Abstract
The female reproductive tract is a major site of mucosa-associated lymphoid tissue and susceptibility to HIV infection, yet the tissue site(s) of infection and the impact of HIV infection on this important mucosal tissue remain poorly understood. CD4+ T-cells and other cell types expressing the major coreceptors for HIV, CCR5 and CXCR4, are abundant in both the lower reproductive tract (endocervix and vagina) and the upper tract (endocervix and uterus), and are highly susceptible to infection. Antiviral defenses in the female reproductive tract are mediated by a variety of soluble factors, and by mucosal effector cells that differ phenotypically from their counterparts in blood. The immunologic characteristics of the female reproductive tract parallel those of the gut, where major HIV-related immunologic injury occurs. The susceptibility of the female reproductive tract to HIV infection and immunopathogenesis suggests important new avenues for further research.
Keywords: HIV, CTL, NK cells, MALT, gastrointestinal tract
Introduction
Mucosal surfaces are the primary sites of most HIV transmission, and thus these tissues are a focus of attention for efforts to prevent HIV infection1. Several mechanisms have been proposed to explain how HIV crosses the epithelial barriers of the female genital tract, including direct passage of virus and/or infected cells through epithelial breaches, transcytosis of free virus across polarized epithelial cells, and binding and/or uptake by mucosal dendritic cells2, 3. Regardless of the mechanism(s) leading to infection via the reproductive mucosa, the rate of HIV transmission per high-risk sexual contact appears to be low4. However, once infection is initiated, virus amplification occurs rapidly, aided by local inflammation5. Systemic dissemination occurs prior to the development of the adaptive immune response6, leading to massive depletion of CD4+ T-cells, notably those lining the gastrointestinal mucosa7–10. Like the gastrointestinal tract, tissues of the lower (ectocervix and vagina) and upper (endocervix and uterus) female reproductive tract contain partially activated, CD4+ memory cells expressing CCR5 and/or CXCR4, which can serve as targets for HIV. However, the effects of acute/early HIV infection on the reproductive tract have not been the focus of intensive study, while much attention has been devoted to the early effects of HIV infection on the gastrointestinal tract. This review summarizes literature on immune responses to HIV/SIV in the female reproductive tract, highlighting immunologic parallels between the reproductive and gastrointestinal mucosal tissues.
HIV infection of the female reproductive tract: local inflammation leads to rapid viral dissemination
Detailed studies of acute simian immunodeficiency virus (SIV) infection in rhesus macaques have revealed the mechanisms that lead to the rapid expansion of HIV/SIV in tissues, beginning with a small “founder” population of cells near the initial site of entry in the endocervix. Li and colleagues employed a combination of in situ hybridization and immunohistochemistry to elucidate the cells and soluble factors participating in the earliest steps of SIV dissemination following cervicovaginal inoculation of macaques5. At just one day post-infection, they found an accumulation in cervical mucosa of CD123+ plasmacytoid dendritic cells (pDC) expressing interferons α and β, and producing the chemokines MIP-1α and MIP-1β. These chemokines, in turn, attract CD4+ T-cells expressing the chemokine receptor CCR5, which serve as targets for SIV infection. This “inside-out” signaling, involving innate and inflammatory responses in mucosal tissues, leads to rapid amplification and expansion of the infection5.
If an inflammatory cascade leads to rapid expansion of HIV/SIV infection in mucosal tissues, might the inhibition of local inflammatory responses prevent viral dissemination following HIV/SIV mucosal exposure? To address this question, Li and colleagues selected an antimicrobial compound, glycerol monolaurate (GML), which inhibits the production of proinflammatory cytokines and chemokines by vaginal epithelial cell cultures in vitro. Strikingly, rhesus macaques treated with 5% GML prior to a high-dose intravaginal challenge with SIV were completely protected from systemic viral infection5. These findings demonstrate that innate immune defenses within mucosal tissues are, paradoxically, critical to the rapid dissemination of HIV/SIV within the host. They also suggest that microbicides designed to limit the host’s inflammatory response, rather than amplifying this response, may prove to be surprisingly effective at reducing HIV transmission. Furthermore, these findings imply that individuals who remain uninfected despite repeated mucosal exposure to HIV may respond to exposure by “immune quiescence” rather than an active inflammatory response. Caution is warranted in the study of topical microbicides, as some inflammatory modulators have an additional, unique role in the female reproductive tract by which they influence the outcome of embryonic implantation and early growth11.
Innate immune defenses in the female reproductive tract
The female reproductive tract is equipped with a variety of physical and chemical defenses against viral infection [recently reviewed by Iwasaki12]. The vagina and ectocervix are lined with multilayered squamous epithelium. In contrast, the upper reproductive tract, including the endocervix, uterine endometrium and fallopian tubes, contains a less resilient monolayer of columnar epithelium. The endocervical canal is lined with mucus, which provides an additional defensive layer by trapping bacteria and viruses. Epithelial cells lining the reproductive tract produce a wide range of soluble factors with documented anti-HIV activity; these include secretory leukocyte protease inhibitor (SLPI)13, lactoferrin14, defensins15, 16, cytokines and chemokines (e.g., RANTES and MIP-1β)17.
The serine protease inhibitor Trappin-2/Elafin has antibacterial activity against Gram-positive and -negative bacteria, as well as fungal pathogens18. Intriguingly, a recent proteomics study identified this protein as a potential biomarker of protection against HIV acquisition19. This study evaluated CVL samples from 315 women, including highly exposed, persistently seronegative (HEPS) commercial sex workers, HIV-positive women, and seronegative controls, using SELDI-TOF mass spectrometry. Trappin-2/Elafin was found to be significantly overexpressed in HEPS women as compared to both HIV-positive and seronegative groups.
A related study found that Trappin-2 was constitutively produced by primary cells from multiple reproductive tract sites, including uterus, Fallopian tube, cervix and ectocervix18. Levels of secreted Trappin-2/Elafin were higher in CVL samples from HIV-negative women than in samples from HIV-positive women, although these differences did not reach statistical significance. Recombinant Trappin-2/Elafin inhibited the infectivity of CXCR4 and CCR5-utilizing HIV strains in a TZM-bl assay18.
Other innate immune factors present in genital fluids include alpha and beta defensins and the antimicrobial peptide cathelicidin (LL-37). One recent study assessed levels of these factors in cervicovaginal secretions from HEPS commercial sex workers20. The presence of multiple sexually transmitted infections (including C. trachomatis, N. gonorrheae, T. vaginalis and candidiasis) was associated with increased levels of human neutrophil peptide-1 (HNP-1), human β-defensin-2-3, and LL-37 in secretions. Levels of HNP-1 and LL-37 were strongly associated with in vitro HIV-neutralizing capacity of cervicovaginal secretions. However, in univariate analysis, the levels of these factors in secretions tended to be increased in high-risk women who eventually seroconverted, relative to those who remained uninfected20.
HIV-specific IgA in cervical secretions
The presence of mucosal IgA at the sites of potential HIV exposure in the female reproductive tract could theoretically provide an important barrier against infection. IgG is also abundant in the reproductive tract, where it may be produced in the vagina or transported from serum by paracellular diffusion12. Intriguingly, several studies have reported the detection of HIV-specific immunoglobulin A (IgA) in cervicovaginal secretions from highly-exposed, persistently seronegative (HEPS) women21. It has also been reported that such antibodies are capable of neutralizing primary strains of HIV in vitro22, and can block transcytosis of HIV across an intact epithelial monolayer23. However, other studies of HEPS cohorts have failed to detect such antibodies, and this topic remains controversial in the HIV field24, 25. Intriguingly, Tudor and colleagues recently described the construction of an antibody expression library derived from B-cells isolated from the cervical mucosa of HEPS women. Antibodies derived from this library included IgA antibodies specific for the membrane-proximal region of gp4126.
HIV-specific T-cells, inflammation, and CD4 depletion in the lower female reproductive tract
Very few studies have characterized HIV-specific T-cell responses in the female reproductive tract of chronically infected individuals, likely due to the challenges associated with obtaining fresh tissues for analysis. Accordingly, it has been difficult to obtain a clear picture of the T-cell repertoire in the female reproductive tract, and the extent to which CD4+ and CD8+ T-cell populations mirror those found elsewhere in the body. Musey and colleagues compared the T cell receptor clonality of HIV-specific CD8+ T-cells from several mucosal sites, and found that the majority of clones were shared between mucosal tissues and PBMC27. They also identified several clones with CTL function that were CD4+, MHC class II-restricted, and responded to peptides within the HIV Env protein28.
Recently, progress has been made in utilizing minimally invasive clinical specimens, notably cervical cytobrushes and cervicovaginal lavage (CVL) for immunological assays29–36. Gumbi and colleagues studied the relationship between mucosal inflammation and HIV-specific CD8+ T-cell responses in the cervix32. They found that increased levels of proinflammatory cytokines (i.e., TNF-α, IL-1β, IL-6 and IL-8) in cervicovaginal lavage fluid were significantly associated with HIV shedding in the genital tract. Plasma viral load was also positively associated with viral shedding from the cervix. However, there was no association between the magnitude of HIV Gag-specific CD8+ T-cell responses in the cervix and viral shedding. There was also no correlation between HIV-specific T-cell response magnitudes at the cervix and in blood. These findings suggest that (1) genital inflammation may promote HIV replication and viral shedding, and (2) HIV-specific T-cell responses in blood do not necessarily predict those in cervical mucosa32.
In a related study, Bebell and colleagues collected cervicovaginal lavage and plasma samples from women in a longitudinal cohort in Durban, South Africa, including 44 women with acute HIV infection29. Levels of IL-6, IL-10 and IL-12p70 in CVL were significantly greater in women with acute HIV infection than in pre-seroconversion samples from the same women, or in CVL from HIV-negative controls. Importantly, cytokine levels in the genital tract were similar in women with acute HIV infection regardless of concurrent STI or bacterial vaginosis. Furthermore, elevated levels of IL-1β, IL-6 and IL-8 correlated with lower systemic CD4+ T-cell counts during acute HIV infection. Unfortunately, mucosal CD4+ T-cell depletion was not measured; nevertheless, these findings reveal that genital inflammation is associated with acute HIV infection and is significantly associated with systemic CD4+ T-cell depletion29.
Nkwanyana and colleagues evaluated the composition and yield of cytobrush-derived T-cells from HIV infected women and uninfected controls34. In the HIV-positive women, yields of CD3+ T-cells as well as CD19+ B-cells were significantly greater than in uninfected controls, although CD4+ T-cells were depleted relative to CD8+ T-cells. The majority of cytobrush T-cells had an effector memory (CD45RA-CCR7-CD27-) phenotype. There was a significant positive correlation between cytobrush T-cell counts and cervical concentrations of IL-1β, TNFα and IL-12.
The low number of viable lymphocytes isolated from cytobrush specimens limits the range of experiments that may be performed to characterize HIV-specific T-cell responses at the cervical mucosa. To address this issue, Bere and colleagues compared several methods for in vitro expansion of cytobrush-derived T-cells30, 31. Expansion with anti-CD3/28 beads and IL-2, IL-7 and IL-15 gave rise to the greatest expansion after 7 days, with an accumulation of central memory T-cells31. The same authors used polyclonally expanded T-cell lines from 16 HIV-positive women to assess the relationship between HIV Gag-specific responses in blood and cervix in Elispot assays30. Both the total response magnitude and the peptide pools targeted were similar in both sites, and cervical responses to HIV Gag peptides were detected only in women with systemic Gag-specific responses >1000 spot-forming units/106 cells30. This finding underscores the limitations inherent in cytobrush sampling, which typically yields between 0.01–1 × 106 viable cells per cytobrush30, 32.
Although highly active antiretroviral therapy (HAART) is associated with control of viremia and variable restoration of CD4+ T-cell counts in the periphery, little is known of the response to HAART in genital mucosa. In a recent cross-sectional study of 62 HIV-positive women, including 35 on HAART, Mkhize and colleagues demonstrated that HAART is associated with higher CD4+ T-cell percentages at the cervical mucosa33. Genital tract HIV shedding was significantly associated with the amount of HIV RNA circulating in blood, and was fully suppressed in HAART-compliant women. As previously reported, HAART was also associated with decreased HIV-specific CD8+ T-cell responses in blood. Surprisingly, however, Gag-specific T-cell responses in cervical mucosa were preserved in women on HAART. These findings may suggest that low levels of virus continue to replicate in cervical mucosa, even when virus reaches very low to undetectable levels in plasma and cervical secretions. Further studies will be required to address this issue.
Sexually transmitted infections alter the mucosal microenvironment
Other sexually transmitted infections are frequently present when HIV exposure occurs, and these infections may alter the mucosal immune environment in ways that facilitate HIV transmission [reviewed by Kaul et al37]. For example, infection with Chlamydia trachomatis, a pathogen of the columnar epithelium, results in increases in the number of CD4+ and CD8+ T cells, neutrophils, CD14+ monocytes, and CD83+ dendritic cells38, 39. Similarly, increased concentrations of a wide range of cytokines are found in genital tract tissues and secretions during infection with C. trachomatis, including INFγ, TNFα, IL-1α, IL-6, IL-8, and IL-10, contributing to an escalating inflammatory response. In contrast, human papillomavirus infections do not elicit an intense inflammatory response, but intraepithelial lesions produced by these viruses may impair barrier function and increase HIV access to susceptible cell types40. Less invasive infections, such as bacterial vaginosis, also produce immunologic responses that may facilitate HIV transmission. St. John and colleagues compared the effects of vaginal fluid from women with bacterial vaginosis or healthy controls on monocyte-derived dendritic cells. Fluids from women with bacterial vaginosis, but not controls, induced production of IL-12, IL-23, increased expression of dendritic cell activation markers (HLA-DR, CD40 and CD83) and stimulated proliferation of T-cells in allogeneic mixed lymphocyte reactions41. Chancroid is one of the STDs most closely associated with HIV transmission, perhaps due to extensive disruption of the epithelial barrier, bleeding and recruitment of susceptible cells42. In addition, Humphreys reported that experimental infection with Haemophilus ducreyi resulted in increased expression of HIV coreceptors by macrophages and CD4+ T-cells cells in chancroid lesions43. Genital infections with herpes simplex viruses (HSV) lead to increases in cervical dendritic cells and CCR5-expressing CD4+ T-cells44. Additionally, some HSV proteins can transactivate HIV gene expression, while others can serve as immune modulators45.
Exposure to semen induces inflammatory responses in the female genital tract
While it is vital to elucidate the immunologic characteristics of the intact female genital tract, HIV exposure often occurs in the context of semen, thus the effects of semen on these immunologic functions must also be understood. In addition to perturbations related to genital tract infections, semen and seminal plasma also alter the immunologic milieu of the female genital tract in a manner that facilitates conception and pregnancy11. The proinflammatory effects of insemination are well documented and referred to as the “post-mating inflammatory response” that is characterized by an increase in the synthesis of cytokines including GM-CSF, MCP-1 and IL-6 and the migration of macrophages, dendritic cells and granulocytes into the endometrial stroma46. Leukocytes also migrate into the cervical epithelium, an occurrence that requires direct contact between seminal plasma and female genital tissues, and is related to increases in GM-CSF, IL-6 and IL-846. Seminal fluid taken from healthy men, and free of leucocytes, significantly increased TGF-β1 and IL-1β levels in the supernatants of endometrial epithelial cell cultures47. In mouse experiments, TGFβ was the principal elicitor of uterine inflammation following mating48. The TGFβ1 content of semen is comparable to that of colostrum, the most potent biological source of this cytokine46. Semen from healthy men also contains significant levels of IL-7, SDF1α, MCP-1 and IL-849. After insemination, TGFβ1 tends to limit Th1-type responses, including those to paternal alloantigens which could undermine fertility50. Robertson reported that exposure to semen results in increases in CD4+CD25+Foxp3+ regulatory T-cells in mice, and tolerance to paternal antigens51. Thus semen is a potent immune modulator in the cervix and uterus, and its effects on susceptibility to HIV infection are largely undefined.
The upper reproductive tract and HIV infection
The common occurrence of sexual transmission of HIV to women via vaginal intercourse was debated early in the AIDS epidemic; and anal intercourse was implicated as a possible mode of male-to-female transmission in the few incidents that were recognized52. Two key reports53, 54 indicated that male-to-female transmission occurred more regularly, but in these studies infection was detected in women who had undergone hysterectomy, and though these cross-sectional studies could not determine that hysterectomy preceded HIV transmission, the studies may have been interpreted as indicating that the upper genital tract was not a common site of HIV transmission. Most studies of HIV transmission to women, including studies of vaccine responses and microbicides, have focused on the lower genital tract.
The assumption that sexual HIV transmission to women occurs in the lower genital tract is not surprising since the upper tract is generally thought to be a sterile environment that is not easily accessed. However, MRI studies demonstrate that intravaginal gels reach the endocervix and uterus within minutes of application.55, 56 Once in the uterus, nonoxynol gels produce significant disruption of the endometrium including focal absence of the uterine epithelium proximal to the cervix55, hemorrhage and areas of necrosis55, as well as increased densities of CD8+ and Foxp3+ cells.57
The uterus contains a variety of cell types that are susceptible to HIV infection, including CD4+ T-cells expressing CXCR4 and/or CCR5 as well as monocyte/macrophages. A polarized human endometrial epithelial cell line, HEC-1, has been shown to efficiently transcytose both X4 and R5 tropic strains of HIV58, 59, allowing transfer of virus to susceptible target cells situated near the basolateral surface. Primary uterine epithelial cells can also transfer virus to CD4+ T-cells in vitro60. In one recent study, primary tissue explants derived from ectocervix and endometrium of HIV-negative premenopausal women were infected ex vivo with R5-tropic HIV, and viral transcription levels in the two tissue types were compared using real-time polymerase chain reaction. Significantly higher levels of HIV transcription were detected in ectocervical tissues as compared to endometrial explants, suggesting that ectocervix may be more permissive than endometrium for HIV replication61. This may be related to a relatively higher density of T cells and dendritic cells in the cervix, particularly the cervical transformation zone, as compared to the upper reproductive tract61, 62.
Few studies have addressed HIV-specific T-cell responses in the upper female reproductive tract. White and colleagues measured HIV-specific cytotoxic T-cell (CTL) activity in PBMC and a variety of upper and lower reproductive tissues from three HIV-positive women, including one long-term nonprogressor (LTNP) and two women on highly active antiretroviral therapy (HAART)63. HIV-specific T-cell responses were found in ovaries, endometrium and endocervix of the LTNP, but such responses were not detected in the two women on HAART. Although limited in scope, these findings demonstrate that HIV-specific T-cell responses are present in the upper reproductive tract under some conditions, and provide a basis for further study.
Changes in the upper reproductive tract during the menstrual cycle
The immune microenvironment of the uterine endometrium fluctuates during the menstrual cycle, and significant changes in cell populations, growth factors, cytokines and adhesion molecules may be required to promote embryo implantation. There is a short window during the mid-secretory phase, limited to about 48h, during which conditions are favorable for implantation (for a review, see Aghajanova et al., 200864). This implantation window typically begins 6–10 days after the surge in luteinizing hormone (LH), roughly days 20–24 of an idealized menstrual cycle. Gene expression changes include upregulation of endometrial growth factors, cytokines and adhesion molecules, coupled with downregulation of genes associated with cell-mediated immunity and the classical complement pathway.
The hormonal fluctuations associated with the menstrual cycle regulate a variety of features of the reproductive tract that may directly impact susceptibility to HIV infection and persistence. Several years ago, White and colleagues demonstrated that cytolytic activity by CD8+ T-cells in the upper reproductive tract, as measured by redirected lysis assays, was present during the proliferative phase of the menstrual cycle (prior to ovulation), yet absent during the secretory phase65. Notably, in contrast to the upper reproductive tract, CTL activity in the cervix and vagina persisted throughout the menstrual cycle66. Postmenopausal women also retained strong cytotoxic T-cell populations in the upper tract. Taken together, these observations suggest tight post-ovulatory regulation of cell-mediated immunity in order to facilitate the implantation of the semi-allogeneic embryo. It is proposed that these fluctuations might contribute to a cycle-dependent “window of vulnerability” to HIV infection in the upper reproductive tract67. Expression of CD4, CCR5 and CXCR4 by uterine epithelial cells and other cell types may also be temporally regulated during the menstrual cycle, with consequences for HIV infection and dissemination68, 69.
Finally, the uterine endometrium also contains hormonally regulated, organized cellular aggregates, consisting of a B-cell core surrounded by CD8+ T-cells and macrophages, which are located in the stratum basalis layer70. Aggregate size is smallest during the proliferative phase of the menstrual cycle, largest during the secretory phase, and absent from postmenopausal women. Whether these structures play an active role in antiviral defense is currently unknown.
Uterine natural killer (NK) cells: A novel cell type with an unusual dual role
Natural killer (NK) cells are distributed throughout the body and play a major role in immunosurveillance of virally infected and neoplastic cells. NK cells utilize a variety of effector mechanisms, including the release of cytolytic granules containing perforin and granzymes, the production and release of cytokines, and antibody-dependent cellular cytotoxicity (ADCC)71. Uterine NK cells (uNK) differ in cell surface phenotype from their counterparts in peripheral blood, typically expressing CD56 but not CD16 or CD57. Their density in the uterine endometrium increases after ovulation. Uterine NK cells appear to secrete a variety of cytokines and growth factors that help prepare the endometrium for embryo implantation. However, high uterine NK activity is also associated with recurrent miscarriage72, 73. The extent to which these unique cells may participate in antiviral defense in the reproductive tract is currently unknown.
Immunological parallels between the reproductive and gastrointestinal tracts
The gut, of course, differs considerably from endometrium in terms of location, morphology and basic function (intake of nutrients and elimination of wastes). The endometrium’s function is to support implantation of the embryo and sustenance until the placenta is fully developed. However, the similarities between the tissues are numerous. Both sites have a dual role in maintaining a tolerogenic microenvironment while retaining rapid responsiveness to pathogens (Table 1)12, 74. The ectocervix and vagina, like the lower anorectal canal, are characterized as Type II mucosal tissues, lined with stratified squamous epithelia and lacking organized mucosa-associated lymphoid tissue (MALT) structures. The endocervix and uterus, as well as the rectum and most of the large and small intestine, are characterized as Type I mucosal tissues, lined with simple columnar epithelium. These tissues produce secretory immunoglobulin, type A (sIgA), and can have organized MALT structures, including hormonally regulated uterine cellular aggregates, lymphoid follicles in the rectum, and intestinal Peyer’s patches.
Table 1.
Immunologic Properties Shared by the Female Reproductive Tract and Gastrointestinal Tract
| Property | Female Reproductive Tract | Gastrointestinal Tract |
|---|---|---|
| Major portal of entry for HIV | Activated CD4+ cells expressing CCR5, CXCR4 | Activated CD4+ cells expressing CCR5, CXCR4 |
Presence of Type I Mucosa
|
Endocervix, Uterus | Rectum, Large and Small Intestine |
Presence of Type II Mucosa
|
Ectocervix, Vagina | Lower Anorectal Canal |
| Mucus contributes to barrier function | Produced by vaginal epithelial cells, cervical crypts | Produced by goblet cells |
| Epithelial cells play multiple roles: Barrier function, antigen presentation, cytokine secretion, transcytosis of antigens |
Yes | Yes |
| Beneficial endogenous flora |
|
|
| Immunologic tolerance |
|
|
| Novel effector cell populations |
|
|
| Innate effector molecules |
|
|
| Sex steroid responsiveness | Significant modulation of tissue architecture, development of inducible MALT | Estrogen receptor ligands affect crypt/villus architecture and may play a role in inflammatory bowel diseases |
Both the reproductive and gastrointestinal tracts are protected by mucus layers, and by epithelial cells that play multiple roles in barrier function, antigen presentation, secretion of cytokines, chemokines and growth factors, and transcytosis of macromolecules. Both tissue systems are extensively colonized by endogenous flora. These flora both prevent the overgrowth of pathogenic bacteria and provide useful “housekeeping” functions; in the case of intestinal flora, these include the synthesis of vitamins and hormones, stimulation of iNOS75 and IgA76, 77 production, and the maintenance of epithelial homeostasis through signaling of Toll-like receptor (TLR) pathways78.
Both the reproductive and gastrointestinal tracts engage in immunological tolerance. In the reproductive tract, tolerance is related to the implantation and preservation of a semi-allogeneic embryo; in the GI tract, tolerance is maintained with respect to food antigens and commensal bacteria. At both sites, the mechanisms regulating tolerance are complex and appear to involve Foxp3+ regulatory T-cells and immunosuppressive cytokines such as TGF-β.
Th17/CD4+ cells and IL-17 play important roles at each site. Depletion of Th17+ cells and lack of IL-17 is associated with impaired intestinal barrier function, as illustrated by increased dissemination of S. typhimurium in simian immunodeficiency virus infected macaques79, 80. In humans, IL-17 and related cytokines are involved in a number of autoimmune and inflammatory disorders, including inflammatory bowel disease81, 82, conditions that, like HIV infection, are characterized by altered gut barrier function83. In the female genital tract, Th17 cells and IL-17 are associated with increased IL-8 and endometriosis,84 and have been implicated in spontaneous abortion85.
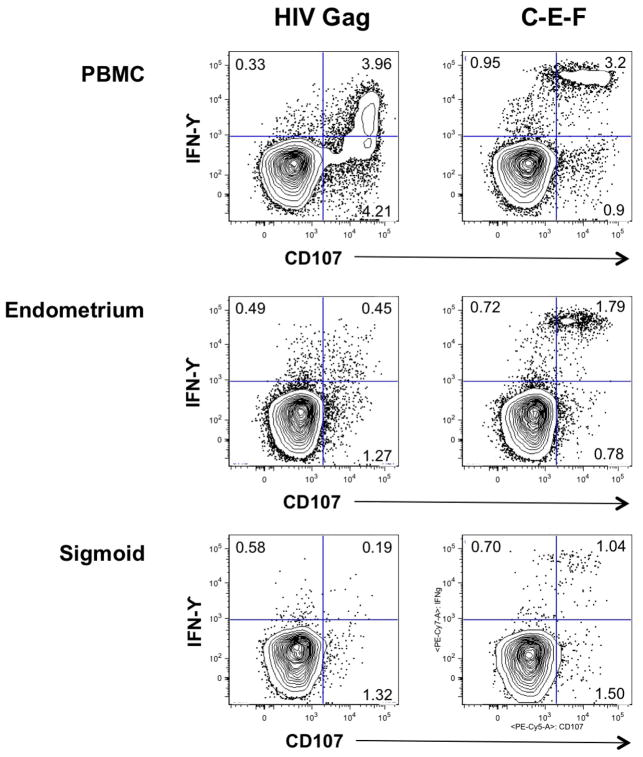
From the perspective of HIV infection, both the reproductive and gastrointestinal tracts serve as important portals of entry. Both tissues are rich in partially activated CD4+ memory T-cells, many of which express CCR5 and/or CXCR4. Both harbor significant populations of HIV-specific memory CD8+ T-cells. Our laboratories are currently studying HIV-specific CD8+ T-cell responses in blood, reproductive mucosa and gastrointestinal mucosa of HIV-infected women74, 86. Although these studies are still preliminary, early results suggest that there are significant differences in antigen responsiveness between CD8+ T-cells isolated from endometrial biopsy as compared to those from PBMC or the gastrointestinal tract of the same individuals (Figure 1). Additional studies will be required to extend these results.
Figure 1.
CD8+ T-cell responses to HIV Gag peptides (left) and a mixture of immunodominant peptides derived from cytomegalovirus, Epstein-Barr virus and Influenza A (C-E-F) (right). Freshly isolated lymphocytes from PBMC, endometrial biopsy, and sigmoid colon were tested in a cytokine flow cytometry assay for production of IFNγ, and the cytolytic granule marker CD107a in response to peptide stimulation. Numbers in individual quadrants indicate the number of cells producing either IFNγ (upper left quadrant), CD107 (lower right quadrant), or both IFNγ and CD107 (upper right quadrant) in response to peptide stimulation. Assays were performed as described elsewhere88, 89. The samples used in this experiment were obtained from a premenopausal HIV-positive woman on long-term HAART (9 years), with plasma viral load <75 copies/mL, blood CD4+ T-cell count 1180, from whom blood and tissue sampled were obtained on day 24 of the menstrual cycle.
Implications for studies on prevention of HIV infection and HIV immunopathogenesis
Women are increasingly affected by the HIV epidemic worldwide, and now account for nearly half the 33 million individuals living with HIV87. In sub-Saharan Africa, women account for approximately 60% of HIV infections87. As the HIV/AIDS epidemic enters its second quarter century, many questions remain unanswered concerning the disease process, and many more studies are needed to advance our understanding of HIV pathogenesis in women.
Due to the susceptibility of endocervical and endometrial cell populations, these tissues are potential sites of HIV infection. Current data do not indicate the relative frequency of any female genital site as the location of initial HIV infection, and it is possible that tissue specific susceptibility to infection depends on the existence of factors such as sexually transmitted infections, trauma, ovulatory cycle, seminal plasma effects or hormonal modulators of genital immune responses. Thus the upper tract tissues should be considered in studies of HIV vaccines, microbicides and other prevention interventions. Several studies point to a potential “window of vulnerability” to HIV infection at 7 to 10 days following ovulation67. Addressing this question in the laboratory will require detailed studies of mucosal immunity in women across the menstrual cycle, and a better understanding of the influence of reproductive hormones on adaptive and innate mucosal immunity. A second, and related area of interest is the potential influence of hormonal contraception on HIV transmission.
Since semen and seminal fluids influence female genital immunology, studies of the tissue effects of prevention modalities must also include evaluations of semen exposure. Studies of the post-menopausal genital tract, and HIV susceptibility and shedding are also needed given the increased survival of HIV patients, and the large number of women who are living with this infection worldwide.
The limited existing evidence indicates that HIV exerts parallel effects on mucosal lymphoid tissues in the gut and endometrium. Although the rapid CD4+ T-cell decline that occurs during acute HIV infection of the GI tract has been extensively studied, much less is known about the effects of acute HIV infection on reproductive tissues. Similarly, the role of immune activation and inflammation within the reproductive tract on HIV disease has not been thoroughly studied, nor have the effects of HAART on CD4+ T-cell repopulation in the reproductive mucosa. These questions should prompt further study since endometrial tissues are readily obtained and no enteric preparation is required. Since tissue sampling in human studies of infrequent HIV phenotypes, such as in acute infection, are often problematic, the use of endometrial sampling may provide an opportunity to obtain MALT tissues more readily.
Acknowledgments
The authors thank Drs. Linda Giudice, Warner C. Greene, Laura Napolitano, and Karen Smith-McCune, University of California at San Francisco, for their ongoing collaboration and Dr. Charles Wira, Dartmouth University, for thought-provoking discussions. B.L.S. thanks Dr. J.W. Critchfield, University of California at Davis, for contributing the data shown in Figure 1. This research is supported by the National Institutes of Health (NIH-NIAID R01 AI-057020 and P01 AI-083050). This research was approved by the Institutional Review Board of UC Davis and the Committee for Human Subjects Research of UC San Francisco.
References
- 1.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 2.Hladik F, Hope TJ. HIV infection of the genital mucosa in women. Curr HIV/AIDS Rep. 2009;6:20–28. doi: 10.1007/s11904-009-0004-1. [DOI] [PubMed] [Google Scholar]
- 3.Hladik F, McElrath MJ. Setting the stage: host invasion by HIV. Nat Rev Immunol. 2008;8:447–457. doi: 10.1038/nri2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, Meehan MO, Lutalo T, Gray RH. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 5.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, Nephew KR, Pambuccian S, Lifson JD, Carlis JV, Haase AT. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reynolds MR, Rakasz E, Skinner PJ, White C, Abel K, Ma ZM, Compton L, Napoe G, Wilson N, Miller CJ, Haase A, Watkins DI. CD8+ T-lymphocyte response to major immunodominant epitopes after vaginal exposure to simian immunodeficiency virus: too late and too little. J Virol. 2005;79:9228–9235. doi: 10.1128/JVI.79.14.9228-9235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Favre D, Lederer S, Kanwar B, Ma ZM, Proll S, Kasakow Z, Mold J, Swainson L, Barbour JD, Baskin CR, Palermo R, Pandrea I, Miller CJ, Katze MG, McCune JM. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5:e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 9.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 10.Veazey R, DeMaria M, Chalifoux L, Shvetz D, Pauley D, Knight H, Rosenzweig M, Johnson R, Desrosiers R, Lackner A. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 11.Schuberth HJ, Taylor U, Zerbe H, Waberski D, Hunter R, Rath D. Immunological responses to semen in the female genital tract. Theriogenology. 2008;70:1174–1181. doi: 10.1016/j.theriogenology.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Iwasaki A. Antiviral immune responses in the genital tract: clues for vaccines. Nat Rev Immunol. 2010;10:699–711. doi: 10.1038/nri2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fahey JV, Wira CR. Effect of menstrual status on antibacterial activity and secretory leukocyte protease inhibitor production by human uterine epithelial cells in culture. J Infect Dis. 2002;185:1606–1613. doi: 10.1086/340512. [DOI] [PubMed] [Google Scholar]
- 14.Berkhout B, Floris R, Recio I, Visser S. The antiviral activity of the milk protein lactoferrin against the human immunodeficiency virus type 1. Biometals. 2004;17:291–294. doi: 10.1023/b:biom.0000027707.82911.be. [DOI] [PubMed] [Google Scholar]
- 15.Quinones-Mateu ME, Lederman MM, Feng Z, Chakraborty B, Weber J, Rangel HR, Marotta ML, Mirza M, Jiang B, Kiser P, Medvik K, Sieg SF, Weinberg A. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS. 2003;17:F39–48. doi: 10.1097/00002030-200311070-00001. [DOI] [PubMed] [Google Scholar]
- 16.Sun L, Finnegan CM, Kish-Catalone T, Blumenthal R, Garzino-Demo P, La Terra Maggiore GM, Berrone S, Kleinman C, Wu Z, Abdelwahab S, Lu W, Garzino-Demo A. Human beta-defensins suppress human immunodeficiency virus infection: potential role in mucosal protection. J Virol. 2005;79:14318–14329. doi: 10.1128/JVI.79.22.14318-14329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fahey JV, Schaefer TM, Channon JY, Wira CR. Secretion of cytokines and chemokines by polarized human epithelial cells from the female reproductive tract. Hum Reprod. 2005;20:1439–1446. doi: 10.1093/humrep/deh806. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh M, Shen Z, Fahey JV, Cu-Uvin S, Mayer K, Wira CR. Trappin-2/Elafin: a novel innate anti-human immunodeficiency virus-1 molecule of the human female reproductive tract. Immunology. 2009;129:207–219. doi: 10.1111/j.1365-2567.2009.03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iqbal SM, Ball TB, Levinson P, Maranan L, Jaoko W, Wachihi C, Pak BJ, Podust VN, Broliden K, Hirbod T, Kaul R, Plummer FA. Elevated elafin/trappin-2 in the female genital tract is associated with protection against HIV acquisition. AIDS. 2009;23:1669–1677. doi: 10.1097/QAD.0b013e32832ea643. [DOI] [PubMed] [Google Scholar]
- 20.Levinson P, Kaul R, Kimani J, Ngugi E, Moses S, MacDonald KS, Broliden K, Hirbod T. Levels of innate immune factors in genital fluids: association of alpha defensins and LL-37 with genital infections and increased HIV acquisition. AIDS. 2009;23:309–317. doi: 10.1097/QAD.0b013e328321809c. [DOI] [PubMed] [Google Scholar]
- 21.Hirbod T, Kaul R, Reichard C, Kimani J, Ngugi E, Bwayo JJ, Nagelkerke N, Hasselrot K, Li B, Moses S, MacDonald KS, Broliden K. HIV-neutralizing immunoglobulin A and HIV-specific proliferation are independently associated with reduced HIV acquisition in Kenyan sex workers. AIDS. 2008;22:727–735. doi: 10.1097/QAD.0b013e3282f56b64. [DOI] [PubMed] [Google Scholar]
- 22.Devito C, Hinkula J, Kaul R, Lopalco L, Bwayo JJ, Plummer F, Clerici M, Broliden K. Mucosal and plasma IgA from HIV-exposed seronegative individuals neutralize a primary HIV-1 isolate. AIDS. 2000;14:1917–1920. doi: 10.1097/00002030-200009080-00006. [DOI] [PubMed] [Google Scholar]
- 23.Belec L, Ghys PD, Hocini H, Nkengasong JN, Tranchot-Diallo J, Diallo MO, Ettiegne-Traore V, Maurice C, Becquart P, Matta M, Si-Mohamed A, Chomont N, Coulibaly IM, Wiktor SZ, Kazatchkine MD. Cervicovaginal secretory antibodies to human immunodeficiency virus type 1 (HIV-1) that block viral transcytosis through tight epithelial barriers in highly exposed HIV-1-seronegative African women. J Infect Dis. 2001;184:1412–1422. doi: 10.1086/324375. [DOI] [PubMed] [Google Scholar]
- 24.Alexander R, Mestecky J. Neutralizing antibodies in mucosal secretions: IgG or IgA? Curr HIV Res. 2007;5:588–593. doi: 10.2174/157016207782418452. [DOI] [PubMed] [Google Scholar]
- 25.Broliden K. Innate molecular and anatomic mucosal barriers against HIV infection in the genital tract of HIV-exposed seronegative individuals. J Infect Dis. 2010;202 (Suppl 3):S351–355. doi: 10.1086/655964. [DOI] [PubMed] [Google Scholar]
- 26.Tudor D, Derrien M, Diomede L, Drillet AS, Houimel M, Moog C, Reynes JM, Lopalco L, Bomsel M. HIV-1 gp41-specific monoclonal mucosal IgAs derived from highly exposed but IgG-seronegative individuals block HIV-1 epithelial transcytosis and neutralize CD4(+) cell infection: an IgA gene and functional analysis. Mucosal Immunol. 2009;2:412–426. doi: 10.1038/mi.2009.89. [DOI] [PubMed] [Google Scholar]
- 27.Musey L, Ding Y, Cao J, Lee J, Galloway C, Yuen A, Jerome KR, McElrath MJ. Ontogeny and specificities of mucosal and blood human immunodeficiency virus type 1-specific CD8(+) cytotoxic T lymphocytes. J Virol. 2003;77:291–300. doi: 10.1128/JVI.77.1.291-300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musey L, Hu Y, Eckert L, Christensen M, Karchmer T, McElrath MJ. HIV-1 induces cytotoxic T lymphocytes in the cervix of infected women. J Exp Med. 1997;185:293–303. doi: 10.1084/jem.185.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bebell LM, Passmore JA, Williamson C, Mlisana K, Iriogbe I, van Loggerenberg F, Karim QA, Karim SA. Relationship between levels of inflammatory cytokines in the genital tract and CD4+ cell counts in women with acute HIV-1 infection. J Infect Dis. 2008;198:710–714. doi: 10.1086/590503. [DOI] [PubMed] [Google Scholar]
- 30.Bere A, Denny L, Burgers WA, Passmore JA. Polyclonal expansion of cervical cytobrush-derived T cells to investigate HIV-specific responses in the female genital tract. Immunology. 2010;130:23–33. doi: 10.1111/j.1365-2567.2009.03172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bere A, Denny L, Hanekom W, Burgers WA, Passmore JA. Comparison of polyclonal expansion methods to improve the recovery of cervical cytobrush-derived T cells from the female genital tract of HIV-infected women. J Immunol Methods. 2010;354:68–79. doi: 10.1016/j.jim.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gumbi PP, Nkwanyana NN, Bere A, Burgers WA, Gray CM, Williamson AL, Hoffman M, Coetzee D, Denny L, Passmore JA. Impact of mucosal inflammation on cervical HIV-1-specific CD8 T cell responses in the female genital tract during chronic HIV infection. J Virol. 2008 doi: 10.1128/JVI.00183-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mkhize NN, Gumbi PP, Liebenberg LJ, Ren Y, Smith P, Denny L, Passmore JA. Persistence of genital tract T cell responses in HIV-infected women on highly active antiretroviral therapy. J Virol. 2010;84:10765–10772. doi: 10.1128/JVI.00518-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nkwanyana NN, Gumbi PP, Roberts L, Denny L, Hanekom W, Soares A, Allan B, Williamson AL, Coetzee D, Olivier AJ, Burgers WA, Passmore JA. Impact of human immunodeficiency virus 1 infection and inflammation on the composition and yield of cervical mononuclear cells in the female genital tract. Immunology. 2009;128:e746–757. doi: 10.1111/j.1365-2567.2009.03077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaul R, Thottingal P, Kimani J, Kiama P, Waigwa CW, Bwayo JJ, Plummer FA, Rowland-Jones SL. Quantitative ex vivo analysis of functional virus-specific CD8 T lymphocytes in the blood and genital tract of HIV-infected women. AIDS. 2003;17:1139–1144. doi: 10.1097/00002030-200305230-00004. [DOI] [PubMed] [Google Scholar]
- 36.Shacklett BL, Cu-Uvin S, Beadle TJ, Pace CA, Fast NM, Donahue SM, Caliendo AM, Flanigan TP, Carpenter CC, Nixon DF. Quantification of HIV-1-specific T-cell responses at the mucosal cervicovaginal surface. AIDS. 2000;14:1911–1915. doi: 10.1097/00002030-200009080-00005. [DOI] [PubMed] [Google Scholar]
- 37.Kaul R, Pettengell C, Sheth PM, Sunderji S, Biringer A, MacDonald K, Walmsley S, Rebbapragada A. The genital tract immune milieu: an important determinant of HIV susceptibility and secondary transmission. J Reprod Immunol. 2008;77:32–40. doi: 10.1016/j.jri.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Agrawal T, Vats V, Salhan S, Mittal A. The mucosal immune response to Chlamydia trachomatis infection of the reproductive tract in women. J Reprod Immunol. 2009;83:173–178. doi: 10.1016/j.jri.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 39.Mittal A, Rastogi S, Reddy BS, Verma S, Salhan S, Gupta E. Enhanced immunocompetent cells in chlamydial cervicitis. J Reprod Med. 2004;49:671–677. [PubMed] [Google Scholar]
- 40.Smith-McCune KK, Shiboski S, Chirenje MZ, Magure T, Tuveson J, Ma Y, Da Costa M, Moscicki AB, Palefsky JM, Makunike-Mutasa R, Chipato T, van der Straten A, Sawaya GF. Type-specific cervico-vaginal human papillomavirus infection increases risk of HIV acquisition independent of other sexually transmitted infections. PLoS One. 2010;5:e10094. doi: 10.1371/journal.pone.0010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.St John EP, Martinson J, Simoes JA, Landay AL, Spear GT. Dendritic cell activation and maturation induced by mucosal fluid from women with bacterial vaginosis. Clin Immunol. 2007;125:95–102. doi: 10.1016/j.clim.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greenblatt RM, Lukehart SA, Plummer FA, Quinn TC, Critchlow CW, Ashley RL, D’Costa LJ, Ndinya-Achola JO, Corey L, Ronald AR, et al. Genital ulceration as a risk factor for human immunodeficiency virus infection. AIDS. 1988;2:47–50. doi: 10.1097/00002030-198802000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Humphreys TL, Schnizlein-Bick CT, Katz BP, Baldridge LA, Hood AF, Hromas RA, Spinola SM. Evolution of the cutaneous immune response to experimental Haemophilus ducreyi infection and its relevance to HIV-1 acquisition. J Immunol. 2002;169:6316–6323. doi: 10.4049/jimmunol.169.11.6316. [DOI] [PubMed] [Google Scholar]
- 44.Rebbapragada A, Wachihi C, Pettengell C, Sunderji S, Huibner S, Jaoko W, Ball B, Fowke K, Mazzulli T, Plummer FA, Kaul R. Negative mucosal synergy between Herpes simplex type 2 and HIV in the female genital tract. AIDS. 2007;21:589–598. doi: 10.1097/QAD.0b013e328012b896. [DOI] [PubMed] [Google Scholar]
- 45.Van de Perre P, Segondy M, Foulongne V, Ouedraogo A, Konate I, Huraux JM, Mayaud P, Nagot N. Herpes simplex virus and HIV-1: deciphering viral synergy. Lancet Infect Dis. 2008;8:490–497. doi: 10.1016/S1473-3099(08)70181-6. [DOI] [PubMed] [Google Scholar]
- 46.Robertson SA. Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res. 2005;322:43–52. doi: 10.1007/s00441-005-1127-3. [DOI] [PubMed] [Google Scholar]
- 47.Ochsenkuhn R, Toth B, Nieschlag E, Artman E, Friese K, Thaler CJ. Seminal plasma stimulates cytokine production in endometrial epithelial cell cultures independently of the presence of leucocytes. Andrologia. 2008;40:364–369. doi: 10.1111/j.1439-0272.2008.00870.x. [DOI] [PubMed] [Google Scholar]
- 48.Tremellen KP, Seamark RF, Robertson SA. Seminal transforming growth factor beta1 stimulates granulocyte-macrophage colony-stimulating factor production and inflammatory cell recruitment in the murine uterus. Biol Reprod. 1998;58:1217–1225. doi: 10.1095/biolreprod58.5.1217. [DOI] [PubMed] [Google Scholar]
- 49.Politch JA, Tucker L, Bowman FP, Anderson DJ. Concentrations and significance of cytokines and other immunologic factors in semen of healthy fertile men. Hum Reprod. 2007;22:2928–2935. doi: 10.1093/humrep/dem281. [DOI] [PubMed] [Google Scholar]
- 50.Robertson SA. Seminal fluid signaling in the female reproductive tract: lessons from rodents and pigs. J Anim Sci. 2007;85:E36–44. doi: 10.2527/jas.2006-578. [DOI] [PubMed] [Google Scholar]
- 51.Robertson SA, Guerin LR, Moldenhauer LM, Hayball JD. Activating T regulatory cells for tolerance in early pregnancy - the contribution of seminal fluid. J Reprod Immunol. 2009;83:109–116. doi: 10.1016/j.jri.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Melbye M, Ingerslev J, Biggar RJ, Alexander S, Sarin PS, Goedert JJ, Zachariae E, Ebbesen P, Stenbjerg S. Anal intercourse as a possible factor in heterosexual transmission of HTLV-III to spouses of hemophiliacs. N Engl J Med. 1985;312:857. doi: 10.1056/NEJM198503283121313. [DOI] [PubMed] [Google Scholar]
- 53.Goedert JJ, Eyster ME, Biggar RJ, Blattner WA. Heterosexual transmission of human immunodeficiency virus: association with severe depletion of T-helper lymphocytes in men with hemophilia. AIDS Res Hum Retroviruses. 1987;3:355–361. doi: 10.1089/aid.1987.3.355. [DOI] [PubMed] [Google Scholar]
- 54.Kreiss JK, Kitchen LW, Prince HE, Kasper CK, Essex M. Antibody to human T-lymphotropic virus type III in wives of hemophiliacs. Evidence for heterosexual transmission. Ann Intern Med. 1985;102:623–626. doi: 10.7326/0003-4819-102-5-623. [DOI] [PubMed] [Google Scholar]
- 55.Dayal MB, Wheeler J, Williams CJ, Barnhart KT. Disruption of the upper female reproductive tract epithelium by nonoxynol-9. Contraception. 2003;68:273–279. doi: 10.1016/s0010-7824(03)00178-1. [DOI] [PubMed] [Google Scholar]
- 56.Barnhart KT, Stolpen A, Pretorius ES, Malamud D. Distribution of a spermicide containing Nonoxynol-9 in the vaginal canal and the upper female reproductive tract. Hum Reprod. 2001;16:1151–1154. doi: 10.1093/humrep/16.6.1151. [DOI] [PubMed] [Google Scholar]
- 57.Rahangdale L, Greenblatt RM, Perry J, Darragh TM, Kobayashi A, Smith-McCune KK. In vivo effects of nonoxynol-9 on endometrial immune cell populations. J Acquir Immune Defic Syndr. 2009;52:137–139. doi: 10.1097/QAI.0b013e3181b05d3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saidi H, Magri G, Nasreddine N, Requena M, Belec L. R5- and X4-HIV-1 use differentially the endometrial epithelial cells HEC-1A to ensure their own spread: implication for mechanisms of sexual transmission. Virology. 2007;358:55–68. doi: 10.1016/j.virol.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 59.Saidi H, Magri G, Carbonneil C, Bouhlal H, Hocini H, Belec L. Apical interactions of HIV type 1 with polarized HEC-1 cell monolayer modulate R5-HIV type 1 spread by submucosal macrophages. AIDS Res Hum Retroviruses. 2009;25:497–509. doi: 10.1089/aid.2008.0156. [DOI] [PubMed] [Google Scholar]
- 60.Asin SN, Fanger MW, Wildt-Perinic D, Ware PL, Wira CR, Howell AL. Transmission of HIV-1 by primary human uterine epithelial cells and stromal fibroblasts. J Infect Dis. 2004;190:236–245. doi: 10.1086/421910. [DOI] [PubMed] [Google Scholar]
- 61.Asin SN, Eszterhas SK, Rollenhagen C, Heimberg AM, Howell AL. HIV type 1 infection in women: increased transcription of HIV type 1 in ectocervical tissue explants. J Infect Dis. 2009;200:965–972. doi: 10.1086/605412. [DOI] [PubMed] [Google Scholar]
- 62.Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005;73:1253–1263. doi: 10.1095/biolreprod.105.043133. [DOI] [PubMed] [Google Scholar]
- 63.White HD, Musey LK, Andrews MM, Yeaman GR, DeMars LR, Manganiello PD, Howell AL, Wira CR, Green WR, McElrath MJ. Human immunodeficiency virus-specific and CD3-redirected cytotoxic T lymphocyte activity in the human female reproductive tract: lack of correlation between mucosa and peripheral blood. J Infect Dis. 2001;183:977–983. doi: 10.1086/319253. [DOI] [PubMed] [Google Scholar]
- 64.Aghajanova L, Hamilton AE, Giudice LC. Uterine receptivity to human embryonic implantation: histology, biomarkers, and transcriptomics. Semin Cell Dev Biol. 2008;19:204–211. doi: 10.1016/j.semcdb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.White HD, Crassi KM, Givan AL, Stern JE, Gonzalez JL, Memoli VA, Green WR, Wira CR. CD3+ CD8+ CTL activity within the human female reproductive tract: influence of stage of the menstrual cycle and menopause. J Immunol. 1997;158:3017–3027. [PubMed] [Google Scholar]
- 66.White HD, Yeaman GR, Givan AL, Wira CR. Mucosal immunity in the human female reproductive tract: cytotoxic T lymphocyte function in the cervix and vagina of premenopausal and postmenopausal women. Am J Reprod Immunol. 1997;37:30–38. doi: 10.1111/j.1600-0897.1997.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 67.Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS. 2008;22:1909–1917. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yeaman GR, Howell AL, Weldon S, Demian DJ, Collins JE, O’Connell DM, Asin SN, Wira CR, Fanger MW. Human immunodeficiency virus receptor and coreceptor expression on human uterine epithelial cells: regulation of expression during the menstrual cycle and implications for human immunodeficiency virus infection. Immunology. 2003;109:137–146. doi: 10.1046/j.1365-2567.2003.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yeaman GR, Asin S, Weldon S, Demian DJ, Collins JE, Gonzalez JL, Wira CR, Fanger MW, Howell AL. Chemokine receptor expression in the human ectocervix: implications for infection by the human immunodeficiency virus-type I. Immunology. 2004;113:524–533. doi: 10.1111/j.1365-2567.2004.01990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yeaman GR, Guyre PM, Fanger MW, Collins JE, White HD, Rathbun W, Orndorff KA, Gonzalez J, Stern JE, Wira CR. Unique CD8+ T cell-rich lymphoid aggregates in human uterine endometrium. J Leukoc Biol. 1997;61:427–435. [PubMed] [Google Scholar]
- 71.Yokoyama WM. Mistaken notions about natural killer cells. Nat Immunol. 2008;9:481–485. doi: 10.1038/ni1583. [DOI] [PubMed] [Google Scholar]
- 72.Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev. 2005;206:306–335. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 73.Saito S, Shiozaki A, Sasaki Y, Nakashima A, Shima T, Ito M. Regulatory T cells and regulatory natural killer (NK) cells play important roles in feto-maternal tolerance. Semin Immunopathol. 2007;29:115–122. doi: 10.1007/s00281-007-0067-2. [DOI] [PubMed] [Google Scholar]
- 74.Shacklett BL. Cell-mediated immunity to HIV in the female reproductive tract. J Reprod Immunol. 2009;83:190–195. doi: 10.1016/j.jri.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tezuka H, Abe Y, Iwata M, Takeuchi H, Ishikawa H, Matsushita M, Shiohara T, Akira S, Ohteki T. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature. 2007;448:929–933. doi: 10.1038/nature06033. [DOI] [PubMed] [Google Scholar]
- 76.He B, Qiao X, Klasse PJ, Chiu A, Chadburn A, Knowles DM, Moore JP, Cerutti A. HIV-1 envelope triggers polyclonal Ig class switch recombination through a CD40-independent mechanism involving BAFF and C-type lectin receptors. J Immunol. 2006;176:3931–3941. doi: 10.4049/jimmunol.176.7.3931. [DOI] [PubMed] [Google Scholar]
- 77.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 78.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 79.Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, Kolls JK, Dandekar S, Baumler AJ. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dandekar S, George MD, Baumler AJ. Th17 cells, HIV and the gut mucosal barrier. Curr Opin HIV AIDS. 2010;5:173–178. doi: 10.1097/COH.0b013e328335eda3. [DOI] [PubMed] [Google Scholar]
- 81.Shen W, Durum SK. Synergy of IL-23 and Th17 cytokines: new light on inflammatory bowel disease. Neurochem Res. 2010;35:940–946. doi: 10.1007/s11064-009-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rovedatti L, Kudo T, Biancheri P, Sarra M, Knowles CH, Rampton DS, Corazza GR, Monteleone G, Di Sabatino A, Macdonald TT. Differential regulation of interleukin 17 and interferon gamma production in inflammatory bowel disease. Gut. 2009;58:1629–1636. doi: 10.1136/gut.2009.182170. [DOI] [PubMed] [Google Scholar]
- 83.Peron JP, de Oliveira AP, Rizzo LV. It takes guts for tolerance: the phenomenon of oral tolerance and the regulation of autoimmune response. Autoimmun Rev. 2009;9:1–4. doi: 10.1016/j.autrev.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 84.Hirata T, Osuga Y, Takamura M, Kodama A, Hirota Y, Koga K, Yoshino O, Harada M, Takemura Y, Yano T, Taketani Y. Recruitment of CCR6-expressing Th17 cells by CCL 20 secreted from IL-1 beta-, TNF-alpha-, and IL-17A-stimulated endometriotic stromal cells. Endocrinology. 2010;151:5468–5476. doi: 10.1210/en.2010-0398. [DOI] [PubMed] [Google Scholar]
- 85.Wang WJ, Hao CF, Yi L, Yin GJ, Bao SH, Qiu LH, Lin QD. Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J Reprod Immunol. 2010;84:164–170. doi: 10.1016/j.jri.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 86.Ferre AL, Lemongello D, Hunt PW, Morris MM, Garcia JC, Pollard RB, Yee HF, Jr, Martin JN, Deeks SG, Shacklett BL. Immunodominant HIV-specific CD8+ T-cell responses are common to blood and gastrointestinal mucosa, and Gag-specific responses dominate in rectal mucosa of HIV controllers. J Virol. 2010;84:10354–10365. doi: 10.1128/JVI.00803-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.UNAIDS. AIDS Epidemic Update: November 2009. Geneva, Switzerland: UNAIDS; 2009. pp. 1–100. [Google Scholar]
- 88.Critchfield JW, Lemongello D, Walker DH, Garcia JC, Asmuth DM, Pollard RB, Shacklett BL. Multifunctional HIVgag Specific CD8+ T-cell Responses in Rectal Mucosa and PBMC During Chronic HIV-1 Infection. J Virol. 2007;81:5460–5471. doi: 10.1128/JVI.02535-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ferre AL, Hunt PW, Critchfield JW, Young DH, Morris MM, Garcia JC, Pollard RB, Yee HF, Jr, Martin JN, Deeks SG, Shacklett BL. Mucosal immune responses to HIV-1 in elite controllers: a potential correlate of immune control. Blood. 2009;113:3978–3989. doi: 10.1182/blood-2008-10-182709. [DOI] [PMC free article] [PubMed] [Google Scholar]