Abstract
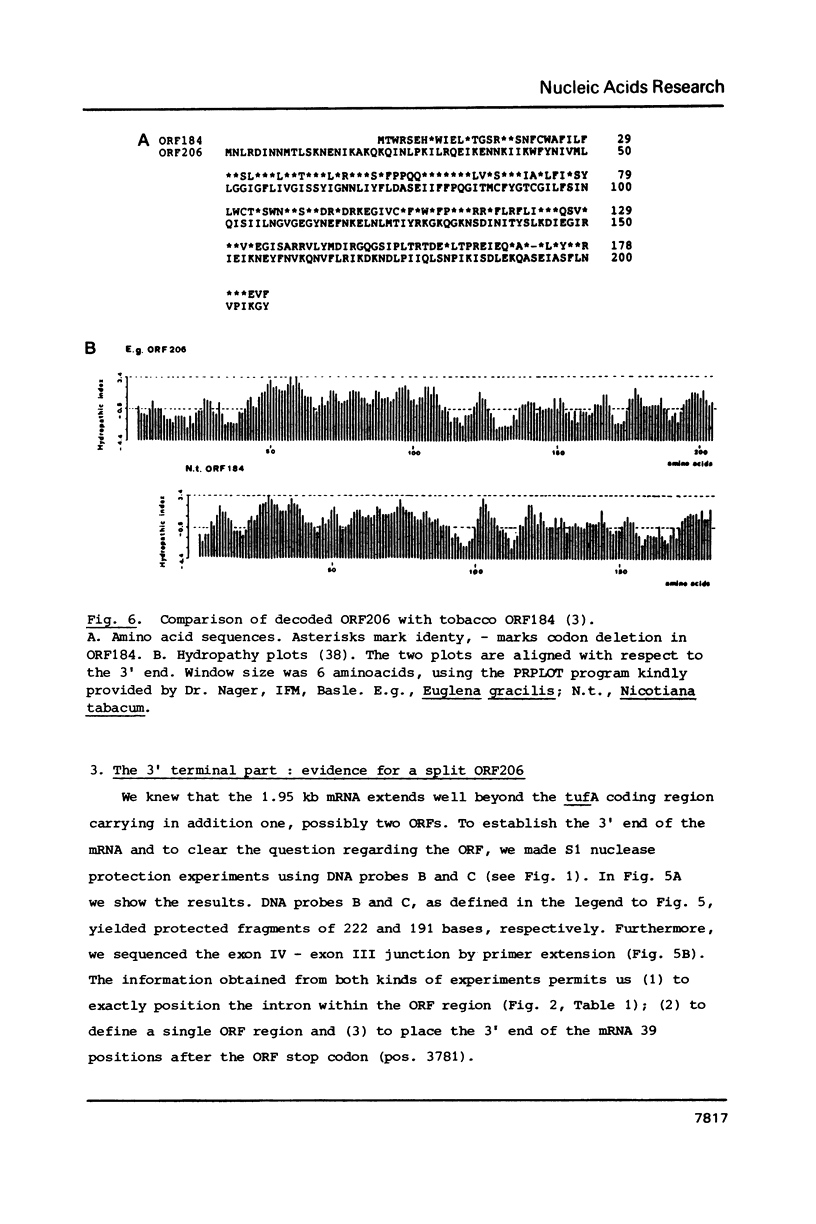
Structural features of a dicistronic 1.95 kb mRNA coding for the chloroplast specific elongation factor Tu and ORF206 are described. The unspliced pre-mRNA is composed of 2562 nucleotides and undergoes four splicing events which remove a total of 606 nucleotides. The first intron splits the untranslated leader, two introns dissect the tufA coding region and the forth intron is within ORF206, which codes for a putative protein that is to 34% homologous with the putative protein of chloroplast ORF184 of tobacco. Introns neither belong to group I nor II, and 5' and 3' intron boundaries do not follow consensus sequences. Potential ribosome binding sites are located 58 and 32 positions upstream of the tufA and ORF206 start codon, respectively.
Full text
PDF



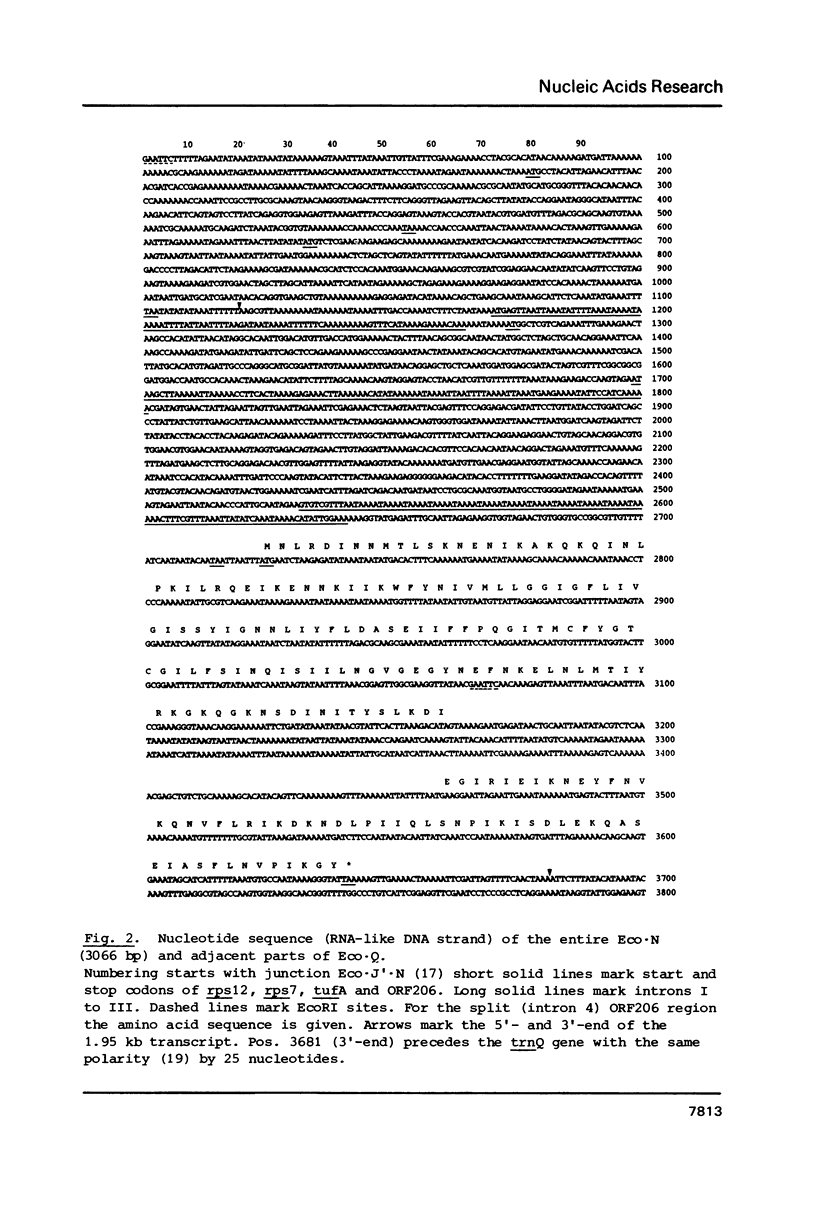

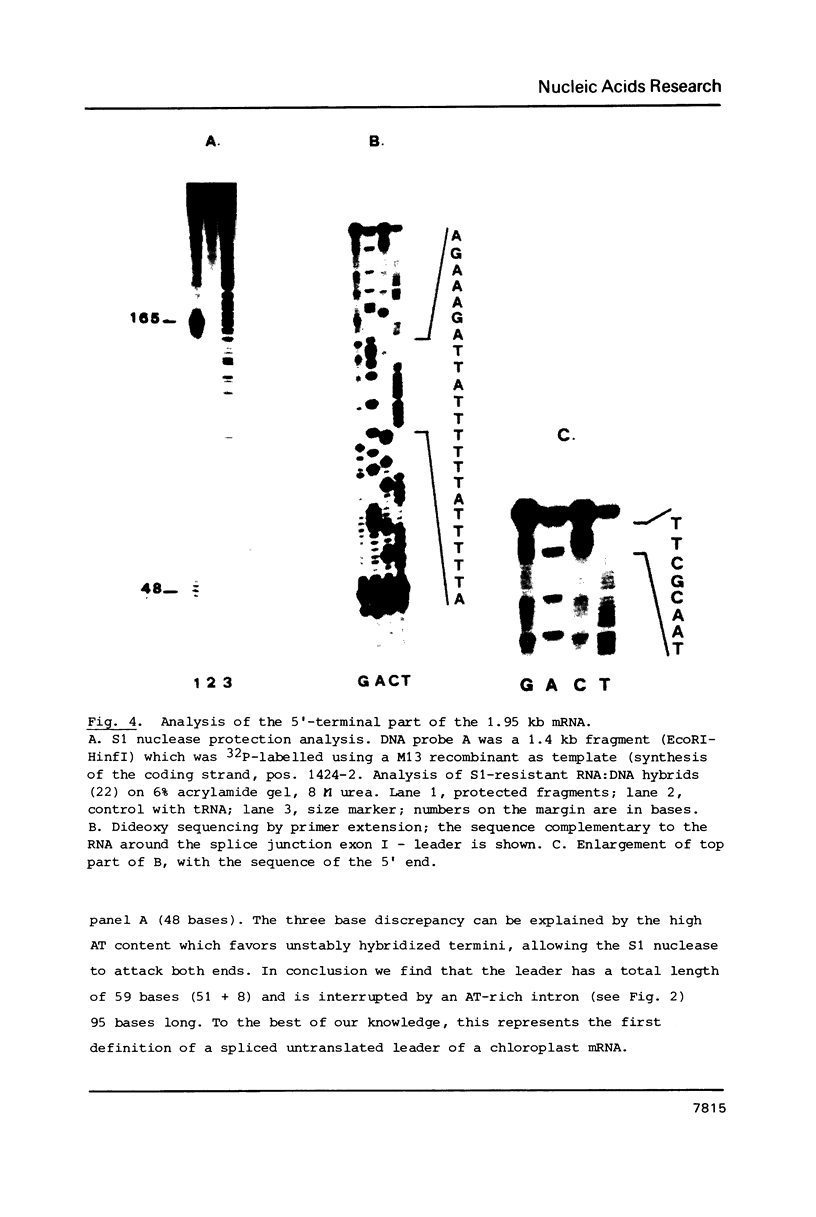
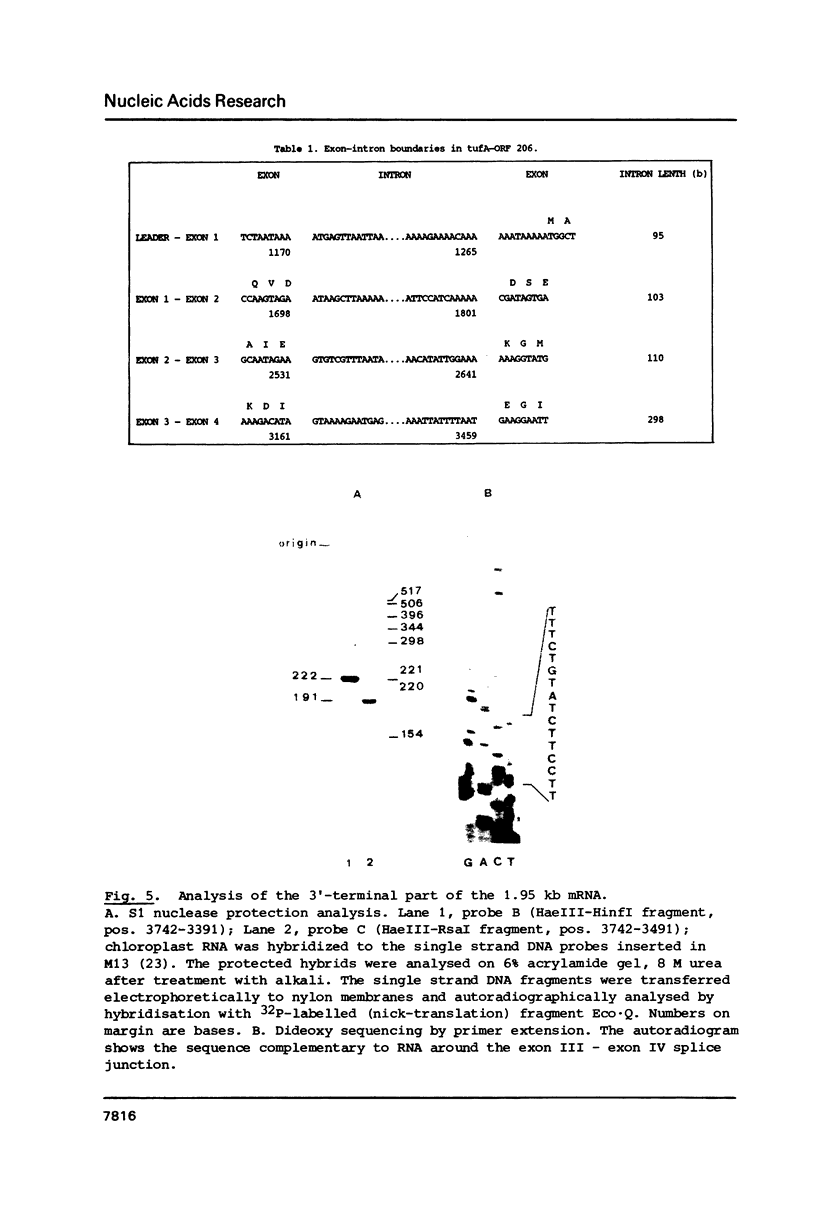





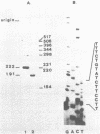
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allet B., Rochaix J. D. Structure analysis at the ends of the intervening DNA sequences in the chloroplast 23S ribosomal genes of C. reinhardii. Cell. 1979 Sep;18(1):55–60. doi: 10.1016/0092-8674(79)90353-2. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Gingrich J. C., Hallick R. B. The Euglena gracilis chloroplast ribulose-1,5-bisphosphate carboxylase gene. II. The spliced mRNA and its product. J Biol Chem. 1985 Dec 25;260(30):16162–16168. [PubMed] [Google Scholar]
- Gold J. C., Spremulli L. L. Euglena gracilis chloroplast initiation factor 2. Identification and initial characterization. J Biol Chem. 1985 Dec 5;260(28):14897–14900. [PubMed] [Google Scholar]
- Goldenberg C. J., Hauser S. D. Accurate and efficient in vitro splicing of purified precursor RNAs specified by early region 2 of the adenovirus 2 genome. Nucleic Acids Res. 1983 Mar 11;11(5):1337–1348. doi: 10.1093/nar/11.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf L., Roux E., Stutz E., Kössel H. Nucleotide sequence of a Euglena gracilis chloroplast gene coding for the 16S rRNA: homologies to E. coli and Zea mays chloroplast 16S rRNA. Nucleic Acids Res. 1982 Oct 25;10(20):6369–6381. doi: 10.1093/nar/10.20.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruissem W., Zurawski G. Analysis of promoter regions for the spinach chloroplast rbcL, atpB and psbA genes. EMBO J. 1985 Dec 16;4(13A):3375–3383. doi: 10.1002/j.1460-2075.1985.tb04093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallick R. B., Chelm B. K., Gray P. W., Orozco E. M., Jr Use of aurintricarboxylic acid as an inhibitor of nucleases during nucleic acid isolation. Nucleic Acids Res. 1977 Sep;4(9):3055–3064. doi: 10.1093/nar/4.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N., Messing J. The making of strand-specific M13 probes. Gene. 1982 Mar;17(3):271–277. doi: 10.1016/0378-1119(82)90143-3. [DOI] [PubMed] [Google Scholar]
- Jaskunas S. R., Lindahl L., Nomura M. Identification of two copies of the gene for the elongation factor EF-Tu in E. coli. Nature. 1975 Oct 9;257(5526):458–462. doi: 10.1038/257458a0. [DOI] [PubMed] [Google Scholar]
- Karabin G. D., Hallick R. B. Euglena gracilis chloroplast transfer RNA transcription units. Nucleotide sequence analysis of a tRNAThr-tRNAGly-tRNAMet-tRNASer-tRNAGln gene cluster. J Biol Chem. 1983 May 10;258(9):5512–5518. [PubMed] [Google Scholar]
- Koller B., Clarke J., Delius H. The structure of precursor mRNAs and of excised intron RNAs in chloroplasts of Euglena gracilis. EMBO J. 1985 Oct;4(10):2445–2450. doi: 10.1002/j.1460-2075.1985.tb03954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller B., Delius H. Intervening sequences in chloroplast genomes. Cell. 1984 Mar;36(3):613–622. doi: 10.1016/0092-8674(84)90341-6. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Manzara T., Hallick R. B. Nucleotide sequence of the Euglena gracilis chloroplast gene for ribosomal protein L20. Nucleic Acids Res. 1987 May 11;15(9):3927–3927. doi: 10.1093/nar/15.9.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mitra G., Warner J. R. A yeast ribosomal protein gene whose intron is in the 5' leader. J Biol Chem. 1984 Jul 25;259(14):9218–9224. [PubMed] [Google Scholar]
- Montandon P. E., Stutz E. Nucleotide sequence of a Euglena gracilis chloroplast genome region coding for the elongation factor Tu; evidence for a spliced mRNA. Nucleic Acids Res. 1983 Sep 10;11(17):5877–5892. doi: 10.1093/nar/11.17.5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon P. E., Stutz E. The genes for the ribosomal proteins S12 and S7 are clustered with the gene for the EF-Tu protein on the chloroplast genome of Euglena gracilis. Nucleic Acids Res. 1984 Mar 26;12(6):2851–2859. doi: 10.1093/nar/12.6.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon P. E., Vasserot A., Stutz E. Euglena gracilis chloroplast DNA: analysis of a 1.6 kb intron of the psb C gene containing an open reading frame of 458 codons. Curr Genet. 1986;11(1):35–39. doi: 10.1007/BF00389423. [DOI] [PubMed] [Google Scholar]
- Müller R., Slamon D. J., Tremblay J. M., Cline M. J., Verma I. M. Differential expression of cellular oncogenes during pre- and postnatal development of the mouse. Nature. 1982 Oct 14;299(5884):640–644. doi: 10.1038/299640a0. [DOI] [PubMed] [Google Scholar]
- Ortiz W., Reardon E. M., Price C. A. Preparation of chloroplasts from euglena highly active in protein synthesis. Plant Physiol. 1980 Aug;66(2):291–294. doi: 10.1104/pp.66.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passavant C. W., Stiegler G. L., Hallick R. B. Location of the single gene for elongation factor Tu on the Euglena gracilis chloroplast chromosome. J Biol Chem. 1983 Jan 25;258(2):693–695. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sagher D., Grosfeld H., Edelman M. Large subunit ribulosebisphosphate carboxylase messenger RNA from Euglena chloroplasts. Proc Natl Acad Sci U S A. 1976 Mar;73(3):722–726. doi: 10.1073/pnas.73.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J. C., Guarente L. The untranslated leader of nuclear COX4 gene of Saccharomyces cerevisiae contains an intron. Nucleic Acids Res. 1987 Apr 24;15(8):3515–3529. doi: 10.1093/nar/15.8.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C., Krainer A., Barry G., Shen W. F., Squires C. L. Nucleotide sequence at the end of the gene for the RNA polymerase beta' subunit (rpoC). Nucleic Acids Res. 1981 Dec 21;9(24):6827–6840. doi: 10.1093/nar/9.24.6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steege D. A., Graves M. C., Spremulli L. L. Euglena gracilis chloroplast small subunit rRNA. Sequence and base pairing potential of the 3' terminus, cleavage by colicin E3. J Biol Chem. 1982 Sep 10;257(17):10430–10439. [PubMed] [Google Scholar]