Testosterone (T) stimulates aromatase expression in rat granulosa cells in the absence of Follicle Stimulating Hormone mediated by an increase in LRH-1 expression.
Abstract
Androgens are crucial for normal folliculogenesis and female fertility as evidenced in androgen receptor-null and granulosa cell conditional knockout mice. It is thought, however, that the multiple effects of androgens in the ovary are mainly complementary to the actions of gonadotropins. Using primary rat granulosa cells, we demonstrated that in the absence of gonadotropins, testosterone (T) increases aromatase (Cyp19) and P450 side-change cleavage expression, two enzymes crucial for normal ovarian function. T can be converted into estradiol, a classical estrogen, by Cyp19 and into 5α-dihydrotestosterone, a pure androgen, by 5α-reductase. However, inhibition of Cyp19 and/or 5α-reductase did not prevent the stimulatory effects of T. In contrast, the effect of this steroid was potentiated by blocking 5α-reductase. Additionally, T, not 5α-dihydrotestosterone, stimulates liver receptor homolog-1 (LRH-1) expression, whereas the expression of steroidogenic factor-1 (SF-1) was not affected by either steroid. LRH-1 and SF-1 are transcription factors known to be involved in the regulation of Cyp19. Accordingly, small interference RNA against LRH-1 prevented Cyp19 and P450 side-change cleavage up-regulation whereas anti-SF-1 small interference RNA had no effects. Chromatin immunoprecipitation demonstrated that T stimulation of LRH-1 leads to the recruitment of LRH-1 to the native Cyp19 promoter, which was not affected by cotreatment with 5α-reductase and Cyp19 inhibitors. Finally, gel shift and supershift analysis demonstrated that the androgen receptor binds to an androgen response element located within the LRH-1 promoter. These results provide novel evidence that T has a direct effect on the expression of genes involved in granulosa cell differentiation.
It has been long recognized that androgens play an important role in the regulation of ovarian function. In addition to their role as substrate for estradiol (E2) production, it has been clearly demonstrated that androgens synergize with FSH to stimulate granulosa cell differentiation (1, 2). Accordingly, granulosa cells of several species including rodents, ruminants, and primates express high levels of androgen receptor (AR) (3). AR-knockout female mice are subfertile, have impaired folliculogenesis, and develop premature ovarian failure (4, 5). These abnormalities were also observed in granulosa cell conditional AR-knockout mice (6). This evidence shows that androgens play an important role in the regulation of female fertility and indicates that AR signaling in granulosa cells is crucial for normal ovarian function.
AR signaling in granulosa cells seems to be necessary for normal follicular growth and progression beyond the preantral stage. Granulosa cell AR-knockout mice contain more preantral and atretic follicles, with fewer antral follicles and corpora lutea (6). In addition, AR antagonists slow mouse follicle growth (7, 8), primate granulosa cell proliferation (9), and prevent primary to secondary follicle transition in cows (10). This supports a role for androgens in follicle growth (11). Strikingly, an excess of androgen enhances apoptosis in granulosa cells of antral follicles (12). These results indicate that androgen's effects on granulosa cell function may depend on the differentiation state of follicular cells.
In the ovary, only a few genes are known to be regulated by androgens via the activation of AR. Androgens increase the expression of FSH receptor (13), IGF-I, and IGF-I receptor (9) in primate follicles and oocytes. Recently, genome-wide expression in whole ovaries of AR-knockout mice revealed that androgens may target genes involved in the oocyte-granulosa cell-regulatory loop including Kit ligand (KitL), bone morphogenetic protein 15 (Bmp15), growth differentiation factor-9 (Gdf9), and hepatocyte growth factor (Hgf) (4). However, it remains to be clarified whether these effects are due to the loss of AR or due to the abnormal population of follicles observed in the ovaries of AR-knockout animals. Therefore, the molecular pathways triggered by the activation of the AR in granulosa cells remains to be unraveled.
The effect of androgens on the regulation of E2 and progesterone production, two steroids essential for normal folliculogenesis and ovulation, has been considered merely complementary to the actions of gonadotropins (1, 2). In this report, we examined the effect of androgens on the expression of aromatase (Cyp19) and P450 side-change cleavage (P450scc), which are crucial for E2 and progesterone production, respectively. These enzymes are stimulated by FSH in granulosa cells as part of the differentiation program induced by this hormone (14). Whether androgens affect the expression of these enzymes in the absence of FSH is not known. Here, it is shown that testosterone (T) has a direct stimulatory effect on Cyp19 and P450scc expression in ovarian granulosa cells. This stimulatory effect is mediated by an increase in liver receptor homolog-1 (LRH-1) expression, which is an exclusive target of T. In summary, our results demonstrate that T not only synergizes with FSH in the regulation of follicle maturation but it also has a direct effect on the expression of genes involved in the differentiation of granulosa cells.
Results
T stimulates Cyp19 and P450scc expression in granulosa cells
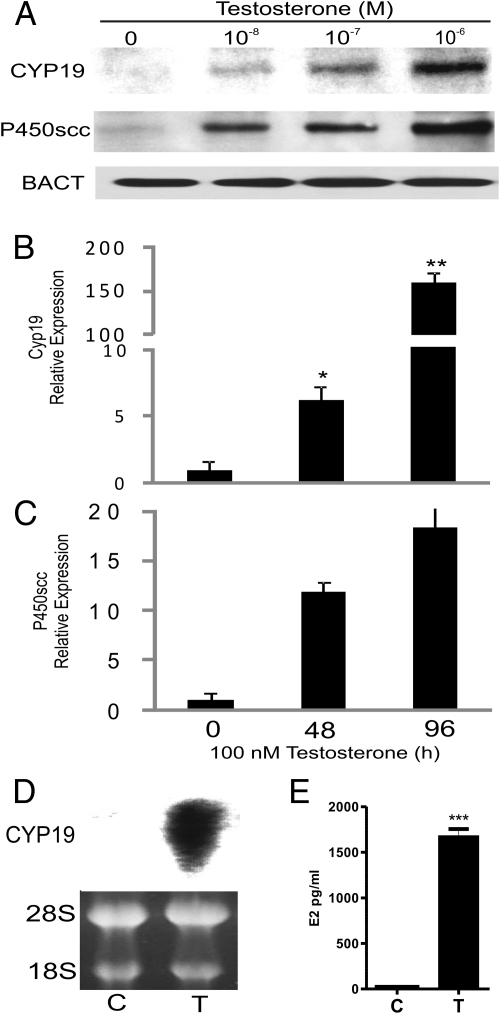
Cyp19 and P450scc protein levels were investigated after 96 h of treatment with increasing doses of T. As shown in Fig. 1A, a dose-dependent stimulation of both Cyp19 and P450scc protein levels was observed. Cyp19 and P450scc mRNA levels increased (5- and 10-fold, respectively) after treatment of granulosa cells with 100 nm T for 48 h (Fig. 1, B and C). Additionally, at the same concentration, Cyp19 mRNA expression was stimulated more than 100-fold after 96 h incubation whereas at this time point P450scc expression showed only a small increase with respect to 48 h (Fig. 1, B and C). Northern blot analysis confirmed the robust stimulation of Cyp19 expression by T (Fig. 1D). The increase in Cyp19 mRNA and protein levels observed in the presence of T was followed by a large increase in E2 production (Fig. 1E).
Fig. 1.
T stimulates Cyp19 and P450scc expression. A, Granulosa cells were cultured in the presence or absence of increasing concentrations of T for 96 h. Cyp19, P450scc, and β-actin (BACT) protein expression was determined by Western blot. Similar results were obtained in three independent experiments. B and C, Granulosa cells were cultured in the presence or absence of T (100 nm) for 48 and 96 h. Cyp19 and P450scc expression was determined by quantitative PCR and expressed as relative expression to ribosomal L19 protein. *P < 0.05, ** P < 0.001 vs. 0. D, Cyp19 mRNA levels were determined by Northern blot analysis after incubation of granulosa cells with vehicle (C) or T (100 nm). E, E2 production was determined by ELISA analysis in cells culture with vehicle (C) or T (100 nm); *** P < 0.001 vs. C.
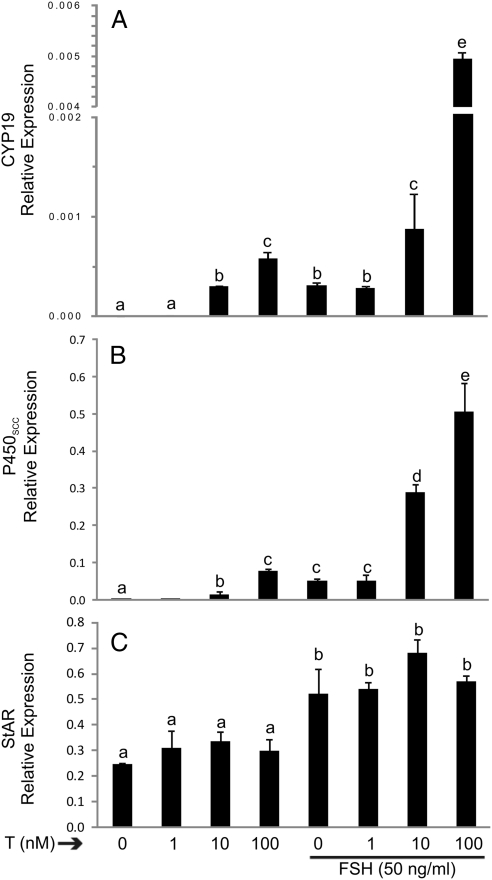
Cyp19 and P450scc mRNA expression was significantly stimulated by a dose as low as 10 nm T whereas steroidogenic acute regulatory protein (StAR) expression was not affected at any of the concentrations used (Fig. 2, A–C). As expected, FSH (50 ng/ml) treatment alone induced the expression of all three genes whereas cotreatment with T maximized Cyp19 and P450scc expression (Fig. 2, A and B). These results confirmed the well-known synergistic effect between these two hormones. However, the stimulatory effect of FSH on StAR expression was not augmented by cotreatment with T (Fig. 2C). These results demonstrate that the effect of T is specific for Cyp19 and P450scc.
Fig. 2.
Synergistic effect of T and FSH on Cyp19 and P450scc but not on StAR expression. Granulosa cells were cultured in the presence or absence of increasing concentrations of T and in the presence or absence of FSH for 96 h. Cyp19 (A), P450scc (B), StAR (C) and Ribosomal L19 mRNA expression was determined by real-time PCR. This experiment was performed five times with identical results. Values are the mean ± sem of a single experiment performed in triplicate. Columns denoted with different letters differ significantly (a and b, P < 0.05; a–d and a–c, P < 0.001; ANOVA I Tukey t test).
T stimulation is not mediated by its conversion to 5α-dihydrotestosterone (DHT) or E2
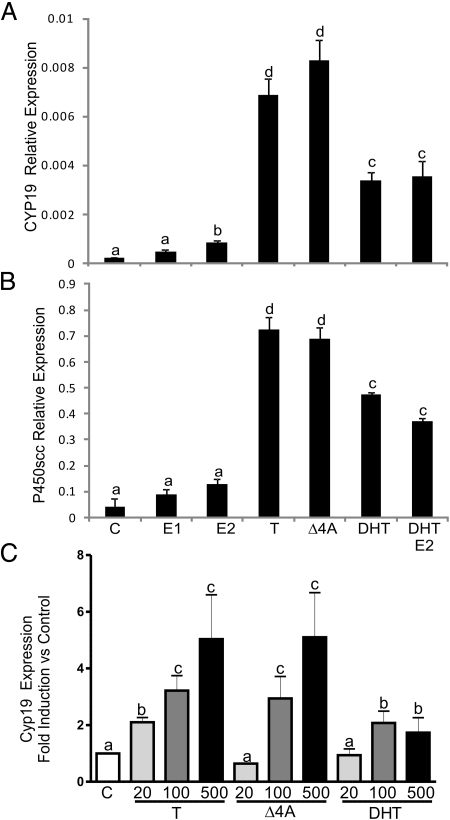
Granulosa cells convert T into E2 or DHT (15); therefore, the effect of these metabolites was examined. T and its precursor, androstenedione (Δ4A), were the most potent stimulators of Cyp19 and P450scc expression (Fig. 3, A and B). Similarly to T, Δ4A stimulated E2 production in granulosa cells (Supplemental Fig. 1 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). However, aromatization of T or Δ4A does not seem to be required for the stimulation of Cyp19 or P450scc because neither E2 nor estrone significantly stimulated the expression of these genes. On the other hand, DHT did stimulate Cyp19 and P450scc, but it was significantly less effective than T or Δ4A (Fig. 3, A and B). Treatment with two metabolites of DHT (5α-androstan-3β,17β-diol and 5α-androstan-3α,17β-diol) also stimulated the expression of Cyp19 and P450scc to levels similar to those reached in the presence of DHT (data not shown).
Fig. 3.
Effect of different steroids on the expression of Cyp19 and P450scc. Cells were cultured in the presence of different steroids (100 nm) for 96 h. Cyp19 (A) and P450scc (B) expression was assessed by real-time PCR. This experiment was performed five times with identical results. Values are the mean ± sem of a single experiment performed in triplicate. C, Granulosa cells were cultured in the presence of increasing concentrations (20, 100, or 500 nm) of T, Δ4A, or DHT. Cyp19 expression was determined using real-time PCR. Columns denoted with different letters differ significantly (a and b, P < 0.05; a–d and a–c, P < 0.001; ANOVA I Tukey t test). E1, Estrone.
Because E2 and DHT can be produced simultaneously from T, whether these two metabolites of T have any additive effects was examined. Treatment of granulosa cells with E2 and DHT simultaneously did not stimulate Cyp19 or P450scc expression beyond the levels observed with DHT alone (Fig. 3, A and B). Next, we compared the response of granulosa cells to increasing doses of T, Δ4A, or DHT. As shown in Fig. 3C, granulosa cells responded to escalating concentrations of T and Δ4A with a progressive increase in Cyp19 expression. However, the effect of DHT on Cyp19 expression did not follow a dose-dependent pattern.
These results demonstrate that T is a powerful stimulant of Cyp19 and P450scc expression in granulosa cells and that this effect is not mediated by T's conversion to E2. Furthermore, these results indicate that T may have a direct effect on ovarian gene expression without conversion into DHT, a classical AR agonist. This suggests the presence of pathways activated exclusively by T.
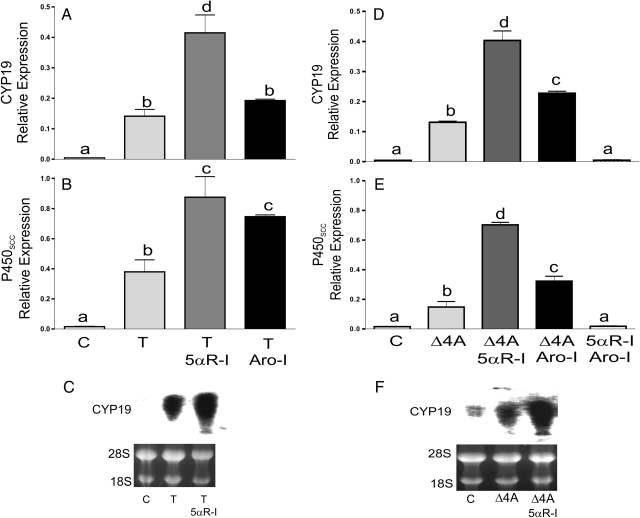
To test this hypothesis, conversion of T to DHT and/or E2 was prevented by treatment with finasteride, a 5α-reductase inhibitor (5αR-I), or with formestane, an aromatase inhibitor (Aro-I). In the presence of finasteride, T (Fig. 4, A and B) as well as Δ4A (Fig. 4, D and E) stimulated Cyp19 and P450scc expression to values that were significantly higher than those found in the presence of either steroid alone. Blocking aromatization did not alter the stimulatory effect of T, although it did synergize with Δ4A on the induction of Cyp19 expression (Fig. 4D). P450scc stimulation by T and Δ4A was clearly potentiated in the presence of the Aro-I (Fig. 4, B and E). Treatment with either inhibitor alone (data not shown) or in combination had no effect on gene expression (Fig. 4, D and E). The synergistic effect observed between finasteride (5αR-I) and both steroids (T and Δ4A) on the stimulation of Cyp19 was confirmed by Northern blot analysis (Fig. 4, C and F).
Fig. 4.
Inhibition of T and Δ4A conversion to DHT or E2 potentiates their stimulatory effect on gene expression. Granulosa cells were treated with vehicle (C), T, or Δ4A in the presence or absence of 5α-R-I (5 μm) or Aro-I (5 μm). mRNA levels were quantified by real-time PCR (A, B, D, and E) or by Northern blot analysis (C and F). These experiments were performed six times with identical results. Values are the mean ± sem of a single experiment performed in triplicate. Columns denoted with different letters differ significantly (a and b, and b–d/c, P < 0.05; a–d and a–c, P < 0.001; ANOVA I Tukey t test).
The efficacy of formestane and finasteride to inhibit Cyp19 and 5α-reductase activities, respectively, in rat granulosa cells was assessed. As shown in Supplemental Fig. 1, treatment with the Aro-I formestane completely inhibited the production of E2 induced by T or Δ4A. Similarly, the accumulation of DHT observed in cells treated with T or Δ4A was prevented by treatment with finasteride, the 5αR-I. These results confirm the efficiency of the inhibitors used. These results also provide further evidence of the capacity of granulosa cells to generate DHT when treated with Δ4A (15). Because DHT can only be produced from T, these results demonstrate that granulosa cells convert Δ4A into T, confirming previous publications (15). Moreover, these findings show that the powerful stimulatory effect of Δ4A, which is a weak activator of the AR, may be mediated by its conversion to T.
T stimulation of Cyp19 and P450scc expression is mediated by AR
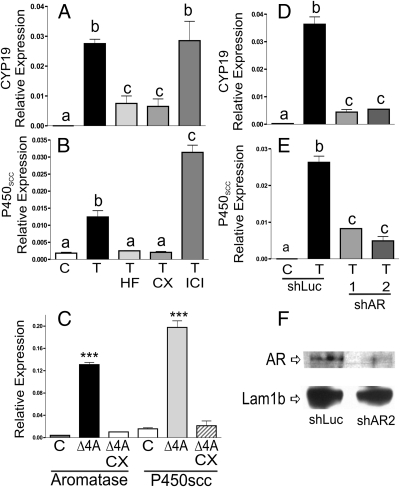
To determine whether the effect of T was mediated by the activation of AR, two antagonists of this receptor: hydroflutamine (5 μm) and bicalutamide 5 μm, were used. We observed that these inhibitors significantly reduced the induction of Cyp19 and P450scc by T and Δ4A(Fig. 5, A–C). However, the use of the pure estrogen receptor antagonist fulvestrant (ICI 182780, 5 μm)) had no effect (Fig. 5, A and B). Similar results were found when AR expression was silenced using small interference RNA targeting two different regions of the coding sequence of the AR receptor (shAR1 and shAR2; Fig. 5, D and E). Figure 5F shows that shAR treatment led to a marked reduction of AR receptor protein expression, whereas the expression of lamin 1B, used as loading control, was not altered.
Fig. 5.
T stimulation of Cyp19 and P450scc expression is mediated by the AR. A–C, Granulosa cells were treated with vehicle, T, or Δ4A in the presence or absence of hydroflutamine (HF) or bicalutamide (CX), which are two antagonists of AR or in the presence of fulvestrant (ICI), which is an ER antagonist. D and E, Granulosa cells were infected with lentivirus carrying shRNA sequences against luciferase (shLUC), used as control, or two regions of the AR-coding sequence (shAR 1 and 2). Cyp19 and P450scc mRNA levels were quantified by real-time PCR. F, AR protein expression after infection with shLuc or shAR2. These experiments were performed three times with identical results. Values are the mean ± sem of a single experiment performed in triplicate. Columns denoted with different letters differ significantly (a–c, P < 0.05; a and b, P < 0.001; ***, P < 0.01 vs. control or Δ4A plus CX; ANOVA I Tukey t test).
T stimulates Cyp19 promoter activity
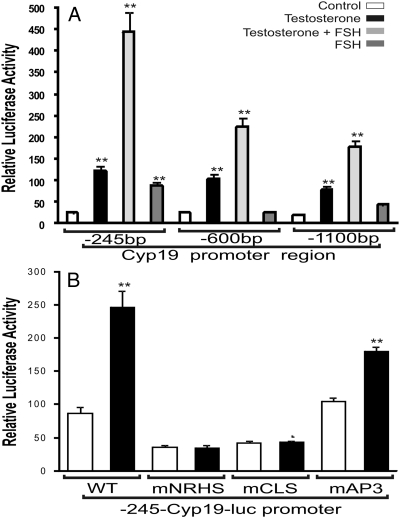
Next, the effect of T on the activity of the Cyp19 promoter was examined. The activity of three reporter vectors containing 245, 600, or 1100 bp of the rat Cyp19 proximal promoter was stimulated by treatment with T (Fig. 6A). As expected, FSH alone increased Cyp19 promoter activity and the presence of T potentiated this effect of FSH (Fig. 6A). T had no effect on the activity of the empty reporter vector, pGL3 (data not shown). The stimulatory effect of T was similar for all the promoters used, suggesting that the shorter region (245 bp) contains the elements necessary for the induction of Cyp19 expression by T. Within the 245-bp region of the Cyp19 promoter, several enhancer elements have been described. Within these elements, a nuclear receptor half-site (NRHS) and a cAMP-responsive element-like sequence (CLS) have been shown to play a key role in the regulation of Cyp19 expression (16–21). Mutation of NRHS and CLS prevented the stimulation of the reporter construct by T. However, a mutation of an AP3 response element, also located within the 245-bp region, had no effect (Fig. 6B). This suggests that activation of NRHS and CLS mediates the stimulation of Cyp19 expression by T.
Fig. 6.
T stimulates Cyp19 promoter activity. A, Granulosa cells were transfected with luciferase reporter constructs containing serial deletions of the Cyp19 proximal promoter: 1100, 600, or 245 bp. B, Granulosa cells were transfected with the 245-Cyp19-luc reporter construct containing no mutations [wild type (WT)], or mutations on the following response elements: NRHS (mNRHS), cAMP-responsive element-like sequence (mCLS), or AP-3 (mAP3). Luciferase activity was determined 48 h after transfection. Results from four experiments performed in triplicate are shown as the mean ± sem. Columns with asterisks (**) differ significantly from control (ANOVA-I Tukey t test).
T, not DHT, stimulates LRH-1 expression in rat granulosa cells
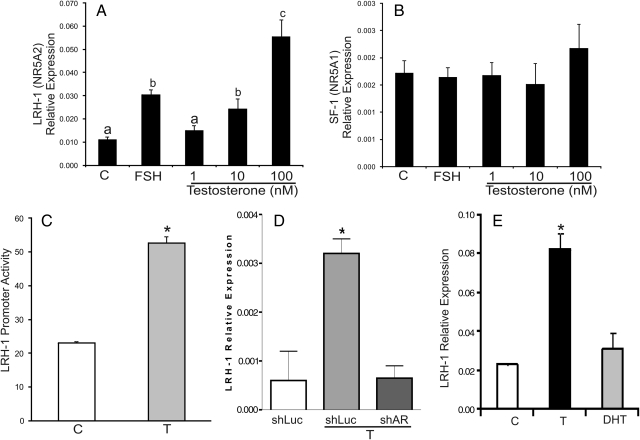
CLS is recognized by the cAMP-responsive element binding protein (CREB). CREB is rapidly phosphorylated by treatment of granulosa cells with FSH, but the expression and subcellular localization of CREB does not change during granulosa cell differentiation (22). In contrast, NRHS is recognized by steroidogenic factor-1 (SF-1) and LRH-1. The expression and/or activity of these factors is regulated during folliculogenesis (23). Consequently, the effect of T on the expression of LRH-1 and SF-1 was examined. T and Δ4A increased the expression of LRH-1 in a dose-dependent manner but had no effect on the expression of SF-1 (Fig. 7, A and B, and data not shown). FSH stimulated the expression of LRH-1 but had no effect on SF-1 mRNA levels. T and Δ4A treatment stimulated the activity of the LRH-1 promoter in granulosa cells (Fig. 7C, and data not shown). Additionally, stimulation of LRH-1 expression by these steroids was prevented by knocking down the AR by using small interference RNA (siRNA) (Fig. 7D and data not shown). In agreement with the limited effect of DHT on Cyp19 expression, this steroid had no effect on the expression of LRH-1 mRNA (Fig. 7E) or on the activity of the LRH-1 promoter (data not shown). This further suggests a differential response of granulosa cells to T and DHT.
Fig. 7.
T, but not DHT, stimulates LRH-1 expression in granulosa cells. Granulosa cells were cultured in the presence of: vehicle, FSH (50 ng/ml) or increasing concentrations of T for 96 h. The expression of LRH-1 (A) and SF-1 (B) was determined by real-time PCR. This experiment was performed three times with identical results. Results from a single experiment performed in triplicate are shown as the mean ± sem. Columns denoted with different letters differ significantly (a and b, P < 0.05; a–c, P < 0.001; ANOVA-I Tukey t test). C, Granulosa cells were transfected with an LRH-1 promoter reporter construct or an empty vector. Cells were cultured in the presence or absence of T (100 nm). Luciferase activity was determined 48 h after transfection. D, Granulosa cells were infected with lentivirus carrying shRNA sequences against luciferase (shLUC), used as control, or against AR (shAR). LRH-1 mRNA levels were quantitated by real-time PCR. Three independent experiments reported identical results. Results from a single experiment performed in triplicate are shown as the mean ± sem. *, P < 0.01 vs. shLUC and shAR+T; ANOVA-I Tukey t test. E, Granulosa cells were cultured in the presence of vehicle, T (100 nm) or DHT (100 mm) for 96 h. The expression of LRH-1 was determined by real-time PCR. *, P < 0.01 vs. C and DHT; ANOVA-I Tukey t test (n = 4).
Δ4A conversion to T is required for LRH-1 stimulation
Δ4A, which binds to the AR with low affinity, was as effective as T in the induction of LRH-1 expression and promoter activity. In granulosa cells, which express 17β-hydroxysteroid dehydrogenase (HSD) type 5 and type 1 (24, 25), Δ4A conversion to T is known to take place due to high 17β-HSD activity (15). To investigate whether this conversion is needed for the induction of LRH-1 expression by Δ4A, we used human embryonic kidney (HEK) 293T cells in which 17β-HSD activity has not been detected (26). HEK 293T cells were cotransfected with the LRH-1 promoter reporter and a rat AR expression vector or an empty vector. As shown in Supplemental Fig. 2, T increased the activity of the LRH-1 promoter in cells expressing the AR whereas Δ4A had no effect. This finding suggests that the stimulatory effect of Δ4A observed in granulosa cells is most likely mediated by its transformation to T. This is further supported by the fact that DHT, a metabolite of T, was present in the culture medium of cells treated with Δ4A (Supplemental Fig. 1).
The effect of T on the binding of LRH-1 to the Cyp19 promoter
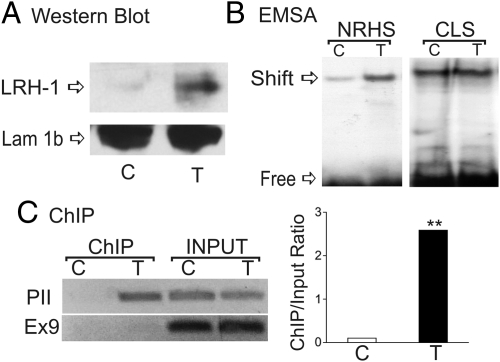
T increased binding of nuclear proteins to the NRHS element as demonstrated by EMSA; however, it had no effect on nuclear protein binding to the CLS element (Fig. 8B). This increase in binding correlates with higher LRH-1 protein levels, whereas lamin 1b, a nuclear envelope membrane used as loading control, remained constant (Fig. 8A). The increase in LRH-1 expression correlates also with the occupancy of the native Cyp19 promoter by LRH-1 (Fig. 8C). Thus, in granulosa cells treated with T, the Cyp19 promoter was enriched after chromatin precipitation with an anti-LRH-1 antibody (Fig. 8C). No enrichment of Cyp19 exon 9 was observed, confirming the specificity of LRH-1 binding for the Cyp19 promoter. Additionally, real-time PCR quantification of chromatin immunoprecipitation (ChIP) results show a 25-fold enrichment of the proximal promoter in samples treated with T when compared with cells treated with vehicle. These results indicate that LRH-1 may mediate the effect of T on the expression of Cyp19 granulosa cells.
Fig. 8.
T induces LRH-1 expression and LRH-1 binding to the Cyp19 promoter. A, LRH-1 expression after treatment of granulosa cells with vehicle or T. B, EMSAs were performed with nuclear extracts of granulosa cells cultured in the presence or absence of T for 96 h and probes containing the NRHS or CLS elements found in the Cyp19 promoter. C, Chromatin was immunoprecipitated with LRH-1 antibodies or normal IgG. Immunoprecipitated (ChIP) or total (Input) DNA was amplified by regular PCR (left) or by real-time PCR (right) using primers that amplified the NRHS region of the Cyp19 proximal promoter (PII). As control, exon 9 (Ex9) of the coding region was also amplified. These experiments were performed four times with identical results. Values are the mean ± sem of a single experiment performed in triplicate. ***, P < 0.01 vs. control Student's t test.
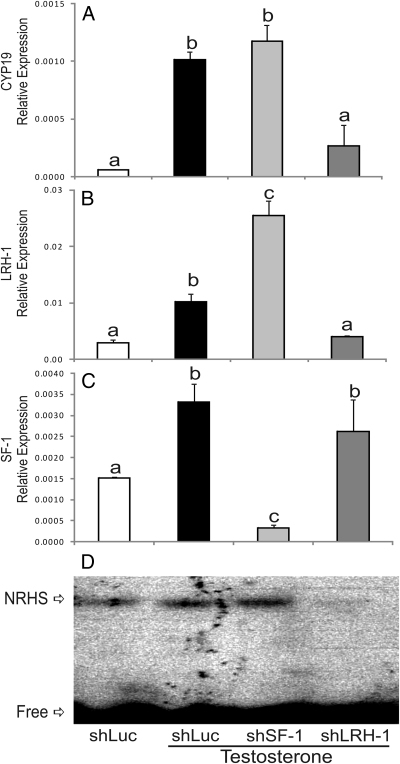
The role of LRH-1 on the induction of Cyp19 expression by T was further investigated by using siRNA. Cyp19 expression was stimulated by T in the presence of a control siRNA (shLUC, Fig. 9A). This stimulatory effect was not affected by the down-regulation of SF-1 (shSF-1, Fig. 9C). However, inhibition of LRH-1 expression (Fig. 9B) completely prevented the increase in Cyp19 expression (shLRH-1, Fig. 9A). Knockdown of LRH-1 and its binding to NRHS was confirmed using gel-shift analysis. As shown in Fig. 9D, T increased protein binding to NRHS. This binding was not affected by treatment with shSF-1, but it was completely abolished by the down-regulation of LRH-1. Western blot analysis confirmed the down-regulation of LRH-1 expression in the presence of shLRH-1 (data not shown). These results demonstrate clearly that LRH-1 is needed for the induction of Cyp19 expression by T in granulosa cells.
Fig. 9.
LRH-1 silencing prevents T stimulation of Cyp19 and P450scc expression. Granulosa cells were infected with lentivirus carrying shRNA sequences against luciferase (shLUC), used as control, SF-1 (shSF-1), or LRH-1 (shLRH-1). Total mRNA or nuclear proteins were isolated 96 h after infection. Total mRNA was used for Cyp19 (A), LRH-1 (B), and SF-1 (C) quantification by real-time PCR, whereas nuclear proteins were used for gel shift analysis using the NRHS element as a probe (D). This experiment was performed three times with identical results. Values are the mean ± sem of a single experiment performed in triplicate. Columns denoted with different letters differ significantly (a and b, P < 0.05; a–c, P < 0.001; ANOVA I Tukey t test).
T actions are not affected by the inhibition of 5α-reductase and Cyp19 activities
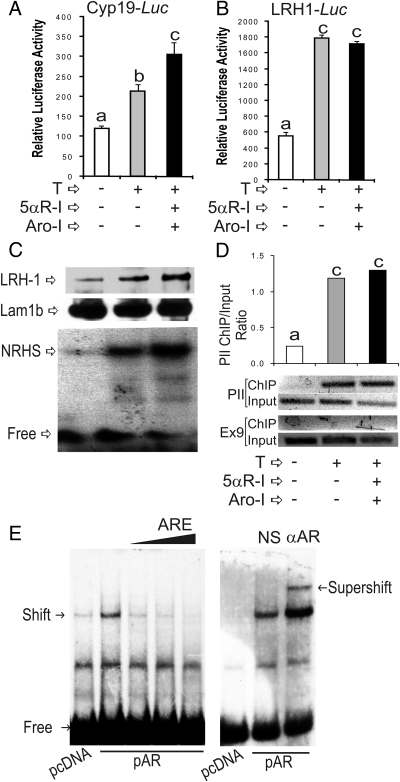
Next, we examined whether inhibition of T metabolism affects Cyp19 and LRH-1 up-regulation, as well as binding of LRH-1 to the Cyp19 promoter. Conversion of T to DHT or E2 was prevented by treatment with finasteride, a 5αR-I, or with formestane, an Aro-I. Treatment with T in the presence of 5αR-I and Aro-I led to a significant increase of Cyp19 promoter activity when compared with cells treated with T alone (Fig. 10A). Accordingly, the stimulation of Cyp19 mRNA accumulation by T was also potentiated by the inhibition of T metabolism by treatment with 5αR-I and Aro-I (data not shown). The induction of LRH-1 promoter activity by T was not affected by the presence of 5αR-I and Aro-I (Fig. 10B). LRH-1 protein levels in the presence of T, 5αR-I, and Aro-I were significantly higher than in the presence of T alone (Fig. 10C, top panel), whereas the expression of lamin 1b was not affected by any treatment (Fig. 10C, middle panel). Moreover, inhibition of T metabolism had no effect on the stimulation of LRH-1 binding to the Cyp19 promoter as determined by gel-shift analysis (Fig. 10C, lower panel) and ChIP (Fig. 10D). These results demonstrate a direct effect of T on the activation of the LRH-1 gene, which in turn binds to the Cyp19 promoter and contributes to the up-regulation of Cyp19 expression.
Fig. 10.
T directly affects LRH-1 expression and LRH-1 binding to the Cyp19 promoter. Granulosa cells were treated with vehicle, T, or with T plus 5α-R-I and Aro-I. Some cells were transfected with Cyp19-Luc (A) or LRH-1-Luc (B) reporter constructs or left without transfection (C and D). In A and B, luciferase activity was determined 48 h after transfection. In C, nuclear extracts were isolated and Western blots using anti-LRH-1 (top) and anti-lamin 1b antibodies (middle), or EMSA using the NRHS as a probe (bottom) were performed. In panel D, ChIP was performed using an anti-LRH-1 antibody. E (left panel), HEK 293T cells were transfected with an empty vector (pcDNA) or an AR expression vector (pAR). Nuclear extracts were obtained after 48 h of transfection and EMSAs performed with a probe containing the AREs found in the LRH-1 promoter. E (right panel), supershift analyses were performed by adding normal antiserum (NS) or an anti-AR antiserum (αAR) to the gel shift reaction. These experiments were performed three times with identical results. Values are the mean ± sem of a single experiment performed in triplicate. Columns denoted with different letters differ significantly (a and b, P < 0.05; a–c, P < 0.001; ANOVA I Tukey t test). PII, Cyp19 proximal promoter; Ex9, Cyp19 exon 9.
Finally, we investigated whether the LRH-1 gene is a direct target of the AR. Analysis of the LRH-1 promoter using a recently published probability matrix to predict AR response elements (27) revealed the presence of a potential androgen response element (ARE) within the region −48 to −61 of the rat LRH-1 promoter where +1 is the transcription start site (28). This ARE, also present in the human LRH-1 promoter, is recognized by the AR as demonstrated by gel-shift analysis. Using a radiolabeled probe spanning −69 to −31 of the rat LRH-1 promoter, a shifted band was observed with nuclear extracts of HEK 293T cells transfected with an AR expression vector (Fig. 10E, left panel). In contrast, no band shift was observed in cells transfected with an empty vector. The formation of the shifted band was decreased by an excess of unlabeled consensus ARE oligonucleotide. Prominently, the shifted band observed in nuclear extract of cells transfected with AR expression vector was supershifted by addition to the binding reaction of an anti-AR antibody (Fig. 10E, right panel). The addition of normal serum (NS) had no effect (Fig. 10E, right panel). These results show that the LRH-1 promoter contains elements that are recognized by the AR, suggesting that this steroid receptor may directly regulate LRH-1 expression.
Discussion
It is widely recognized that FSH is the main regulator of Cyp19 expression in granulosa cells (14). However, studies in FSH-β subunit (29) and FSH receptor-knockout mice (30) revealed the involvement of other factors in the regulation of ovarian E2 production. In FSH-β subunit-knockout mice, Cyp19 expression is reduced (6-fold), although these low levels of expression are enough to maintain normal circulatory levels of E2 (31). Also, mice null for the FSH receptor have normal Cyp19 mRNA and protein levels in the ovary (30). These findings, although rather controversial, clearly established the role of factors other than FSH in the activation of the Cyp19 gene in the ovary. Our results demonstrate, for the first time, that androgens, in particular T, provide a powerful stimulation of not only Cyp19 but also P450scc expression in rat granulosa cells.
Previous reports have examined the effect of androgens on the expression of Cyp19. In fact, androgens were found to stimulate Cyp19 expression in the brain of zebrafish (32), human endometrial cells (33), and bovine granulosa cells (34). However, estrogens had an equal or greater effect than T, and these effects were prevented by ER antagonists but not by AR antagonists; therefore, these reports concluded that androgens need to be converted to E2 to stimulate Cyp19 expression. A second report using bovine granulosa cells showed that T, but not DHT, up-regulated Cyp19 expression (35), suggesting that the effect of T may be mediated by its conversion to E2. Surprisingly, in the same study, E2 treatment had no effect. Because high concentrations of E2 (1 and 10 μm) were used in the study, this could have prevented the stimulation of Cyp19 as the authors demonstrated is the case for high concentrations of T (35).
It is interesting that Δ4A, which binds to the AR with low affinity, is as effective as T in the induction of Cyp19. However, this is not surprising because murine granulosa cells have high 17β-HSD activity (15) and express both 17β-HSD type 5 and type 1 (24, 25). Thus they readily convert Δ4A into T as well as estrone into E2 (15). It is most likely that the stimulatory effect of Δ4A on Cyp19 and LRH-1 expression is mediated by its prior conversion to T. This conclusion is further supported by the fact that Δ4A has no stimulatory effect on LRH-1 promoter activity in HEK 293T cells, which are void of 17β-HSD activity.
One striking finding in our study is that T seems to be the most active androgen in granulosa cells. The role and specific mechanisms of T action in the ovary are difficult to understand because in this tissue T can be metabolized into E2, which signals via estrogen receptors (ERs) and into DHT, which can activate the AR. Moreover, DHT itself can be reduced into compounds such as 5α-androstan-3β,17β-diol, which has estrogenic activity (36). Using specific inhibitors for these metabolic pathways, we have demonstrated that T increases Cyp19 and P450scc expression without its conversion to either E2 or DHT.
T and DHT bind to the AR with similar affinity (37, 38). This is supported by crystallographic studies showing nearly identical interactions of T and DHT with the AR ligand-binding pocket (38). On the other hand, it is known that the DHT-AR complex is more potent than the T-AR complex in the activation of a number of prostate genes. Notably, this decreased potency of T can be overcome by mass action. Thus, early studies demonstrated that T bound to the AR is 10-fold less potent than the DHT-AR complex in activating some androgen-responsive genes such as murine mammary tumor virus. However, both steroids reach identical maximal activities at higher concentrations (39). In contrast, our results indicate that in granulosa cells T is a greater activator of the AR than DHT. These findings are in accordance with previous reports in which it is suggested that the specific effects of a particular ligand-AR complex are likely to vary depending on the promoter context and the cell type. For instance, recent studies showed that T is more potent than DHT in the induction of a subset of androgen-responsive genes during rat prostate regrowth. In this experimental model, it was demonstrated that T enhances the expression of both U19/Eaf2 and prostate-specific antigen genes, whereas coadministration of finasteride, which prevents DHT formation, further enhanced T-induced expression of U19/Eaf2 but not that of prostate-specific antigen (40, 41). Using differential display PCR, Avila et al. (42) demonstrated that a subset of genes expressed in the rat prostate are up-regulated by T treatment in the presence of DHT synthesis inhibitors. These authors also demonstrated that T, but not DHT, seems to increase the expression of the prostate C3 gene. Interestingly, it has been demonstrated that C3 promoter stimulation by T is 2-fold higher than by DHT, whereas, in the same experimental model the stimulation of the murine mammary tumor virus promoter by DHT was severalfold higher than that of T (43). T was also found to be more effective than DHT in the repression of other androgen target genes such as TDD5 (44). These findings clearly indicated that many androgen-responsive genes may be regulated in fundamentally different ways in response to T and DHT.
The mechanism(s) of these contrasting effects of T and DHT is not well understood. However, differential effects of T and DHT on the expression of various genes are most likely due to different conformations of the liganded AR resulting in differences in AR-DNA and AR-protein interactions at AREs associated with specific genes. In fact, crystallographic studies suggest that the interactions of the AR activation function 2 domain with coactivators is slightly different between the T-AR complex and the DHT-AR complex (38). It is possible that the greater stimulatory effect of T on LRH-1 expression is due to the recruitment of granulosa cell-specific cofactors by the T-AR complex. Gel shift analyses indicate the presence of ARE in the LRH-1 promoter. However, future studies are needed to clarify the molecular mechanisms of T activation of gene expression in ovarian granulosa cells and, in particular, on the regulation of the LRH-1 gene.
Interestingly, the stimulatory effect of T on Cyp19 gene expression was most pronounced in the presence of 5αR-I. This finding is in accordance with the high levels of E2 observed in the 5α-reductase-1-knockout mice (45). The elevated levels of E2 found in 5α-reductase-1-knockout animals are probably due to an excess of estrogen precursors (T and Δ4A) and an increase in Cyp19 expression. This idea is supported by the fact that in 5α-reductase-1-knockout animals, E2 levels are elevated before there is an increase in Cyp19 substrates. The mechanism by which inhibition of 5α-reductase brings about the major effect of T remains unknown. If we consider that DHT is less effective in inducing Cyp19 and P450scc expression, it is possible to suggest that the lack of metabolism increases the active concentration of T.
Androgens are associated with polycystic ovarian syndrome (PCOS), a common condition that features excessive ovarian androgen production, infertility, acne, and hirsutism (46). Interestingly, PCOS patients have low levels of Cyp19 expression in the ovary (47). One common characteristic of PCOS patients is the development of insulin resistance (46), which seems to be the cause of hyperandrogenism (48, 49). Preliminary results in our laboratory indicate that the stimulatory effect of T in granulosa cells is potentiated by the presence of insulin in the medium. This evidence suggests that, in PCOS patients, a decrease in insulin signaling within the ovary may lead to an attenuated response of granulosa cells to T. This would result in low levels of Cyp19 expression.
We have clearly demonstrated that T has a direct stimulatory effect on Cyp19 and P450scc expression in ovarian granulosa cells. This stimulatory effect is mediated, in part, by an increase in LRH-1 expression, which is an exclusive target of T and is not affected by DHT. These novel findings indicate that androgens not only synergize with FSH in the stimulation of Cyp19 and P450scc but also have a direct effect on the expression of these genes, which are involved in the differentiation of granulosa cells.
Materials and Methods
Animals and granulosa cell culture
Immature female Sprague Dawley rats were purchased (Charles River Laboratories Inc., Wilmington, MA) and housed in the Biological Resources Laboratory. Animals were treated in accordance with the NIH Guide for Care and Use of Laboratory Animals, and all protocols were approved by the University of Illinois at Chicago Animal Care Committee. Granulosa cells were isolated from immature rats treated with E2 (1.5 mg/d for 3 d) and cultured as previously described (50).
EMSAs
Nuclear protein extracts from granulosa cells cultured in the absence and presence of T were prepared as described previously (50). Double-stranded oligonucleotide probes containing the NRHS- or the CLS-binding sites labeled γ32P-ATP were used as probes. The oligonucleotide GAC AGC AAG AGA GAT AAG AAC TCT GGG CAG ATA ATG GC (the potential ARE is underlined) spanning from −69 to −31b of the rat LRH-1 promoter was used in the experiments presented in Fig. 10E. For competition experiments, increasing concentrations of a consensus ARE oligonucleotide were used (27). Supershift experiments were carried out by adding to the binding reaction 2 μl of the AR antibody (PG21; Millipore Corp.) or 2 μl of a normal serum 20 min before the addition of the labeled probe.
Western blot analysis
To determine the expression levels of Cyp19 and P450scc, cytosolic protein extract of granulosa cells was subjected to SDS-PAGE. Proteins were transferred to nitrocellulose membranes (Bio-Rad Laboratories, Inc., Hercules, CA) and processed by routine procedures. Immunoreactive bands were visualized by blotting with primary antibodies against Cyp19 (1:500, Serotec, Ltd., Oxford, UK), P450scc (1:1000, Millipore Corp.), lamin 1b (1:2000; Abcam, Cambridge, MA), LRH-1 (1:1000, Abcam), or AR (1:500, Millipore), followed by incubation with horseradish peroxidase-conjugated secondary antibodies and detection with enhanced chemiluminescence (Thermo Scientific, Rockford, IL).
RNA interference
Short hairpin RNAs (shRNAs) under the control of the H1 promoter were used to specifically knock down the expression of the AR, LRH-1, and SF-1 genes. The target recognition sequences used were shAR1: ctgctccgcagacattaaagacatc; shAR2: cagtcccagttgtgttaaaa gtgaa; shLRH-1: cgatgagcctcaagttcaagcgaaa; and shSF-1: gaaggtgcatggtatttaaggagct. shRNA against luciferase (shLUC): gcctgaagtctctgattaagtacaa, was used as control. Oligonucleotides containing the target recognition sequence and its corresponding antisense sequence separated by a short spacer sequence were chemically synthesized (Integrated DNA Technologies, Inc., Coralville, IA). These oligonucleotides were inserted into pTRIP plasmid to produce shRNA lentiviral vectors (51). Viral stocks were generated in HEK 293T cells (Invitrogen) cotransfected with the shRNA lentiviral vector along with the packaging and envelope plasmids psPAX2 and pMD2G (Addgene, Inc., Cambridge, MA). Cell supernatants were concentrated by ultracentrifugation. Viral stocks were titrated in HEK 293T cells. Viral stocks carrying shRNA were then added directly to the granulosa cells at a multiple of infection of 20.
Promoter reporter constructs and cell transfection
The rat Cyp19 promoter was previously described (52, 53). The promoter region rat LRH-1 gene was cloned from genomic DNA using the following primers: forward, TAC TGT TCC AGC CCT ATG ACC; reverse, AAG CTT TCA ATA GGC TTT GAC TGG TC. These primers amplify the region between −507 to +39 of the rat LRH-1 gene, where +1 is the transcription initiation site (28). PCR products were cloned into the pGL3 Basic luciferase report vector (Promega Corp., Madison, WI) by using XhoI and HindIII restriction sites. This construct was named –507LRH-1pr-luc. All cloning was confirmed by bidirectional sequencing. These promoters were used to transfect granulosa cells 48 h after plating using Fugene HD (Roche). Luciferase activity was assessed 48 h after transfection using the dual luciferase assay kit (Promega, Madison, WI).
RNA quantitation
Total RNA was isolated using TRIzol-Reagent (Invitrogen) following the manufacturer's instructions. mRNA was quantified as previously described (54). The relative expression of target genes was expressed in reference to ribosomal L19 expression.
Steroid determinations
E2 and DHT concentrations in the cell culture medium were determined using ELISA. For E2 determination, the Estradiol ELISA kit (catalog no. 582251; Cayman Chemical Co., Ann Arbor, MI) was used. For DHT determination, the DHT ELISA kit form BioVendor (catalog no. RCAN-DHT-280R) was used. Both determinations were performed following manufacturer's recommendations using undiluted, 1:50, or 1:150 dilutions of the cell culture medium.
ChIP assay
ChIP assays were performed using a MAGNA ChIP kit (Millipore) according to the manufacturer's protocol. Immunoprecipitations were carried out using 6 μg of an anti-LRH-1 antibody (Abcam, catalog no. 18293) or normal IgG. Immunoprecipitated DNA (ChIP DNA) and input DNA were amplified by PCR using the primers spanning the NRHS found in the Cyp19 promoter or primers that amplify exon 9 of the Cyp19 gene as control.
Statistics
Data are expressed as the mean ± sem. Two-group comparisons were performed using a t test for independent samples. Multiple group statistical analyses were performed by one-way ANOVA followed by the Tukey t test for multiple comparisons. Statistics were calculated with GraphPad Prism 5 (GraphPad, La Jolla, CA).
Acknowledgments
This work was supported by National Institutes Health grant R01HD057110.
Disclosure Summary: The authors declare no conflict of interest.
NURSA Molecule Pages:
Nuclear Receptors: SF-1 | LRH-1 | AR
Ligands: Testosterone | Dihydrotestosterone
Footnotes
- Δ4A
- Androstenedione
- AR
- androgen receptor
- ARE
- androgen response element
- Aro-I
- aromatase inhibitor
- ChIP
- chromatin immunoprecipitation
- CLS
- cAMP-responsive element-like sequence
- CREB
- cAMP-responsive element binding protein
- Cyp19
- aromatase
- DHT
- 5α-dihydrotestosterone
- E2
- estradiol
- HEK
- human embryonic kidney
- HSD
- hydroxysteroid dehydrogenase
- LRH-1
- liver receptor homolog-1
- NRHS
- nuclear receptor half-site
- P450scc
- P450 side-change cleavage
- PCOS
- polycystic ovarian syndrome
- 5αR-I
- 5α-reductase inhibitor
- SF-1
- steroidogenic factor-1
- shRNA
- short hairpin RNA
- siRNA
- small interference RNA
- T
- testosterone.
References
- 1. Armstrong DT, Dorrington JH. 1976. Androgens augment FSH-induced progesterone secretion by cultured rat granulosa cells. Endocrinology 99:1411–1414 [DOI] [PubMed] [Google Scholar]
- 2. Daniel SA, Armstrong DT. 1980. Enhancement of follicle-stimulating hormone-induced aromatase activity by androgens in cultured rat granulosa cells. Endocrinology 107:1027–1033 [DOI] [PubMed] [Google Scholar]
- 3. Walters KA, Allan CM, Handelsman DJ. 2008. Androgen actions and the ovary. Biol Reprod 78:380–389 [DOI] [PubMed] [Google Scholar]
- 4. Shiina H, Matsumoto T, Sato T, Igarashi K, Miyamoto J, Takemasa S, Sakari M, Takada I, Nakamura T, Metzger D, Chambon P, Kanno J, Yoshikawa H, Kato S. 2006. Premature ovarian failure in androgen receptor-deficient mice. Proc Natl Acad Sci USA 103:224–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hu YC, Wang PH, Yeh S, Wang RS, Xie C, Xu Q, Zhou X, Chao HT, Tsai MY, Chang C. 2004. Subfertility and defective folliculogenesis in female mice lacking androgen receptor. Proc Natl Acad Sci USA 101:11209–11214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sen A, Hammes SR. 2010. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Mol Endocrinol 24:1393–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spears N, Murray AA, Allison V, Boland NI, Gosden RG. 1998. Role of gonadotrophins and ovarian steroids in the development of mouse follicles in vitro. J Reprod Fertil 113:19–26 [DOI] [PubMed] [Google Scholar]
- 8. Murray AA, Gosden RG, Allison V, Spears N. 1998. Effect of androgens on the development of mouse follicles growing in vitro. J Reprod Fertil 113:27–33 [DOI] [PubMed] [Google Scholar]
- 9. Vendola K, Zhou J, Wang J, Famuyiwa OA, Bievre M, Bondy CA. 1999. Androgens promote oocyte insulin-like growth factor I expression and initiation of follicle development in the primate ovary. Biol Reprod 61:353–357 [DOI] [PubMed] [Google Scholar]
- 10. Yang MY, Fortune JE. 2006. Testosterone stimulates the primary to secondary follicle transition in bovine follicles in vitro. Biol Reprod 75:924–932 [DOI] [PubMed] [Google Scholar]
- 11. Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. 1998. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest 101:2622–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Okutsu Y, Itoh MT, Takahashi N, Ishizuka B. 2010. Exogenous androstenedione induces formation of follicular cysts and premature luteinization of granulosa cells in the ovary. Fertil Steril 93:927–935 [DOI] [PubMed] [Google Scholar]
- 13. Weil S, Vendola K, Zhou J, Bondy CA. 1999. Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab 84:2951–2956 [DOI] [PubMed] [Google Scholar]
- 14. Hunzicker-Dunn M, Maizels ET. 2006. FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A. Cell Signal 18:1351–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bogovich K, Richards JS. 1984. Androgen synthesis during follicular development: evidence that rat granulosa cell 17-ketosteroid reductase is independent of hormonal regulation. Biol Reprod 31:122–131 [DOI] [PubMed] [Google Scholar]
- 16. Fitzpatrick SL, Richards JS. 1994. Identification of a cyclic adenosine 3′,5′-monophosphate-response element in the rat aromatase promoter that is required for transcriptional activation in rat granulosa cells and R2C Leydig cells. Mol Endocrinol 8:1309–1319 [DOI] [PubMed] [Google Scholar]
- 17. Michael MD, Michael LF, Simpson ER. 1997. A CRE-like sequence that binds CREB and contributes to cAMP-dependent regulation of the proximal promoter of the human aromatase P450 (CYP19) gene. Mol Cell Endocrinol 134:147–156 [DOI] [PubMed] [Google Scholar]
- 18. Fitzpatrick SL, Richards JS. 1993. cis-acting elements of the rat aromatase promoter required for cyclic adenosine 3′,5′-monophosphate induction in ovarian granulosa cells and constitutive expression in R2C Leydig cells. Mol Endocrinol 7:341–354 [DOI] [PubMed] [Google Scholar]
- 19. Carlone DL, Richards JS. 1997. Functional interactions, phosphorylation, and levels of 3′,5′-cyclic adenosine monophosphate-regulatory element binding protein and steroidogenic factor-1 mediate hormone-regulated and constitutive expression of aromatase in gonadal cells. Mol Endocrinol 11:292–304 [DOI] [PubMed] [Google Scholar]
- 20. Carlone DL, Richards JS. 1997. Evidence that functional interactions of CREB and SF-1 mediate hormone regulated expression of the aromatase gene in granulosa cells and constitutive expression in R2C cells. J Steroid Biochem Mol Biol 61:223–231 [PubMed] [Google Scholar]
- 21. Hinshelwood MM, Repa JJ, Shelton JM, Richardson JA, Mangelsdorf DJ, Mendelson CR. 2003. Expression of LRH-1 and SF-1 in the mouse ovary: localization in different cell types correlates with differing function. Mol Cell Endocrinol 207:39–45 [DOI] [PubMed] [Google Scholar]
- 22. Mukherjee A, Park-Sarge OK, Mayo KE. 1996. Gonadotropins induce rapid phosphorylation of the 3′,5′-cyclic adenosine monophosphate response element binding protein in ovarian granulosa cells. Endocrinology 137:3234–3245 [DOI] [PubMed] [Google Scholar]
- 23. Falender AE, Lanz R, Malenfant D, Belanger L, Richards JS. 2003. Differential expression of steroidogenic factor-1 and FTF/LRH-1 in the rodent ovary. Endocrinology 144:3598–3610 [DOI] [PubMed] [Google Scholar]
- 24. Pelletier G, Luu-The V, Li S, Labrie F. 2005. Localization of type 5 17β-hydroxysteroid dehydrogenase mRNA in mouse tissues as studied by in situ hybridization. Cell Tissue Res 320:393–398 [DOI] [PubMed] [Google Scholar]
- 25. Pelletier G, Luu-The V, Li S, Ren L, Labrie F. 2004. Localization of 17β-hydroxysteroid dehydrogenase type 1 mRNA in mouse tissues. J Mol Endocrinol 33:459–465 [DOI] [PubMed] [Google Scholar]
- 26. Dufort I, Rheault P, Huang XF, Soucy P, Luu-The V. 1999. Characteristics of a highly labile human type 5 17β-hydroxysteroid dehydrogenase. Endocrinology 140:568–574 [DOI] [PubMed] [Google Scholar]
- 27. Denayer S, Helsen C, Thorrez L, Haelens A, Claessens F. 2010. The rules of DNA recognition by the androgen receptor. Mol Endocrinol 24:898–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang CK, Lin W, Cai YN, Xu PL, Dong H, Li M, Kong YY, Fu G, Xie YH, Huang GM, Wang Y. 2001. Characterization of the genomic structure and tissue-specific promoter of the human nuclear receptor NR5A2 (hB1F) gene. Gene 273:239–249 [DOI] [PubMed] [Google Scholar]
- 29. Burns KH, Yan C, Kumar TR, Matzuk MM. 2001. Analysis of ovarian gene expression in follicle-stimulating hormone β knockout mice. Endocrinology 142:2742–2751 [DOI] [PubMed] [Google Scholar]
- 30. Danilovich N, Babu PS, Xing W, Gerdes M, Krishnamurthy H, Sairam MR. 2000. Estrogen deficiency, obesity, and skeletal abnormalities in follicle-stimulating hormone receptor knockout (FORKO) female mice. Endocrinology 141:4295–4308 [DOI] [PubMed] [Google Scholar]
- 31. Kumar TR, Wang Y, Lu N, Matzuk MM. 1997. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet 15:201–204 [DOI] [PubMed] [Google Scholar]
- 32. Mouriec K, Gueguen MM, Manuel C, Percevault F, Thieulant ML, Pakdel F, Kah O. 2009. Androgens upregulate cyp19a1b (aromatase B) gene expression in the brain of zebrafish (Danio rerio) through estrogen receptors. Biol Reprod 80:889–896 [DOI] [PubMed] [Google Scholar]
- 33. Bukulmez O, Hardy DB, Carr BR, Auchus RJ, Toloubeydokhti T, Word RA, Mendelson CR. 2008. Androstenedione up-regulation of endometrial aromatase expression via local conversion to estrogen: potential relevance to the pathogenesis of endometriosis. J Clin Endocrinol Metab 93:3471–3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Luo W, Wiltbank MC. 2006. Distinct regulation by steroids of messenger RNAs for FSHR and CYP19A1 in bovine granulosa cells. Biol Reprod 75:217–225 [DOI] [PubMed] [Google Scholar]
- 35. Hamel M, Vanselow J, Nicola ES, Price CA. 2005. Androstenedione increases cytochrome P450 aromatase messenger ribonucleic acid transcripts in nonluteinizing bovine granulosa cells. Mol Reprod Dev 70:175–183 [DOI] [PubMed] [Google Scholar]
- 36. Omoto Y, Lathe R, Warner M, Gustafsson JA. 2005. Early onset of puberty and early ovarian failure in CYP7B1 knockout mice. Proc Natl Acad Sci USA 102:2814–2819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wilson EM, French FS. 1976. Binding properties of androgen receptors. Evidence for identical receptors in rat testis, epididymis, and prostate. J Biol Chem 251:5620–5629 [PubMed] [Google Scholar]
- 38. Askew EB, Gampe RT, Jr, Stanley TB, Faggart JL, Wilson EM. 2007. Modulation of androgen receptor activation function 2 by testosterone and dihydrotestosterone. J Biol Chem 282:25801–25816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Deslypere JP, Young M, Wilson JD, McPhaul MJ. 1992. Testosterone and 5α-dihydrotestosterone interact differently with the androgen receptor to enhance transcription of the MMTV-CAT reporter gene. Mol Cell Endocrinol 88:15–22 [DOI] [PubMed] [Google Scholar]
- 40. Gupta S, Wang Y, Ramos-Garcia R, Shevrin D, Nelson JB, Wang Z. 2010. Inhibition of 5α-reductase enhances testosterone-induced expression of U19/Eaf2 tumor suppressor during the regrowth of LNCaP xenograft tumor in nude mice. Prostate 70:1575–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dadras SS, Cai X, Abasolo I, Wang Z. 2001. Inhibition of 5α-reductase in rat prostate reveals differential regulation of androgen-response gene expression by testosterone and dihydrotestosterone. Gene Expr 9:183–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Avila DM, Fuqua SA, George FW, McPhaul MJ. 1998. Identification of genes expressed in the rat prostate that are modulated differently by castration and finasteride treatment. J Endocrinol 159:403–411 [DOI] [PubMed] [Google Scholar]
- 43. Hsiao PW, Thin TH, Lin DL, Chang C. 2000. Differential regulation of testosterone vs. 5α-dihydrotestosterone by selective androgen response elements. Mol Cell Biochem 206:169–175 [DOI] [PubMed] [Google Scholar]
- 44. Lin TM, Chang C. 1997. Cloning and characterization of TDD5, an androgen target gene that is differentially repressed by testosterone and dihydrotestosterone. Proc Natl Acad Sci USA 94:4988–4993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mahendroo MS, Cala KM, Landrum DP, Russell DW. 1997. Fetal death in mice lacking 5α-reductase type 1 caused by estrogen excess. Mol Endocrinol 11:917–927 [DOI] [PubMed] [Google Scholar]
- 46. Ehrmann DA. 2005. Polycystic ovary syndrome. N Engl J Med 352:1223–1236 [DOI] [PubMed] [Google Scholar]
- 47. Guzick DS. 2007. Ovulation induction management of PCOS. Clin Obstet Gynecol 50:255–267 [DOI] [PubMed] [Google Scholar]
- 48. Nestler JE, Barlascini CO, Matt DW, Steingold KA, Plymate SR, Clore JN, Blackard WG. 1989. Suppression of serum insulin by diazoxide reduces serum testosterone levels in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab 68:1027–1032 [DOI] [PubMed] [Google Scholar]
- 49. Geffner ME, Kaplan SA, Bersch N, Golde DW, Landaw EM, Chang RJ. 1986. Persistence of insulin resistance in polycystic ovarian disease after inhibition of ovarian steroid secretion. Fertil Steril 45:327–333 [PubMed] [Google Scholar]
- 50. Kwintkiewicz J, Cai Z, Stocco C. 2007. Follicle-stimulating hormone-induced activation of Gata4 contributes in the up-regulation of Cyp19 expression in rat granulosa cells. Mol Endocrinol 21:933–947 [DOI] [PubMed] [Google Scholar]
- 51. Stove V, Smits K, Naessens E, Plum J, Verhasselt B. 2006. Multiple gene knock-down by a single lentiviral vector expressing an array of short hairpin RNAs. Electronic J Biotechnol 9:572–579 [Google Scholar]
- 52. Stocco C, Kwintkiewicz J, Cai Z. 2007. Identification of regulatory elements in the Cyp19 proximal promoter in rat luteal cells. J Mol Endocrinol 39:211–221 [DOI] [PubMed] [Google Scholar]
- 53. Cai Z, Kwintkiewicz J, Young ME, Stocco C. 2007. Prostaglandin E2 increases cyp19 expression in rat granulosa cells: implication of GATA-4. Mol Cell Endocrinol 263:181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stocco C. 2004. In Vivo and In Vitro Inhibition of cyp19 Gene expression by prostaglandin F2α in murine luteal cells: implication of GATA-4. Endocrinology 145:4957–4966 [DOI] [PubMed] [Google Scholar]