ELK1 and SRF sites. Although ELK1 site is not required, upon GnRH stimulation, ELK1 interacts with SRF and is recruited to the SRF site. GnRH phosphorylates ELK1 through ERK1/2 and p38 MAPK, which correlates with the signaling pathways necessary for c-Fos and FSHβ induction. GnRH also causes phosphorylation of SRF through calmodulin-dependent kinase II (CamKII), which leads to increased binding to its site. CamKII activation is sufficient for phosphorylation of SRF and for induction of the c-Fos gene through the SRF site. Thus, GnRH uses a combination of growth factor signaling and the CamKII pathway to induce c-Fos to regulate FSHβ gene expression in gonadotrope cells.
Extensive investigation has been devoted to the mechanisms of GnRH signaling in the pituitary gonadotrope due to the central role it plays in reproductive function. Much is known about the signaling pathways and cis elements important for LH and FSH regulation by GnRH (1). However, this regulation is not direct; immediate early genes induced by GnRH signaling act at the LH and FSH genes. For example, GnRH regulates FSHβ synthesis through induction of an activator protein 1 (AP-1) transcription factor that binds the promoter of the FSHβ-subunit gene (2). FSHβ is normally expressed at a very low basal level. Its induction by GnRH and/or activin leads to higher FSH concentrations after heterodimerization of the FSHβ subunit with α-glycoprotein subunit, which is available in excess (3, 4). Because FSH can be constitutively secreted (5), induction of the β-subunit is the critical regulatory step that controls FSH concentrations, and, thus, follicular growth in the ovary.
The only factor currently known to be involved in induction of the mouse and human FSHβ-subunit genes by GnRH is AP-1 (1). AP-1 is comprised of a heterodimer of the immediate early proteins, c-Fos and c-Jun. We have previously demonstrated that the AP-1 isoforms c-Fos, c-Jun, FosB, and JunB (but not JunD), are rapidly induced by GnRH in a model of the mature gonadotropes, the mouse LβT2 cell line (2). c-Fos is one of the most highly GnRH-induced genes in the gonadotrope (6, 7), whereas other AP-1 isoforms including c-Jun increase less with hormone treatment. In vivo, c-Fos-deficient mice are infertile and show phenotypes similar to the FSHβ-null mice, indicating the importance of c-Fos for fertility (8).
c-Fos is an immediate-early gene that is activated rapidly and transiently in most cell types by growth factor signaling. It is induced by a plethora of physiological signals: growth factors, cytokines and hormones, and environmental stimuli. In turn, c-Fos regulates a wide range of cellular processes, including cell proliferation, death, survival, and differentiation. Although a role for c-Fos was reported in a variety of processes, its primary role is dependent on the cell type and stimuli (9, 10). Surprisingly, although gonadotropes express certain growth factor receptors, such as epidermal growth factor (EGF) receptor, which in other cell types induce c-Fos, in the gonadotropes EGF treatment does not induce c-Fos (11).
Induction of the c-Fos gene by growth factor signaling occurs by formation of an active complex of a subfamily of Ets factors, of which the prototypical member is ELK1, with serum response factor (SRF) (12). Both transcription factors bind directly to the promoter in the basal state but are activated after growth factor-induced phosphorylation of ELK1/Ets, preferentially by the MAPK pathway, which leads to ELK1/Ets association with SRF and c-Fos gene induction (13, 14). The cAMP-signaling pathway can activate c-Fos by phosphorylating cAMP response element-binding protein, which also constitutively binds the c-Fos promoter at cAMP response element sites (15, 16), whereas cytokines upon activation of the JAK/STAT pathway activate c-Fos through Stat-inducible element (17).
Induction of c-Fos expression by GnRH signaling is partially dependent on p38 and ERK1/2 MAPKs (18), which are the same pathways activated by growth factor signaling. Induction is also dependent on protein kinase C (PKC) (19, 20) and calcium influx (21, 22) through L-type voltage-gated channels, but not from intracellular calcium stores (22). However, the GnRH-responsive elements of the c-Fos promoter have not been examined nor have the transcription factors and pathways that activate them been identified.
Given that c-Fos is induced in a variety of cell types by growth factors, some of which have receptors on gonadotrope cells, but do not normally induce c-Fos or FSHβ, we determined the specificity of GnRH signaling to the c-Fos promoter. The same signaling pathways are necessary for induction of both c-Fos and FSHβ, and c-Fos induction precedes that of FSHβ, implying that c-Fos is upstream of FSHβ. Similar to growth factor signaling, GnRH leads to phosphorylation of ELK1, through ERK1/2 and p38, the same MAPK pathways necessary for c-Fos and FSHβ induction. Surprisingly, although ELK1 interacts with SRF after GnRH treatment, the ELK1 site is not necessary for induction by GnRH, whereas it is necessary for the induction by 12-tetradecanoylphorbol-13-acetate (TPA) (diacylglycerol analog) and constitutively active MAPK kinase (MEK). However, because the SRF site is necessary and sufficient for c-Fos induction by GnRH, but not by TPA, we concentrated on examination of SRF activation. GnRH phosphorylates SRF via calcium/calmodulin-dependent kinase II (CamKII), which is sufficient to induce c-Fos through the SRF site. Thus, the CamKII pathway provides the specificity of GnRH signaling to the c-Fos gene.
Results
c-Fos mRNA is induced by GnRH, and its induction precedes induction of FSHβ
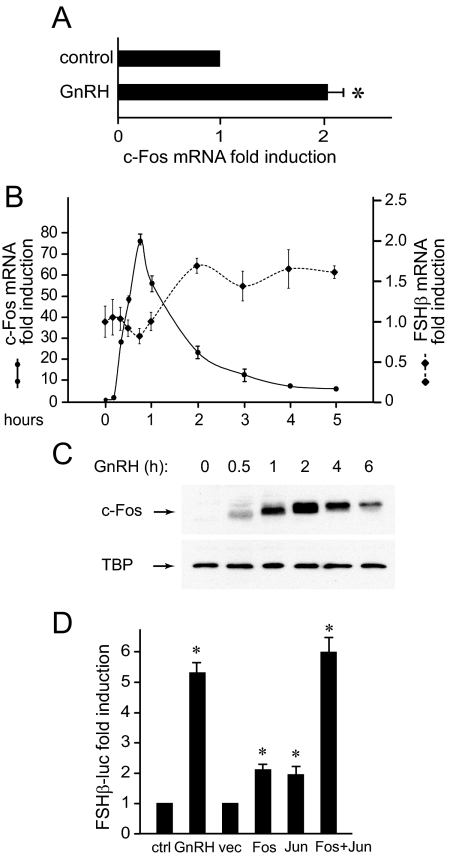
We previously identified an AP-1 site as the critical site for GnRH induction of the FSHβ gene (2). The −76 element is still the only site known to be involved in GnRH induction, although questions have been raised as to whether the AP-1 factor is indeed the key activator at this element (23). Although AP-1 isoforms are the most highly induced mRNAs in LβT2 gonadotrope cells after treatment with GnRH, and c-Fos induction by GnRH is observed in vivo in sheep pituitaries (24), another report using rat pituitary cells in culture failed to detect an increase in c-Fos with hormonal treatment over that of media replacement (11). Indeed, as an immediate-early gene that responds to stress signals, simple media change can induce c-Fos, albeit to a low level. Thus, we determined whether c-Fos induction could be detected in primary mouse pituitary cells after GnRH treatment, using a more sensitive method, quantitative RT-PCR. Dispersed mouse pituitary cells were incubated overnight in serum-free media and then treated with a pulse of GnRH or vehicle. After 1 h, the mRNA was extracted, and c-Fos induction was quantified and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). After further normalization to the induction by media change alone, we observed a 2-fold induction by hormone over control vehicle-treated cells (Fig. 1A). Because gonadotrope cells comprise only up to 10% in a mixed-cell pituitary population, in a pure culture of immortalized gonadotrope cells, c-Fos is robustly induced by GnRH. Its induction precedes that of FSHβ (Fig. 1B). c-Fos induction in LβT2 cells can be detected after 15 min of treatment, reaches maximum at 45 min, and declines thereafter. FSHβ mRNA induction by GnRH occurs by 2 h of GnRH treatment, which coincides with the maximal c-Fos protein levels (Fig. 1C). We further established that overexpression of c-Fos is sufficient to induce FSHβ, and, when overexpressed with its heterodimeric partner c-Jun, the induction of the FSHβ reporter in the gonadotrope cell line, LβT2, reaches the level of GnRH treatment (Fig. 1D).
Fig. 1.
c-Fos induction by GnRH precedes induction of FSHβ. A, Mouse primary pituitary cells were dispersed in culture and treated with a 5-min pulse of vehicle of 10 nm GnRH. mRNA was extracted 1 h later and subjected to quantitative RT-PCR. c-Fos mRNA concentration was normalized to GAPDH mRNA in the same sample and then to the ratio in vehicle-treated cells. B, LβT2 cells were treated with GnRH for different lengths of time, after which the mRNA was extracted and the amount of c-Fos and FSHβ mRNA was analyzed by real-time quantitative RT-PCR. C, Whole-cell extracts from LβT2 cells treated with GnRH, time indicated above each lane, were subjected to Western blots for c-Fos and TATA-binding protein (TBP) as loading control. D, LβT2 cells transiently transfected with a 1-kb mouse FSHβ-luciferase reporter plasmid were treated for 5 h with 10 nm GnRH or cotransfected with expression vectors for c-Fos and c-Jun (Fos, Jun) or empty vector control (vec), as indicated. The luciferase/β-gal normalized values were determined and presented as fold induction from vehicle-treated cells. *, Significant induction with P < 0.05. ctrl, Control.
GnRH utilizes the same signaling pathways to regulate c-Fos and FSHβ
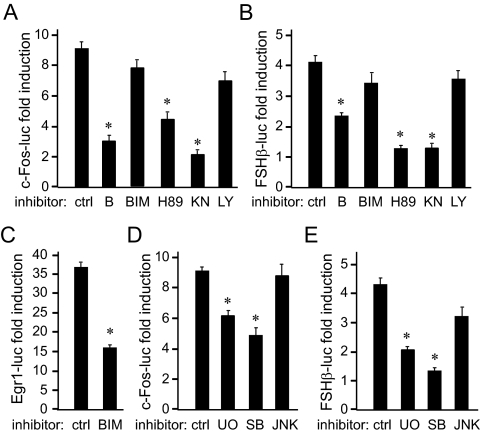
Because, in other cell types, c-Fos is induced by a multitude of stimuli via several signaling pathways, we used selective inhibitors of signaling pathways to determine which pathways are necessary for GnRH induction of c-Fos and FSHβ. To analyze transcriptional responses of either gene, 1 kb of the mouse c-Fos proximal promoter linked to a luciferase reporter, and 1 kb of the mouse FSHβ-proximal regulatory region fused to a luciferase reporter, were transfected into the LβT2 gonadotrope cell line. Chelation of intracellular calcium by BAPTA decreases GnRH induction of both the c-Fos and FSHβ reporters (Fig. 2, A and B, respectively). Thus, calcium increase is necessary for both genes to be induced by GnRH. Inhibitors H89, likely inhibiting protein kinase A (PKA) action, and KN-93, which inhibits primarily CamKII, decreased induction of both c-Fos and FSHβ, indicating a role for these pathways in GnRH signaling. Inhibition of phosphatidylinositol 3-kinase by LY 294002 did not have an effect, demonstrating that the phosphatidylinositol 3-kinase pathway does not play a role in GnRH signaling to c-Fos or FSHβ. Inhibition of classical PKC isoforms by bis-indolylmaleimide (BIM) failed to reduce induction by GnRH of either c-Fos or FSHβ (Fig. 2, A and B). This is surprising because PKC is thought to be important for GnRH activation of MAPK pathway (25, 26). To determine whether a sufficient concentration of the inhibitor was used, GnRH induction of another immediate-early gene, Egr-1, was analyzed. The Egr-1 promoter is robustly induced by GnRH, and inhibition of the PKC pathway by BIM reduced induction by 60%, indicating that BIM effectively inhibited PKC signaling and that classical PKC isoforms play a role in Egr-1 induction by GnRH (Fig. 3C). The lack of an effect of BIM on induction of c-Fos or FSHβ may indicate that PKC does not play a role in GnRH signaling to these genes or that a different pool of PKC isoforms that are not inhibited by BIM may be required. Indeed, previous studies determined that several PKC isoforms are present in the LβT2 cells (27), some of which are not inhibited by BIM.
Fig. 2.
GnRH uses the same signaling pathways for induction of the c-Fos and FSHβ genes. LβT2 cells were transfected with 1 kb mouse c-Fos-luciferase (A and D); 1 kb mouse FSHβ-luciferase (B and E), or 1 kb mouse Egr-1-luciferase (C), and then treated with 10 nm GnRH for 5 h. BAPTA (B, 50 μm) (to chelate intracellular calcium), 1 μm BIM (to inhibit PKC), 10 μm H89 (to inhibit PKA), 10 μm KN-93 (KN; to inhibit CamKII), 50 μm LY 294002 (LY; to inhibit phosphatidylinositol 3 kinase), 5 μm UO126 (UO; to inhibit MEK1 and activation of ERK1/2), 20 μm SB 202190 (SB; to inhibit p38), or 10 μm SP 600125 (JNK; to inhibit JNK), were added to cells as indicated 10 min before hormone treatment. The results were represented as fold induction by GnRH relative to the control cells treated with the same inhibitor; *, Significant decrease in fold induction from GnRH-treated cells without inhibitor (ctrl); P < 0.05. ctrl, Control.
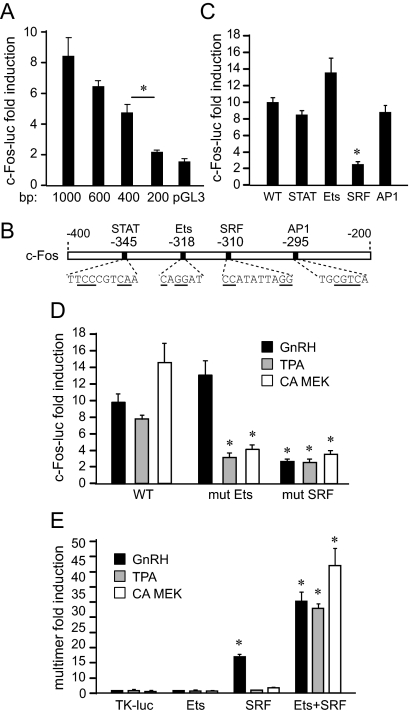
Fig. 3.
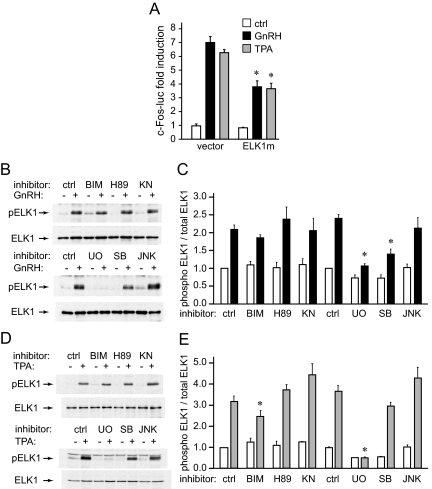
The SRF site is necessary and sufficient for c-Fos induction by GnRH. A, Different lengths of c-Fos luciferase were transfected into LβT2 cells and then treated with vehicle or GnRH. For each truncation, luciferase expression after GnRH treatment was normalized to control-treated cells and results are presented as fold induction. *, Significant decrease in fold induction from the previous truncation. B, Known regulatory elements in the −400 to −200 region of the c-Fos promoter are presented on the schematic below the graph. Mutated residues used in C, are underlined. C, Reporters containing mutations were transfected into LβT2 cells and treated with vehicle or GnRH. *, Statistically significant decrease in fold induction caused by mutation compared with the induction of the wild type (WT). D, Mutations in the Ets site (mut Ets) and SRF site (mut SRF) were transfected to analyze their effect on induction by TPA and constitutively active MEK1 (CA MEK), in addition to GnRH. *, Significant decrease in fold induction. E, Four copies of individual promoter elements (multimers), indicated underneath the bars, were linked to the minimal TK promoter luciferase (TK-luc) reporter and analyzed for sufficiency for a response to GnRH, TPA, or CA MEK. *, Significant induction with GnRH, TPA, or CA MEK of the multimer over TK-luc control.
We further analyzed MAPK pathways using inhibitors for specific branches: UO126 (UO) to inhibit ERK1/2, SB 202190 to inhibit p38, and JNK Inhibitor II, SP600125 (JNK), to inhibit JNK. Both c-Fos and FSHβ gene induction by GnRH were reduced by inhibition of the ERK1/2 and p38 pathways (Fig. 2, D and E). Taken together, these results corroborate that c-Fos is upstream of FSHβ in the GnRH cascade, in addition to our previous finding that c-Fos binds the FSHβ promoter (2), because both genes require the same signaling pathways for their induction by GnRH.
The c-Fos SRF site is necessary and sufficient for induction by GnRH
Because c-Fos induction is an intermediary of FSHβ induction by GnRH, and the FSHβ gene is specifically expressed in the anterior pituitary gonadotrope, we set out to determine the molecular mechanism of GnRH induction of the c-Fos gene. To identify regions of the c-Fos promoter critical for induction by GnRH, truncations consisting of different promoter lengths were created and analyzed in transient transfection assays. For each truncation, GnRH-treated values were normalized to vehicle control, and results are presented as fold induction, and a significant drop in induction occurred when the promoter was truncated from −400 to −200 bp (Fig. 3A), after which the responsiveness was lost. Thus, GnRH activity on the c-Fos promoter maps to the region between −400 and −200 bp from the transcription start site.
Four putative binding elements for STAT, Ets, SRF, and AP-1 transcription factors have been identified in this region (Fig. 3B), and all were previously reported to play a role in c-Fos induction by selective stimuli (28). Site-directed mutagenesis in the −1000 bp c-Fos reporter was performed to specifically mutate each of these sites, at the underlined residues. Mutations in the STAT and AP-1 sites had no significant affect on induction by GnRH, indicating that these sites do not play a role in GnRH induction (Fig. 3C). Surprisingly, mutation in the Ets site that affects induction by GH and growth factors in other cell types did not decrease GnRH induction either. However, mutation in the SRF site resulted in an 80% decrease in induction by GnRH.
Due to the unexpected result that the Ets site mutation did not reduce c-Fos induction by GnRH, we investigated this element further. Growth factor activation of c-Fos occurs through MAPK pathway activation and phosphorylation of Ets subfamily members, which then bind the Ets site and interact with SRF to activate transcription. To mimic growth factor activation, LβT2 cells were treated with TPA or cotransfected with constitutively active MEK1 (CA MEK), which activate the PKC or diacylglycerol kinases, and ERK1/2 pathways, respectively. Transient transfections were performed with the c-Fos promoter containing mutations in the Ets or SRF sites and compared with the induction of the wild type (Fig. 3D). Both mimics of growth factor signaling are capable of inducing the c-Fos reporter, similarly to GnRH. SRF1 mutation abrogates induction by each of the treatments. Consistent with the role of the Ets site in other cell types, the Ets mutation prevented induction by TPA and CA MEK1, but not GnRH. Thus, the Ets and SRF sites are both required for activation of c-Fos through TPA and the ERK1/2 pathway, but only the SRF element is critical for induction by GnRH.
Although SRF is constitutively bound to its SRF site in the c-Fos promoter, binding is not sufficient to induce transcription of c-Fos, but rather it requires interaction with an Ets factor through the Ets site for activation (29, 30). To assess the sufficiency of this site in GnRH activation of c-Fos in the gonadotropes, we created luciferase reporters containing four copies of each site (multimers), fused to a heterologous thymidine kinase (TK) promoter. The SRF site alone is sufficient for induction by GnRH, evident by the 17-fold increase after treatment, whereas the Ets site is not (Fig. 3E). Furthermore, neither the Ets nor SRF sequences alone are responsive to induction by TPA and CA MEK1, but together, the multimer containing both Ets+SRF elements is robustly induced by all treatments. This result is consistent with the results obtained with the mutations in the c-Fos promoter, in which both the Ets and SRF sites were necessary for induction by TPA and CA MEK1. The presence of the Ets site augments the GnRH response, because induction with the Ets+SRF multimer is considerably higher than with SRF alone. Taken together, both the Ets and SRF sites are required for growth factor activation of c-Fos, whereas, in contrast, the SRF site is necessary and sufficient for induction by GnRH.
ELK1 and SRF are phosphorylated after GnRH treatment
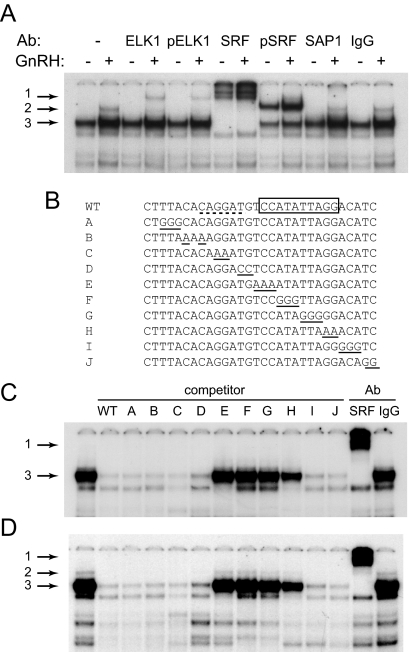
EMSAs were performed to identify complexes that bind the c-Fos promoter, using a region that contains the Ets- and SRF-binding sites as a probe. Nuclear extracts from LβT2 cells, treated with vehicle or GnRH for 15 min, were incubated in binding reactions with the c-Fos probe. Antibodies to proteins that bind these sites: ELK1 (a prototypical member of Ets subfamily transcription factors), phospho-ELK1, SRF, phospho-SRF, or stress-activated protein (SAP)1 (closely related to ELK1), were included to determine the identity of the different transcription factors binding to the probe (Fig. 4A). GnRH treatment of LβT2 cells caused changes in two complexes, indicated with arrows 2 and 3. Complex 2 is present only after GnRH treatment, whereas complex 3 increases in intensity after GnRH treatment. Complex 3 is supershifted with antibodies to SRF, indicating that SRF is constitutively bound to the c-Fos promoter, consistent with previously published reports (31). The difference in binding intensity from control vs. GnRH-treated extracts is supershifted with antibodies specific for the phosphorylated form of SRF, indicating that the increase in SRF binding after GnRH treatment is due to SRF phosphorylation. The complex indicated by arrow 2 only appears after GnRH treatment and is supershifted with the ELK1, phospho-ELK1, and with SRF antibodies (supershift arrow 1), suggesting that it is an ELK1/SRF complex that forms after GnRH-induced phosphorylation of ELK1, which promotes its interaction with SRF. Under these conditions, we could not detect a complex containing SAP1, the protein related to ELK1, nor could we detect ELK1 binding alone. Either the binding reaction was not optimal for ELK1 or ELK1 is always present in the complex with SRF on the c-Fos promoter. Thus, we can conclude that this increase in binding to the c-Fos promoter as a result of GnRH treatment is due to the phosphorylation of both SRF and ELK1.
Fig. 4.
ELK1 and SRF bind to the c-Fos promoter. Nuclear extracts from LβT2 cells treated with vehicle control or GnRH were incubated with the probe, indicated as WT (wild type) in panel B, encompassing the −335/−305 elements and antibodies (Ab) for ELK1, phospho-ELK1 (pELK1), SRF, phospho-SRF (pSRF), SAP1, or IgG control. Arrow 1 indicates a supershift of the complex by the addition of an antibody, whereas arrows 2 and 3 represent complexes that change after GnRH treatment. B, Unlabeled oligonucleotides (A–J) were used in 200-fold excess in EMSA to compete with the radiolabeled probe (WT) for binding nuclear extracts from LβT2 cells. The 2- or 3-bp residues from the wild-type sequence that are mutated in each competitor are underlined. The Ets site bound by ELK1 has a dotted underline in WT, whereas the SRF site is indicated with a box. C and D, Unlabeled oligonucleotides A–J, presented above the corresponding lane, were used as competitors with radioactively labeled WT oligonucleotide as a probe, for complexes from the vehicle-treated extracts (C) or GnRH-treated extracts (D). Oligonucleotides with mutations that prevent binding of the complex of interest are unable to compete with the wild-type probe. SRF or nonimmune IgG antibodies (Ab) are included in the last two binding reactions instead of competitors to determine the identity of the complex.
To evaluate which base pairs in the Ets- and SRF-binding elements are needed for protein interaction with the c-Fos promoter, oligonucleotides with scanning mutations (Fig. 4B) were used in 200-fold excess as competitors in EMSAs. Nuclear extracts were collected from LβT2 cells and incubated in the binding reaction with oligonucleotides containing scanning mutations A–J, either after vehicle treatment (Fig. 4C) or after GnRH treatment (Fig. 4D). Oligonucleotides E–H, with scanning mutations in the SRF 10-bp core binding site, indicated with a box in Fig. 4B, are not able to compete for the SRF complex (arrow 3), illustrating that these base pairs are necessary for binding of the SRF complex. The identity of the complex was confirmed with a supershift with SRF antibodies (arrow 1). After GnRH treatment, the ELK1/SRF complex (arrow 2; Fig. 4D) requires only the SRF sequence for binding, because mutations in the SRE prevent competition. Wild type and competitors with mutations in the Ets site (A–D), were able to compete for the ELK1/SRF complex (arrow 3) present in the first lane. Therefore, the SRF site is required for ELK1/SRF complex binding, but the Ets sequence is not essential, consistent with the transfection experiments. Overall, these results demonstrate a requirement for the SRF sequence for both SRF and ELK1/SRF complex interaction with the promoter, and interestingly, that the Ets site is not necessary for ELK1/SRF binding.
GnRH phosphorylates ELK1 through the ERK1/2 and p38 pathways
From the EMSA, it is clear that GnRH causes ELK1 phosphorylation, which induces ELK1 recruitment to the promoter. To analyze whether phosphorylation of ELK1 is critical for c-Fos induction by GnRH, we mutated a serine in the ELK1 phosphorylation site to alanine and cotransfected the mutant with the c-Fos reporter. Because TPA treatment required the ELK1 binding site (the Ets element) for c-Fos induction, whereas GnRH treatment caused ELK1 recruitment to the promoter through interaction with SRF, we used both TPA and GnRH treatment to analyze the role of ELK1 phosphorylation. Overexpression of the phosphorylation-resistant mutant reduced c-Fos fold induction by both GnRH and TPA compared with the controls (Fig. 5A), indicating that phosphorylation of ELK1 is important for both TPA and GnRH induction of c-Fos.
Fig. 5.
GnRH phosphorylates ELK1 through ERK1/2 and p38 MAPK. A, A serine to alanine mutant of ELK1 that prevents its phosphorylation was cloned into an expression vector and cotransfected with the c-Fos reporter. Fold induction in response to GnRH and TPA was compared with controls. *, Statistically significant decrease in fold induction. B and D, Whole-cell extracts after GnRH (B) or TPA (D) treatment, with or without addition of signaling pathway inhibitors (as in Fig. 3), were subjected to Western blotting with antibodies specific for phosphorylated ELK1. After quantification, Western blots were stripped and reprobed with antibodies to total ELK1. C and E, The ratio of phospho-ELK1 to total ELK1 in each sample treated with vehicle (white bars) or GnRH (black bars) in panel C; or vehicle and TPA (gray bars) in panel E, was quantified. Four separate experiments were averaged and an asterisk represents statistically significant decrease in ELK1 phosphorylation caused by inhibition of specific signaling pathways. ctrl, Control.
Western blots with antibodies specific for the phosphorylated form of ELK1 on whole-cell extracts from cells treated with vehicle, GnRH, or TPA, with or without inhibitors for specific signaling pathways, were performed to identify pathways employed by GnRH and TPA to phosphorylate ELK1 (Fig. 5, B and D, respectively). The amount of phospho-ELK1, after quantification, was normalized to the total ELK1 concentration in each lane, and four experiments, each normalized to the their control, were averaged and presented in graph form: GnRH treatment in Fig. 5C and TPA treatment in Fig. 5E. GnRH phosphorylates ELK1 through the ERK1/2 and p38 pathways, because its inhibitors, UO126 and SB 202190, respectively, lower ELK1 phosphorylation by GnRH compared with control. Phosphorylation of ELK1 by TPA, on the other hand, was lowered by the PKC inhibitor BIM and completely abrogated with the inhibition of ERK1/2 pathway with UO126. A role for p38 in GnRH signaling that leads to ELK1 phosphorylation, but not in TPA signaling, may account for differences in Ets site utilization, because p38 has been implicated in differential posttranslational modification of ELK1 (32).
GnRH phosphorylates SRF through the CamKII pathway
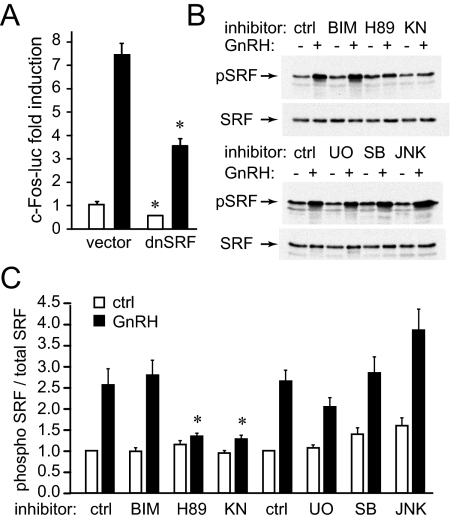
We next analyzed the role of SRF, because its site is necessary and sufficient for GnRH induction of c-Fos. As mentioned earlier, SRF activation of the c-Fos promoter was thought to be due to interaction with phosphorylated ELK1. However, using EMSA, we observed that SRF was phosphorylated by GnRH treatment and that this phosphorylation augmented its affinity to its binding site. Overexpression of dominant-negative SRF lowers both basal expression and GnRH induction of c-Fos (Fig. 6A), indicating an important role for SRF protein. Western blots with phospho-specific antibodies normalized to blots with antibodies to total SRF (Fig. 6B) were used to analyze pathways that GnRH activates to phosphorylate SRF (Fig. 6C). Phosphorylation of SRF increased with GnRH treatment and was diminished with inhibitors for PKA (H89) and CamKII (KN93), the same pathways that were established as important for GnRH induction of c-Fos and FSHβ (Fig. 2, A and B).
Fig. 6.
GnRH phosphorylates SRF through the PKA and CamKII pathways. A, LβT2 cells were transfected with an expression vector containing a cDNA for a dominant-negative SRF or empty vector control. The cells were treated with vehicle (white bars) or GnRH (black bars) for 5 h, and results are presented as fold induction over vector control treated with vehicle. *, Significant decrease in both basal expression for vehicle-treated cells (white bars) and GnRH induction (black bars). B, Whole-cell extracts from LβT2 cells treated with GnRH with or without inhibitors as indicated above the corresponding lanes were used in Western blots and probed with antibodies specific for phosphorylated SRF. Blots were stripped and reprobed with antibodies for total SRF and the ratio of phosphorylated to total SRF in each lane from three different experiments were averaged and presented in panel C. *, Statistically significant decrease in SRF phosphorylation. ctrl, Control.
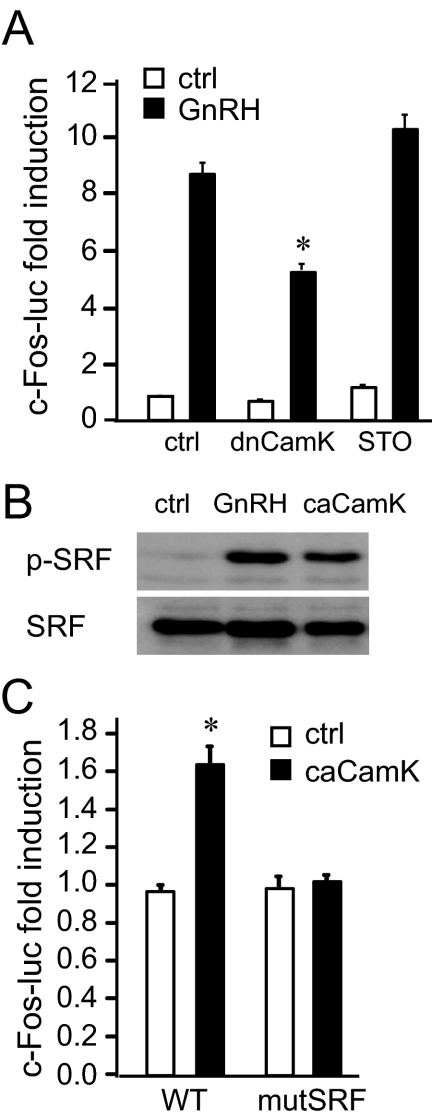
Because the inhibitor KN93 has the potential to inhibit CamKK, which activates CamKI and CamKIV, in addition to inhibition of CamKII, to determine the specificity of the inhibitor, we used a dominant-negative CamKII and a specific inhibitor for CamKK. The CamKK inhibitor, STO 609, does not have an effect on c-Fos induction by GnRH, whereas the dominant-negative CamKII reduced induction by GnRH (Fig. 7A), indicating that indeed CamKII, and not CamKK, plays a role in GnRH signaling. We then overexpressed constitutively active CamKII and determined that it is sufficient to cause phosphorylation of SRF (Fig. 7B). CamKII is also sufficient for c-Fos induction, because overexpression of constitutionally active CamK increased c-Fos reporter expression. Furthermore, induction occurs through the SRF site, because the SRF mutation abrogated induction of c-Fos by constitutively active CamKII (Fig. 7C). Thus, GnRH activation of CamKII causes phosphorylation of SRF, which increases binding of SRF to its site and is necessary and sufficient for induction of the c-Fos gene in gonadotrope cells.
Fig. 7.
Activation of CamKII is necessary and sufficient for induction of c-Fos. A, To confirm the specificity of the KN-93 inhibitor, we cotransfected a dominant-negative CamKII (dnCamK) or treated the LβT2 cells with the inhibitor of CamKK, STO-609, and treated with vehicle (white bars) or GnRH (black bars). *, Significant decrease in fold induction in the cells cotransfected with the dominant-negative CamKII. B, Western blot of the whole-cell lysates treated with GnRH or cotransfected with constitutively active CamKII (caCamK), and probed with antibodies for phospho-SRF and total SRF. C, LβT2 cells were cotransfected with the wild-type (WT) c-Fos reporter or with c-Fos containing a mutation in the SRF-binding site (mutSRF), and constitutively active CamKII (caCamK; black bars) or vector control (white bars). *, Significant induction by caCamK, not observed in the cells cotransfected with c-Fos reporter containing a mutation in the SRF-binding site. ctrl, Control.
Discussion
GnRH regulation of FSH is critical for follicular development. GnRH induces transcription of the FSHβ gene; however, this regulation is indirect, because it occurs through an induction of the immediate-early genes that comprise AP-1 (2). Because c-Fos is an immediate-early gene that is rapidly and robustly induced by a variety of stimuli specific to individual cell types, but in gonadotropes is only induced by GnRH, we determined the specificity of GnRH induction of c-Fos in the gonadotrope cells. We established that the SRF site is necessary and sufficient for GnRH induction, and that GnRH activates SRF protein through CamKII. GnRH also phosphorylates ELK1, which activates c-Fos through interaction with SRF on the SRF site, whereas the presence of the ELK1 site augments the response, but is not necessary. Most importantly, GnRH phosphorylates ELK1 and SRF through the same pathways that are necessary for c-Fos and FSHβ induction. Thus, this study increased our understanding of the specificity of GnRH induction of the c-Fos gene, as an intermediary for GnRH induction of FSHβ expression, and elucidated the signaling pathways necessary for FSHβ up-regulation by this key physiological activator.
Previously, we established that c-Fos, after induction by GnRH, binds the FSHβ promoter and that the AP-1 site is critical for GnRH induction (2). Initially, in this study, to support that c-Fos is the key mediator of GnRH induction of FSHβ, we showed that it precedes FSHβ induction and determined that the same signaling pathways are required for GnRH induction of c-Fos and FSHβ. GnRH induction of either gene requires calcium, CamKII, and PKA. PKC is not necessary for c-Fos or FSHβ induction, contrary to previous reports (19), which may differ from ours, either due to differences in inhibitor specificity or in the cell type. However, PKC is necessary for induction of Egr-1, which conveys GnRH induction of LHβ. Instead, it is more likely that the requirement for calcium for c-Fos and FSHβ induction is upstream of CamKII activation. As for a role of PKA, because GnRH activates Gs in addition to Gq/11 (33, 34) and its inhibitors affecting c-Fos induction, this may be due to lack of specificity of the inhibitor or may indicate that PKA is upstream of CamKII, as has been shown in other cell types (35, 36), because GnRH induction does not map to PKA-responsive cAMP response element sites.
Additionally, GnRH induction of both genes requires the ERK1/2 and p38 branches of MAPK. Involvement of ERK1/2 in FSHβ induction is in agreement with previous studies in rat pituitaries (37). However, p38 was not involved in pulsatile GnRH stimulation of FSHβ transcription in rat primary cells (38). Whether the difference in the observed role of p38 stems from the species differences (rat vs. mouse), analysis of the primary transcript vs. a transcriptional reporter, concentration of the inhibitor, treatment paradigm, or the effect of p38 on mRNA stability, translation, or turnover [not only transcription (39)] remains to be determined. The mechanism by which GnRH activates MAPK is still not clear. Three pathways have been proposed. Some studies have shown that GnRH induction of MAPK requires PKC (25, 26). Others demonstrate that GnRH activates Ras, via GnRH receptor cross talk with EGF receptor, which then leads to MAPK activation (40). A third mechanism, shown in studies by Roberson et al. (41), demonstrated that the GnRH receptor is associated with c-Raf kinase and calmodulin in lipid rafts which then induces MAPK. In our study, the PKC inhibitor, BIM, had no effect on GnRH phosphorylation of either ELK1 or SRF or on induction of c-Fos. Therefore, our results support the findings that GnRH receptors are associated in lipid rafts with c-Raf kinase and calmodulin. The activation of both c-Raf kinase, which activates Ras and through it ERK1/2 and p38, and the release of calmodulin, in addition to calcium influx, would explain the phosphorylation of ELK1 through MAPKs, and phosphorylation of SRF through calmodulin and calcium activation of CaMKII, independently of PKC.
Surprisingly, induction of c-Fos by GnRH differs from growth factors in that GnRH does not require the Ets element that normally binds ELK1 (or SAP). Growth factor induction of c-Fos results from SRF activation via interaction with ELK1 or SAP, after their phosphorylation by one of the MAPKs (28, 42). The c-Fos promoter Ets consensus sequence CAGGAT/A (43), in cooperation with the adjacent SRF site, is essential for GH- (17) and growth factor- (44) mediated transcription of c-Fos. Thus, growth factor activation of c-Fos involves activation of ELK1 or SAP to interact with SRF and requires both factors to bind their adjacent cognate promoter elements, the Ets and SRF elements (28, 31, 42, 45, 46). In contrast, the Ets-binding site is not necessary for GnRH induction of c-Fos, whereas the SRF site is both necessary and sufficient. Although ELK1 is phosphorylated after GnRH treatment, it is associated with DNA only indirectly through interaction with SRF binding to the SRF site. Gel shifts demonstrate that ELK1 forms a complex with SRF on the SRF-binding site in the c-Fos promoter, which is in agreement with the finding that the SRF site, but not the Ets site, is important.
Although its site is not necessary, GnRH phosphorylates ELK1 through ERK1/2 and p38 MAPK. In growth factor signaling, ELK1 is also activated through MAPK-signaling pathways (31, 47, 48). Although the JNK MAPK pathway is capable of activating ELK1 (42, 47), and JNK can be activated by GnRH (38, 49), JNK does not appear to have a role in GnRH activation of ELK1. TPA treatment, which also induces c-Fos, but requires both the Ets and SRF sites, phosphorylates ELK1 only through ERK1/2 MAPK. Thus, p38 is required for GnRH (but not TPA) phosphorylation of ELK1. p38 activation sustains sumoylation of ELK1, in addition to phosphorylation, through activation of protein inhibitor of activated STATxα small ubiquitin-like modifier-ligase (32). Sumoylation causes different conformational changes in ELK1, and thus, may contribute to the ability of GnRH-activated ELK1 to induce c-Fos transcription without direct DNA binding, through interaction with SRF. Although SAP and ELK1 are homologous, their DNA-binding properties are different, because SAP binds to the c-Fos promoter with high affinity whereas ELK1 binds poorly (43, 50). Thus, growth factors may activate SAP preferentially, whereas GnRH acts through ELK1, potentially contributing to the differences in requirement for the Ets-binding site. Therefore, differential use of signaling pathways by GnRH and TPA may lead to varied modification of ELK1 and its behavior, either as a DNA-binding transcription factor or as a cofactor for SRF.
The SRF protein and its site are both necessary and sufficient to convey GnRH responsiveness to the c-Fos promoter. Our data indicate that SRF and ELK1 are phosphorylated by GnRH signaling. SRF may also be further activated by complex formation with GnRH-phosphorylated ELK1. In differentiated cell types, like muscle, it is possible for SRF to be activated independently of ELK1 or SAP. In muscle-specific gene promoters, SRF binds the CArG element, CC(A/T)6GG, SRF site, which lacks an adjacent Ets site, and undergoes site-specific phosphorylation (28, 51, 52). SRF activation in PC12 cells, similar to what we observe, is also sufficient for c-Fos induction and is activated by calcium influx due to depolarization, whereas NGF induction of c-Fos in the same cells requires the Ets site. GnRH stimulation of gonadotrope cells causes calcium influx that is necessary for c-Fos induction (21, 22). Surprisingly, GnRH induction of c-Fos is similar to cell depolarization rather than to hormone/growth factor signaling through growth factor receptors, despite GnRH activation of the MAPK pathway. GnRH activation of SRF requires CaMKII, which is activated in part by calcium influx. CamKII is sufficient to induce c-Fos, because it phosphorylates SRF and SRF is sufficient to activate the c-Fos gene. Given that GnRH induction of the Ets+SRF multimer is significantly higher than the SRF multimer alone, the Ets site may augment the affinity of the SRF/ELK1 complex. Thus, GnRH may activate c-Fos through a combination of growth factor signaling through ELK1 activation, and the depolarization and calcium increase pathway, that activates CamKII and SRF.
Regulation of gonadotropin genes by GnRH in gonadotropes is through the direct action of GnRH receptor activation of kinase cascades on immediate-early genes such as c-Fos, c-Jun, Egr-1, and ATF-3. Through understanding the mechanisms of GnRH activation of c-Fos, we have begun to delineate the mechanism of activation of FSHβ by AP-1 at the AP-1 binding element in the FSHβ promoter. However, because c-Fos requires dimerization with c-Jun to form the active transcriptional regulator, AP-1, to fully understand the regulation of FSHβ by AP-1 will require further investigations into the induction of c-Jun by GnRH. Thus far, it is known that β-catenin is important for c-Jun induction by GnRH (53, 54) but the mechanism is unknown. It will be of particular interest to determine whether c-Jun induction also requires the signaling cascades that we have now shown to be important for c-Fos.
Materials and Methods
Plasmids
The 1-kb murine c-Fos promoter was cloned by PCR from genomic DNA and linked to the luciferase reporter in the pGL3 basic plasmid. 5′-Truncations of the c-Fos 1-kb promoter were created by PCR subcloning. Mutagenesis of the c-Fos promoter luciferase plasmid was performed using the QuikChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA) following the manufacturer's instructions. Mutations were confirmed by dideoxyribonucleotide sequencing performed by the DNA Sequencing Shared Resource, UCSD Cancer Center (P30 CA023100). The mouse FSHβ luciferase reporter was described previously (55). For the multimer reporter, four copies of the individual response elements found in the c-Fos promoter, with 2-bp spacers (Ets: caCAGGATgt; SRF: tCCATATTAGGat; Ets+SRF: aCAGGATGTCCATATTAGGac) were cloned into the multiple cloning site in front of the thymidine kinase minimal promoter in the pGL3 basic luciferase reporter. MEK1 expression vectors and constitutively active mutants were kindly provided by Dr. Michael Weber. The dominant-negative expression vector for calcium/calmodulin II kinase (CaMKII N) and the constitutively active mutant CaMKII H282R, with vector control, were kindly provided by Dr. Tom Soderling. Constitutively active calcium/calmodulin II kinase, CaMKII 1-290, was generously provided by Dr. Steven Green. The SRF expression vector was provided by Dr. Ron Prywes.
Cell culture and transfections
LβT2 and Cos-1 cells were maintained in DMEM (Mediatech, Inc., Herndon, VA) with 10% fetal bovine serum (Gemini Bio-Products, West Sacramento, CA) and penicillin/streptomycin antibiotics (Life Technologies, Gaithersburg, MD/Invitrogen, Carlsbad, CA) at 37 C and 5% CO2. For transfection, LβT2 cells were plated in 12-well plates 1 d before transfection in DMEM 10% fetal bovine serum. FuGENE 6 transfection reagent (Roche Molecular Biochemicals, Indianapolis, IN) was used for transfecting cells following the manufacturer's instructions. Each well was transfected with 0.5 μg of a luciferase-reporter plasmid and 0.1 μg of TK β-galactosidase, a reporter plasmid driven by a Herpes virus TK promoter as a control for transfection efficiency. Forty-eight hours after transfection and after overnight starvation in serum-free medium with treatments as indicated in each figure, cells were rinsed with 1 × PBS and lysed with 100 nm KPO4 buffer containing 0.2% Triton X-100. Cell lysate (20 μl) was used to measure luciferase activity on a Veritas Microplate luminometer (Turner Biosystems, Sunnyvale, CA) by injecting 100 μl of buffer containing 25 mm Tris (pH 7.8), 15 mm MgSO4, 10 mm ATP, and 65 μm luciferin into each well. Using the Tropix Galacto-light β-galactosidase assay (Applied Biosystems, Foster City, CA) and following the manufacturer's instructions, β-galactosidase activity was measured subsequently. Transfections were performed in triplicate and repeated a minimum of three times. One-way ANOVA statistical analysis with Tukey's post hoc test was performed using the JMP program with significance set at P < 0.05.
Reverse transcription and PCR
RNA was obtained with Trizol reagent (Invitrogen, Carlsbad, CA/Life Technologies) according to the manufacturer's instructions. Contaminating DNA was removed with DNA-free reagent (Ambion, Austin, TX) and 2 μg RNA was reverse transcribed using Superscript III First-Strand Synthesis System (Invitrogen). Quantitative Real-Time PCR was performed in an iCycler from Bio-Rad Laboratories, Inc. (Hercules, CA), using QuantiTect SYBR Green PCR Kit (QIAGEN, Valencia, CA) and the following primers: c-Fos forward, GGCAAAGTAGAGCAGCTATCTCCT; c-Fos reverse, TCAGCTCCCTCCTCCGATTC; FSH forward, GCCGTTTCTGCATAAGC; FSH reverse, CAATCTTACGGTCTCGTATACC; GAPDH forward, TGCACCACCAACTGCTTAG; GAPDH reverse, GGATGCAGGGATGATGTTC; under the following conditions: 95 C for 15 min, followed by 40 cycles at 95 C for 15 sec, 54 C for 30 sec, and 72 C for 30 sec. Each sample was assayed in triplicate, and the experiment was repeated four times. Standard curves with dilutions of a plasmid containing cFos, FSHβ, or GAPDH cDNA were generated with the samples in each run. In each experiment, the amount of cFos or FSHβ was calculated by comparing the threshold cycle obtained for each sample with the standard curve generated in the same run. Replicates were averaged and divided by the mean value of GAPDH in the same sample. After each run, a melting curve analysis was performed to confirm that a single amplicon was generated in each reaction.
Whole-cell extracts and Western blotting
After overnight starvation in serum-free DMEM supplemented with 0.1% BSA, LβT2 cells were treated as indicated in each figure. The cells were rinsed with 1× PBS and lysed with lysis buffer containing 20 mm Tris (pH 7.4), 140 mm NaCl, protease inhibitors from Sigma (St. Louis, MO), 1 mm phenylmethylsulfonyl fluoride (PMSF), 10 mm NaF, 1% Nonidet P-40, 0.5 mm EDTA, and 1 mm EGTA. Protein concentrations were determined using Bradford reagent (Bio-Rad Laboratories) in which protein concentrations were calculated using a standard curve. Equal amounts of protein per sample were loaded and resolved on a SDS-PAGE and transferred to a polyvinylidene fluoride membrane. The membrane was blocked with 10% milk in wash buffer (20 mm Tris, pH 7.4; 0.1% Tween; 150 mm NaCl; and 0.5% BSA) and then probed with antibodies to: phospho-Elk-1 (Abcam, Cambridge, MA), phospho-SRF (Cell Signaling Technology, Danvers, MA), Elk-1 (Abcam), or SRF (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Bands were detected using goat antirabbit IgG linked to horseradish peroxidase secondary antibody and Enhanced Chemiluminescence Western Blotting Detection Reagent (GE Healthcare, Piscataway, NJ). To measure total protein, membranes were stripped by rotating the membrane at 60 C for 1 h with strip buffer (50 mm Tris, pH 6.8; 5% sodium dodecyl sulfate; and 100 mm β-mercaptoethanol). After stripping, the membrane was reexposed to Enhanced Chemiluminescence and autoradiography to ensure complete removal of the antibody and then blocked again with milk and reprobed for the total protein. The GeneGnome Bio Imaging Chemiluminescence (Syngene, Frederick, MD) reader was used to quantify the bands. For each experiment the results of the phospho-specific blot were normalized to the total protein amount and the average of all experiments was presented in the graphs. Statistical significance was determined by using ANOVA and Tukey's post hoc test and indicated with an asterisk in the figure.
Nuclear extract and EMSA
To obtain nuclear extracts after treatments, the cells were allowed to swell in hypotonic buffer [20 mm Tris (pH 7.4), 10 mm NaCl, 1 mm MgCl2, 1 mm PMSF, protease inhibitor cocktail from Sigma-Aldrich, 10 mm NaF, 0.5 mm EDTA, and 0.1 mm EGTA] and then passed three times though a 255/8 G needle. The pelleted nuclei were resuspended in hypertonic buffer [20 mm HEPES (pH 7.8), 20% glycerol, 420 mm KCl, 1.5 mm MgCl2, 1 mm PMSF, protease inhibitor cocktail (Sigma-Aldrich), 10 mm NaF, 0.5 mm EDTA, and 0.1 mm EGTA]. After incubation nuclear extracts were obtained by centrifugation. Protein determination was performed using the Bradford reagent, as described above for the whole-cell extracts. Oligonucleotides probes were obtained from Integrated DNA Technologies (Coralville, IA) and annealed and then labeled with [γ32P]ATP using T4 Polynucleotide Kinase (New England Biolabs, Inc., Beverly, MA) and column purified using Micro Bio-Spin Chromatography Columns (Bio-Rad Laboratories, Inc.). Binding reactions were 20 μl and contained 2 μg of nuclear extract, 10 mm HEPES (pH 7.8), 50 mm KCl, 0.5 mm MgCl2, 10% glycerol, 0.1% Nonidet P-40, 0.25 μg dIdC, 5 mm dithiothreitol, and 5 fmol of probe. In competition or antibody shift assays, 1 nm of unlabeled oligonucleotide or 1 μg antibody was added to the binding reaction. Electrophoresis was carried out on a 5% nondenaturing polyacrylamide gel. Gels were run at 250 V/cm2 and dried, and autoradiography was performed to identify complexes.
Acknowledgments
We thank Drs. Michael Weber (University of Virginia, Virginia), Tom Soderling (Vollum Institute, Oregon), Steven Green (University of Iowa, Iowa) and Ron Prywes (Columbia University, New York) for various expression vectors. We greatly appreciate thoughtful discussions and suggestions from Drs. Kellie Breen-Church, Varykina G. Thackray, and Mark A. Lawson (University of California, San Diego, California).
This work was supported by National Institutes of Health (NIH) Grants R01 HD057549, R21 HD058752, and R03 HD054595 (to D.C.); NIH Grants R01 HD020377 and R01 DK044838 (to P.L.M.) and National Institutes of Child Health and Human Development/NIH cooperative agreement U54 HD012303 as part of the Specialized Cooperative Centers Program in Reproduction Research. H.E. was supported by the Howell Foundation and the Endocrine Society Summer Research Fellowships.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AP-1
- Activator protein 1
- BIM
- bis-indolylmaleimide
- CA MEK
- constitutively active MEK1
- CamKII
- calmodulin-dependent kinase II
- EGF
- epidermal growth factor
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- MEK
- MAPK kinase
- PKA
- protein kinase A
- PKC
- protein kinase C
- PMSF
- phenylmethylsulfonyl fluoride
- SAP
- stress-activated protein
- SRF
- serum response factor
- TK
- thymidine kinase
- TPA
- 12-tetradecanoylphorbol-13-acetate.
References
- 1. Thackray VG, Mellon PL, Coss D. 2010. Hormones in synergy: regulation of the pituitary gonadotropin genes. Mol Cell Endocrinol 314:192–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coss D, Jacobs SB, Bender CE, Mellon PL. 2004. A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone β gene by gonadotropin-releasing hormone. J Biol Chem 279:152–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaiser UB, Conn PM, Chin WW. 1997. Studies of gonadotropin-releasing hormone (GnRH) action using GnRH receptor-expressing pituitary cell lines. Endocr Rev 18:46–70 [DOI] [PubMed] [Google Scholar]
- 4. Weiss J, Guendner MJ, Halvorson LM, Jameson JL. 1995. Transcriptional activation of the follicle-stimulating hormone β-subunit gene by activin. Endocrinology 136:1885–1891 [DOI] [PubMed] [Google Scholar]
- 5. Grady RR, Shin L, Charlesworth MC, Cohen-Becker IR, Smith M, Rivier C, Rivier J, Vale W, Schwartz NB. 1985. Differential suppression of follicle-stimulating hormone and luteinizing hormone secretion in vivo by a gonadotropin-releasing hormone antagonist. Neuroendocrinology 40:246–252 [DOI] [PubMed] [Google Scholar]
- 6. Wurmbach E, Yuen T, Ebersole BJ, Sealfon SC. 2001. Gonadotropin-releasing hormone receptor-coupled gene network organization. J Biol Chem 276:47195–47201 [DOI] [PubMed] [Google Scholar]
- 7. Kakar SS, Winters SJ, Zacharias W, Miller DM, Flynn S. 2003. Identification of distinct gene expression profiles associated with treatment of LβT2 cells with gonadotropin-releasing hormone agonist using microarray analysis. Gene 308:67–77 [DOI] [PubMed] [Google Scholar]
- 8. Johnson RS, Spiegelman BM, Papaioannou V. 1992. Pleiotropic effects of a null mutation in the c-fos proto-oncogene. Cell 71:577–586 [DOI] [PubMed] [Google Scholar]
- 9. Shaulian E, Karin M. 2002. AP-1 as a regulator of cell life and death. Nat Cell Biol 4:E131–E136 [DOI] [PubMed] [Google Scholar]
- 10. Wagner EF, Eferl R. 2005. Fos/AP-1 proteins in bone and the immune system. Immunol Rev 208:126–140 [DOI] [PubMed] [Google Scholar]
- 11. Armstrong JL, Childs GV. 1998. Regulation of c-fos expression by EGF and GnRH in specific anterior pituitary cells from proestrous female rats. J Histochem Cytochem 46:935–944 [DOI] [PubMed] [Google Scholar]
- 12. Robertson LM, Kerppola TK, Vendrell M, Luk D, Smeyne RJ, Bocchiaro C, Morgan JI, Curran T. 1995. Regulation of c-fos expression in transgenic mice requires multiple interdependent transcription control elements. Neuron 14:241–252 [DOI] [PubMed] [Google Scholar]
- 13. Sharrocks AD. 2001. The ETS-domain transcription factor family. Nat Rev Mol Cell Biol 2:827–837 [DOI] [PubMed] [Google Scholar]
- 14. Hazzalin CA, Mahadevan LC. 2002. MAPK-regulated transcription: a continuously variable gene switch? Nat Rev Mol Cell Biol 3:30–40 [DOI] [PubMed] [Google Scholar]
- 15. Ahn S, Olive M, Aggarwal S, Krylov D, Ginty DD, Vinson C. 1998. A dominant-negative inhibitor of CREB reveals that it is a general mediator of stimulus-dependent transcription of c-fos. Mol Cell Biol 18:967–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sassone-Corsi P, Visvader J, Ferland L, Mellon PL, Verma IM. 1988. Induction of proto-oncogene fos transcription through the adenylate cyclase pathway: characterization of a cAMP-responsive element. Genes Dev 2:1529–1538 [DOI] [PubMed] [Google Scholar]
- 17. Liao J, Hodge C, Meyer D, Ho PS, Rosenspire K, Schwartz J. 1997. Growth hormone regulates ternary complex factors and serum response factor associated with the c-fos serum response element. J Biol Chem 272:25951–25958 [DOI] [PubMed] [Google Scholar]
- 18. Roberson MS, Zhang T, Li HL, Mulvaney JM. 1999. Activation of the p38 mitogen-activated protein kinase pathway by gonadotropin-releasing hormone. Endocrinology 140:1310–1318 [DOI] [PubMed] [Google Scholar]
- 19. Cesnjaj M, Catt KJ, Stojilkovic SS. 1994. Coordinate actions of calcium and protein kinase-C in the expression of primary response genes in pituitary gonadotrophs. Endocrinology 135:692–701 [DOI] [PubMed] [Google Scholar]
- 20. Cesnjaj M, Krsmanovic LZ, Catt KJ, Stojilkovic SS. 1993. Autocrine induction of c-fos expression in GT1 neuronal cells by gonadotropin-releasing hormone. Endocrinology 133:3042–3045 [DOI] [PubMed] [Google Scholar]
- 21. Liu F, Austin DA, Mellon PL, Olefsky JM, Webster NJ. 2002. GnRH activates ERK1/2 leading to the induction of c-fos and LHβ protein expression in LβT2 cells. Mol Endocrinol 16:419–434 [DOI] [PubMed] [Google Scholar]
- 22. Mulvaney JM, Zhang T, Fewtrell C, Roberson MS. 1999. Calcium influx through L-type channels is required for selective activation of extracellular signal-regulated kinase by gonadotropin-releasing hormone. J Biol Chem 274:29796–29804 [DOI] [PubMed] [Google Scholar]
- 23. Ciccone NA, Lacza CT, Hou MY, Gregory SJ, Kam KY, Xu S, Kaiser UB. 2008. A composite element that binds basic helix loop helix and basic leucine zipper transcription factors is important for gonadotropin-releasing hormone regulation of the follicle-stimulating hormone β gene. Mol Endocrinol 22:1908–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Padmanabhan V, Dalkin A, Yasin M, Haisenleder DJ, Marshall JC, Landefeld TD. 1995. Are immediate early genes involved in gonadotropin-releasing hormone receptor gene regulation? Characterization of changes in GnRH receptor (GnRH-R), c-fos, and c-jun messenger ribonucleic acids during the ovine estrous cycle. Biol Reprod 53:263–269 [DOI] [PubMed] [Google Scholar]
- 25. Benard O, Naor Z, Seger R. 2001. Role of dynamin, Src, and Ras in the protein kinase C-mediated activation of ERK by gonadotropin-releasing hormone. J Biol Chem 276:4554–4563 [DOI] [PubMed] [Google Scholar]
- 26. Dobkin-Bekman M, Naidich M, Pawson AJ, Millar RP, Seger R, Naor Z. 2006. Activation of mitogen-activated protein kinase (MAPK) by GnRH is cell-context dependent. Mol Cell Endocrinol 252:184–190 [DOI] [PubMed] [Google Scholar]
- 27. Vasilyev VV, Pernasetti F, Rosenberg SB, Barsoum MJ, Austin DA, Webster NJ, Mellon PL. 2002. Transcriptional activation of the ovine follicle-stimulating hormone-β gene by gonadotropin-releasing hormone involves multiple signal transduction pathways. Endocrinology 143:1651–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hill CS, Treisman R. 1995. Differential activation of c-fos promoter elements by serum, lysophosphatidic acid, G proteins and polypeptide growth factors. EMBO J 14:5037–5047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sharrocks AD. 2002. Complexities in ETS-domain transcription factor function and regulation: lessons from the TCF (ternary complex factor) subfamily. The Colworth Medal Lecture. Biochem Soc Trans 30:1–9 [DOI] [PubMed] [Google Scholar]
- 30. Hodge C, Liao J, Stofega M, Guan K, Carter-Su C, Schwartz J. 1998. Growth hormone stimulates phosphorylation and activation of elk-1 and expression of c-fos, egr-1, and junB through activation of extracellular signal-regulated kinases 1 and 2. J Biol Chem 273:31327–31336 [DOI] [PubMed] [Google Scholar]
- 31. Yordy JS, Muise-Helmericks RC. 2000. Signal transduction and the Ets family of transcription factors. Oncogene 19:6503–6513 [DOI] [PubMed] [Google Scholar]
- 32. Yang SH, Sharrocks AD. 2006. PIASxα differentially regulates the amplitudes of transcriptional responses following activation of the ERK and p38 MAPK pathways. Mol Cell 22:477–487 [DOI] [PubMed] [Google Scholar]
- 33. Liu F, Usui I, Evans LG, Austin DA, Mellon PL, Olefsky JM, Webster NJ. 2002. Involvement of both Gq/11 and Gs proteins in gonadotropin-releasing hormone receptor-mediated signaling in Lβ T2 cells. J Biol Chem 277:32099–32108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsutsumi R, Mistry D, Webster NJ. 2010. Signaling responses to pulsatile gonadotropin-releasing hormone in LβT2 gonadotrope cells. J Biol Chem 285:20262–20272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lohse MJ, Engelhardt S, Eschenhagen T. 2003. What is the role of β-adrenergic signaling in heart failure? Circ Res 93:896–906 [DOI] [PubMed] [Google Scholar]
- 36. Bers DM. 2008. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70:23–49 [DOI] [PubMed] [Google Scholar]
- 37. Burger LL, Haisenleder DJ, Aylor KW, Marshall JC. 2008. Regulation of intracellular signaling cascades by GNRH pulse frequency in the rat pituitary: roles for CaMK II, ERK, and JNK activation. Biol Reprod 79:947–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haisenleder DJ, Burger LL, Walsh HE, Stevens J, Aylor KW, Shupnik MA, Marshall JC. 2008. Pulsatile gonadotropin-releasing hormone stimulation of gonadotropin subunit transcription in rat pituitaries: evidence for the involvement of Jun N-terminal kinase but not p38. Endocrinology 149:139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Clark AR, Dean JL, Saklatvala J. 2003. Post-transcriptional regulation of gene expression by mitogen-activated protein kinase p38. FEBS Lett 546:37–44 [DOI] [PubMed] [Google Scholar]
- 40. Kraus S, Benard O, Naor Z, Seger R. 2003. c-Src is activated by the epidermal growth factor receptor in a pathway that mediates JNK and ERK activation by gonadotropin-releasing hormone in COS7 cells. J Biol Chem 278:32618–32630 [DOI] [PubMed] [Google Scholar]
- 41. Roberson MS, Bliss SP, Xie J, Navratil AM, Farmerie TA, Wolfe MW, Clay CM. 2005. Gonadotropin-releasing hormone induction of extracellular-signal regulated kinase is blocked by inhibition of calmodulin. Mol Endocrinol 19:2412–2423 [DOI] [PubMed] [Google Scholar]
- 42. Cavigelli M, Dolfi F, Claret FX, Karin M. 1995. Induction of c-fos expression through JNK-mediated TCF/Elk-1 phosphorylation. EMBO J 14:5957–5964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Verger A, Duterque-Coquillaud M. 2002. When Ets transcription factors meet their partners. Bioessays 24:362–370 [DOI] [PubMed] [Google Scholar]
- 44. Treisman R. 1995. Journey to the surface of the cell: Fos regulation and the SRE. EMBO J 14:4905–4913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Karin M, Liu Z, Zandi E. 1997. AP-1 function and regulation. Curr Opin Cell Biol 9:240–246 [DOI] [PubMed] [Google Scholar]
- 46. Hill CS, Treisman R. 1995. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell 80:199–211 [DOI] [PubMed] [Google Scholar]
- 47. Tanos T, Marinissen MJ, Leskow FC, Hochbaum D, Martinetto H, Gutkind JS, Coso OA. 2005. Phosphorylation of c-Fos by members of the p38 MAPK family. Role in the AP-1 response to UV light. J Biol Chem 280:18842–18852 [DOI] [PubMed] [Google Scholar]
- 48. Knebel B, Kotzka J, Avci H, Schiller M, Brüning JC, Hafner M, Krone W, Müller-Wieland D. 2000. Characterization of a postreceptor signaling defect that impairs cfos expression in cultured fibroblasts of a patient with insulin resistance. Biochem Biophys Res Commun 268:577–582 [DOI] [PubMed] [Google Scholar]
- 49. Bonfil D, Chuderland D, Kraus S, Shahbazian D, Friedberg I, Seger R, Naor Z. 2004. Extracellular signal-regulated kinase, Jun N-terminal kinase, p38, and c-Src are involved in gonadotropin-releasing hormone-stimulated activity of the glycoprotein hormone follicle-stimulating hormone β-subunit promoter. Endocrinology 145: 2228–2244 [DOI] [PubMed] [Google Scholar]
- 50. Mo Y, Vaessen B, Johnston K, Marmorstein R. 2000. Structure of the elk-1-DNA complex reveals how DNA-distal residues affect ETS domain recognition of DNA. Nat Struct Biol 7:292–297 [DOI] [PubMed] [Google Scholar]
- 51. Iyer D, Chang D, Marx J, Wei L, Olson EN, Parmacek MS, Balasubramanyam A, Schwartz RJ. 2006. Serum response factor MADS box serine-162 phosphorylation switches proliferation and myogenic gene programs. Proc Natl Acad Sci USA 103:4516–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Miranti CK, Ginty DD, Huang G, Chatila T, Greenberg ME. 1995. Calcium activates serum response factor-dependent transcription by a Ras- and Elk-1-independent mechanism that involves a Ca2+/calmodulin-dependent kinase. Mol Cell Biol 15:3672–3684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Salisbury TB, Binder AK, Grammer JC, Nilson JH. 2007. Maximal activity of the luteinizing hormone β-subunit gene requires β-catenin. Mol Endocrinol 21:963–971 [DOI] [PubMed] [Google Scholar]
- 54. Salisbury TB, Binder AK, Nilson JH. 2008. Welcoming β-catenin to the GnRH transcriptional network in gonadotropes. Mol Endocrinol 22:1295–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Corpuz PS, Lindaman LL, Mellon PL, Coss D. 2010. FoxL2 is required for activin induction of the mouse and human follicle-stimulating hormone β-subunit genes. Mol Endocrinol 24:1037–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]