Thyroid hormone metabolism regulated by deiodinase type 3 is dependent on the thyroid hormone receptor alpha isoform.
Abstract
Mice deficient in thyroid hormone receptor α (TRα) display hypersensitivity to thyroid hormone (TH), with normal serum TSH but diminished serum T4. Our aim was to determine whether altered TH metabolism played a role in this hypersensitivity. TRα knockout (KO) mice have lower levels of rT3, and lower rT3/T4 ratios compared with wild-type (WT) mice. These alterations could be due to increased type 1 deiodinase (D1) or decreased type 3 deiodinase (D3). No differences in D1 mRNA expression and enzymatic activity were found between WT and TRαKO mice. We observed that T3 treatment increased D3 mRNA in mouse embryonic fibroblasts obtained from WT or TRβKO mice, but not in those from TRαKO mice. T3 stimulated the promoter activity of 1.5 kb 5′-flanking region of the human (h) DIO3 promoter in GH3 cells after cotransfection with hTRα but not with hTRβ. Moreover, treatment of GH3 cells with T3 increased D3 mRNA after overexpression of TRα. The region necessary for the T3-TRα stimulation of the hD3 promoter (region −1200 to −1369) was identified by transfection studies in Neuro2A cells that stably overexpress either TRα or TRβ. These results indicate that TRα mediates the up-regulation of D3 by TH in vitro. TRαKO mice display impairment in the regulation of D3 by TH in both brain and pituitary and have reduced clearance rate of TH as a consequence of D3 deregulation. We conclude that the absence of TRα results in decreased clearance of TH by D3 and contributes to the TH hypersensitivity.
Thyroid hormone (TH, here denoting the precursor T4 and active hormone T3) action is mediated through nuclear TH receptors (TRs). The latter function as ligand-dependent transcription factors that bind to target gene promoters and constitutively or through ligand binding either repress or activate gene expression. The TRs are encoded by two distinct but related genes, TRα and TRβ. The TRα gene locus encodes TRα-1 and three other isoforms that do not bind the ligand T3, TRα-2, TRΔα-1, and TRΔα-2. The physiological role of TRα-2, which has a wide tissue distribution (1), and of TRΔα-1 and TRΔα-2, found mainly in brain, gut, and lung (2), is still unknown. However, all have been shown to inhibit ligand-dependent transactivation of TRβ and TRα1 (2–5). Although both TRα-1 and TRβ bind T3 with high affinity and recognize the same TH response elements (TREs) on DNA (6, 7), some studies have suggested that α and β TRs may show preferential activation of certain target genes (8–11).
Impairment of TRβ action by mutations or deletion results in a state of reduced responsiveness to TH, termed “resistance to TH” (12). This condition manifests, in both humans and in mice, as increased concentration of all serum iodothyronines with normal or elevated TSH concentrations. On the other hand, deficiency of TRα in mouse models (no human examples have been described) results in apparent hypersensitivity to TH (13). In fact, mice lacking all isoforms of TRα [(known as TRα0/0 mice, here referred to as TRα knockout (KO) mice], have similar concentrations of serum TSH compared with wild-type (WT) mice despite lower T4 concentrations (13). In addition, as shown for the first time in this communication, TRαKO mice also display much lower levels of reverse T3 (rT3) that results in lower rT3/T4 ratios, suggesting an alteration in TH metabolism. Metabolism of TH is regulated by three iodothyronine deiodinases. Type 1 (D1) and type 2 (D2) deiodinases serve to generate bioactive T3 by outer ring deiodination of T4. In contrast, type 3 iodothyronine deiodinase (D3) inactivates THs by inner-ring deiodination of T4 to rT3 and T3 to 3,3′-diiodothyronine (T2), both biologically inactive compounds (14). The decreased serum levels of rT3, found in TRαKO mice, could be explained either by increased D1 or decreased D3. The purpose of this study was to evaluate whether an alteration of TH metabolism contributed to the increased sensitivity to TH observed in TRαKO mice.
This work demonstrates that the up-regulation of D3 transcript levels by T3 is dependent on TRα isoform. The data support the conclusion that the absence of TRα results in decreased clearance of TH and is in part responsible for the TH hypersensitivity.
Results
Baseline thyroid function tests, liver D1 mRNA levels, and enzymatic activity in TRαKO and WT mice
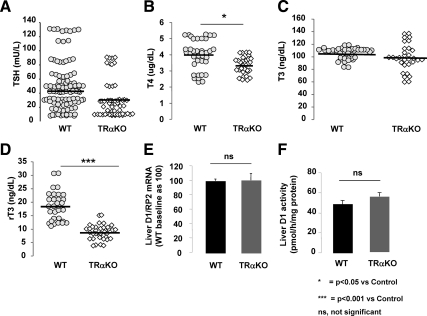
In agreement with previous data from our laboratory (13), TRαKO mice had similar levels of TSH (30.06 ± 6.05 vs. 43.4 ± 7.0 mU/liter; P > 0.05) and yet significantly lower levels of T4 compared with WT animals (3.3 ± 0.1 vs. 4.0 ± 0.2 μg/dl; P < 0.05) (Fig. 1, A and B). T3 concentrations were not different in the two groups (Fig. 1C). On the other hand, TRαKO mice display significantly lower levels of rT3 compared with WT animals (8.7 ± 0.71 vs. 18.5 ± 1.18 ng/dl; P < 0.001) (Fig. 1D) and as a consequence, significantly lower rT3/T4 ratios (2.63 ± 0.18 vs. 5.09 ± 0.22; P < 0.001) that suggest a potential alteration in the metabolism of TH. The decreased serum rT3 levels in TRαKO mice could be due to either increased D1 or decreased D3. Liver D1 mRNA levels (Fig. 1E) and enzymatic activity (Fig. 1F) were similar in WT and TRαKO animals, suggesting that abnormality in the TRαKO mice might be in D3.
Fig. 1.
Serum tests of thyroid function and liver D1 mRNA content and enzymatic activity in TRαKO and WT animals. Serum TSH (A), T4 (B), T3 (C), and rT3 (D) concentrations in TRαKO and WT at baseline. D1 mRNA levels (E) and enzymatic activity (F) in liver of WT (black bars) and TRαKO (gray bars) mice at baseline. Data on D1 are expressed as mean ± sem; n = 7 animals per group. Statistical differences between groups are indicated.
D3 mRNA levels in mouse embryonic fibroblasts (MEFs) from TRαKO, TRβKO, and WT mice
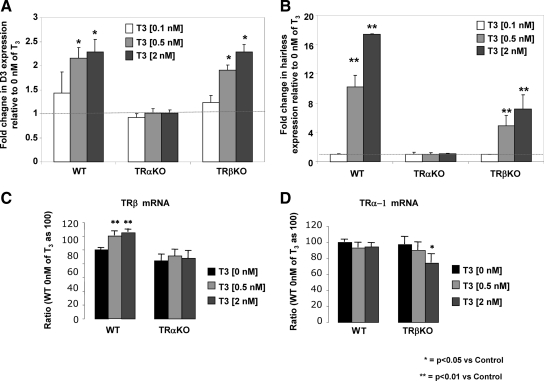
Previous studies have shown that D3 mRNA levels increase in brain and placenta during hyperthyroidism and decrease during hypothyroidism (15–17). However, the mechanism of this regulation remains unknown. D3 mRNA increased after T3 treatment (2 nm) of MEFs from WT and TRβKO mice, (2.29 ± 0.10-fold, P < 0.05; and 2.29 ± 0.06-fold, P < 0.05, respectively), whereas up-regulation of D3 mRNA by T3 was absent in the MEFs from TRαKO mice (1.01 ± 0.03-fold) (Fig. 2A). Expression of the TRα-regulated gene, hairless (18), corresponded to that of D3 (Fig. 2B). These findings suggest that T3-mediated increase in D3 mRNA is TRα dependent. To rule out possible reciprocal TR regulations in MEFs from TRαKO or TRβKO mice, TRα-1 and TRβ mRNA levels were analyzed both at baseline and after treatment with T3. There were no differences in TRβ mRNA levels between TRαKO and WT MEFs at baseline (0 nm T3) (Fig. 2C). MEFs from TRβKO animals expressed similar levels of TRα-1 mRNA as WT mice (Fig. 2D). This indicates that there was no change in the expression in one isoform when the other was absent. T3 treatment (0.5 and 2 nm) induced a slight but significant increase in TRβ mRNA in MEFs from WT mice (120 ± 4.9, P < 0.05; and 125 ± 7.4, P < 0.05, respectively) but not in MEFs from TRαKO animals (Fig. 2C). No significant differences in TRα-1 mRNA were found in MEFs from WT mice after T3 treatment as compared with baseline, whereas in MEFs from TRβKO mice, TRα-1 mRNA was reduced after treatment with 2 nm T3 (74 ± 11 vs. 97 ± 10; P < 0.05) (Fig. 2D). Even when a decrease in TRα mRNA was detected in MEFs from TRβKO mice, D3 mRNA was up-regulated after T3 treatment as observed in MEFs from WT animals. These findings suggest that the slight but significant differences observed in the TR isoforms expression after treatment with T3 could not explain the total absence of D3 mRNA response in MEFs from TRαKO animals and support the hypothesis that D3 is TRα dependent.
Fig. 2.
D3 mRNA levels after T3 treatment of MEFs from TRαKO, TRβKO, and WT mice. Fold change of D3 (A) and hairless (B) mRNA levels in MEFs from the three genotypes after treatment with increasing doses of T3 for 24 h (0.1, 0.5, and 2 nm) in relation to control (MEFs of their respective genotype treated with vehicle). TRβ mRNA levels in MEFs from WT or TRαKO mice (C) and TRα-1 mRNA levels of in MEFs from WT or TRβKO mice (D) after treatment with increasing doses of T3 for 24 h (0.5 and 2 nm) as compared with control (vehicle). The experiment was repeated three times in triplicate. Data are expressed as mean ± sem. Significant differences as compared with control are indicated.
Overexpression of TRα increases D3 mRNA levels and stimulates the promoter activity of the hDIO3 gene in GH3 cells
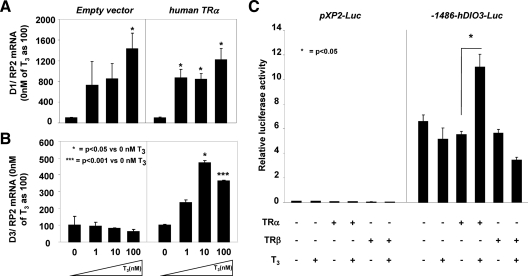
To investigate the role of TRα in the transcriptional regulation of D3 by T3 in vitro, pituitary GH3 cells were transfected with the hTRα plasmid or with the empty pcDNA vector and treated with increasing doses of T3 (0, 1, 10, 100 nm) for 24 h. D1 mRNA levels served as positive control. D1 was stimulated by 14.3 ± 2.9-fold (P < 0.05) after T3 treatment (100 nm) of GH3 cells transfected with the empty vector or with one expressing human TRα (11.45 ± 2.11-fold; P < 0.05) (Fig. 3A). In contrast, D3 mRNA levels did not respond to T3 treatment (100 nm) in the presence of the empty pcDNA vector but did show a 3.7 ± 0.5-fold increase in the presence of the hTRα plasmid (P < 0.001) (Fig. 3B). These results indicate that T3 treatment up-regulates D3 mRNA through TRα, but not through endogenous TRβ present in pituitary GH3 cells.
Fig. 3.
Overexpression of TRα induces D3 mRNA levels in GH3 cells after T3 treatment. D1 (A) and D3 (B) mRNA levels in pituitary GH3 cells after transfection with the empty pcDNA vector (left panel) or with hTRα plasmid (right panel) and treatment with increasing doses of T3 for 24 h (0, 1, 10, 100 nm). The experiment was repeated twice in triplicate. Data are expressed as mean ± sem. Significant differences between groups are indicated. C, T3-TRα regulation of human DIO3 promoter activity in GH3 cells: −1486 bp of the human DIO3 promoter or pXP2-Luc empty vector and hTRα or TRβ were cotransfected into GH3 cells and analyzed for luciferase activity after T3 treatment as described in Materials and Methods. Bars represent the mean ± sem of three different experiments, each performed in triplicate. Significant differences between groups are indicated.
The role of the −1486 bp 5′-flanking region (FR) on the promoter of the hDIO3 gene was evaluated in GH3 cells. Under basal conditions, −1486-hDIO3-Luc construct showed 65.8 ± 10.5-fold more reporter activity than the pXP2-Luc plasmid. Compared with basal activity, T3 stimulation of the promoter activity occurred only after cotransfection with hTRα (2.14 ± 0.02-fold increase; P < 0.05), but not hTRβ (0.67 ± 0.01 fold increase) (Fig. 3C), suggesting the presence of a TRα-specific site in the hDIO3 promoter.
Delimitation of the region in the hDIO3 promoter containing a potential TRα-specific site
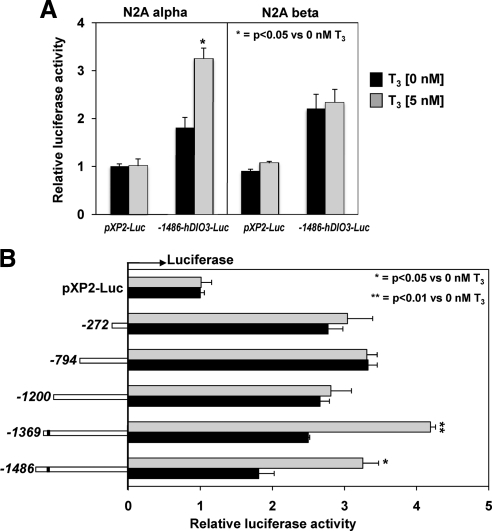
To localize the putative TRα-specific site in the human D3 promoter we transfected constructs containing fragments from the positions −1486, −1369, −1200, −794, and −272 bp 5′-flanking sequences of the hDIO3 promoter in N2A cells that stably overexpress either TRα (N2A α) or TRβ (N2A β). In agreement with the results observed in GH3 cells, after transfection of the −1486-DIO3-Luc construct, T3 stimulation (5 nm) of the promoter activity occurred only in the N2A cell line that overexpress TRα, but not in those that overexpressed the TRβ (1.8 ± 0.03; P < 0.05 vs. 1.05 ± 0. 12-fold increase, respectively) (Fig. 4A).
Fig. 4.
T3 response of deletion constructs of the hDIO3 promoter in N2A α and N2A β cells. A, −1486 bp of the hDIO3 promoter-Luc reporter or pXP2-Luc empty vector were transfected into N2A α and N2A β cells and analyzed for luciferase activity after T3 treatment as described in Materials and Methods. Bars represent the mean ± sem of three different experiments, each performed in triplicate. Significant differences between groups are indicated. B, Human DIO3 promoter containing −1486, −1369, −1200, −794, and −272 positions 5′-FR to +72 location at 3′ (just before the native translational start codon) or pXP2-Luc empty vector were transfected into N2A α cells and analyzed for luciferase activity after T3 treatment as described in Materials and Methods. Bars represent the mean ± sem of the three different experiments, each performed in triplicate. Significant differences are indicated. Black bars, T3, 0 nm; shaded bars, T3, 5 nm.
As shown in the Fig. 4B, T3 treatment (5 nm) significantly stimulated the promoter activity of the −1486-DIO3-Luc and −1369-DIO3-Luc constructs in N2A α cells. However, the T3-dependent response was lost in the fragments containing −1200, −794, and −272 bp DIO3-Luc. This result suggests that the −1200 to −1369 region of the human DIO3 promoter contains a potential TRα-specific site that is necessary for T3 stimulation of the DIO3 gene. The sequence of 1.5 kb 5′-FR of the hDIO3 gene was analyzed to map potential TREs by using the Transcriptional Element Search Software (TESS) (19). TESS search revealed a potential TRα-specific site with 100% homology to the TRE-binding sequence (AGGTCA) at the position −1350 (reversed), which might explain the results of the functional localization of the TRα-specific site in N2A α cells.
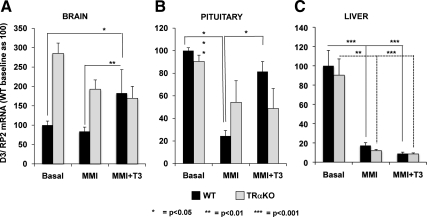
D3 mRNA levels in brain, pituitary, and liver of TRαKO and WT mice
To investigate the role of T3 in the TRα-mediated transcriptional regulation of D3 in vivo, D3 mRNA levels were measured by real-time quantitative PCR (qPCR) in the brain, pituitary, and liver of TRαKO and WT mice during TH deprivation (induced by treatment with methimazole (MMI, 0.05% wt/vol) and sodium perchlorate (1.0% wt/vol)) and after supplementation with T3 [2 μg of T3/100 g body weight (BW)/d for 4 d]. The results are shown in Fig. 5. In brain, D3 mRNA was induced by T3 treatment in WT and TRαKO animals by 2.2 ± 0.2 vs. 0.9 ± 0.04-fold, respectively (P < 0.05) (Fig. 5A) and in pituitary 3.4 ± 1.1 vs. 0.8 ± 0.4-fold (P < 0.05) as compared with the levels observed after TH depletion (Fig. 5B). Although TH depletion reduced liver D3 mRNA levels similarly in WT and TRαKO mice (17.27 ± 2.99 vs. 11.96 ± 1.38) (Fig. 5C), after T3 treatment, D3 mRNA levels were not induced in either WT or TRαKO animals (8.90 ± 1.49 vs. 8.05 ± 1.10, respectively) (Fig. 5C).
Fig. 5.
D3 mRNA levels in brain, pituitary, and liver of TRαKO (gray bars) and WT mice (black bars) after T3 treatment. Mice from the two genotypes were TH-deprived (MMI and potassium perchlorate) and then treated with T3 (MMI and potassium perchlorate + 2 μg of T3/ 100 g BW). D3 mRNA levels in brain (A), pituitary (B), and liver (C) of TRαKO and WT mice at baseline, during hypothyroidism, and after T3 treatment by qPCR. Bars represent the mean ± sem. Significant differences between groups are indicated; n = 7 animals per group.
These results indicate that the transcriptional regulation of D3 by TH is TRα dependent in vivo, as we demonstrated in vitro, but this mechanism of regulation seems to be restricted to the tissues where TH stimulates D3 mRNA.
Serum T3 and rT3/T4 ratios after administration of T3 or T4, respectively, in hypothyroid TRαKO and WT mice
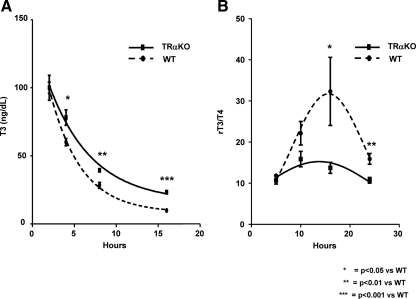
To analyze whether the TH clearance was affected in TRαKO mice due to the impairment in the transcriptional regulation of D3 by TH, T3 levels were determined in TRαKO and WT mice deprived of TH by feeding with a low-iodine (LoI) diet supplemented with 0.15% propylthiouracil (PTU), at different times after the administration of 2 μg of T3/100 g BW. TRαKO mice had significantly higher levels of T3 at 4, 8, and 16 h after the last injection of T3 compared with WT animals (Fig. 6A). In another group of animals, rT3 and T4 were measured in TH-deprived animals (see above) after the administration of 10 μg of T4 /100 g BW. As compared with WT mice, TRαKO animals have 42.9 ± 4.16% (P < 0.05) and 67.0 ± 4.2% (P < 0.01) lower rT3/T4 ratios at 16 and 24 h, respectively, after the last injection of T4 (Fig. 6B). These results support the hypothesis that TRαKO mice have reduced clearance of TH associated with a decrease in D3 content.
Fig. 6.
Serum T3 concentrations and rT3/T4 ratios after administration of T3 (A) or T4 (B), respectively, in hypothyroid TRαKO and WT mice. A, Serum T3 concentrations at different times after the administration of 2 μg of T3 /100 g BW. Blood was obtained at the indicated times after the last ip dose of T3. T3 serum levels were represented in relation to the first point of the curve (2 h). Data are expressed as mean ± sem. Differences between groups are indicated. B, Serum rT3/T4 ratios at different times after the administration of 10 mg of T4 /100 g BW. Blood was obtained at the indicated times after the last ip dose of T4. Mean values ± sem are depicted. Differences between groups are indicated; n = 7 animals per group.
Discussion
In a previous report we demonstrated that TRαKO mice have increased sensitivity to TH (13). It was speculated that a potential mechanism for hypersensitivity in these animals would be the elimination of the inhibitory effect of TRα-2, the TR isoform that binds DNA but does not bind the hormone. However, because the ablation of TRα-2 in mice was accompanied by an increase in TRα-1 (20), it was not possible to directly prove that the observed hypersensitivity to TH is due to the silencing effect of TRα-2 alone. In the present study we observed that, at baseline, the serum levels of rT3 and the rT3/T4 ratios are significantly lower in TRαKO mice compared with WT animals, an observation that further suggests a disruption in TH metabolism, either as a result of increased D1 or decreased D3 enzymatic activity. In agreement with a previous report (21), no differences were found in liver D1 mRNA expression or enzymatic activity between WT and TRαKO mice at baseline, thus suggesting that TRαKO mice might display a potential alteration in D3. The main objective of this study was to determine whether the hypersensitivity to TH of TRαKO mice is partly due to alterations in TH metabolism mediated by D3.
D3 mRNA levels are up-regulated during hyperthyroidism, contributing to TH homeostasis by protecting tissues from an excess of active T3 (14). No TH response element has been identified in the promoter of DIO3 gene. However, TH up-regulation of D3 expression could be mediated by TRα. In that case, the lack of TRα may cause an impairment of D3 transcriptional regulation by TH, which will lead to low T3 clearance, T3 overexposure, and hypersensitivity to TH as found in the TRαKO animals.
Using MEFs, which express both isoforms of TH receptors (22, 23), we show that TH induction of D3 mRNA is ablated in cells from TRαKO mice, but not in cells from TRβKO mice. This finding cannot be explained by a change in the expression of TH receptors, indicating that the TH up-regulation of D3 is specifically mediated by TRα in these cells. That Dio3 is a target gene of TRα was confirmed by using the rat pituitary tumor cell line GH3, in which TRβ-2 is the most highly expressed TR isoform (24). When TRα-1 was overexpressed in GH3 cells, D3 mRNA increased significantly after T3 treatment, suggesting that D3 mRNA can be up-regulated by TRα but not by endogenous TRβ. In contrast, TH-induced expression of another TH target gene, Dio1, did not require the presence of TRα.
We observed that a 1.5-kb fragment containing the hDIO3 promoter and 5′-FR is markedly responsive to T3 but only when TRα, and not TRβ, is overexpressed in GH3 cells. Analysis of the promoter activity of smaller fragments of the hDIO3 5′-FR in N2A cells revealed that the response to T3 was lost when a region between −1369 and −1200 bp was deleted. Although TESS search predicted a potential TRα-specific site at position −1350, which is conserved in the mouse and has 100% homology to the TRE-binding sequence (AGGTCA), we could not identify, by in silico analysis, paired hexamer half-sites in a described spacing and configuration for a TRE.
The specificity of the DIO3 gene for TRα is not surprising, because some studies have shown that α and β TRs may exhibit preferential activation of certain target genes (8–11). Katz and Koenig (25) have shown that the optimal binding site for TRα1 is 1 or 2 bp larger than previously thought (hexamer AGGTCA) and that a single binding site can function as a response element. The synthetic octamer TAAGGTCA had the high-affinity-binding site for TRα-1, and substitutions in the two bases upstream of the TRE hexamer alter the TRα1 affinity (25). However, the octamer resulting after the addition of 2 bp upstream to the potential TRα-specific site that we identified in the human DIO3 promoter (ctAGGTCA), was not studied in the cited report. Further studies are needed to verify the binding of TRα-1 to this binding site.
Additional evidence for the role of TRα in the regulation of D3 comes from our studies in vivo. D3 expression is regulated by the thyroid status, being increased in hyperthyroidism and decreased in hypothyroidism (16, 26). Thus, T3 treatment caused an increase in the transcript levels of D3 in brain and pituitary of WT mice. However, no effect was noted in TRαKO animals. This lack of regulation cannot be attributed to a reduced half-life of the hormone in TRαKO mice, as we show that the opposite is true. Thyroid function tests in TRαKO and WT mice were measured after TH deprivation and supplementation with T3 or T4. In the first case, T3 serum levels were significantly higher in TRαKO mice 5 h after the last injection. Similar results were obtained in our previous report in which serum T3 concentrations were measured at different times after the administration of T3 (13). Although values are not significantly different among TRαKO and WT mice at each point, significant differences were found when the serum levels of T3 were represented in relation to the first point of the curve (2 h), because the serum T3 levels at 2 h were lower in the TRαKO mice. In the other group of animals, D1 activity was inhibited with PTU administration (14) and D2 with T4 treatment at high dose (27) with the aim to analyze the functionality of D3 in the clearance of TH. As expected, the ratio rT3/T4 was significantly lower in the TRαKO mice compared with WT animals. These results confirmed that, as demonstrated in vitro, the transcriptional regulation of D3 by TH is dependent on TRα in vivo as well. Indeed, TRαKO mice exhibit reduced clearance of both T3 and T4, a finding consistent with the impairment in D3 stimulation by TH.
Recently Kwakkel et al. (21) reported a higher level of liver D3 mRNA in TRαKO animals compared with WT mice. Because TRβ is the predominant receptor in hepatic tissue, this finding could indicate that this receptor mediates the induction of D3 by TH in this tissue. However, we found no differences in liver D3 mRNA expression between TRαKO and WT mice. Furthermore, liver D3 mRNA was not induced after T3 treatment, either in WT or in TRαKO animals. These results suggest that TRα mediates the transcriptional regulation of D3 by T3 only in tissues where TH stimulates D3 mRNA.
The increased sensitivity to TH seen in TRαKO has been shown in pituitary and liver (13). Our data indicate that whereas in pituitary D3 is TRα dependent, liver D3 is not up-regulated after treatment with TH. As Macchia et al. (13) have previously shown, T3 suppression of TSH is greater in TRαKO than in WT mice. This effect might be due to the impairment in D3 up-regulation by T3 observed in TRαKO. This will increase the local concentration of active TH and cause a larger suppression of TSH. On the other hand, although the level of D3 mRNA was not up-regulated in liver after T3 treatment in neither TRαKO nor WT animals, TRαKO mice showed a greater increase in liver T3-inducible mRNAs (D1 and malic enzyme) as compared with WT animals (13). This may be due to increased availability of coactivators for the TRβ. In this regard, Sadow et al. (28) reported that double TRα/steroid receptor coactivator-1 (SRC-1) KO mice show TH inductions of liver D1 mRNA that are similar to those of WT and SRC-1 KO animals. This indicates that the hypersensitivity conferred upon TRα-deficient mice is abolished in the absence of SRC-1. Thus, the liver hypersensitivity to TH of TRαKO mice might be due to an increased availability of SRC-1 to interact with the TRβ, an event that may be controlled in TR-competent mice by squelching of the coactivator by the TRα. Alternatively, the increased sensitivity to TH in the liver of TRαKO mice is that this organ reflects the higher T3 serum levels observed in these animals after TH treatment due to their alteration in the clearance rate.
In summary, we show that the up-regulation of D3 expression by T3 is mediated by TRα in vivo and in vitro, a finding consistent with the impaired regulation of D3 expression observed in TRαKO mice. This may explain, at least in part, the deficit in TH clearance and the increased sensitivity to TH that are characteristic of this mouse model. The specific role of the TRα in D3 expression may have important implications for the physiological processes affected by this enzyme.
Materials and Methods
Mice and MEFs
TRαKO and TRβKO mice were previously described (29, 30). The WT mice used in this study as controls were littermates of the KO mice. Mice were 70–90 d of age at the time of analyses. Blood was obtained from the tail veins under light anesthesia. Serum was separated by centrifugation and stored at −20 C until analyzed in the same assay for each experiment. All blood samples were obtained between 1000 h and 1300 h. Experiments were terminated by exsanguinations under isoflurane anesthesia (Pitman-Moore, Mundelein, IL). MEFs were isolated from embryonic d 17.5 mouse embryos of TRαKO, TRβKO, and WT mice and used at their early passages (one to three passages). After removal of the head and inner organs and digestion with 0.1% trypsin (30 min at 37 C), cells were cultured in low-glucose DMEM with 10% fetal calf serum, antibiotics (penicillin/streptomycin), and nonessential amino acids (31). All animal experiments were performed at The University of Chicago according to protocols approved by the Institutional Animal Care and Use Committee.
Measurements of TH and TSH concentrations in serum
Serum TSH was measured in 50 μl of serum using a sensitive, heterologous, disequilibrium double-antibody precipitation RIA, and results were expressed in bioassayable TSH units (32). Serum T4 and T3 concentrations were measured by a double-antibody precipitation RIA (Diagnostic Products Corp.) using 25 and 50 μl of serum, respectively. rT3 was measured in 35 μl of serum by RIA using reagents from Adaltis Italia (Rino, Italy). All samples were individually analyzed for each mouse.
Induction of hypothyroidism and treatment with TH
TH deprivation was produced in five to nine male TRαKO and WT mice after treatment with MMI (0.05% wt/vol) and sodium perchlorate (1.0% wt/vol) in the drinking water for 21 d. On the 21st day, animals of each genotype were split into two groups. One group received daily ip injections of 2 μg of T3/100 g BW/d for 4 d, whereas the other group received only the vehicle (PBS). The concentration of T3 was confirmed by RIA. Experiments were terminated 6 h after the last injection for tissue collection. In another group of animals, TH depletion was induced by feeding with a LoI diet supplemented with 0.15% PTU (Harlan Teklad, Madison, WI). On the 11th day, seven to eight male TRαKO and WT mice were injected once daily for 7 d with the vehicle only (PBS), and others received 10 μg of T4/100 g BW while maintained on the LoI/PTU diet. Blood samples were obtained for rT3 and T4 measurement at baseline, on the 10th day after the initiation of the LoI/PTU diet, and 5 h, 10 h, 16 h, and 24 h after last T4 injection.
In a separate experiment, a group of six to seven TH-depleted TRαKO and WT animals, treated with PTU as described above, received daily ip injections of 2 μg of T3/100 g BW/d for 4 d, whereas the other group received only the vehicle. The PTU diet was given throughout the T3 and vehicle treatment period. Experiments were terminated 16 h after the last injection. Blood was collected for serum T3 determination 24 h after the penultimate T3 dose and at 2, 4, 8, and 16 h after the last T3 injection. The concentration of T3 was confirmed by RIA. Note that separate animals were used for the 2- and 8-h and 4- and 16-h posttreatment blood sampling.
D1 enzymatic activity
D1 enzymatic activity in liver was measured using [125I]T4 as previously described (33) with the following modifications: tissue homogenates (20 μg of protein) in 100 μl reaction mixture containing 0.1 m phosphate buffer (pH 7), 1 mm EDTA, 10 mm dithiothreitol, 100,000 cpm [125I]T4, and 1 μm unlabeled T4 were incubated at 37 C for 30 min. The enzymatic activity was expressed in picomoles per hour and milligram of protein and was corrected for nonenzymatic deiodination observed in the tissue-free controls.
Isolation of mRNA and qPCR
Total RNA was extracted from brain, pituitary glands, liver, MEFs, and pituitary GH3 cells, using phenol/guanidine isothiocyanate (TRIZOL; Invitrogen, Carlsbad, CA), and 2 μg total RNA was reverse transcribed using Superscript III ribonuclease H reverse transcriptase kit (Invitrogen) in the presence of 100 ng random hexamers. Reactions for the quantification of mRNAs by qPCR were performed in an ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA), using SYBR Green I as detector dye. The oligonucleotide primers were designed to cross introns. Primers used for the qPCR of type 1 and 3 iodothyronine deiodinases, TRα-1, TRβ, and hairless (Hr) mRNAs are available on request. Amplification of the housekeeping genes RNA polymerase II or 18S RNA was used as internal control.
Cell culture and transfection experiments
Neuro2A cells stably transfected with either chicken TRα-1 (here referred as N2A α) or with human TRβ-1 (here referred to as N2A β) were previously described (34, 35). For transient transfections, pituitary GH3 cells and Neuro-2A cell lines (N2A α and N2A β) were seeded in 24-well plates at a density of 5 × 104 cells per well. Lipofectamine reagent (Invitrogen) was used as described in the manufacturer's protocol.
Human DIO3 promoter constructs were previously described (36, 37). Briefly, fragments of the hDIO3 promoter from positions −1486, −1369, −1200, −794, and −272 bp from the 5′FR to +72 bp location at 3′ (just before the native translational start codon) were subcloned in pXP2, a plasmid containing the luciferase reporter gene (38). Human TRβ and TRα expression vectors were previously described (39).
Each well received 250 ng of each promoter construct. GH3 cells were cotransfected with 50 ng of hTRα or hTRβ plasmid. Renilla luciferase activity from cotransfected pRL-Tk plasmid (Promega Corp., Madison, WI) was used to monitor transfection efficiency in cell lysates. The total amount of DNA transfected was kept constant by adjusting with empty pcDNA vector. Cells were incubated for 4 h with the DNA/lipid complexes in serum-free medium, and the medium was then replaced by DMEM supplemented with 10% of bovine thyroidectomized serum (40). T3 was added at the indicated doses 48 h after transfection. Cells were lysed in 150 μl lysis buffer 24 h later. Luciferase activity was determined in cell lysates by using the Dual Glo luciferase assay system (Promega).
Statistical analysis
Values are reported as mean ± sem. The number of mice is indicated. Statistical analysis was performed with Student's t test. P > 0.05 was considered not to be significant.
Acknowledgments
Current address for M.A.: Hospital Clinico Universitario de Santiago, 15706 Santiago de Compostela, Spain.
This work was supported by National Institutes of Health Grants 4R37-DK15070, DK20595, and 2P60-DK020595 and the Esformes Endowment.
Disclosure Summary: The authors have declared that no conflict of interest exists.
NURSA Molecule Pages:
Nuclear Receptors: TR-α
Ligands: Thyroid hormone
Footnotes
- BW
- Body weight
- D1
- type 1 deiodinase
- D3
- type 3 deiodinase
- FR
- flanking region
- KO
- knockout
- LoI
- low iodine
- MEF
- mouse embryonic fibroblast
- MMI
- methimazole
- PTU
- propylthiouracil
- qPCR
- quantitative PCR
- rT3
- reverse T3
- SRC-1
- steroid receptor coactivator-1
- TH
- thyroid hormone
- TR
- TH receptor
- TRE
- TH response element
- WT
- wild type.
References
- 1. Hodin RA, Lazar MA, Chin WW. 1990. Differential and tissue-specific regulation of the multiple rat c-erbA messenger RNA species by thyroid hormone. J Clin Invest 85:101–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chassande O, Fraichard A, Gauthier K, Flamant F, Legrand C, Savatier P, Laudet V, Samarut J. 1997. Identification of transcripts initiated from an internal promoter in the c-erbA α locus that encode inhibitors of retinoic acid receptor-α and triiodothyronine receptor activities. Mol Endocrinol 11:1278–1290 [DOI] [PubMed] [Google Scholar]
- 3. Lazar MA, Hodin RA, Chin WW. 1989. Human carboxyl-terminal variant of α-type c-erbA inhibits trans-activation by thyroid hormone receptors without binding thyroid hormone. Proc Natl Acad Sci USA 86:7771–7774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burgos-Trinidad M, Koenig RJ. 1999. Dominant negative activity of thyroid hormone receptor variant α2 and interaction with nuclear corepressors. Mol Cell Endocrinol 149:107–114 [DOI] [PubMed] [Google Scholar]
- 5. Tagami T, Kopp P, Johnson W, Arseven OK, Jameson JL. 1998. The thyroid hormone receptor variant α2 is a weak antagonist because it is deficient in interactions with nuclear receptor corepressors. Endocrinology 139:2535–2544 [DOI] [PubMed] [Google Scholar]
- 6. Glass CK. 1994. Differential recognition of target genes by nuclear receptor monomers, dimers, and heterodimers. Endocr Rev 15:391–407 [DOI] [PubMed] [Google Scholar]
- 7. Lazar MA. 1993. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev 14:184–193 [DOI] [PubMed] [Google Scholar]
- 8. Denver RJ, Ouellet L, Furling D, Kobayashi A, Fujii-Kuriyama Y, Puymirat J. 1999. Basic transcription element-binding protein (BTEB) is a thyroid hormone-regulated gene in the developing central nervous system. Evidence for a role in neurite outgrowth. J Biol Chem 274:23128–23134 [DOI] [PubMed] [Google Scholar]
- 9. Lezoualc'h F, Hassan AH, Giraud P, Loeffler JP, Lee SL, Demeneix BA. 1992. Assignment of the β-thyroid hormone receptor to 3,5,3′-triiodothyronine-dependent inhibition of transcription from the thyrotropin-releasing hormone promoter in chick hypothalamic neurons. Mol Endocrinol 6:1797–1804 [DOI] [PubMed] [Google Scholar]
- 10. Strait KA, Zou L, Oppenheimer JH. 1992. β 1 Isoform-specific regulation of a triiodothyronine-induced gene during cerebellar development. Mol Endocrinol 6:1874–1880 [DOI] [PubMed] [Google Scholar]
- 11. Zavacki AM, Harney JW, Brent GA, Larsen PR. 1993. Dominant negative inhibition by mutant thyroid hormone receptors is thyroid hormone response element and receptor isoform specific. Mol Endocrinol 7:1319–1330 [DOI] [PubMed] [Google Scholar]
- 12. Weiss RE, Refetoff S. 2000. Resistance to thyroid hormone. Rev Endocr Metab Disord 1:97–108 [DOI] [PubMed] [Google Scholar]
- 13. Macchia PE, Takeuchi Y, Kawai T, Cua K, Gauthier K, Chassande O, Seo H, Hayashi Y, Samarut J, Murata Y, Weiss RE, Refetoff S. 2001. Increased sensitivity to thyroid hormone in mice with complete deficiency of thyroid hormone receptor α. Proc Natl Acad Sci USA 98:349–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. 2002. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23:38–89 [DOI] [PubMed] [Google Scholar]
- 15. Kaplan MM, Yaskoski KA. 1980. Phenolic and tyrosyl ring deiodination of iodothyronines in rat brain homogenates. J Clin Invest 66:551–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Escobar-Morreale HF, Obregon MJ, Hernandez A, Escobar del Rey F, Morreale de Escobar G. 1997. Regulation of iodothyronine deiodinase activity as studied in thyroidectomized rats infused with thyroxine or triiodothyronine. Endocrinology 138:2559–2568 [DOI] [PubMed] [Google Scholar]
- 17. Mori K, Yoshida K, Fukazawa H, Kiso Y, Sayama N, Kikuchi K, Aizawa Y, Abe K. 1995. Thyroid hormone regulates rat placental type III iodothyronine deiodinase activity by inducing kinetic changes different from those in the same isozyme in rat brain. Endocr J 42:753–760 [DOI] [PubMed] [Google Scholar]
- 18. Ramos HE, Weiss RE. 2006. Regulation of nuclear coactivator and corepressor expression in mouse cerebellum by thyroid hormone. Thyroid 16:211–216 [DOI] [PubMed] [Google Scholar]
- 19. Schug J. 2008. Using TESS to predict transcription factor binding sites in DNA sequence. Curr Protoc Bioinformatics. Philadelphia, PA: John Wiley and Sons: Chapter 2:1–15 [DOI] [PubMed] [Google Scholar]
- 20. Saltó C, Kindblom JM, Johansson C, Wang Z, Gullberg H, Nordström K, Mansén A, Ohlsson C, Thorén P, Forrest D, Vennström B. 2001. Ablation of TRα2 and a concomitant overexpression of α1 yields a mixed hypo- and hyperthyroid phenotype in mice. Mol Endocrinol 15:2115–2128 [DOI] [PubMed] [Google Scholar]
- 21. Kwakkel J, Chassande O, van Beeren HC, Fliers E, Wiersinga WM, Boelen A. 2010. Thyroid hormone receptor α modulates lipopolysaccharide-induced changes in peripheral thyroid hormone metabolism. Endocrinology 151:1959–1969 [DOI] [PubMed] [Google Scholar]
- 22. Murray MB, Zilz ND, McCreary NL, MacDonald MJ, Towle HC. 1988. Isolation and characterization of rat cDNA clones for two distinct thyroid hormone receptors. J Biol Chem 263:12770–12777 [PubMed] [Google Scholar]
- 23. Too CK, Guernsey DL. 1992. Increased messenger RNA levels of the antagonist thyroid hormone receptor erbA-α 2 and decreased levels of erbA-α 1 and erbA-β 1 receptor messenger RNAs in neoplastic rodent cells. Cancer Res 52:2186–2190 [PubMed] [Google Scholar]
- 24. Hahn CG, Pawlyk AC, Whybrow PC, Tejani-Butt SM. 1999. Differential expression of thyroid hormone receptor isoforms by thyroid hormone and lithium in rat GH3 and B103 cells. Biol Psychiatry 45:1004–1012 [DOI] [PubMed] [Google Scholar]
- 25. Katz RW, Koenig RJ. 1994. Specificity and mechanism of thyroid hormone induction from an octamer response element. J Biol Chem 269:18915–18920 [PubMed] [Google Scholar]
- 26. Tu HM, Legradi G, Bartha T, Salvatore D, Lechan RM, Larsen PR. 1999. Regional expression of the type 3 iodothyronine deiodinase messenger ribonucleic acid in the rat central nervous system and its regulation by thyroid hormone. Endocrinology 140:784–790 [DOI] [PubMed] [Google Scholar]
- 27. Farwell AP, Lynch RM, Okulicz WC, Comi AM, Leonard JL. 1990. The actin cytoskeleton mediates the hormonally regulated translocation of type II iodothyronine 5′-deiodinase in astrocytes. J Biol Chem 265:18546–18553 [PubMed] [Google Scholar]
- 28. Sadow PM, Chassande O, Gauthier K, Samarut J, Xu J, O'Malley BW, Weiss RE. 2003. Specificity of thyroid hormone receptor subtype and steroid receptor coactivator-1 on thyroid hormone action. Am J Physiol Endocrinol Metab 284:E36–E46 [DOI] [PubMed] [Google Scholar]
- 29. Gauthier K, Plateroti M, Harvey CB, Williams GR, Weiss RE, Refetoff S, Willott JF, Sundin V, Roux JP, Malaval L, Hara M, Samarut J, Chassande O. 2001. Genetic analysis reveals different functions for the products of the thyroid hormone receptor α locus. Mol Cell Biol 21:4748–4760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gauthier K, Chassande O, Plateroti M, Roux JP, Legrand C, Pain B, Rousset B, Weiss R, Trouillas J, Samarut J. 1999. Different functions for the thyroid hormone receptors TRα and TRβ in the control of thyroid hormone production and post-natal development. EMBO J 18:623–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lu X, Kambe F, Cao X, Yoshida T, Ohmori S, Murakami K, Kaji T, Ishii T, Zadworny D, Seo H. 2006. DHCR24-knockout embryonic fibroblasts are susceptible to serum withdrawal-induced apoptosis because of dysfunction of caveolae and insulin-Akt-Bad signaling. Endocrinology 147:3123–3132 [DOI] [PubMed] [Google Scholar]
- 32. Pohlenz J, Maqueem A, Cua K, Weiss RE, Van Sande J, Refetoff S. 1999. Improved radioimmunoassay for measurement of mouse thyrotropin in serum: strain differences in thyrotropin concentration and thyrotroph sensitivity to thyroid hormone. Thyroid 9:1265–1271 [DOI] [PubMed] [Google Scholar]
- 33. Balzano S, Bergmann BM, Gilliland MA, Silva JE, Rechtschaffen A, Refetoff S. 1990. Effect of total sleep deprivation on 5′-deiodinase activity of rat brown adipose tissue. Endocrinology 127:882–890 [DOI] [PubMed] [Google Scholar]
- 34. Pastor R, Bernal J, Rodríguez-Peña A. 1994. Unliganded c-erbA/thyroid hormone receptor induces trkB expression in neuroblastoma cells. Oncogene 9:1081–1089 [PubMed] [Google Scholar]
- 35. Lebel JM, Dussault JH, Puymirat J. 1994. Overexpression of the β 1 thyroid receptor induces differentiation in neuro-2a cells. Proc Natl Acad Sci USA 91:2644–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hernandez A, St Germain DL. 2003. Activity and response to serum of the mammalian thyroid hormone deiodinase 3 gene promoter: identification of a conserved enhancer. Mol Cell Endocrinol 206:23–32 [DOI] [PubMed] [Google Scholar]
- 37. Hernandez A, Park JP, Lyon GJ, Mohandas TK, St Germain DL. 1998. Localization of the type 3 iodothyronine deiodinase (DIO3) gene to human chromosome 14q32 and mouse chromosome 12F1. Genomics 53:119–121 [DOI] [PubMed] [Google Scholar]
- 38. Nordeen SK. 1988. Luciferase reporter gene vectors for analysis of promoters and enhancers. Biotechniques 6:454–458 [PubMed] [Google Scholar]
- 39. Hayashi Y, Sunthornthepvarakul T, Refetoff S. 1994. Mutations of CpG dinucleotides located in the triiodothyronine (T3)-binding domain of the thyroid hormone receptor (TR) β gene that appears to be devoid of natural mutations may not be detected because they are unlikely to produce the clinical phenotype of resistance to thyroid hormone. J Clin Invest 94:607–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moeller LC, Wardrip C, Niekrasz M, Refetoff S, Weiss RE. 2009. Comparison of thyroidectomized calf serum and stripped serum for the study of thyroid hormone action in human skin fibroblasts in vitro. Thyroid 19:639–644 [DOI] [PMC free article] [PubMed] [Google Scholar]