Abstract
Neurotrophins are initially synthesized as larger precursors (proneurotrophins), which undergo proteolytic cleavage to yield mature forms. Although the functions of the mature neurotrophins have been well established during neural development and in the adult nervous system, roles for the proneurotrophins in developmental and injury-induced cell death, as well as in synaptic plasticity, have only recently been appreciated. Interestingly, both mature neurotrophins and proneurotrophins utilize dual-receptor complexes to mediate their actions. The mature neurotrophin coreceptors consist of the Trk receptor tyrosine kinases and p75NTR, wherein Trk transduces survival and differentiative signaling, and p75NTR modulates the affinity and selectivity of Trk activation. On the other hand, proneurotrophins engage p75NTR and the structurally distinct coreceptor sortilin, to initiate p75NTR-dependent signal transduction cascade. Although the specificity of mature neurotrophins vs. proneurotrophins actions is due in part to the formation of distinct coreceptor complexes, a number of recent studies highlight how different p75NTR-mediated cellular actions are modulated. Here, we review emerging evidence for a novel transmembrane mechanism for ligand-specific p75NTR activation and several mechanisms by which p75NTR-dependent apoptotic and nonapoptotic responses can be selective activated.
Keywords: ProNGF, ProBDNF, neurodegenerative diseases, neural development, neuronal apoptosis
INTRODUCTION
The neurotrophins are a four-member peptide growth factor family that includes nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT-3), and neurotrophin 4 (NT-4), which exhibit well-described actions in the nervous system (Snider, 1994; Lewin and Barde, 1996). Classically defined as target-derived survival factors for developing neuronal populations, roles of the neurotrophins now include growth cone guidance, synaptic modulation, injury protection, and influence on memory and behavior (Glebova and Ginty, 2005; Lu et al., 2005; Zweifel et al., 2005; Schecterson and Bothwell, 2008; Lessmann and Brigadski, 2009). Indeed one of the most intriguing aspects of neurotrophin physiology is that only four neurotrophin genes are found in mammals and yet they appear to modulate a diverse repertoire of critical functions both in and outside of the nervous system.
All neurotrophins, translated from single coding exons, are synthesized as larger precursors (proneurotrophins) of ~30–34 kDa that rapidly associate as noncovalent homodimers (Suter et al., 1991; Kolbeck et al., 1994; Heymach and Shooter, 1995; Ibanez, 2002). Proneurotrophins can be cleaved by furin and proconvertases in the ER and Golgi to produce C-terminal mature neurotrophins (~13 kDa). The prodomains of neurotrophins have been demonstrated to play a critical role in ensuring proper protein folding and dimerization of the nascent molecules(Suter et al., 1991; Heymach and Shooter, 1995), while the mature domains are traditionally viewed as the secreted ligands responsible for neurotrophins’ diverse biological effects. At the molecular level, mature neurotrophins interact with two distinct receptors: p75NTR and Trk (Kaplan and Miller, 2000; Huang and Reichardt, 2003; Segal, 2003; Teng and Hempstead, 2004; Reichardt, 2006). Indeed most neurotrophin actions on neuronal differentiation and survival can be ascribed to this coreceptor system; with Trk receptor tyrosine kinase being the signaling entity and p75NTR serving to restrict and augment ligand recognition specificity.
Unexpectedly, NGF or BDNF activation of p75NTR has been found to induce apoptosis when Trk signaling is absent (Casaccia-Bonnefil et al., 1996; Frade et al., 1996; Bamji et al., 1998). Despite these elegant studies, the ability of neurotrophins to act both as pro-survival and pro-death ligands was perplexing; high concentrations of ligands were required to induce modest levels of cell death in in vitro paradigms (see Casaccia-Bonnefil et al., 1996; Friedman, 2000 for examples), leading to the hypothesis of additional p75NTR ligands that selectively trigger apoptosis. The findings that proNGF and proBDNF selectively bind p75NTR but not Trk receptors to elicit cell killing provided a mechanism by which different forms of neurotrophins can initiate diverse actions (Lee et al., 2001; Ibanez, 2002; Teng et al., 2005). Interestingly a third structurally unrelated receptor, sortilin, specifically recognizes the pro-domains of proNGF and proBDNF, and forms a high affinity coreceptor complex with p75NTR to convey proneurotrophin-induced apoptotic signaling (Nykjaer et al., 2004; Teng et al., 2005; Jansen et al., 2007; Willnow et al., 2008). Thus, the opposing effects of neurotrophins on neuronal survival/death depend on whether proneurotrophins or mature neurotrophins are released, and bind to Trk receptors or p75NTR:sortilin receptor complex to elicit distinct cellular responses (see Fig. 1).
Figure 1.
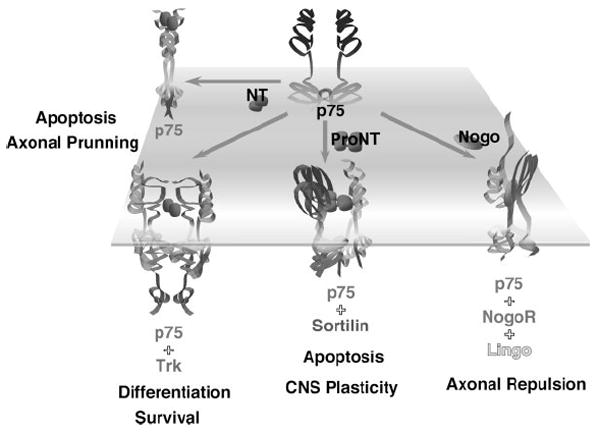
Schematic representation of mature neurotrophins (NT) and proneurotrophin (proNT) actions as well as the diversity of coreceptor interactions. See text for detail discussion.
ROLES OF PRONEUROTROPHINS IN NERVOUS SYSTEM DEVELOPMENT AND FOLLOWING INJURY
Following the initial description of proNGF induced p75NTR-mediated apoptosis in vitro, multiple in vivo models have validated the role of proNGF during periods of naturally occurring developmental cell death and in injury and disease progression (Hempstead, 2009). Building upon the observation that proNGF binds to sortilin in biochemical analysis (Nykjaer et al., 2004), as well as p75NTR and sortilin colocalization in the developing retina by immuno-histological study (Nakamura et al., 2007), genetic ablation of sortilin was found to result in a significant reduction of retinal ganglion cell apoptosis in embryos (Jansen et al., 2007), comparable to that observed in p75NTR- and NGF-null animals (Frade and Barde, 1998, 1999). Somewhat surprisingly, no reduction in sympathetic neuron death in sortilin-deficient mice was observed at early postnatal time points when p75NTR has been shown to be critical for sympathetic neuron elimination (Bamji et al., 1998). Instead, an increase in sympathetic neuron survival in aged (>1-year-old) animals was demonstrated (Jansen et al., 2007), suggesting a causal role for sortilin, and potentially proneurotrophins, in neuronal loss with aging (Bierl and Isaacson, 2007). These findings are intriguing because proNGF levels are elevated in Alzheimer’s Disease patients (Fahnestock et al., 2001; Peng et al., 2004; Pedraza et al., 2005) and more recently, implicated in spongiform encephalomyelopathy (Stoica et al., 2008) and Parkinson’s disease progression (Chen et al., 2008). Better understanding of a mechanistic role for proNGF in slow onset neurodegenerative diseases awaits further experimental validation and identification of pharmacological agents that selectively block proNGF actions. Both p75NTR and proNGF have been clearly implicated in acute neural tissue damage resulting from spinal cord injury (Beattie et al., 2002; Harrington et al., 2004) and seizure models (Volosin et al., 2006, 2008). Significantly, infusion of anti-proNGF antibody improves neuronal survival in these studies. Future studies will be required to determine whether blockade of proNGF:sortilin interactions might be a means to prevent age-associated neuronal loss, as has been proposed for proNGF:p75NTR antagonists (Massa et al., 2006; Hempstead, 2009).
Mature BDNF is well documented to modulate synaptic efficacy in the hippocampus, and neurotransmitter release at the neuromuscular junction (Nagappan and Lu, 2005; Cohen and Greenberg, 2008; Lu et al., 2009; Waterhouse and Xu, 2009). Like NGF, the unprocessed pro-form of BDNF was originally thought to be a precursor with no biological function of its own. However, multiple reports suggest that both proNGF and proBDNF can escape intracellular processing and be secreted from neurons (Lee et al., 2001; Pang et al., 2004; Woo et al., 2005; Bruno and Cuello, 2006; Yang et al., 2009b), although the efficiency of intracellular processing remains a point of controversy (Matsumoto et al., 2008; Yang et al., 2009b). Following secretion, cleavage of proNGF or proBDNF by enzymes in the extracellular milieu, such as matrix metalloproteases or plasmin, can occur, allowing for highly localized activation of receptors on adjacent cells (Pang et al., 2004; Bruno and Cuello, 2006). In contrast to proNGF that induces neuronal apoptosis upon injury, a primary role of proBDNF may be to modulate synaptic efficacy during development (Woo et al., 2005; Yang et al., 2009a). Thus, extracellular conversion of proBDNF to mature BDNF by the tPA/plasmin protease cascade promotes TrkB-dependent late phase-long term potentiation (L-LTP) (Pang et al., 2004). In contrast, application of proBDNF to p75NTR-expressing hippocampal slices enhanced long-term depression (LTD) (Woo et al., 2005), suggesting that proBDNF can also act as an endogenous ligand to directly regulate LTD. Since p75NTR expression is developmentally regulated, with highest expression in early postnatal stages that decreases at later times (Rosch et al., 2005; Woo et al., 2005; Yang et al., 2009b), these studies suggest that different forms of synaptic plasticity may be modulated by BDNF isoforms; with proBDNF enhancing LTD in the p75NTR-expressing neonatal and juvenile hippocampus, whereas intracellular or extracellular conversion to mature BDNF may enhance hippocampal L-LTP in the adult (Lu et al., 2005). More recently, a cleavage-resistant BDNF (CR-proBDNF), encoded by two rare single nucleotide polymorphisms in humans, has been shown to promote cerebellar granule neuron apoptosis and to reduce dendritic spine density in hippocampal neurons in vitro (Koshimizu et al., 2009), suggesting that even among CNS neuron classes, proneurotrophins can elicit cell type-specific responses.
At present, it is unclear how p75NTR can distinguish between proNGF and proBDNF to execute downstream signaling events unique to a given proneurotrophin. In addition, although both secreted proNGF and proBDNF can be internalized by the glia and rereleased as mature neurotrophins (Althaus and Kloppner, 2006; Bergami et al., 2008; Boutilier et al., 2008), the physiological significance of this novel neuron-glia interplay is currently unknown.
RECENT ADVANCES IN UNDERSTANDING p75NTR ACTIVATION
Historically, p75NTR was the first neurotrophin receptor identified (Johnson et al., 1986; Radeke et al., 1987); however, its biological actions and signaling mechanisms remain incompletely defined due in part to the ability of p75NTR to act as a coreceptor for diverse ligands that exhibit distinct biological activities (Dechant and Barde, 2002; Hempstead, 2002; Roux and Barker, 2002; Chao, 2003; Barker, 2004; Gentry et al., 2004; Teng and Hempstead, 2004). p75NTR can interact with Trk receptor kinases to enhance the binding specificity and affinity of mature neurotrophins (Esposito et al., 2001), with NogoR and Lingo-1 to mediate the axonal growth inhibitory effects of CNS myelin (Wang et al., 2002; Mi et al., 2004), with Neuropilin-1 to modulate Semaphorin3A-mediated axonal growth inhibition (Ben-Zvi et al., 2007) and with Ephrin-A for reverse signaling upon Eph receptor activation (Lim et al., 2008). In addition to being the “bridesmaid” to these receptors, p75NTR also acts to trigger apoptosis during development and following injury (Bamji et al., 1998; Majdan et al., 2001; Beattie et al., 2002; Harrington et al., 2004; Pedraza et al., 2005; Volosin et al., 2006, 2008; Jansen et al., 2007), to promote myelination of DRG axons (Chan et al., 2006; Xiao et al., 2009), to induce axonal retraction (Singh et al., 2008) and to modulate synaptic plasticity (Woo et al., 2005; Yang et al., 2009a). How does p75NTR mediate these distinct biological outcomes? A unifying theory has yet to emerge; however, a number of significant advances, summarized below, have begun to shed light on the mechanisms of p75NTR activation.
Structural Insights
Structurally, p75NTR belongs to the tumor necrosis factor receptor (TNFR) superfamily and like other members of this family, does not possess intrinsic catalytic activity (Hempstead, 2002; Bandtlow and Dechant, 2004; Gentry et al., 2004; Hasegawa et al., 2004). Thus, upon activation, p75NTR relies on the recruitment of adaptor protein complexes for downstream actions. However, p75NTR differs from other TNF receptor members in two aspects, the most notable of which is that p75NTR binds dimeric neurotrophins whereas receptors for TNF and Fas-L exist as preformed homotrimers that bind cognate trimeric ligands. Second, the globular intracellular “death domain” of p75NTR does not self-associate in solution (Liepinsh et al., 1997), consistent with the hypothesis that p75NTR is distinct from other TNFRs in its recruitment of downstream effector molecules not shared by the other family members (Schutze et al., 2008; Guicciardi and Gores, 2009).
Although p75NTR can bind to all four neurotrophins, both kinetic and mutagenesis studies have demonstrated that NGF and NT-3 interact with p75NTR distinctively (Rodriguez-Tebar et al., 1992; Urfer et al., 1994; Ryden et al., 1995). To date, the cocrystal structures for p75NTR:NGF and p75NTR:NT-3 have been solved (He and Garcia, 2004; Gong et al., 2008). Surprisingly while the former represents a single p75NTR in complex with dimeric NGF (He and Garcia, 2004), p75NTR binds to NT-3 in a 2:2 stoichiometry (Gong et al., 2008). Biochemical analysis to resolve this discrepancy has suggested that the glycosylation state of p75NTR can influence the stoichiometry of p75NTR:neurotrophin complex (Gong et al., 2008); however, a direct structural comparison of glycosylated vs. nonglycosylated p75NTR in complex with NGF is not yet available for evaluation. The co-crystal structure of NGF with TrkA (Wehrman et al., 2007) reveals a 2:2 stoichiometry; however, the NGF dimer is oriented in an opposite direction as compared to the NGF dimer bound to p75NTR. Thus while p75NTR enhances the affinity of NGF binding to TrkA (Mahadeo et al., 1994), the mechanism by which this occurs is not apparent from the crystal structure data (Barker, 2007), and may reflect interactions between the transmembrane domains of Trk and p75NTR (Esposito et al., 2001).
In addition to the structural analyses, a recent study highlights the dynamic nature of p75NTR clustering and offers intriguing insights into how p75NTR might be activated upon ligand binding. Vilar et al. (2009) have identified an intermolecular disulfide bond utilizing a conserved cysteine residue (Cys257) in the transmembrane domain of p75NTR, as well as additional amino acids (AxxxG266) in the transmembrane domain that maintain a proportion of p75NTR as preformed dimers. Mutagenesis of the G266 to isoleucine results in monomeric p75NTR, while mutation of Cys257 to alanine renders p75NTR incapable of neurotrophin signaling without affecting its dimerization status. Thus, the authors propose a “snail-tong” mechanism for p75NTR activation whereby dimeric ligand binding to predimerized p75NTR, brings about a Cys257-dependent conformational change in the intracellular regions (including the death domain) that permits effector molecule recruitment. Interestingly, the p75NTR Cys257 mutant is still functional in RhoA activation in response to MAG, suggesting that p75NTR may signal through dimeric or monomeric modes of activation. In addition, the dependence of neurotrophin signaling on Cys257 raises the possibility that p75NTR activation can be modulated by “cysteine modifying” cellular redox pathways such as protein S-nitrosylation (Hess et al., 2005; Forrester and Stamler, 2007).
Roles of the Coreceptor Sortilin
Although p75NTR was the first proneurotrophin receptor to be identified (Lee et al., 2001), subsequent studies revealed that proneurotrophins also bind to sortilin via their pro-domains (Nykjaer et al., 2004; Teng et al., 2005). Indeed, interactions of proNGF with a coreceptor complex of p75NTR and sortilin initiate apoptosis in cultured sympathetic neurons and genetic deletion of either sortilin or p75NTR, or inclusion of a sortilin antagonist, neurotensin, impairs proNGF-induced apoptosis (Nykjaer et al., 2004).
Sortilin is a member of the Vps10p domain containing family of proteins that includes sorLA and sorCS1-3 (Petersen et al., 1997; Willnow et al., 2008). It is dynamically regulated in the CNS during development, with expression observed in neuronal precursors beginning at E9.5 and high levels in the cortex, hippocampus, and neural retinal at E14.5. Expression persists in these areas in adulthood and is particularly high in the pyramidal cells of the hippocampus (Hermans-Borgmeyer et al., 1999; Sarret et al., 2003).
Recent elucidation of the crystal structure of the sortilin ectodomain in complex with neurotensin provides some insight into its interactions with diverse binding partners. Quistgaard et al. (2009) observed that sortilin is folded into a novel 10 bladed β propeller and identified neurotensin binding in the tunnel formed by the blades. The structure also reveals two cysteine-rich regions (10CC) that interact extensively with the propeller and two protruding hydrophobic loops, providing possible interactions with hydrophobic patches of receptors, ligands, or with membranes.
Although neurotensin blocks proneurotrophin-induced apoptosis (Nykjaer et al., 2004; Teng et al., 2005), competition assays suggest that the binding site for the proneurotrophins may not precisely overlap that of neurotensin (Quistgaard et al., 2009). This is in agreement with the observation that 10,000-fold molar excess of neurotensin is required to effectively inhibit proNGF-induced apoptosis. The authors suggest that the narrow tunnel restricts access to binding sites such that sortilin interaction with one ligand precludes binding of another. Although the resolution of a sortilin crystal improves our understanding of the sortilin structure and possible functional relationships, several questions are unanswered. These include the possibility that ligand binding alters the conformation of the extracellular domain, and to this end, the structure of the proneurotrophins in a complex with sortilin and p75NTR would be highly desirable. In addition, the Quistgaard model needs to be reconciled with the studies published by Westergaard et al. (2004), which demonstrated that the 10CC domain dictates ligand specificity.
NRH2: Modulator of Proneurotrophin Actions
Prior studies have identified a gene structurally related to p75NTR in mammals, named NRH2 (neurotrophin receptor homolog 2, also termed PLAIDD and NRADD) (Frankowski et al., 2002; Kanning et al., 2003). NRH2 shares sequence homology to p75NTR in the transmembrane and cytoplasmic domains, but contains a unique truncated extracellular domain that does not bind to neurotrophin ligands (Kanning et al., 2003). Although NRH2 lacks a neurotrophin binding domain, it can associate with TrkA receptor and enhances NGF binding to TrkA receptor (Murray et al., 2004). This action is similar to that observed when p75NTR is coexpressed with TrkA to form a high-affinity NGF binding site (Hempstead et al., 1991), suggesting that NRH2 may function in some regards like p75NTR.
Interestingly, NRH2 is expressed in subpopulations of cells in the developing spinal cord, retina, dorsal root ganglion, or by sympathetic neurons (Kanning et al., 2003; Murray et al., 2004), regions where p75NTR and sortilin are coexpressed, and where proneurotrophin-induced cell death has been reported (Sarret et al., 2003; Nykjaer et al., 2004; Domeniconi et al., 2007; Jansen et al., 2007; Nakamura et al., 2007). Recently a novel mechanism for NRH2 in regulating proneurotrophin-induced neuronal death has been described (Kim and Hempstead, 2009), which is selective for NRH2 and is not mediated by p75NTR. In this study, NRH2 was found to interact with sortilin through its intracellular juxtamembrane region, and specifically retargets sortilin to the cell surface (Kim and Hempstead, 2009).
Unlike the other neurotrophin receptors, p75NTR and Trks, which are highly expressed on the cell membrane, sortilin is predominantly intracellular in location, particularly in the trans-Golgi network, endosomes and lysosomes, and less than 10% of the total sortilin pool is localized on the plasma membrane (Willnow et al., 2008). These observations raised the question of whether a cell intrinsic mechanism regulates sortilin localization to the cell surface and thus controls cellular responsiveness to proneurotrophins. Upon expression of NRH2, sortilin relocalizes to the cell surface, and promotes the formation of a dual receptor complex of p75NTR and sortilin, which renders neurons sensitive to the apoptotic actions of proneurotrophins (Kim and Hempstead, 2009). NRH2 expression is also dynamically regulated during development, with increased expression during periods when proneurotrophin-induced apoptosis is robust. Thus, NRH2 may act as a developmentally regulated component that co-opts sortilin from an intracellular trafficking chaperone to become a part of the death receptor complex. In the future, it will be interesting to determine whether NRH2 is upregulated under pathological conditions, similar to the induced expression of p75NTR and proNGF (Beattie et al., 2002; Harrington et al., 2004; Volosin et al., 2006) and how NRH2 expression is regulated during neural development.
Negative Modulator of Nerve Growth
In a series of elegant analyses, Miller and coworkers have previously demonstrated that excess sympathetic neurons in newborn animals are eliminated by BDNF via a p75NTR-dependent mechanism (Bamji et al., 1998; Majdan et al., 2001). In addition to inducing apoptosis, p75NTR activation by neurotrophins can also inhibit axonal growth and induces dendritic retraction (Yamashita et al., 1999, 2002; Zagrebelsky et al., 2005). In a recent study, activity-dependent synthesis and release of BDNF was found to induce p75NTR-dependent axon pruning that occurs in postnatal life, unless TrkA was concomitantly activated by NGF (Singh and Miller, 2005; Singh et al., 2008). These studies provide a model of how the fidelity of neural circuitry can be established by a coordinated mechanism that involves (i) target-derived neurotrophic support to the winning axons and (ii) active elimination of strayed nascent axons. Although the authors did not distinguish whether the in vivo effects on p75NTR-dependent axon pruning were due to secreted mature BDNF or proBDNF, the possible role for proBDNF as the p75NTR ligand that causes axonal retraction was investigated in another recent study. Using the neuromuscular synapse to probe proBDNF actions and function blocking antibody against proBDNF, the work identified target-derived proBDNF as a critical ligand to induce presynaptic terminal retraction (Yang et al., 2009a).
Since mature BDNF does not bind sortilin (Teng et al., 2005) and high concentrations of BDNF are required to observe axonal degeneration in vitro (Singh et al., 2008), it is unclear how mature BDNF triggers p75NTR signaling in vivo to promote pruning. Nevertheless, the dissociation of BDNF from p75NTR is the slowest among the neurotrophins (Rodriguez-Tebar et al., 1992), and it interacts distinctively with p75NTR, based on mutagenesis studies (Ryden et al., 1995); which may provide a mechanism by which mature BDNF signals without a p75NTR coreceptor. Interestingly, BDNF is retrogradedly transported along with p75NTR in sympathetic neurons (Hibbert et al., 2006) where it triggers apoptosis (Bamji et al., 1998; Majdan et al., 2001). Further studies are necessary to understand how BDNF, presented at the axons, selectively activates retrograde p75NTR-dependent transport or mediates localized pruning actions (Glebova and Ginty, 2005; Zweifel et al., 2005; Ibanez, 2007; Deppmann et al., 2008).
It is likely that activation of specific p75NTR effector pathways is modulated by both the coreceptor with which it partners, and by the ligand to which it binds (Dechant and Barde, 2002; Hempstead, 2002; Roux and Barker, 2002; Chao, 2003; Teng and Hempstead, 2004). Prior studies have identified a number of downstream pathways that play critical roles in p75NTR-mediated apoptosis (Gentry et al., 2004), including the stress-induced MAP kinase member JNK (Harrington et al., 2002; Bhakar et al., 2003; Becker et al., 2004), which can be activated by either high concentrations of mature BDNF or lesser amount of proneurotrophins. More recent findings suggest that proneurotrophins and mature BDNF activate γ-secretase mediated cleavage of p75NTR to release its intracellular domain, resulting in the ubiquitination and nuclear translocation of NRIF, a zinc finger transcription factor that binds p75NTR (Linggi et al., 2005; Kenchappa et al., 2006; Volosin et al., 2006, 2008). Although p75NTR cleavage and NRIF nuclear translocation may be required for p75NTR-dependent apoptosis, it is not known whether NRIF transcriptionally activates “pro-apoptotic genes” upon nuclear translocation. In addition, Bertrand et al. (2008) has provided genetic evidence that another p75NTR binding protein NRAGE participates in developmental cell death of the sympathetic ganglia and in neurotrophin-induced JNK activation. However, NRAGE null animals do not fully phenocopy the defects in embryonic development exhibited by the p75NTR and NRIF knockout mice (Casademunt et al., 1999; Bertrand et al., 2008), perhaps reflecting the diverse actions of different p75NTR ligands, or possible interactions of NRAGE with other receptors.
FUTURE CHALLENGES AND PROMISES
Since the discovery of NGF as a growth promoting factor some 50 years ago (Levi-Montalcini and Angeletti, 1968), there have been many advancements in the field including the identification of other neurotrophin family members, their individual roles in neural development and in adulthood, as well as the underlying mechanisms of how neurotrophins signal through different receptors. The unexpected finding that neurotrophins can elicit both pro-survival and pro-death pathways offers new possibilities for therapeutic intervention of acute neuronal injuries and slow onset neurodegenerative diseases. Central to this endeavor will be determining whether apoptotic signaling induced by the proneurotrophins can be selectively attenuated. To this end, the finding that p75NTR can differentiate between different ligands (e.g., BDNF vs. MAG) (Vilar et al., 2009) suggests that a better structural understanding of p75NTR bound to individual ligands, and in complex with sortilin, may lead to promising venues for drug discovery. Thus, an emerging challenge is to identify the in vivo triggers that activate p75NTR-mediated apoptosis under pathological conditions and to differentiate these apoptotic signaling events from those that modulate axonal growth and synaptic plasticity.
Acknowledgments
Contract grant sponsor: NIH/NINDS; contract grant numbers: NS057627, NS30687, NS064114.
References
- Althaus HH, Kloppner S. Mature pig oligodendrocytes rapidly process human recombinant pro-nerve growth factor and do not undergo cell death. J Neurochem. 2006;98:506–517. doi: 10.1111/j.1471-4159.2006.03891.x. [DOI] [PubMed] [Google Scholar]
- Bamji SX, Majdan M, Pozniak CD, Belliveau DJ, Aloyz R, Kohn J, Causing CG, et al. The p75 neurotrophin receptor mediates neuronal apoptosis and is essential for naturally occurring sympathetic neuron death. J Cell Biol. 1998;140:911–923. doi: 10.1083/jcb.140.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandtlow C, Dechant G. From cell death to neuronal regeneration, effects of the p75 neurotrophin receptor depend on interactions with partner subunits. Sci STKE 2004. 2004:pe24. doi: 10.1126/stke.2352004pe24. [DOI] [PubMed] [Google Scholar]
- Barker PA. p75NTR is positively promiscuous: Novel partners and new insights. Neuron. 2004;42:529–533. doi: 10.1016/j.neuron.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Barker PA. High affinity not in the vicinity? Neuron. 2007;53:1–4. doi: 10.1016/j.neuron.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, et al. ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron. 2002;36:375–386. doi: 10.1016/s0896-6273(02)01005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker EB, Howell J, Kodama Y, Barker PA, Bonni A. Characterization of the c-Jun N-terminal kinase-BimEL signaling pathway in neuronal apoptosis. J Neurosci. 2004;24:8762–8770. doi: 10.1523/JNEUROSCI.2953-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zvi A, Ben-Gigi L, Klein H, Behar O. Modulation of semaphorin3A activity by p75 neurotrophin receptor influences peripheral axon patterning. J Neurosci. 2007;27:13000–13011. doi: 10.1523/JNEUROSCI.3373-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergami M, Santi S, Formaggio E, Cagnoli C, Verderio C, Blum R, Berninger B, et al. Uptake and recycling of pro-BDNF for transmitter-induced secretion by cortical astrocytes. J Cell Biol. 2008;183:213–221. doi: 10.1083/jcb.200806137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand MJ, Kenchappa RS, Andrieu D, Leclercq-Smekens M, Nguyen HN, Carter BD, Muscatelli F, et al. NRAGE, a p75NTR adaptor protein, is required for developmental apoptosis in vivo. Cell Death Differ. 2008;15:1921–1929. doi: 10.1038/cdd.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakar AL, Howell JL, Paul CE, Salehi AH, Becker EB, Said F, Bonni A, et al. Apoptosis induced by p75NTR overexpression requires Jun kinase-dependent phosphorylation of Bad. J Neurosci. 2003;23:11373–11381. doi: 10.1523/JNEUROSCI.23-36-11373.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierl MA, Isaacson LG. Increased NGF proforms in aged sympathetic neurons and their targets. Neurobiol Aging. 2007;28:122–134. doi: 10.1016/j.neurobiolaging.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Boutilier J, Ceni C, Pagdala PC, Forgie A, Neet KE, Barker PA. Proneurotrophins require endocytosis and intracellular proteolysis to induce TrkA activation. J Biol Chem. 2008;283:12709–12716. doi: 10.1074/jbc.M710018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno MA, Cuello AC. Activity-dependent release of precursor nerve growth factor, conversion to mature nerve growth factor, and its degradation by a protease cascade. Proc Natl Acad Sci USA. 2006;103:6735–6740. doi: 10.1073/pnas.0510645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaccia-Bonnefil P, Carter BD, Dobrowsky RT, Chao MV. Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature. 1996;383:716–719. doi: 10.1038/383716a0. [DOI] [PubMed] [Google Scholar]
- Casademunt E, Carter BD, Benzel I, Frade JM, Dechant G, Barde YA. The zinc finger protein NRIF interacts with the neurotrophin receptor p75(NTR) and participates in programmed cell death. EMBO J. 1999;18:6050–6061. doi: 10.1093/emboj/18.21.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JR, Jolicoeur C, Yamauchi J, Elliott J, Fawcett JP, Ng BK, Cayouette M. The polarity protein Par-3 directly interacts with p75NTR to regulate myelination. Science. 2006;314:832–836. doi: 10.1126/science.1134069. [DOI] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Chen LW, Yung KK, Chan YS, Shum DK, Bolam JP. The proNGF-p75NTR-sortilin signalling complex as new target for the therapeutic treatment of Parkinson’s disease. CNS Neurol Disord Drug Targets. 2008;7:512–523. doi: 10.2174/187152708787122923. [DOI] [PubMed] [Google Scholar]
- Cohen S, Greenberg ME. Communication between the synapse and the nucleus in neuronal development, plasticity, and disease. Annu Rev Cell Dev Biol. 2008;24:183–209. doi: 10.1146/annurev.cellbio.24.110707.175235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechant G, Barde YA. The neurotrophin receptor p75(NTR): Novel functions and implications for diseases of the nervous system. Nat Neurosci. 2002;5:1131–1136. doi: 10.1038/nn1102-1131. [DOI] [PubMed] [Google Scholar]
- Deppmann CD, Mihalas S, Sharma N, Lonze BE, Niebur E, Ginty DD. A model for neuronal competition during development. Science. 2008;320:369–373. doi: 10.1126/science.1152677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeniconi M, Hempstead BL, Chao MV. Pro-NGF secreted by astrocytes promotes motor neuron cell death. Mol Cell Neurosci. 2007;34:271–279. doi: 10.1016/j.mcn.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito D, Patel P, Stephens RM, Perez P, Chao MV, Kaplan DR, Hempstead BL. The cytoplasmic and transmembrane domains of the p75 and Trk A receptors regulate high affinity binding to nerve growth factor. J Biol Chem. 2001;276:32687–32695. doi: 10.1074/jbc.M011674200. [DOI] [PubMed] [Google Scholar]
- Fahnestock M, Michalski B, Xu B, Coughlin MD. The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer’s disease. Mol Cell Neurosci. 2001;18:210–220. doi: 10.1006/mcne.2001.1016. [DOI] [PubMed] [Google Scholar]
- Forrester MT, Stamler JS. A classification scheme for redox-based modifications of proteins. Am J Respir Cell Mol Biol. 2007;36:135–137. doi: 10.1165/rcmb.2006-001ED. [DOI] [PubMed] [Google Scholar]
- Frade JM, Barde YA. Microglia-derived nerve growth factor causes cell death in the developing retina. Neuron. 1998;20:35–41. doi: 10.1016/s0896-6273(00)80432-8. [DOI] [PubMed] [Google Scholar]
- Frade JM, Barde YA. Genetic evidence for cell death mediated by nerve growth factor and the neurotrophin receptor p75 in the developing mouse retina and spinal cord. Development. 1999;126:683–690. doi: 10.1242/dev.126.4.683. [DOI] [PubMed] [Google Scholar]
- Frade JM, Rodriguez-Tebar A, Barde YA. Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature. 1996;383:166–168. doi: 10.1038/383166a0. [DOI] [PubMed] [Google Scholar]
- Frankowski H, Castro-Obregon S, del Rio G, Rao RV, Bredesen DE. PLAIDD, a type II death domain protein that interacts with p75 neurotrophin receptor. Neuromolecular Med. 2002;1:153–170. doi: 10.1385/NMM:1:3:153. [DOI] [PubMed] [Google Scholar]
- Friedman WJ. Neurotrophins induce death of hippocampal neurons via the p75 receptor. J Neurosci. 2000;20:6340–6346. doi: 10.1523/JNEUROSCI.20-17-06340.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry JJ, Barker PA, Carter BD. The p75 neurotrophin receptor: Multiple interactors and numerous functions. Prog Brain Res. 2004;146:25–39. doi: 10.1016/S0079-6123(03)46002-0. [DOI] [PubMed] [Google Scholar]
- Glebova NO, Ginty DD. Growth and survival signals controlling sympathetic nervous system development. Annu Rev Neurosci. 2005;28:191–222. doi: 10.1146/annurev.neuro.28.061604.135659. [DOI] [PubMed] [Google Scholar]
- Gong Y, Cao P, Yu HJ, Jiang T. Crystal structure of the neurotrophin-3 and p75NTR symmetrical complex. Nature. 2008;454:789–793. doi: 10.1038/nature07089. [DOI] [PubMed] [Google Scholar]
- Guicciardi ME, Gores GJ. Life and death by death receptors. FASEB J. 2009;23:1625–1637. doi: 10.1096/fj.08-111005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington AW, Kim JY, Yoon SO. Activation of Rac GTPase by p75 is necessary for c-jun N-terminal kinase-mediated apoptosis. J Neurosci. 2002;22:156–166. doi: 10.1523/JNEUROSCI.22-01-00156.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington AW, Leiner B, Blechschmitt C, Arevalo JC, Lee R, Morl K, Meyer M, et al. Secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. Proc Natl Acad Sci USA. 2004;101:6226–6230. doi: 10.1073/pnas.0305755101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y, Yamagishi S, Fujitani M, Yamashita T. p75 neurotrophin receptor signaling in the nervous system. Biotechnol Annu Rev. 2004;10:123–149. doi: 10.1016/S1387-2656(04)10005-7. [DOI] [PubMed] [Google Scholar]
- He XL, Garcia KC. Structure of nerve growth factor complexed with the shared neurotrophin receptor p75. Science. 2004;304:870–875. doi: 10.1126/science.1095190. [DOI] [PubMed] [Google Scholar]
- Hempstead BL. The many faces of p75NTR. Curr Opin Neurobiol. 2002;12:260–267. doi: 10.1016/s0959-4388(02)00321-5. [DOI] [PubMed] [Google Scholar]
- Hempstead BL. Commentary: Regulating proNGF action: Multiple targets for therapeutic intervention. Neurotox Res. 2009;16:255–260. doi: 10.1007/s12640-009-9054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempstead BL, Martin-Zanca D, Kaplan DR, Parada LF, Chao MV. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991;350:678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- Hermans-Borgmeyer I, Hermey G, Nykjaer A, Schaller C. Expression of the 100-kDa neurotensin receptor sortilin during mouse embryonal development. Brain Res Mol Brain Res. 1999;65:216–219. doi: 10.1016/s0169-328x(99)00022-4. [DOI] [PubMed] [Google Scholar]
- Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: Purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- Heymach JV, Jr, Shooter EM. The biosynthesis of neurotrophin heterodimers by transfected mammalian cells. J Biol Chem. 1995;270:12297–12304. doi: 10.1074/jbc.270.20.12297. [DOI] [PubMed] [Google Scholar]
- Hibbert AP, Kramer BM, Miller FD, Kaplan DR. The localization, trafficking and retrograde transport of BDNF bound to p75NTR in sympathetic neurons. Mol Cell Neurosci. 2006;32:387–402. doi: 10.1016/j.mcn.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: Roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Ibanez CF. Jekyll-Hyde neurotrophins: The story of proNGF. Trends Neurosci. 2002;25:284–286. doi: 10.1016/s0166-2236(02)02169-0. [DOI] [PubMed] [Google Scholar]
- Ibanez CF. Message in a bottle: Long-range retrograde signaling in the nervous system. Trends Cell Biol. 2007;17:519–528. doi: 10.1016/j.tcb.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Jansen P, Giehl K, Nyengaard JR, Teng K, Lioubinski O, Sjoegaard SS, Breiderhoff T, et al. Roles for the pro-neurotrophin receptor sortilin in neuronal development, aging and brain injury. Nat Neurosci. 2007;10:1449–1457. doi: 10.1038/nn2000. [DOI] [PubMed] [Google Scholar]
- Johnson D, Lanahan A, Buck CR, Sehgal A, Morgan C, Mercer E, Bothwell M, et al. Expression and structure of the human NGF receptor. Cell. 1986;47:545–554. doi: 10.1016/0092-8674(86)90619-7. [DOI] [PubMed] [Google Scholar]
- Kanning KC, Hudson M, Amieux PS, Wiley JC, Bothwell M, Schecterson LC. Proteolytic processing of the p75 neurotrophin receptor and two homologs generates C-terminal fragments with signaling capability. J Neurosci. 2003;23:5425–5436. doi: 10.1523/JNEUROSCI.23-13-05425.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Kenchappa RS, Zampieri N, Chao MV, Barker PA, Teng HK, Hempstead BL, Carter BD. Ligand-dependent cleavage of the P75 neurotrophin receptor is necessary for NRIF nuclear translocation and apoptosis in sympathetic neurons. Neuron. 2006;50:219–232. doi: 10.1016/j.neuron.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Kim T, Hempstead BL. NRH2 is a trafficking switch to regulate sortilin localization and permit proneurotrophin-induced cell death. EMBO J. 2009;28:1612–1623. doi: 10.1038/emboj.2009.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbeck R, Jungbluth S, Barde YA. Characterisation of neurotrophin dimers and monomers. Eur J Biochem. 1994;225:995–1003. doi: 10.1111/j.1432-1033.1994.0995b.x. [DOI] [PubMed] [Google Scholar]
- Koshimizu H, Kiyosue K, Hara T, Hazama S, Suzuki S, Uegaki K, Nagappan G, et al. Multiple functions of precursor BDNF to CNS neurons: Negative regulation of neurite growth, spine formation and cell survival. Mol Brain. 2009;2:27. doi: 10.1186/1756-6606-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Lessmann V, Brigadski T. Mechanisms, locations, and kinetics of synaptic BDNF secretion: An update. Neurosci Res. 2009;65:11–22. doi: 10.1016/j.neures.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R, Angeletti PU. Nerve growth factor. Physiol Rev. 1968;48:534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- Liepinsh E, Ilag LL, Otting G, Ibanez CF. NMR structure of the death domain of the p75 neurotrophin receptor. EMBO J. 1997;16:4999–5005. doi: 10.1093/emboj/16.16.4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YS, McLaughlin T, Sung TC, Santiago A, Lee KF, O’Leary DD. p75(NTR) mediates ephrin-A reverse signaling required for axon repulsion and mapping. Neuron. 2008;59:746–758. doi: 10.1016/j.neuron.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linggi MS, Burke TL, Williams BB, Harrington A, Kraemer R, Hempstead BL, Yoon SO, et al. Neurotrophin receptor interacting factor (NRIF) is an essential mediator of apoptotic signaling by the p75 neurotrophin receptor. J Biol Chem. 2005;280:13801–13808. doi: 10.1074/jbc.M410435200. [DOI] [PubMed] [Google Scholar]
- Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- Lu B, Wang KH, Nose A. Molecular mechanisms underlying neural circuit formation. Curr Opin Neurobiol. 2009;19:162–167. doi: 10.1016/j.conb.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadeo D, Kaplan L, Chao MV, Hempstead BL. High affinity nerve growth factor binding displays a faster rate of association than p140trk binding. Implications for multi-subunit polypeptide receptors. J Biol Chem. 1994;269:6884–6891. [PubMed] [Google Scholar]
- Majdan M, Walsh GS, Aloyz R, Miller FD. TrkA mediates developmental sympathetic neuron survival in vivo by silencing an ongoing p75NTR-mediated death signal. J Cell Biol. 2001;155:1275–1285. doi: 10.1083/jcb.200110017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa SM, Xie Y, Yang T, Harrington AW, Kim ML, Yoon SO, Kraemer R, et al. Small, nonpeptide p75NTR ligands induce survival signaling and inhibit proNGF-induced death. J Neurosci. 2006;26:5288–5300. doi: 10.1523/JNEUROSCI.3547-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Rauskolb S, Polack M, Klose J, Kolbeck R, Korte M, Barde YA. Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat Neurosci. 2008;11:131–133. doi: 10.1038/nn2038. [DOI] [PubMed] [Google Scholar]
- Mi S, Lee X, Shao Z, Thill G, Ji B, Relton J, Levesque M, et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- Murray SS, Perez P, Lee R, Hempstead BL, Chao MV. A novel p75 neurotrophin receptor-related protein, NRH2, regulates nerve growth factor binding to the Trk A receptor. J Neurosci. 2004;24:2742–2749. doi: 10.1523/JNEUROSCI.3960-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagappan G, Lu B. Activity-dependent modulation of the BDNF receptor TrkB: Mechanisms and implications. Trends Neurosci. 2005;28:464–471. doi: 10.1016/j.tins.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Namekata K, Harada C, Harada T. Intracellular sortilin expression pattern regulates proNGFinduced naturally occurring cell death during development. Cell Death Differ. 2007;14:1552–1554. doi: 10.1038/sj.cdd.4402173. [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, et al. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- Pedraza CE, Podlesniy P, Vidal N, Arevalo JC, Lee R, Hempstead B, Ferrer I, et al. Pro-NGF isolated from the human brain affected by Alzheimer’s disease induces neuronal apoptosis mediated by p75NTR. Am J Pathol. 2005;166:533–543. doi: 10.1016/S0002-9440(10)62275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Wuu J, Mufson EJ, Fahnestock M. Increased proNGF levels in subjects with mild cognitive impairment and mild Alzheimer disease. J Neuropathol Exp Neurol. 2004;63:641–649. doi: 10.1093/jnen/63.6.641. [DOI] [PubMed] [Google Scholar]
- Petersen CM, Nielsen MS, Nykjaer A, Jacobsen L, Tommerup N, Rasmussen HH, Roigaard H, et al. Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography. J Biol Chem. 1997;272:3599–3605. doi: 10.1074/jbc.272.6.3599. [DOI] [PubMed] [Google Scholar]
- Quistgaard EM, Madsen P, Groftehauge MK, Nissen P, Petersen CM, Thirup SS. Ligands bind to Sortilin in the tunnel of a ten-bladed β-propeller domain. Nat Struct Mol Biol. 2009;16:96–98. doi: 10.1038/nsmb.1543. [DOI] [PubMed] [Google Scholar]
- Radeke MJ, Misko TP, Hsu C, Herzenberg LA, Shooter EM. Gene transfer and molecular cloning of the rat NGF receptor. Nature. 1987;325:593–597. doi: 10.1038/325593a0. [DOI] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos TransRSoc LondBBiol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Tebar A, Dechant G, Gotz R, Barde YA. Binding of neurotrophin-3 to its neuronal receptors and interactions with nerve growth factor and brain-derived neurotrophic factor. EMBO J. 1992;11:917–922. doi: 10.1002/j.1460-2075.1992.tb05130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosch H, Schweigreiter R, Bonhoeffer T, Barde YA, Korte M. The neurotrophin receptor p75NTR modulates long-term depression and regulates the expression of AMPA receptor subunits in the hippocampus. Proc Natl Acad Sci USA. 2005;102:7362–7367. doi: 10.1073/pnas.0502460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67:203–233. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Ryden M, Murray-Rust J, Glass D, Ilag LL, Trupp M, Yancopoulos GD, McDonald NQ, et al. Functional analysis of mutant neurotrophins deficient in low-affinity binding reveals a role for p75LNGFR in NT-4 signalling. EMBO J. 1995;14:1979–1990. doi: 10.1002/j.1460-2075.1995.tb07190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarret P, Krzywkowski P, Segal L, Nielsen MS, Petersen CM, Mazella J, Stroh T, et al. Distribution of NTS3 receptor/sortilin mRNA and protein in the rat central nervous system. J Comp Neurol. 2003;461:483–505. doi: 10.1002/cne.10708. [DOI] [PubMed] [Google Scholar]
- Schecterson LC, Bothwell M. An all-purpose tool for axon guidance. Sci Signal. 2008;1:pe50. doi: 10.1126/scisignal.147pe50. [DOI] [PubMed] [Google Scholar]
- Schutze S, Tchikov V, Schneider-Brachert W. Regulation of TNFR1 and CD95 signalling by receptor compartmentalization. Nat Rev Mol Cell Biol. 2008;9:655–662. doi: 10.1038/nrm2430. [DOI] [PubMed] [Google Scholar]
- Segal RA. Selectivity in neurotrophin signaling: Theme and variations. Annu Rev Neurosci. 2003;26:299–330. doi: 10.1146/annurev.neuro.26.041002.131421. [DOI] [PubMed] [Google Scholar]
- Singh KK, Miller FD. Activity regulates positive and negative neurotrophin-derived signals to determine axon competition. Neuron. 2005;45:837–845. doi: 10.1016/j.neuron.2005.01.049. [DOI] [PubMed] [Google Scholar]
- Singh KK, Park KJ, Hong EJ, Kramer BM, Greenberg ME, Kaplan DR, Miller FD. Developmental axon pruning mediated by BDNF-p75NTR-dependent axon degeneration. Nat Neurosci. 2008;11:649–658. doi: 10.1038/nn.2114. [DOI] [PubMed] [Google Scholar]
- Snider WD. Functions of the neurotrophins during nervous system development: What the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Stoica G, Lungu G, Kim HT, Wong PK. Up-regulation of pro-nerve growth factor, neurotrophin receptor p75, and sortilin is associated with retrovirus-induced spongiform encephalomyelopathy. Brain Res. 2008;1208:204–216. doi: 10.1016/j.brainres.2008.02.085. [DOI] [PubMed] [Google Scholar]
- Suter U, Heymach JV, Jr, Shooter EM. Two conserved domains in the NGF propeptide are necessary and sufficient for the biosynthesis of correctly processed and biologically active NGF. EMBO J. 1991;10:2395–2400. doi: 10.1002/j.1460-2075.1991.tb07778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng KK, Hempstead BL. Neurotrophins and their receptors: Signaling trios in complex biological systems. Cell Mol Life Sci. 2004;61:35–48. doi: 10.1007/s00018-003-3099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urfer R, Tsoulfas P, Soppet D, Escandon E, Parada LF, Presta LG. The binding epitopes of neurotrophin-3 to its receptors trkC and gp75 and the design of a multifunctional human neurotrophin. EMBO J. 1994;13:5896–5909. doi: 10.1002/j.1460-2075.1994.tb06935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilar M, Charalampopoulos I, Kenchappa RS, Simi A, Karaca E, Reversi A, Choi S, et al. Activation of the p75 neurotrophin receptor through conformational rearrangement of disulphide-linked receptor dimers. Neuron. 2009;62:72–83. doi: 10.1016/j.neuron.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volosin M, Song W, Almeida RD, Kaplan DR, Hempstead BL, Friedman WJ. Interaction of survival and death signaling in basal forebrain neurons: Roles of neurotrophins and proneurotrophins. J Neurosci. 2006;26:7756–7766. doi: 10.1523/JNEUROSCI.1560-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volosin M, Trotter C, Cragnolini A, Kenchappa RS, Light M, Hempstead BL, Carter BD, et al. Induction of proneurotrophins and activation of p75NTR-mediated apoptosis via neurotrophin receptor-interacting factor in hippocampal neurons after seizures. J Neurosci. 2008;28:9870–9879. doi: 10.1523/JNEUROSCI.2841-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- Waterhouse EG, Xu B. New insights into the role of brain-derived neurotrophic factor in synaptic plasticity. Mol Cell Neurosci. 2009;42:81–89. doi: 10.1016/j.mcn.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrman T, He X, Raab B, Dukipatti A, Blau H, Garcia KC. Structural and mechanistic insights into nerve growth factor interactions with the TrkA and p75 receptors. Neuron. 2007;53:25–38. doi: 10.1016/j.neuron.2006.09.034. [DOI] [PubMed] [Google Scholar]
- Westergaard UB, Sorensen ES, Hermey G, Nielsen MS, Nykjaer A, Kirkegaard K, Jacobsen C, et al. Functional organization of the sortilin Vps10p domain. J Biol Chem. 2004;279:50221–50229. doi: 10.1074/jbc.M408873200. [DOI] [PubMed] [Google Scholar]
- Willnow TE, Petersen CM, Nykjaer A. VPS10P-domain receptors—Regulators of neuronal viability and function. Nat Rev Neurosci. 2008;9:899–909. doi: 10.1038/nrn2516. [DOI] [PubMed] [Google Scholar]
- Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, et al. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- Xiao J, Wong AW, Willingham MM, Kaasinen SK, Hendry IA, Howitt J, Putz U, et al. BDNF exerts contrasting effects on peripheral myelination of NGF-dependent and BDNF-dependent DRG neurons. J Neurosci. 2009;29:4016–4022. doi: 10.1523/JNEUROSCI.3811-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Higuchi H, Tohyama M. The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho. J Cell Biol. 2002;157:565–570. doi: 10.1083/jcb.200202010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Tucker KL, Barde YA. Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron. 1999;24:585–593. doi: 10.1016/s0896-6273(00)81114-9. [DOI] [PubMed] [Google Scholar]
- Yang F, Je HS, Ji Y, Nagappan G, Hempstead B, Lu B. Pro-BDNF-induced synaptic depression and retraction at developing neuromuscular synapses. J Cell Biol. 2009a;185:727–741. doi: 10.1083/jcb.200811147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Siao CJ, Nagappan G, Marinic T, Jing D, McGrath K, Chen ZY, et al. Neuronal release of proBDNF. Nat Neurosci. 2009b;12:113–115. doi: 10.1038/nn.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagrebelsky M, Holz A, Dechant G, Barde YA, Bonhoeffer T, Korte M. The p75 neurotrophin receptor negatively modulates dendrite complexity and spine density in hippocampal neurons. J Neurosci. 2005;25:9989–9999. doi: 10.1523/JNEUROSCI.2492-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Kuruvilla R, Ginty DD. Functions and mechanisms of retrograde neurotrophin signalling. Nat Rev Neurosci. 2005;6:615–625. doi: 10.1038/nrn1727. [DOI] [PubMed] [Google Scholar]


