Abstract
Objective
To investigate the incidence of noncardiac vascular disease in patients with rheumatoid arthritis (RA) and its relationship to systemic extraarticular disease in a community-based cohort.
Methods
A retrospective medical record review of 609 patients with incident RA diagnosed during 1955–1994 was carried out in Olmsted County, Minnesota. Patients were followed up from 1955 to 2000 (median followup 11.8 years). Incident noncardiac vascular disease and severe extraarticular RA manifestations (including pericarditis, pleuritis, and vasculitis) were recorded according to predefined criteria, and incidence rates were estimated. Using Cox proportional hazards models, the risk (hazard ratio [HR]) of developing vascular events was assessed in patients with and without severe extraarticular RA.
Results
Cerebrovascular and peripheral arterial events occurred in 139 patients (22.8%). The 30-year cumulative incidence rates of peripheral arterial events, cerebrovascular events, and venous thromboembolic events were estimated to be 19.6%, 21.6%, and 7.2%, respectively. The presence of severe extraarticular RA manifestations was found to be associated with all subgroups of noncardiac vascular disease except cerebrovascular disease alone (HR 2.3, 95% confidence interval [95% CI] 1.2–4.3 for peripheral arterial events; HR 3.7, 95% CI 1.3–10.3 for venous thromboembolic events; HR 1.5, 95% CI 0.7–3.2 for cerebrovascular events) after adjusting for age, sex, body mass index, smoking, and rheumatoid factor status.
Conclusion
This is the first study to assess the incidence of noncardiac vascular disease in RA. Severe extraarticular RA was associated with all forms of noncardiac vascular disease except cerebrovascular disease alone. Similar to cardiac disease, the excess risk of noncardiac vascular disease in RA is likely to be related, in part, to the systemic inflammation associated with the extraarticular manifestations of RA.
Rheumatoid arthritis (RA) is a systemic inflammatory disease with various extraarticular manifestations. Apart from leading to severe disability in many cases, it is also associated with an increased mortality, some of which is due to the 2-fold increased risk of cardiovascular disease in RA (1–9). The reasons for increased mortality from cardiovascular causes are not fully known. RA itself seems to represent an independent risk factor for development of accelerated atherosclerosis, supported by the finding that even patients with early seropositive arthritis are at increased risk for premature cardiovascular-related mortality (3,8). This increased risk may be attributable both to disease-related factors, including the presence of systemic inflammatory disease and the concomitant vascular endothelial activation from immune dysregulation and inflammation, and to treatment-related factors, such as accelerated atherosclerotic disease associated with the use of corticosteroids (6,10–12).
Extraarticular manifestations of the systemic disease can occur in up to 46% of patients with RA over a 30-year period from disease onset (13). A subset of patients may develop severe extraarticular RA, with manifestations such as pericarditis, pleuritis, scleritis, vasculitis, and Felty’s syndrome. The incidence rate of severe extraarticular manifestations in patients with RA has been estimated to be 1 per 100 person-years at risk (4). Patients with severe extraarticular RA have a significantly increased risk of premature mortality compared with those without extraarticular RA (13–18). It has been suggested that this excess mortality is mainly due to cardiovascular disease (14).
The occurrence of cardiovascular disease in RA has been well studied in several different populations (1,5–7,9,11,12), but little is known about the impact of extraarticular manifestations on cardiovascular comorbidity. The purpose of this study was to investigate the incidence of noncardiac vascular disease in RA and its relationship to systemic extraarticular disease in a community-based cohort of patients with RA.
PATIENTS AND METHODS
The population of Rochester, Minnesota is well suited for an investigation of the epidemiology of RA and associated extraarticular features because comprehensive medical records on all residents seeking medical care are available. A record linkage system allows ready access to the medical records from all health care providers for the local population, including the Mayo Clinic and its affiliated hospitals, the Olmsted Medical Group, the Olmsted Community Hospital, local nursing homes, and the few private practitioners. The potential of this data system for use in population-based studies has been described previously (19,20). This system ensures virtually complete ascertainment of all clinically recognized cases of RA among the residents of Rochester, Minnesota.
In previous surveys (21,22), all diagnosed cases of RA among subjects ages ≥18 years between January 1, 1955 and December 31, 1994 (n = 609) were identified using the computerized diagnostic index and a review of the complete medical record in each potential case, as previously described (22). The incidence date was defined as the earliest date at which the patient fulfilled at least 4 of the 7 American College of Rheumatology (formerly, the American Rheumatism Association) 1987 classification criteria for RA (23). The population of the area was 92,000 in 1980, and the overall incidence of RA during the study period has been estimated to be 44.6 in 100,000, with a progressive decline in the RA incidence rate (22). The occurrence of extraarticular manifestations of RA in this cohort was assessed using a structured protocol as previously described (4,13). For patients who moved away from the area, the date of the last physical examination noted in the case record was used as the date at which the patient was lost to followup. In a previous study using the same cohort of patients, the date of death according to the death certificate was already noted (13).
To further characterize vascular morbidity in this cohort of patients with RA, the date of first occurrence of noncardiac vascular disease after the diagnosis of RA was recorded. Noncardiac vascular disease included hemorrhagic stroke, nonhemorrhagic or nonspecified stroke, transient ischemic attack (TIA), amaurosis fugax, aortic aneurysm, renal artery stenosis (RAS), peripheral vascular disease (PVD) or atherosclerosis obliterans (ASO), arterial thromboembolism, deep vein thrombosis (DVT), and pulmonary embolism, as determined by satisfaction of the criteria for each disease entity (as set forth in Table 1) upon review of the complete medical record. Disease prevalence was assessed by recording those cases in which a vascular disease event occurred prior to the diagnosis of RA. Prevalent cases were excluded from the incidence analyses. For verification of completeness of the abstracted records, the records of 280 of the 609 patients initially reviewed (by KPL and KVL) were reviewed a second time by different investigators (ELM and CT), with concordance in 96% of the abstracted data. Discrepancies were resolved through a consensus discussion.
The cumulative incidence of noncardiac vascular disease was estimated using Kaplan-Meier methods, and results are presented as the 30-year cumulative incidence rate. In addition, the cumulative incidence of related diagnoses of vascular disease entities was estimated, including cerebrovascular and peripheral arterial events (events 1–8 in Table 1), venous thromboembolic phenomena (events 9 and 10 in Table 1), cerebrovascular events (events 1–4 in Table 1), and peripheral arterial events (events 5–8 in Table 1). In addition, the 15-year cumulative incidence of noncardiac vascular disease by decade of RA diagnosis (i.e., 1955–1964, 1965–1974, 1975–1984, and 1985–1994) was estimated using Kaplan-Meier methods. All rates were adjusted for the competing risk of death (24).
Noncardiac vascular disease–free survival curves were estimated using the Kaplan-Meier product limit method (25), with allowance for transferral of patients from the non–extraarticular RA group to the mild or severe extraarticular RA group. This was done for all patients with RA, for patients with severe extraarticular RA as previously defined (pericarditis, pleuritis, Felty’s syndrome, major cutaneous or other organ vasculitis, neuropathy, scleritis, episcleritis, retinal vasculitis, and glomerulonephritis), and for patients with mild extraarticular RA as previously defined (amyloidosis, kerato-conjunctivitis sicca, xerostomia, secondary Sjögren’s syndrome, pulmonary fibrosis, bronchiolitis obliterans organizing pneumonia, cervical myelopathy, subcutaneous rheumatoid nodules, and rheumatoid nodules in other locations) (4,14). This allowed for comparison of vascular morbidity between patients with non–extraarticular RA and those patients with severe extraarticular RA and mild extraarticular RA, incorporating the time-varying nature of extraarticular RA manifestations. The distinction between severe and mild extraarticular RA manifestations was based on epidemiologic and pathogenetic concepts as previously discussed (4,26).
To control for other potential confounding variables, Cox proportional hazards models of the time to development of noncardiac vascular disease were used. The presence of extraarticular RA manifestations was treated as a time-dependent variable in both the unadjusted models and the models controlling for age, sex, smoking history at the time of RA diagnosis (ever versus never smokers), body mass index (BMI), and rheumatoid factor (RF) status at RA diagnosis. Patients were followed up until onset of noncardiac vascular disease, death, or loss to followup.
RESULTS
The RA cohort consisted of 609 patients with RA who first received their diagnosis between January 1, 1955 and December 31, 1994. The patients, ages 18 years or older, were residents of Rochester, Minnesota. There were 445 women and 164 men. The median age at diagnosis was 58.1 years. The median followup period was 11.8 years (range 0.1–42.8 years, interquartile range 6.8–18.9 years). Extraarticular RA manifestations occurred in 247 patients (30-year cumulative incidence 46.0%), and 78 patients developed severe extraarticular RA (30-year cumulative incidence 16.7%) (13). During the followup period to December 31, 2000, a total of 139 patients (22.8%) developed cerebrovascular and peripheral arterial events, including hemorrhagic stroke, nonhemorrhagic stroke, TIA, amaurosis fugax, aortic aneurysm, RAS, PVD or ASO, and arterial thromboembolism (events 1–8 in Tables 1 and 2). Notably, 32 patients (5.3%) had a vascular event prior to the diagnosis of RA and were therefore removed from the incidence analysis. Similarly, prevalent cases were removed from the incidence analysis for each individual vascular disease entity and combination of entities (Table 2).
Table 1.
Criteria for inclusion in the study of noncardiac vascular disease in patients with rheumatoid arthritis*
| 1. Hemorrhagic stroke | Clinical diagnosis + verified by CT/MRI or autopsy or cerebrospinal fluid analysis |
| 2. Nonhemorrhagic or nonspecified stroke | Clinical diagnosis by neurologist; clinical diagnosis + verified by CT/MRI or autopsy |
| 3. Transient ischemic attack | Clinical diagnosis |
| 4. Amaurosis fugax | Clinical diagnosis |
| 5. Aortic aneurysm | Diameter increased >50% compared with normal values; diameter ≥3.0 cm in abdominal aorta (38); verified by ultrasound/CT or at autopsy |
| 6. Renal artery stenosis | Verified by ultrasound/renal scintigraphy/angiography; assumed clinically significant if detected |
| 7. Peripheral vascular disease or atherosclerosis obliterans | Clinical diagnosis (supported by documented vascular physical examination); ankle/brachial index <0.9 or angiography confirming disease, if performed |
| 8. Arterial thromboembolism | Clinical diagnosis supported by angiography or autopsy |
| 9. Deep vein thrombosis | Verified by phlebography/venography or ultrasound or autopsy |
| 10. Pulmonary embolism | Verified by angiography, CT, scintigraphy, or autopsy |
CT = computed tomography; MRI = magnetic resonance imaging.
Table 2.
Cumulative incidence rates of noncardiac vascular disease events over 30 years in patients with rheumatoid arthritis (RA)*
| Event | Prior to RA diagnosis, no. (%)† | After RA diagnosis, no. | 30-year cumulative incidence |
|---|---|---|---|
| 1. Hemorrhagic stroke | 0 | 18 | 4.3 |
| 2. Nonhemorrhagic or nonspecified stroke | 11 (1.8) | 68 | 15.8 |
| 3. Transient ischemic attack | 5 (0.8) | 28 | 6.9 |
| 4. Amaurosis fugax | 2 (0.3) | 7 | 1.5 |
| 5. Aortic aneurysm | 3 (0.5) | 18 | 4.0 |
| 6. Renal artery stenosis | 1 (0.2) | 4 | 1.0 |
| 7. Peripheral vascular disease or atherosclerosis obliterans | 15 (2.5) | 68 | 16.1 |
| 8. Arterial thromboembolism | 1 (0.2) | 14 | 3.1 |
| 9. Deep vein thrombosis | 1 (0.2) | 21 | 4.9 |
| 10. Pulmonary embolism | 2 (0.3) | 17 | 3.8 |
| Cerebrovascular events (events 1–4 above) | 15 (2.5) | 92 | 21.6 |
| Peripheral arterial events (events 5–8 above) | 19 (3.1) | 84 | 19.6 |
| Venous thromboembolic events (events 9 and 10 above) | 3 (0.5) | 31 | 7.2 |
| Cerebrovascular and peripheral arterial events (events 1–8 above) | 32 (5.3) | 139 | 32.2 |
All rates are adjusted for the competing risk of death. First event is used in the grouped events.
Excluded from the incidence analyses.
The most common incident vascular disease entities in the cohort were nonhemorrhagic or nonspecified strokes (n = 68) and PVD or ASO (n = 68). Other vascular disease entities, in descending frequency, were TIA (n = 28), DVT (n = 21), hemorrhagic stroke (n = 18), aortic aneurysm (n = 18), pulmonary embolism (n = 17), arterial thromboembolism (n = 14), amaurosis fugax (n = 7), and RAS (n = 4). The 30-year cumulative incidence of cerebrovascular events, comprising hemorrhagic stroke, nonhemorrhagic or nonspecific stroke, TIA, and amaurosis fugax, was estimated to be 21.6%. The 30-year cumulative incidence of peripheral arterial events, comprising aortic aneurysm, RAS, PVD or ASO, and arterial thromboembolism, was estimated to be 19.6%, and that of venous thromboembolic events, comprising DVT and pulmonary embolism, was estimated to be 7.2%. The combined 30-year cumulative incidence of cerebrovascular and peripheral arterial events was 32.2%. The prevalence of individual disease entities followed a pattern similar to that of the cumulative incidences.
There was a trend toward a higher incidence of noncardiac vascular disease for those diagnosed later during the study period, but it did not reach statistical significance. We observed that the 15-year cumulative incidence of cerebrovascular or peripheral arterial events was 10.4% for those with RA onset in 1955–1964, 15.1% for those with disease onset in 1965–1974, 14.5% for those with disease onset in 1975–1984, and 25.7% for those with disease onset in 1985–1994 (P = 0.11). Similarly, there was no significant difference in the incidence, by decade, of venous thromboembolic events, cerebrovascular events, or peripheral arterial events, when analyzed separately (data not shown).
Unadjusted Cox proportional hazards models were used to assess the influence of all potential confounding variables on noncardiac vascular disease. In the unadjusted analysis, male sex (hazard ratio [HR] 1.60, 95% confidence interval [95% CI] 1.02–2.51) and being a smoker at the time of RA diagnosis (HR 1.89, 95% CI 1.17–3.05) were significant predictors of peripheral arterial disease (events 5–8 in Table 1), but these variables were not predictive of cerebrovascular events or venous thromboembolic events. BMI and presence of RF were not significantly associated with the risk of noncardiac vascular disease in this cohort (data not shown). As expected, with increasing age, increased numbers of patients developed cerebrovascular and peripheral arterial events (HR 1.07/year, 95% CI 1.05–1.08); the effect of age was similar among all noncardiac vascular disease subgroups.
Table 3 summarizes the results of the unadjusted and multivariable Cox proportional hazards model analyses of the effect of severe extraarticular RA on the risk of all cerebrovascular and peripheral arterial events, as well as peripheral arterial events, cerebrovascular events, and venous thromboembolic events alone.
Table 3.
Multivariable analysis of severe extraarticular disease manifestations as predictors of various groups of noncardiac vascular disease events*
| Group of noncardiac vascular disease events† | Unadjusted analysis |
Adjusted for age and sex |
Adjusted for age, sex, BMI, smoking, and RF status |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P | Hazard ratio | 95% CI | P | Hazard ratio | 95% CI | P | |
| Cerebrovascular and peripheral arterial events | 1.91 | 1.14–3.19 | 0.014 | 2.30 | 1.37–3.85 | 0.0016 | 2.13 | 1.26–3.62 | 0.0049 |
| Peripheral arterial events | 2.35 | 1.26–4.37 | 0.007 | 2.99 | 1.59–5.59 | 0.0006 | 2.29 | 1.20–4.34 | 0.012 |
| Cerebrovascular events | 1.27 | 0.61–2.63 | 0.53 | 1.50 | 0.72–3.12 | 0.28 | 1.52 | 0.72–3.21 | 0.27 |
| Venous thromboembolic events | 2.70 | 1.02–7.11 | 0.045 | 3.56 | 1.33–9.55 | 0.012 | 3.68 | 1.32–10.25 | 0.013 |
95% CI = 95% confidence interval; BMI = body mass index; RF = rheumatoid factor.
Cerebrovascular and peripheral arterial events combined comprise events 1–8 in Table 1, peripheral arterial events comprise events 5–8 in Table 1, cerebrovascular events comprise events 1–4 in Table 1, and venous thromboembolic events comprise events 9 and 10 in Table 1.
In the unadjusted analysis, presence of severe extraarticular RA was predictive of all cerebrovascular and peripheral arterial events, with an HR of 1.91 (95% CI 1.14–3.19). After adjusting for age and sex, the presence of severe extraarticular RA continued to be a significant predictor (HR 2.30, 95% CI 1.37–3.85). After adjusting for age, sex, BMI, smoking exposure, and RF status, the presence of severe extraarticular RA was still strongly associated with the risk of cerebrovascular and peripheral arterial events (HR 2.13, 95% CI 1.26–3.62).
The presence of severe extraarticular RA was also a predictor of peripheral arterial events alone, with an HR of 2.35 (95% CI 1.26–4.37) in unadjusted analyses, an age- and sex-adjusted HR of 2.99 (95% CI 1.59–5.59), and an HR (adjusted for age, sex, BMI, smoking exposure, and RF status) of 2.29 (95% CI 1.20–4.34). Furthermore, the presence of severe extraarticular RA was a significant predictor of venous thromboembolic events in the unadjusted analysis (HR 2.70, 95% CI 1.02–7.11), in the analysis adjusted for age and sex (HR 3.56, 95% CI 1.33–9.55), and after adjusting for age, sex, BMI, smoking exposure, and RF status (HR 3.68, 95% CI 1.32–10.25). The presence of severe extraarticular RA was not a significant predictor of cerebrovascular events alone in the unadjusted analysis or in the multivariable models (Table 3). This was the only subgroup of noncardiac vascular events that was not significantly associated with the presence of severe extraarticular RA.
In the fully adjusted model, smoking was a significant predictor of peripheral arterial events (HR 2.63, 95% CI 1.52–4.56); this was the only subgroup of noncardiac vascular events in which smoking proved to be a significant predictor. In addition, male sex was a predictor of peripheral arterial events (HR 1.64, 95% CI 1.04–2.58) in the model adjusted for age and extraarticular RA, but not in the fully adjusted model. Age was a highly significant predictor of all noncardiac vascular events, regardless of the presence of covariates.
Figure 1 shows the disease-free survival curves for those patients with mild extraarticular RA, those with severe extraarticular RA, and those without extraarticular RA in relation to the potential risk of developing each subgroup of noncardiac vascular disease (cerebrovascular and peripheral arterial events, venous thromboembolic events, cerebrovascular events, and peripheral arterial events). Notably, patients with severe extraarticular RA, as compared with those with mild extraarticular RA and those without extraarticular RA, had worse rates of disease-free survival for every vascular disease subgroup except cerebrovascular events.
Figure 1.
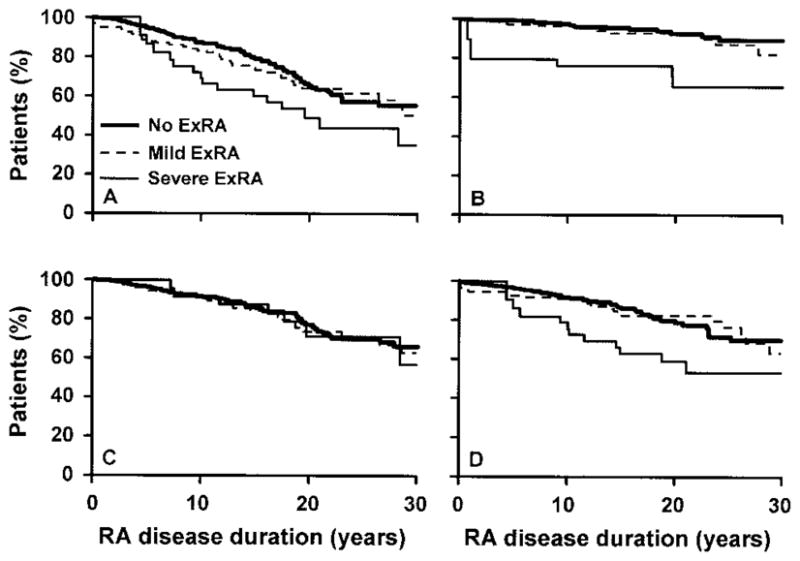
Survival curves in relation to the development of noncardiac vascular disease in patients with mild extraarticular rheumatoid arthritis (ExRA) or severe extraarticular RA as compared with those without extraarticular RA. A, Survival free of cerebrovascular and peripheral arterial disease (events 1–8 in Table 1). B, Survival free of venous thromboembolic events (events 9 and 10 in Table 1). C, Survival free of cerebrovascular disease (events 1–4 in Table 1). D, Survival free of peripheral arterial disease (events 5–8 in Table 1). The presence of severe extraarticular RA was associated with worse disease-free survival for all noncardiac vascular disease subgroups except cerebrovascular disease.
DISCUSSION
In this community-based cohort study of RA, which included patients with incident RA from 1955 to 1994, the 30-year cumulative incidence of cerebrovascular and peripheral arterial disease was estimated to be 32.2% and that of venous thromboembolic disease was estimated to be 7.2%. In this same cohort of patients, previous studies have shown the 30-year cumulative incidence of any extraarticular disease manifestation to be 46.0% and that of severe extraarticular disease to be 16.7% (13,14). The presence of severe extraarticular RA was a predictor of all noncardiac vascular disease subtypes, except for cerebrovascular disease alone. Male sex and being a smoker at RA diagnosis were predictors of peripheral arterial disease. Severe extraarticular RA was a statistically significant predictor of peripheral arterial events and venous thromboembolic events in multivariable models adjusted for age, sex, BMI, smoking exposure, and RF status, indicating that the association between severe extraarticular RA and vascular morbidity was not explained by these factors. We were unable to assess the impact of antirheumatic treatment on vascular morbidity, but the lack of decline in the incidence of vascular disease over time suggests that changing practices during the study period did not reduce the risk of vascular complications.
Patients with RA, and particularly those with extraarticular RA, have an increased mortality due to cardiovascular disease (3,8,14,22). In patients with severe extraarticular RA, an increased mortality specifically due to heart disease has been suggested (14), and data from studies of larger patient samples indicate an increased mortality (27) and morbidity (28) from cardiovascular disease in this subset. This is the first study to indicate that severe extraarticular RA is associated with an increase in noncardiac vascular morbidities.
Prior to this study, there have been few studies assessing the relationship between noncardiac vascular disease and RA. Watson et al recently examined the incidence of all-cause mortality, myocardial infarction, sudden/unknown cause of death, cerebrovascular events, vascular death, and all vascular events among patients with RA 40 years or older in the UK General Practice Research Database (8). Patients with RA had a higher age- and sex-adjusted incidence of all-cause mortality and of major vascular events during almost 5 years of followup compared with patients with osteoarthritis (OA) and patients with neither RA nor OA.
Review of the medical literature provides estimates of rates of noncardiac vascular disease in various populations, which may serve as a framework from which to compare the incidence and prevalence rates with those reported in our RA cohort. The incidence of DVT and pulmonary embolism in Olmsted County, Minnesota during the 25-year period from 1966 through 1990 has been studied, using the same data resources of the Rochester Epidemiology Project as used in our study (29). The overall average age- and sex-adjusted annual incidence of venous thromboembolism was 117 per 100,000. This would correspond to an average 30-year cumulative incidence of venous thromboembolic events of 3.5%, which is lower than the 30-year cumulative incidence of venous thromboembolic events of 7.2% found in our study of RA patients.
The prevalence of peripheral arterial disease was studied in a multicenter, cross-sectional study of 350 local primary care practices in the United States from June to October 1999 (30). That study included 6,979 patients ages 70 years or older or ages 50–69 years with a history of smoking or diabetes. In this large at-risk population, the prevalence of clinically recognized peripheral arterial disease was 14.9%, which is lower than the 30-year cumulative incidence of peripheral arterial disease of 19.6% in our study.
Epidemiologic investigations of stroke in the late twentieth century, including a review of 15 population-based stroke incidence studies, has recently been reported by Feigin et al (31). The age-standardized incidence of total stroke (including both ischemic and hemorrhagic strokes) per 1,000 person-years for subjects ages 55 years or older was in the range of 4.2–6.5 in 12 of the 15 studies. This corresponds to a 30-year cumulative incidence of 12.6–19.5% for total stroke, which is slightly lower than the 21.6% cumulative incidence of cerebrovascular events found in our study of RA patients.
The basis of the increased risk of vascular disease in patients with RA and other rheumatic conditions is not completely understood. However, accumulating evidence indicates that inflammation is important in the pathogenesis of atherosclerosis. Inflammatory biomarkers, such as elevated levels of high-sensitivity C-reactive protein, are predictive of the occurrence of myocardial infarction (32,33) and also of peripheral vascular disease (34) in the general population. In patients with RA, a higher erythrocyte sedimentation rate has been reported to be predictive of cardiovascular comorbidity (35). Suggested shared disease mechanisms in severe extraarticular RA and cardiovascular disease include T cell abnormalities (36) and systemic endothelial activation (37). The relationship between systemic rheumatic disease and atherosclerotic and thromboembolic vascular disorders should be further studied.
Assessment of vascular disease in this study relied on a combination of clinical and imaging data. The clinical examinations and imaging studies were not performed to systematically screen patients with RA for the presence of these noncardiac vascular conditions. The retrospective medical record review is likely to have resulted in a conservative estimate of incidence rates. Although it is possible that detection was biased toward the presence of extraarticular RA, we consider it unlikely. If these patients had more severe disease, they would have received medical attention more frequently; however, we are unable to support or refute this contention. Finally, we did not directly compare rates of noncardiac vascular disease in these patients with those in patients without RA.
Based on our findings, we suggest that some of the increased risk of vascular disease in RA is attributable to disease-related factors, particularly severe systemic disease. Severe extraarticular disease in RA has been shown to be associated with accelerated atherosclerosis (11), which is likely to be the result of an increase in inflammatory markers, activation of T cells, and possibly steroid usage (6). Traditional risk factors such as smoking, hyperlipidemia, elevated homocysteine, hypertension, and diabetes may play a concomitant role in exacerbating vascular disease in RA patients. However, our study suggests that even after adjusting for smoking at RA onset, obesity, and age, the presence of severe extraarticular RA is an independent risk factor for the development of noncardiac vascular disease, excluding cerebrovascular events. Since we did not adjust for other cardiovascular risk factors, we cannot exclude the possibility that unequal distribution of factors such as diabetes and hypertension or differences in total smoking exposure in patients with and without extraarticular RA influenced our results. Further studies that would include these factors and the effect of treatment are necessary. In addition, further analyses to determine the impact of noncardiac vascular disease on mortality in patients with extraarticular RA are indicated.
Acknowledgments
Supported in part by the Swedish Rheumatism Association, Lund University, the NIH, the USPHS (grant AR-30582), and the Mayo Clinic.
We are grateful to Dr. Sherine E. Gabriel for her support and advice regarding this study.
References
- 1.Gabriel SE, Crowson CS, Kremers HM, Doran MF, Turesson C, O’Fallon WM, et al. Survival in rheumatoid arthritis: a population-based analysis of trends over 40 years. Arthritis Rheum. 2003;48:54–8. doi: 10.1002/art.10705. [DOI] [PubMed] [Google Scholar]
- 2.Myllykangas-Luosujarvi R, Aho AK, Isomaki HA. Mortality in rheumatoid arthritis. Semin Arthritis Rheum. 1995;25:193–202. doi: 10.1016/s0049-0172(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 3.Goodson NJ, Wiles NJ, Lunt M, Barrett EM, Silman AJ, Symmons DP. Mortality in early inflammatory polyarthritis: cardiovascular mortality is increased in seropositive patients. Arthritis Rheum. 2002;46:2010–9. doi: 10.1002/art.10419. [DOI] [PubMed] [Google Scholar]
- 4.Turesson C, O’Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002;29:62–7. [PubMed] [Google Scholar]
- 5.Park YB, Ahn CW, Choi HK, Lee SH, In BH, Lee HC, et al. Atherosclerosis in rheumatoid arthritis: morphologic evidence obtained by carotid ultrasound. Arthritis Rheum. 2002;46:1714–9. doi: 10.1002/art.10359. [DOI] [PubMed] [Google Scholar]
- 6.Alkaabi JK, Ho M, Levison R, Pullar T, Belch JJ. Rheumatoid arthritis and macrovascular disease. Rheumatology (Oxford) 2003;42:292–7. doi: 10.1093/rheumatology/keg083. [DOI] [PubMed] [Google Scholar]
- 7.Jonsson SW, Backman C, Johnson O, Karp K, Lundstrom E, Sundqvist KG, et al. Increased prevalence of atherosclerosis in patients with medium term rheumatoid arthritis. J Rheumatol. 2001;28:2597–602. [PubMed] [Google Scholar]
- 8.Watson DJ, Rhodes T, Guess HA. All-cause mortality and vascular events among patients with rheumatoid arthritis, osteoarthritis, or no arthritis in the UK General Practice Research Database. J Rheumatol. 2003;30:1196–202. [PubMed] [Google Scholar]
- 9.Turesson C, Jarenros A, Jacobsson L. Increased incidence of cardiovascular disease in patients with rheumatoid arthritis: results from a community based study. Ann Rheum Dis. 2004;63:952–5. doi: 10.1136/ard.2003.018101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan MJ, McCune WJ. New evidence for vascular disease in patients with early rheumatoid arthritis. Lancet. 2003;361:1068–9. doi: 10.1016/S0140-6736(03)12901-7. [DOI] [PubMed] [Google Scholar]
- 11.Van Doornum S, McColl G, Wicks IP. Accelerated atherosclerosis: an extraarticular feature of rheumatoid arthritis? [review] Arthritis Rheum. 2002;46:862–73. doi: 10.1002/art.10089. [DOI] [PubMed] [Google Scholar]
- 12.Pasceri V, Yeh ET. A tale of two diseases: atherosclerosis and rheumatoid arthritis. Circulation. 1999;100:2124–6. doi: 10.1161/01.cir.100.21.2124. [DOI] [PubMed] [Google Scholar]
- 13.Turesson C, O’Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Extraarticular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years. Ann Rheum Dis. 2003;62:722–7. doi: 10.1136/ard.62.8.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turesson C, Jacobsson L, Bergstrom U. Extraarticular rheumatoid arthritis: prevalence and mortality. Rheumatology (Oxford) 1999;38:668–74. doi: 10.1093/rheumatology/38.7.668. [DOI] [PubMed] [Google Scholar]
- 15.Erhardt CC, Mumford PA, Venables PJ, Maini RN. Factors predicting a poor life prognosis in rheumatoid arthritis: an eight year prospective study. Ann Rheum Dis. 1989;48:7–13. doi: 10.1136/ard.48.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hara KS, Ballard DJ, Ilstrup DM, Connolly DC, Vollertsen RS. Rheumatoid pericarditis: clinical features and survival. Medicine (Baltimore) 1990;69:81–91. [PubMed] [Google Scholar]
- 17.Vollertsen RS, Conn DL, Ballard DJ, Ilstrup DM, Kazmar RE, Silverfield JC. Rheumatoid vasculitis: survival and associated risk factors. Medicine (Baltimore) 1986;65:365–75. [PubMed] [Google Scholar]
- 18.Foster CS, Forstotm SL, Wilson LA. Mortality rate in rheumatoid arthritis patients developing necrotizing scleritis or peripheral ulcerative keratitis: effects of systemic immunosuppression. Ophthalmology. 1984;91:1253–63. doi: 10.1016/s0161-6420(84)34160-4. [DOI] [PubMed] [Google Scholar]
- 19.Kurland LT, Molgaard CA. The patient record in epidemiology. Sci Am. 1981;245:54–63. doi: 10.1038/scientificamerican1081-54. [DOI] [PubMed] [Google Scholar]
- 20.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 21.Gabriel SE, Crowson CS, O’Fallon WM. The epidemiology of rheumatoid arthritis in Rochester, Minnesota, 1955–1985. Arthritis Rheum. 1999;42:415–20. doi: 10.1002/1529-0131(199904)42:3<415::AID-ANR4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 22.Doran MF, Pond GR, Crowson CS, O’Fallon WM, Gabriel SE. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year period. Arthritis Rheum. 2002;46:625–31. doi: 10.1002/art.509. [DOI] [PubMed] [Google Scholar]
- 23.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 24.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 25.Turnbull B. The empirical distribution function with arbitrarily grouped, censored and truncated data. J Royal Stat Soc. 1976;38:290–5. [Google Scholar]
- 26.Turesson C, Jacobsson LT. Epidemiology of extraarticular manifestations in rheumatoid arthritis. Scand J Rheumatol. 2004;33:65–72. doi: 10.1080/03009740310004621. [DOI] [PubMed] [Google Scholar]
- 27.Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2005;52:722–32. doi: 10.1002/art.20878. [DOI] [PubMed] [Google Scholar]
- 28.Turesson C, McClelland RL, Christianson TJ, Matteson EL. Severe extraarticular disease manifestations are associated with an increased risk of first ever cardiovascular events in patients with rheumatoid arthritis [abstract] Arthritis Rheum. 2004;50 (Suppl 9):S380–1. doi: 10.1136/ard.2006.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., III Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158:585–93. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 30.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–24. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 31.Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43–53. doi: 10.1016/s1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- 32.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–9. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–3. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- 34.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998;97:425–8. doi: 10.1161/01.cir.97.5.425. [DOI] [PubMed] [Google Scholar]
- 35.Wallberg-Jonsson S, Johansson H, Ohman ML, Rantapaa-Dahlqvist S. Extent of inflammation predicts cardiovascular disease and overall mortality in seropositive rheumatoid arthritis: a retrospective cohort study from disease onset. J Rheumatol. 1999;26:2562–71. [PubMed] [Google Scholar]
- 36.Liuzzo G, Goronzy JJ, Yang H, Kopecky SL, Holmes DR, Frye RL, et al. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation. 2000;101:2883–8. doi: 10.1161/01.cir.101.25.2883. [DOI] [PubMed] [Google Scholar]
- 37.Turesson C. Endothelial expression of MHC class II molecules in autoimmune disease. Curr Pharm Des. 2004;10:129–43. doi: 10.2174/1381612043453414. [DOI] [PubMed] [Google Scholar]
- 38.Van der Vliet JA, Boll AP. Abdominal aortic aneurysm. Lancet. 1997;349:863–6. doi: 10.1016/s0140-6736(96)07282-0. [DOI] [PubMed] [Google Scholar]


