SUMMARY
This study investigated the influence of fiber size on the distribution of nuclei and fiber growth patterns in white muscle of black sea bass, Centropristis striata, ranging in body mass from 0.45 to 4840 g. Nuclei were counted in 1 μm optical sections using confocal microscopy of DAPIand Acridine-Orange-stained muscle fibers. Mean fiber diameter increased from 36±0.87 μm in the 0.45 g fish to 280±5.47 μm in the 1885 g fish. Growth beyond 2000 g triggered the recruitment of smaller fibers, thus significantly reducing mean fiber diameter. Nuclei in the smaller fibers were exclusively subsarcolemmal (SS), whereas in larger fibers nuclei were more numerous and included intermyofibrillar (IM) nuclei. There was a significant effect of body mass on nuclear domain size (F=118.71, d.f.=3, P<0.0001), which increased to a maximum in fish of medium size (282–1885 g) and then decreased in large fish (>2000 g). Although an increase in the number of nuclei during fiber growth can help preserve the myonuclear domain, the appearance of IM nuclei during hypertrophic growth seems to be aimed at maintaining short effective diffusion distances for nuclear substrates and products. If only SS nuclei were present throughout growth, the diffusion distance would increase in proportion to the radius of the fibers. These observations are consistent with the hypothesis that changes in nuclear distribution and fiber growth patterns are mechanisms for avoiding diffusion limitation during animal growth.
KEY WORDS: white muscle, nuclear distribution, diffusion constraint, hypertrophic growth
INTRODUCTION
Cells are generally small (Prescott, 1956; Conlon and Raff, 1999), and size limitation is thought to be due to diffusion constraints (Johnston, 2006; Kinsey et al., 2007; Kinsey et al., 2011). Exceptions include skeletal muscle fibers, which are some of the largest cells found in the animal kingdom (Kinsey et al., 2007). In mammalian embryonic development, primordial muscle cells (myoblasts) fuse to form myotubes (Pizon et al., 2005). Myotubes have striations, an axial core of pale cytoplasm and centrally located nuclei (Boyd, 1968). When these cells attain complete transverse striations and their nuclei become located at the periphery they are considered muscle fibers (Boyd, 1968), which are generally categorized by a variety of criteria including: fiber color (white, red or pink), myosin isoforms, metabolic capacity (oxidative versus glycolytic) and contractile rate (fast versus slow). The frequency and distribution of these various muscle fiber types have been reviewed by Sänger and Stoiber (Sänger and Stoiber, 2001) and Rowlerson and Veggetti (Rowlerson and Veggetti, 2001). In fish, muscle fiber types tend to occur in discrete patterns with the majority of myotomal muscle consisting of fast-contracting white glycolytic fibers for burst swimming and slow-contracting red oxidative muscle fibers for cruising (Goldspink et al., 2001).
These syncytial cells continue to grow by the fusion of satellite cells to the muscle fiber (Mauro, 1961; Moss and Leblond, 1970). The nuclei of these fused satellite cells become attached to the sarcolemma (Allen et al., 1999) and are therefore referred to as subsarcolemmal (SS). The resulting addition of nuclei increases the growth potential and synthetic capability of the muscle cell, allowing it to add more myofibrils and thus increase in size. This type of growth is termed hypertrophic growth. In fishes, red muscle fibers typically have relatively small diameters whereas most white fibers have larger diameters (Sänger and Stoiber, 2001). As an alternative to growing through an increase of cell size, muscle tissue can grow by the addition of new muscle fibers, referred to as hyperplasic growth. In certain fishes, the continuous growth of existing muscle fibers occurs simultaneously with the appearance of new, smaller fibers, a phenomenon referred to as mosaic hyperplasia (Rowlerson and Veggetti, 2001; Johnston et al., 2004; Johnston, 2006). Weatherley and Gill reported that, in rainbow trout up to 50 cm in body length, most of the increase in anaerobic white muscle is due to recruitment of new fibers (hyperplasia), but beyond 50 cm muscle growth is a result of increasing the diameter of existing fibers (hypertrophy) (Weatherley and Gill, 1981; Weatherley and Gill, 1987).
In multi-nucleated muscle fibers it is thought that each nucleus services a volume of cytoplasm known as the myonuclear domain (MND) (Hall and Ralston, 1989). It was once believed that, because the DNA content increased with cell size (Gregory, 2001), there was a fixed cytoplasm to nucleus ratio. Recent studies, however, have demonstrated that MND size is not always constant (Allen et al., 1995; Allen et al., 1996; Ohira et al., 1999; Ohira et al., 2001; Rosser et al., 2002; Wada et al., 2003; Liu et al., 2008). Wada et al. reported that MND size was not conserved during hypertrophic growth and that MND size was not always a determinant factor of muscle fiber size (Wada et al., 2003). Liu et al. reported that MND scales positively with body mass (Liu et al., 2008). Rosser et al. demonstrated variation in MND size along the length of muscle fibers and an increase in MND size with age (Rosser et al., 2002). Furthermore, several studies have revealed a decrease in MND size with muscle wasting or atrophy (Allen et al., 1995; Allen et al., 1996; Ohira et al., 1999; Ohira et al., 2001; Bruusgaard and Gundersen, 2008; Gundersen and Bruusgaard, 2008).
Early studies found that mRNA and large proteins remain close to the nuclei of origin while smaller, more soluble proteins can readily diffuse throughout the cell (Hall and Ralston, 1989; Pavlath et al., 1989; Russell and Dix, 1992). These studies emphasized the significance of diffusion of nuclear products and how diffusion constraints may limit the range over which an individual nucleus can influence cellular processes. Because most of the increase in muscle tissue mass typically occurs by hypertrophy rather than hyperplasia, avoiding a metabolically limiting nuclear domain size requires increasing the number of nuclei with increasing fiber volume (Enesco and Puddy, 1964; Moss, 1968; Goldspink, 1964; Stickland et al., 1975; Weatherley et al., 1979; Weatherley and Gill, 1981; Weatherley and Gill, 1985; Allen et al., 1995; Allen et al., 1996; McCall et al., 1998; Roy et al., 1999).
Although in embryonic mammals myonuclei are found in the interior portion of the muscle cell [intermyofibrillar (IM) nuclei] (Boyd, 1968; Rosser et al., 2002), in post-embryonic mammals myonuclei generally have an exclusively SS distribution. IM nuclei are rare and usually associated with diseases (Bruusgaard et al., 2003; Ralston et al., 2006). However, IM nuclei have also been reported in cases of extreme muscle fiber hypertrophy in humans (Kadi et al., 1999), chickens (Rosser et al., 2002) and crustaceans (Hardy et al., 2009; Hardy et al., 2010). Furthermore, in mature avian muscle, IM nuclei are normally present along with SS nuclei (George and Berger, 1966).
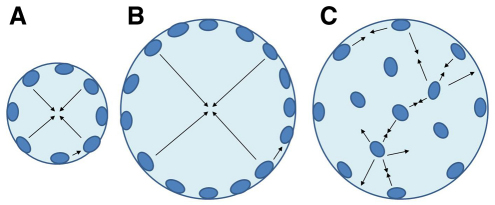
The regulatory mechanisms that govern the location of nuclei are unresolved, but recent studies indicate that the anchoring and spacing of nuclei is controlled by binding between nuclear envelope membrane proteins and cytoskeletal elements (Apel et al., 2000; Bruusgaard et al., 2003; Bruusgaard et al., 2006; Grady et al., 2005; Starr, 2007; Gundersen and Bruusgaard, 2008; Starr, 2009). Maximal intracellular diffusion distances within the MND have, in fact, been implicated in the control of SS nuclear distribution in mammalian muscle (Bruusgaard et al., 2003; Bruusgaard et al., 2006), although it is not possible to fully preserve both MND size and diffusion distances with exclusively SS nuclei. That is, as muscle fibers grow, the increase in SS nuclei due to satellite cell recruitment can promote a conservation of MND volume, but the maximal diffusion distances within that domain will increase with fiber size, being essentially equal to the radius of the fiber (Fig. 1A,B).
Fig. 1.
Schematic of a black sea bass small muscle fiber with only subsarcolemmal (SS) nuclei (A) and larger fibers with only SS nuclei (B) or with SS and intermyofibrillar (IM) nuclei (C). Arrows indicate diffusion distances.
Most studies of muscle growth have examined mammalian muscle cells that have maximal diameters of <100 μm. In contrast, many teleost fishes have post-embryonic increases in body mass that span several orders of magnitude, and when this is coupled to hypertrophic growth, anaerobic white muscle fibers from adult animals may have diameters exceeding 400 μm (Battram and Johnston, 1991; Johnston, 2006; Nyack et al., 2007). Thus, intracellular diffusion distances in fish anaerobic white muscle increase dramatically during animal growth.
Crustaceans also undergo an extreme increase in body mass and white muscle fiber size during growth, and the increase in fiber diameter is associated with a shift in the distribution of both mitochondria and nuclei (Boyle et al., 2003; Hardy et al., 2009; Hardy et al., 2010). As the muscle fiber diameter in crabs increases and exceeds a certain size, mitochondria change their distribution from being evenly distributed across the cell to being restricted to the SS region in large cells. This was interpreted as being a response to oxygen diffusion constraints (Boyle et al., 2003; Hardy et al., 2009). An opposing pattern has been seen with nuclei, which are not directly dependent on oxygen. Their distribution was found to shift from being exclusively SS in small, white muscle fibers to being evenly distributed across large fibers (Hardy et al., 2009). Using reaction-diffusion mathematical models, Hardy et al. demonstrated that rates of both mitochondrial and nuclear processes would be much slower in the large fibers if not for these shifts in organelle distribution, further implying that diffusion influences both mitochondrial and nuclear positioning (Hardy et al., 2009). Mitochondrial and nuclear distributions have since been found to be dependent on fiber size in both anaerobic and aerobic fibers in a number of species of crustaceans (Hardy et al., 2010). The addition of SS nuclei contributes to reducing the MND size, but in the absence of IM nuclei, the maximal diffusion distance for SS nuclei is still equal to the radius of the fiber (Fig. 1B), which would be >100 μm in the largest fibers of fishes and crustaceans. However, the appearance of IM nuclei as fibers increase in diameter would greatly reduce the maximal diffusion distance for nuclear products (Fig. 1C) (Hardy et al., 2009; Hardy et al., 2010).
Black sea bass, Centropristis striata (Linnaeus 1758), have white muscle fibers that also undergo extreme hypertrophic growth that is comparable to that seen in crustaceans. In fact, previously reported reaction-diffusion mathematical modeling of aerobic metabolism suggested that anaerobic white muscle fibers in adults of this species are near the brink of diffusion limitation (Kinsey et al., 2007; Locke and Kinsey, 2008). Furthermore, Nyack et al. found an ontogenetic shift in mitochondrial distribution in black sea bass anaerobic white muscle fibers that was similar to that observed in crustaceans (Nyack et al., 2007). The present study was designed to determine whether black sea bass anaerobic white muscle fibers also undergo a change in nuclear distribution during hypertrophic growth.
MATERIALS AND METHODS
Animal maintenance
Black sea bass ranging from 0.45 to 4840 g (N=35) in body mass were obtained from the University of North Carolina Wilmington (UNCW) Aquaculture Facility (Wrightsville Beach, NC, USA) and wild fish were provided by Dr F. S. Scharf (UNCW). Fish were maintained and processed according to UNCW Institutional Animal Care and Use Committee standards. For ontogenetic comparison of nuclear distribution 16 fish were used, and for growth rate comparison 22 fish were used (three of these fish were the same for both studies).
Dissection
Animals were anesthetized with tricaine methanesulfonate (MS-222; Argent Laboratories, Redmond, WA, USA) diluted in filtered seawater to <1000 mg l–1. Fish body mass and total length were recorded and anesthetized animals were killed via decapitation. Scales were removed from the dorsolateral surface and a rectangular section of skin parallel to the dorsal fin was cut and peeled away. Small, rectangular pieces of epaxial white muscle were excised from the frontal region parallel to the fiber's long-axis orientation and fixed for 24 h in 4% paraformaldehyde in Sörensen's phosphate buffered saline (PBS), pH 7.4. To determine fiber type, frozen sections of fresh unfixed tissue were stained for succinate dehydrogenase (Presnell and Schreibman, 1997; Hardy et al., 2010) and myosin ATPase (Hermanson and Hurley, 1990; Dearolf et al., 2000; Etnier et al., 2004) (data not shown). Both stains confirmed that all the fibers were anaerobic, fast glycolytic white fibers. To confirm the presence of IM nuclei, teased fibers were fixed in 2.5% glutaraldehyde and 1.5% paraformaldehyde in PBS and processed for transmission electron microscopy (TEM) using the protocol from Nyack et al. (Nyack et al., 2007). We also sampled two cohorts of fish of the same age (∼500 days old) but of different body mass, thereby allowing for comparisons between fish with fast (N=10; mean body mass=357 g) and slow (N=10; mean body mass=90 g) growth rates. Additionally, we obtained fish from the same cohort of slow-growing fish 100 days later (N=2; mean body mass=282 g, age=600 days old), allowing for a comparison of fish of similar body size but of different ages.
Sectioning and staining
Fixed tissue destined for frozen sectioning was rinsed in a 5% sucrose solution followed by overnight infiltration in 30% sucrose. Tissue was trimmed, embedded in Tissue-Tek optimum cutting temperature compound (Sakura Finetek Inc., Torrance, CA, USA) and frozen for 20–40 s in isopentane (2-methylbutane; Fisher Scientific, Pittsburgh, PA, USA) cooled with liquid nitrogen. Frozen tissue was sectioned at 30 μm at –19°C with a Reichert-Jung Cryocut 1800 cryostat (Leica Microsystems Inc., Bannockburn, IL, USA). Sections were placed on Superfrost PLUS slides (Fischer Scientific, Pittsburgh, PA, USA) and allowed to air dry for ∼15 min. A systematic random sampling method (Howard and Reed, 1998) was used to ensure accurate representation of the nuclear distribution across the entire length of the fiber.
Sections were rinsed for 5 min in deionized (DI) water, incubated for 5 min in 0.001% Acridine Orange (Sigma, St Louis, MO, USA) in DI water at pH 4.0 to label the myofilaments in fibers, rinsed for 5 min in PBS, incubated for 15 min in 300 nmol l–1 4′,6-diamidino-2-phenylindole (DAPI) (Molecular Probes, Eugene, OR, USA) in PBS to label the nuclei, and then rinsed for 15 min in PBS. Coverslips were then mounted using 1:9 tris:glycerol.
Imaging and measurements
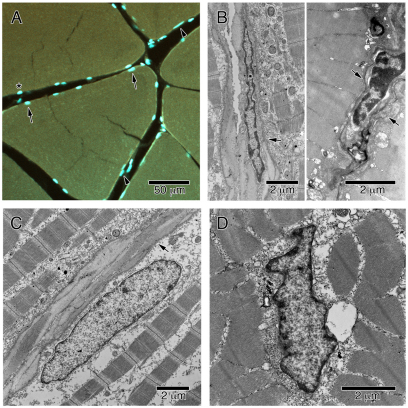
Sections were examined with an Olympus FV1000 laser scanning confocal microscope (Olympus, Center Valley, PA, USA). Optical slices 1 μm thick were simultaneously collected on three channels: one for the 405 nm (DAPI) signal, one for the 488 nm (Acridine Orange) signal and one for the differential interference contrast (DIC) signal. Stacks of 20–25 images of fiber cross sections were collected and viewed using Olympus Fluoview v1.6a software. Nuclei from complete fibers (∼100 fibers per fish) were classified as either SS, if they were intracellular, surrounded by myofibrils and in direct contact with the sarcolemma, or IM, if they were not in contact with the sarcolemma throughout the stack. Using our criteria, SS nuclei could be distinguished from satellite cells and connective tissue cells all but 0.25% of the time. Acridine Orange, which stained myofibrils, assisted in identification of intracellular versus extracellular nuclei because intracellular nuclei were at least partially surrounded by myofibrils (Fig. 2A). Furthermore, the DIC images collected simultaneously helped delineate the fiber boundary. Acridine Orange has been shown to provide good contrast between cytoplasm and fluorescently labeled nuclei in whole (isolated) muscle cells (Allen et al., 1996; Allen et al., 1999; Ohira et al., 1999; Roy et al., 1999) and the same was found to be true for tissue sections. In addition, the morphology of each nucleus helped determine whether a peripherally located nucleus was truly SS. Nuclei with a thin, spindle-shape morphology tended to be extracellular (Fig. 2B) whereas SS nuclei tended to be more rod shaped (Fig. 2C), as confirmed by TEM. Finally, if the exact location of the nucleus could not be determined, a classification was systematically assigned. The fiber was divided into four quadrants, and nuclei that were not completely surrounded by Acridine Orange and appeared to be intracellular on the DIC channel were classified based on their quadrant as either intracellular or extracellular, thus randomizing the error to prevent systematic bias. Fiber ends (adjacent to myosepta) and obviously damaged areas of muscle were also excluded from analysis. These above criteria were used to differentiate SS nuclei from satellite cells despite the fact that satellite cells have been reported to represent only 1–5% of all myonuclei (Edgerton and Roy, 1991; Rosser et al., 2002; Liu et al., 2008). Furthermore, Battram and Johnston found that satellite cells were extremely rare in muscle from an Antarctic teleost fish (Notothenia neglecta), suggesting that this may not be a major source of error in fish muscle (Battram and Johnston, 1991). TEM was also used to confirm that DAPI-stained organelles in the interior of the cell (see below) were IM nuclei (Fig. 2D).
Fig. 2.
Laser confocal micrograph of black sea bass anaerobic white muscle fibers with SS and satellite cell nuclei (A). TEM micrographs of satellite cell (B), SS nucleus (C) and IM nucleus (D). Arrows in A indicate SS nuclei, arrowheads are satellite cells and the asterisk is an example of a nucleus that was difficult to characterize and was therefore classified as either intraor extra-fiber depending on the image quadrant in which it occurred (see Materials and methods for additional details). Arrows in B and C indicate the sarcolemma.
Images of muscle fibers were outlined with an Intuos 3 tablet (Wacom Co., Ltd, Vancouver, WA, USA) in Adobe Photoshop (version 7.0; Adobe Systems Inc., San Jose, CA, USA) and resulting polygons were analyzed with ImagePro Plus (v. 6.1.0.346; Media Cybernetics Inc., Bethesda, MD, USA) to determine their cross-sectional area (CSA), perimeter and mean diameter. The mean diameter was determined by taking the mean of the distances across the cell through the centroid in 2 deg increments around the perimeter of the cell.
Myonuclei per millimeter of fiber, myonuclear domain and nuclear density
To calculate the number of myonuclei per millimeter of fiber (X), we used the formula of Schmalbruch and Hellhammer (Schmalbruch and Hellhammer, 1977):
| (1) |
where N is the number of myonuclei in a fiber cross-section, L is the desired length of segment, d is the thickness of the section and l is the mean length of a muscle nucleus. L was set at 1000 μm. The optical thickness of each image was used as d (1 μm) and l was calculated from longitudinal sections for each individual class size. From this X-value we calculated the volume of cytoplasm per myonucleus (myonuclear domain) (Y):
| (2) |
where C is the CSA of the muscle fiber, L is the length of the fiber segment to be considered (1000 μm) and X is the number of myonuclei per millimeter of fiber determined from Eqn 1. Nuclear density (number of nuclei per μm3 of fiber) was calculated as the reciprocal of myonuclear domain (1/Y).
Myonuclear length
To determine the length of myonuclei, longitudinal sections (stained in the same manner as the cross sections) were used. The length of myonuclei was measured for fish weighing 4, 30, 310, 1885 and 3200 g. Between 68 and 99 nuclei were measured per fish. The lowest nuclear length was 9.39±2.78 μm for fish with a body mass of 3200 g, and the highest nuclear length was 13.02±2.81 μm for fish with a body mass of 4 g. A slight but significant negative linear relationship was found; therefore, we used the equation Ln=11.92–0.00091Mb (r2=0.11, P<0.0001), where Ln is nuclear length and Mb is body mass, to calculate nuclear lengths for the other fish rather than using a grand mean value. In the same manner, cross sections were used to determine the mean diameter of myonuclei. Between 103 and 119 nuclei were measured per fish. A slight but significant positive linear relationship was found (nuclear diameter=3.94+0.00061Mb) (r2=0.39; P<0.0001). Together these results indicate that nuclei in juvenile fish were longer and narrower than in adults.
Statistical analysis
All statistical analyses were performed using JMP 7.0.7 (SAS Institute, Cary, NC, USA). ANOVA was used to test for group effects and, where differences were detected, t-tests were used to make pairwise comparisons of the means. Bartlett's test was used to assess differences between variances (Zar, 1999). Linear regression analysis was used to evaluate continuous data. A non-linear model was used to evaluate the relationship of cell diameter to body mass, in which the line is defined by the dependence of the diameter of cylindrical muscle fibers on body mass, assuming hypertrophic muscle growth (M1/3b×b1), where b1 is a regression coefficient. P<0.05 was considered significant in all cases. For ease of visualization of plots of fiber size, myonuclear domain and number volume by body size, animals were grouped in four classes according to body mass: extra small (0.4–4 g), small (30–114.33 g), medium (279–1885 g) and large (2258–4840 g).
RESULTS
Body mass and fiber diameter
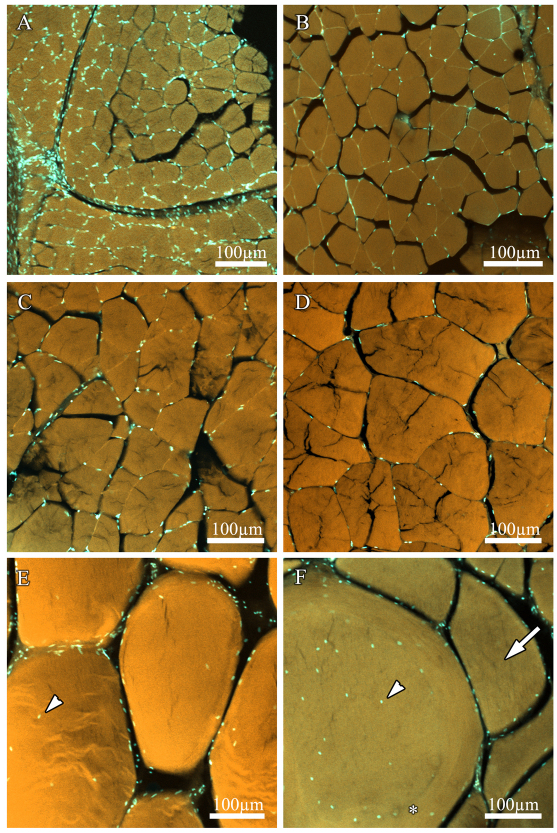
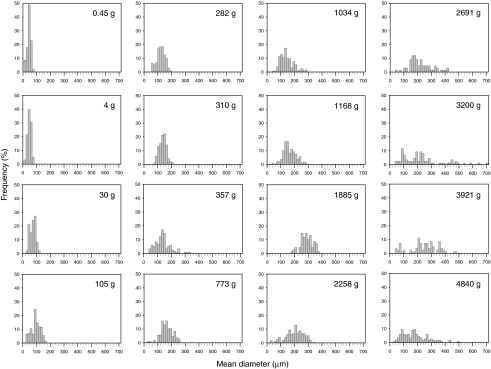
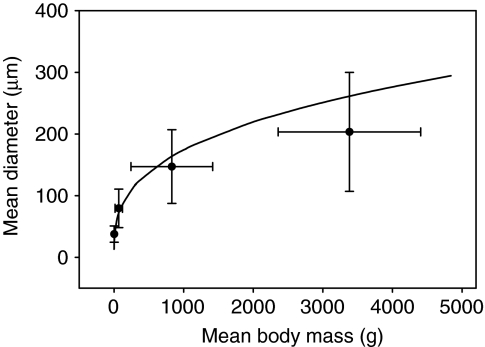
There was a significant positive relationship between body mass and mean fiber diameter (ANOVA, F=167.78, d.f.=15, P<0.0001). Figs 3, 4, 5 demonstrate that the increase in muscle mass in smaller fish occurred via hypertrophic fiber growth. Hypertrophic growth and a relatively uniform fiber diameter are illustrated in Fig. 3A–E, whereas a notable difference in fiber diameter can be seen in Fig. 3F, indicating that the addition of smaller fibers is occurring in large fish. As black sea bass increased in body size, mean muscle cell diameter proportionally increased from 36±0.87 μm in the 0.45 g fish to 280±5.47 μm in the 1885 g fish (Fig. 4). Fig. 4 also demonstrates that the frequencies of fiber diameters approached a bimodal distribution in fish ranging from 2691 to 4840 g, indicating the addition of small fibers (<100 μm in diameter) or the splitting of larger fibers. This resulted in a significant increase in fiber size variation in fish from the large size class (Bartlett's test, F=101.59, d.f.=15, P<0.0001). It also resulted in a decrease in the mean fiber diameter as fish increased in body mass from 1885 to 2258 g (Fig. 4). Fig. 5 shows that mean fiber diameter initially increased with increasing body mass as predicted for a cylindrical fiber (M1/3b×b1) until reaching 1885 g (within the medium size class). Fitting the fiber growth curve to the first three size classes (Mb<1885 g), where growth occurs through hypertrophy only, yields a predicted fiber size that compares well to experimental data (r.m.s.e.=41.68, b1=17.41) (Fig. 5). However, mean fiber diameters of the fish in the large size class with body mass >2258 g fall below the projected hypertrophic growth line and have increased variance due to the onset of either fiber splitting or hyperplasia.
Fig. 3.
Anaerobic white muscle fiber cross sections from black sea bass with body masses of 0.45 g (A), 4 g (B), 30 g (C), 310 g (D), 1885 g (E) and 3200 g (F) with DAPI-stained nuclei and Acridine-Orange-stained fiber cytoplasm. Note that fibers from smaller fish grew hypertrophically into larger fibers, and hyperplasia (appearance of new, smaller fibers) and/or fiber splitting was observed in the largest size classes (arrow in F). Changes in nuclear distribution were also apparent. Fibers from juvenile fish (A–D) contained low numbers of almost exclusively SS nuclei, whereas as the fish and muscle fibers grew, numbers of IM nuclei increased (arrowheads in E,F). Scale bar, 100 μm.
Fig. 4.
Frequency of anaerobic white muscle fiber mean diameters in black sea bass ranging from 0.45 to 4840 g (N=16).
Fig. 5.
Mean fiber diameters in anaerobic white muscle of black sea bass with body masses ranging from 0.45 to 4840 g (N=16). The solid line represents a projected hypertrophic growth line (r.m.s.e.=41.68). Note that large fish (>2000 g) have mean diameters falling below the projected hypertrophic growth line and have a greater standard deviation due to the appearance of small fibers. The line is defined by the dependence of the diameter of a cylinder on its volume (Mb1/3×b1, where b1=17.41). See Materials and methods for additional details. Data are means ± s.d. of the four size classes: very small (0.4–4 g), small (30–105 g), medium (282–1885 g) and large (2258–4840 g).
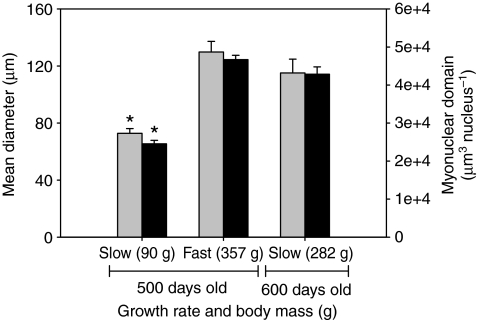
Examination of fiber diameters of ageversus size-matched fish indicated that mass, not age, determines fiber diameter (Fig. 6). The mean fiber diameter for white muscle fibers of slow-growing fish with a mean mass of 90 g was significantly lower than that of white muscle fibers of slow-growing fish with a mean mass of 282 g and fast-growing fish with a mean mass of 357 g (ANOVA, F=23.61, d.f.=2, P<0.0001).
Fig. 6.
Mean fiber diameters (gray bars) and myonuclear domain (MND; black bars) in anaerobic white muscle fibers of black sea bass grouped as slow-growing with a mean body mass of 90 g, slow-growing with a mean body mass of 282 g and fast-growing with a mean body mass of 357 g. Fish with mean body masses of 90 and 357 g were the same age (500 days), whereas fish with a mean body mass of 282 g were 100 days older. Asterisks indicate that mean diameter or MND is significantly different than that of the other groups. Data are means ± s.e.m.
Myonuclear domain size and nuclear distribution
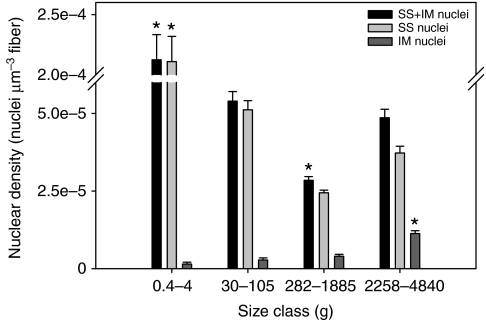
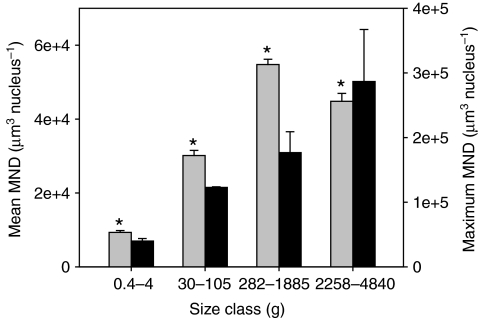
Fig. 3A–E also illustrates that nuclei are almost exclusively SS in the small fibers of juvenile fish (<120 μm), whereas IM nuclei become obvious in the largest fibers (Fig. 3F). Myonuclear domain calculations depend on the length of the nuclei; therefore, ∼100 nuclear lengths were measured for each of five fish with body masses of 4, 30, 320, 1885 and 3200 g. We found a slight but significant negative linear relationship, with the slope of the line approaching zero (Ln=11.92–0.00091Mb; slope=0.0009, r2=0.11, r.m.s.e.=3.09, P<0.0001). We therefore used the regression equation to predict the nuclear length for each animal as a function of its body mass. An ANOVA indicated a significant effect of body mass on nuclear density for total (F=113.19, d.f.=3, P<0.0001), SS (F=124.27, d.f.=3, P<0.0001) and IM nuclei (F=41.86, d.f.=3, P<0.0001) and mean myonuclear domain size (F=118.71, d.f.=3, P<0.0001) (Figs 7, 8). Maximum MND size increased with body mass (F=3.28, d.f.=3, P=0.048) (Fig. 8), as would be expected if some fibers continue to grow hypertrophically. However, mean MND size increased to a maximum in fish of medium size (279–1885 g) and then decreased in large fish (>2000 g) (Fig. 8). This pattern resulted from an increase in the density of both IM and SS nuclei in larger fish. The increase in SS nuclear density in the larger fish was primarily the result of a proliferation of small fibers that have less cytoplasmic volume, whereas the appearance of numerous IM nuclei coupled with hyperplasia/fiber splitting lowered the domain size in the largest cells. In agreement with the fiber diameter data, the MND size seems to be correlated with fish mass and not age (Fig. 6). Myonuclear domain was not different for fish with a mean mass of 282 or 357 g but was significantly smaller for fish with a mean mass of 90 g (ANOVA, F=125.46, d.f.=2, P<0.0001).
Fig. 7.
Comparison of nuclear density (number of nuclei per μm3 of fiber) in anaerobic white muscle from black sea bass ranging from 0.45 to 4840 g. Within a group (i.e. SS+IM, SS or IM), asterisks indicate a significant difference between size classes. Data are means ± s.e.m.
Fig. 8.
Comparison of mean MND (gray bars, left y-axis) and maximum MND (black bars, right y-axis) in anaerobic white muscle from black sea bass ranging from 0.45 to 4840 g. Asterisks indicate that the mean MND size from each size class is significantly different from all other size classes. For maximum MND, the only size classes different from each other are ‘very small’ and ‘large’. Data are means ± s.e.m.
The most remarkable finding in this study was that IM nuclei appeared in fibers with diameters greater than ∼120 μm. In addition, the IM nuclei of large anaerobic white muscle fibers of black sea bass were not as evenly distributed nor as numerous as in the largest anaerobic white muscle fibers of the blue crab (Hardy et al., 2009). IM nuclei were either randomly distributed or arranged in a ring pattern (asterisk in Fig. 3F).
DISCUSSION
The present study demonstrates that as anaerobic white muscle fibers from black sea bass undergo extreme hypertrophic growth they seem to reach a size beyond which myonuclei of large fibers change their distribution and IM nuclei become abundant. There appears to be a threshold (mean fiber diameter of ∼120 μm) beyond which SS nuclei are apparently unable to adequately service the entire MND, leading to the appearance of IM nuclei. Our observations also suggest that after black sea bass reach a critical mass (>2000 g), new small diameter fibers are formed. However, it is not known whether the small fibers that appear in large fish are the result of fiber splitting or mosaic hyperplasia. Consequently, the black sea bass white muscle fibers are similar to crustacean white muscle fibers (Hardy et al., 2009; Hardy et al., 2010) in that the distribution of nuclei changes with ontogeny. Furthermore, the MND increased during hypertrophic muscle growth and then decreased in the largest fish because of a combination of proliferation of SS and IM nuclei and the appearance of smaller fibers. We propose that these structural alterations in adult animals are consistent with a response to diffusion constraints, and that it is the maximal diffusion distances within the MND, and not the MND size per se, that is restrictive and leads to the appearance of IM nuclei in this model of extreme hypertrophic growth.
We found a significant positive relationship between body mass and mean fiber diameter, which is consistent with previous studies (Goldspink, 1964; Stickland et al., 1975; Weatherley et al., 1979; Weatherley and Gill, 1981; Weatherley and Gill, 1985; Johnston, 2006). Our observation that an increase in the variability of fiber size and a decrease in the mean fiber diameter in the largest black sea bass individuals (Figs 3, 4, 5), as a result of both small fiber formation (hyperplasia or fiber splitting) and continued growth of older fibers (hypertrophy), is a rare demonstration that both can be important in the adult stages of fish. Mosaic hyperplasia, the formation of new muscle fibers amongst old fibers, is typically seen in anaerobic white muscle but is the primary mechanism for increasing muscle fiber number during growth in juveniles and young adults in most species of teleost fishes (Weatherley and Gill, 1981; Weatherley and Gill, 1987; Rowlerson and Veggetti, 2001; Johnston, 2006; Johnston et al., 2004). Hyperplasia is typically found to cease when fish reach ∼44% of the final length, after which growth occurs by hypertrophy only (Weatherley et al., 1979; Weatherley and Gill, 1981; Weatherley and Gill, 1985; Weatherley and Gill, 1987; Weatherley, 1990). It should be noted that Zimmerman and Lowery reported hyperplasia in large white sea bass that had attained 61% of the maximum length for that species (Zimmerman and Lowery, 1999). However, in that study hyperplasia was observed in all size classes of fish, with a decrease in the percent of fibers displaying hyperplasia as the fish attained larger sizes (e.g. <1% in the largest specimen measuring 91.8 cm). In contrast, the present study found that black sea bass larger than 2000 g displayed both largeand small-diameter fibers. In our study, hyperplasia seemed to have ceased before fish had reached 1885 g, but then small fibers reappeared in fish larger than 2000 g. In adult fish, the appearance of small fibers can result from cell splitting, where portions of large fibers break off to form new fibers (Koumans and Akster, 1995), or from myogenesis from the fusion of muscle precursor cells (satellite cells) (Anderson, 2006). In the present study we were not able to determine whether the small fibers observed in extremely large fish were the result of fiber splitting or hyperplasia. According to Johnston, maximum fiber diameters in fishes, and therefore the extent and timing of mosaic hyperplasia, are thought to be determined by body size, activity levels and limits to the diffusion of metabolites and macromolecules (Johnston, 2006).
Results from this study's comparison of fastand slow-growing fish are in accordance with the literature (Goldspink, 1964; Stickland et al., 1975; Weatherley et al., 1979; Weatherley and Gill, 1981; Weatherley and Gill, 1985; Johnston, 2006) insofar as they indicated that fiber size correlates with fish total body mass and not with age. This is also consistent with the findings of Johnston et al., who observed that the cessation of fiber recruitment (mosaic hyperplasia) in Arctic char was body-length dependent and not age dependent (Johnston et al., 2004).
Johnston et al. speculated that the benefit of having fibers as large as possible was to minimize fiber surface area and therefore decrease the cost of maintaining the membrane potential (Johnston et al., 2004). Black sea bass anaerobic white muscle fibers are <50 μm in diameter in juveniles, but can exceed 400 μm in diameter in adults (Nyack et al., 2007). As the fibers increased in size, Nyack et al. found that mitochondria shifted from a largely IM distribution to a nearly exclusively SS distribution (Nyack et al., 2007). The authors concluded that this was in response to the need to maintain short diffusion distances for O2 at the expense of relatively long diffusion distances for small mitochondrial substrates and products, such as ADP and ATP. Similarly, in blue crab swimming muscle, small fibers (<120 μm) have IM mitochondria, whereas in large fibers (>120 μm) the mitochondria are almost exclusively SS (Boyle et al., 2003; Hardy et al., 2009). A reaction–diffusion mathematical model indicated that this change in distribution of mitochondria was necessary to support the observed rates of aerobic metabolism in adult animals (Hardy et al., 2009). However, in the same study the authors observed the opposite pattern shift for the distribution of nuclei in the blue crabs, where the nuclei were SS in small fibers and IM in large fibers. Nuclei have no direct need for O2, and their function is dependent on the relatively slow diffusion of large nuclear products. Reaction–diffusion analyses predicted that if nuclear distribution had not changed, the rates of nuclear processes would have to have been reduced by three orders of magnitude (Hardy et al., 2009). Thus, mitochondria and nuclei respond differently to changing diffusion distances, and ontogenetic shifts in organelle distribution during extreme hypertrophic growth may help reveal underlying diffusion constraints that dictate basic muscle design and function. Although we did not directly measure diffusion of large molecules or nuclear products in the present study, the redistribution of nuclei we observed in larger fibers was consistent with the concept of diffusion constraints influencing fiber design (Kinsey et al., 2007; Locke and Kinsey, 2008; Kinsey et al., 2011). In muscle fibers, nuclei supply the mRNA required for the formation of myofilaments and other proteins. Thus, if the repair of myofibrils is necessary in large fibers, having centrally located nuclei would allow for timely movement of mRNA to the regions requiring repair, assuming that proteins are synthesized at the site of replacement, as suggested by Briggs et al. (Briggs et al., 1995). If only SS nuclei were present in extremely large fibers, the periphery of the fibers would have easy access to mRNA and proteins, but the center of the fiber would have a significantly delayed or diminished supply of the nuclear products necessary to carry out protein synthesis and repair.
In agreement with the more recent literature, myonuclear domain was not conserved across all developmental sizes during extreme hypertrophic growth of black sea bass in the present study. Prior studies of mammalian muscle have used growth hormone (McCall et al., 1998) or exercise (Allen et al., 1995; Roy et al., 1999) to induce modest hypertrophy. McCall et al. found a linear relationship between fiber CSA and myonuclear number, but the hypertrophic fibers observed were only 38% larger than the control fibers (McCall et al., 1998). This is in striking contrast to the 27-fold increase in mean CSA observed in fibers from the smallest and largest black sea bass in the present study. Recent studies have shown that MND size does vary with hypertrophic growth, as well as with muscle atrophy (Allen et al., 1995; Allen et al., 1996; Ohira et al., 1999; Ohira et al., 2001; Rosser et al., 2002; Wada et al., 2003; Bruusgaard et al., 2006; Bruusgaard and Gundersen, 2008; Gundersen and Bruusgaard, 2008; Liu et al., 2008). Rosser et al. found that MND size increased during development in chick muscle, which undergoes a relatively large increase in fiber size (∼14-fold increase in CSA) during muscle growth (Rosser et al., 2002). The authors observed smaller MNDs at the terminal tips of maturing chicken pectoralis muscle fibers (Rosser et al., 2002), which are the sites of longitudinal growth according to Swatland (Swatland, 1994) and Zhang and McLennan (Zhang and McLennan, 1995). Rosser et al. also hypothesized that this may correlate with the need for greater protein synthesis at these growing tips of muscle (Rosser et al., 2002). In the blue crab, another animal with anaerobic white muscle fibers that undergo extreme hypertrophic growth, MND was conserved between juveniles and adults (Hardy et al., 2009). It must be noted that animals of intermediate size were not used in that study, only small juveniles and very large adults. In the present study, the domain increased from the smallest to the medium size classes of fish and then decreased slightly, but significantly, in the largest size class (N=5). The reduced mean MND in the largest fish (N=5) was due in part to the increased frequency of small fibers that have a smaller MND. However, the MND reduction also coincided with an increased frequency of IM nuclei. Because the calculation of MND is based on cell volume divided by total nuclei, either increasing nuclei numbers (both SS and IM) or decreasing the mean volume of a cell could reduce the MND. It is likely that both contributed to this decrease in black sea bass.
Most studies on MND during animal growth in a single species found no difference in nuclear length with body mass (Galavazi and Szirmai, 1971; Manta et al., 1987; Matthew and Moore, 1987; Rosser et al., 2002). Rosser et al. found that throughout chicken development, from neonatal to adult, myonuclei had very similar mean lengths (Rosser et al., 2002). In contrast, our study found a slight negative linear relationship between mean myonuclear length and body mass, although we do not have an obvious explanation for this pattern. Because we also found a slight but significant positive linear relationship between nuclear mean cross-sectional diameter and body mass, it appears that there are changes in nuclear shape during the very large (four orders of magnitude) increase in body mass that occurs in this species, although the functional advantage of such a change, if any, is unclear.
The distribution of nuclei in anaerobic white fibers changes markedly over the lifespan of black sea bass, with juvenile fish having small diameter fibers and low numbers of almost exclusively SS nuclei (Fig. 3A–C), whereas in the largest fibers, high numbers of IM nuclei were seen and relatively low numbers of SS nuclei were present. The 1885 g fish was the smallest animal that had numerous fibers with IM nuclei. Thus, there appears to be a threshold (mean diameter of ∼120 μm) beyond which SS nuclei are unable to carry out cellular processes, thereby leading to the appearance of IM nuclei. As fibers grow their diameters increase, and if only SS nuclei were present in large fibers, the maximal diffusion distance would be the radius of the fiber. When both SS and IM nuclei are present, the maximal diffusion distance is reduced; it is no longer the radius of the fiber, but rather a smaller distance between each nucleus and the boundary of its domain. Additionally, the observation that mosaic hyperplasia in black sea bass follows the proliferation of IM nuclei supports the notion that diffusion constraints may also trigger the formation of new fibers (Kinsey et al., 2007).
Although the 120 μm fiber diameter threshold for nuclear distribution changes is an approximation, the non-linear relationship between diffusion distance and diffusion time suggests that this is a reasonable estimate. The one-dimensional root mean square displacement of a molecule (λ) can be calculated as λ=√2Dt, where D is the diffusion coefficient and t is the diffusion time. If we assume a D of 1×10–7 cm2 s–1 for a typical macromolecule in a muscle fiber, then it will take 40 s for this molecule to diffuse across the MND of a 40-μm fiber from a juvenile fish with SS nuclei. As the fiber grows to a diameter of 120 μm, the diffusion time increases to 360 s, 320 s longer than in the juvenile. However, if the fibers were to continue to grow hypertrophically while having only SS nuclei, it would take more than 37 min for that same macromolecule to diffuse across the MND in an adult fiber with a diameter of 300 μm. The appearance of IM nuclei as fibers exceed ∼120 μm is therefore not surprising, as it reduces the diffusion distances within the MND and preserves relatively short diffusion times for nuclear products.
The presence of IM nuclei in large muscle cells and relatively low numbers of SS nuclei represent a pronounced deviation from the typical arrangement in adult mammalian skeletal muscle, where myonuclei are normally found only at the fiber periphery (Bruusgaard et al., 2003; Bruusgaard et al., 2006). This may be because the average mammalian skeletal muscle fiber diameter is <100 μm and therefore is not diffusion limited.
In contrast to the uniform pattern of IM nuclei seen in large white muscle fibers of blue crabs (Hardy et al., 2009), the IM nuclei of black sea bass had two distinct patterns: random (non-uniform) and arranged in a circular array. We suspect that the random distribution of IM nuclei arose from the release of individual SS nuclei from the sarcolemma, but not from the existing cytoskeletal elements present in the muscle fiber (microtubules). This would result in randomly distributed myonuclei in the middle of the fiber with new myofilaments being added around and peripheral to them. Alternatively, the circular pattern may be the result of a single ‘event’ that released multiple SS nuclei from the sarcolemma simultaneously. It is improbable that SS nuclei were moved or actively transported towards the center of the fiber as there are many physical barriers inside a muscle cell, such as densely packed myofibrils, mitochondria and cytoskeletal elements (Luby-Phelps, 2000; Kinsey et al., 2011). We are currently investigating the distribution and ultrastructure of the microtubule network in the anaerobic white muscle fibers of black sea bass because microtubule networks have been shown to surround nuclei in muscle fibers (Rahkila et al., 1997; Bugnard et al., 2005; Bruusgaard et al., 2006; Scholz et al., 2008).
The fact that nuclear distribution and, by extension, MND are regulated during hypertrophic growth in muscle suggests that there is an underlying mechanism governing nuclear spacing. Bruusgaard et al. (Bruusgaard et al., 2003; Bruusgaard et al., 2006) have shown that the SS distribution of nuclei in mammalian muscle is not random, and that nuclei appear to be equidistantly spaced from each other, with the exception of neuromuscular junctions, where several nuclei are anchored in close proximity to each other (Apel et al., 2000; Grady et al., 2005). Additionally, it has been proposed that diffusion of activating and/or inhibiting molecules may allow a nucleus to ‘sense’ the proximity of adjacent nuclei, which would permit the control of nuclear distribution (Bruusgaard et al., 2003). It is known that nuclei, including myonuclei, are anchored to cytoskeletal elements as well as to the cell membrane (Apel et al., 2000; Grady et al., 2005; Starr, 2007; Starr, 2009). However, the existence of IM nuclei observed in crustaceans and fish means that a mechanism for spacing and anchoring those nuclei must be extended to the interior of the muscle fiber.
Finally, our documentation of the existence of IM nuclei in large anaerobic white fibers of black sea bass is important because these IM nuclei have the effect of simultaneously reducing the MND while also decreasing the effective diffusion distance for nuclear substrates and products. Increased numbers of SS nuclei alone could accomplish the former but not the latter because the maximal diffusion distance for SS nuclei would still be the radius of the muscle cell. These changes in muscle structure and growth observed here appear to be part of a suite of responses aimed at reducing possible diffusion constraints on metabolism during hypertrophic fiber growth. By keeping diffusion distances within certain limits, the control of metabolic fluxes will continue to be dictated by the rates of reactions and not by diffusion.
ACKNOWLEDGEMENTS
The authors are grateful for the technical assistance of Mark Gay, and use of equipment from Dr Ann Pabst.
LIST OF ABBREVIATIONS
- CSA
cross-sectional area
- DAPI
4′,6-diamidino-2-phenylindole
- DI
deionized
- DIC
differential interference contrast
- IM
intermyofibrillar
- MND
myonuclear domain
- PBS
phosphate buffered saline
- SS
subsarcolemmal
- TEM
transmission electron microscopy
Footnotes
This research was supported by National Science Foundation grant IOS-0719123 (to S.T.K. and R.M.D.) and National Institute of Arthritis and Musculoskeletal and Skin Diseases grant R15-AR-052708 (to S.T.K.). Deposited in PMC for release after 12 months.
REFERENCES
- Allen D. L., Monke S. R., Talmadge R. J., Roy R. R., Edgerton V. R. (1995). Plasticity of myonuclear number in hypertrophied and atrophied mammalian skeletal muscle fibers. J. Appl. Physiol. 78, 1969-1976 [DOI] [PubMed] [Google Scholar]
- Allen D. L., Yasui W., Tanaka T., Ohira Y., Nagaoka S., Sekiguchi E., Hinds W. E., Roy R. R., Edgerton V. R. (1996). Myonuclear number and myosin heavy chain expression in rat soleus single muscle fibers after spaceflight. J. Appl. Physiol. 81, 145-151 [DOI] [PubMed] [Google Scholar]
- Allen D. L., Roy R. R., Edgerton V. R. (1999). Myonuclear domains in muscle adaptation and disease. Muscle Nerve 22, 1350-1360 [DOI] [PubMed] [Google Scholar]
- Anderson J. E. (2006). The satellite cell as a companion in skeletal muscle plasticity: currency, conveyance, clue, connector and colander. J. Exp. Biol. 209, 2276-2292 [DOI] [PubMed] [Google Scholar]
- Apel E. D., Lewis R. M., Grady R. M., Sanes J. R. (2000). Syne-1, a dystrophin- and klarsicht-relate protein ssociated with synaptic nuclei at the neuromuscular junction. J. Biol. Chem. 275, 31986-31995 [DOI] [PubMed] [Google Scholar]
- Battram J. C., Johnston I. A. (1991). Muscle growth in the Antarctic teleost, Notothenia neglecta (Nybelin). Antarct. Sci. 3, 229-233 [Google Scholar]
- Boyd J. D. (1968). Development of striated muscle. In The Structure and Function of Muscle. Vol. I, Structure (ed. Bourne G. H.), pp. 62-85 New York: Academic Press; [Google Scholar]
- Boyle K. L., Dillaman R. M., Kinsey S. T. (2003). Mitochondrial distribution and glycogen dynamics suggest diffusion constraints in muscle fibers of the blue crab, Callinectes sapidus. J. Exp. Zool. 297A, 1-16 [DOI] [PubMed] [Google Scholar]
- Briggs R. T., Scordilis S. P., Powell J. A. (1995). Myofibrillogenesis in rodent skeletal muscle in vitro: two pathways involving thick filament aggregates. Tissue Cell 27, 91-104 [DOI] [PubMed] [Google Scholar]
- Bruusgaard J. C., Gundersen K. (2008). In vivo time-lapse microscopy reveals no loss of murine myonuclei during weeks of muscle atrophy. J. Clin. Invest. 118, 1450-1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruusgaard J. C., Liestøl K., Ekmark M., Kollstad K., Gundersen K. (2003). Number and spatial distribution of nuclei in the muscle fibers of normal mice studied in vivo. J. Physiol. 551, 467-478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruusgaard J. C., Liestøl K., Gundersen K. (2006). Distribution of myonuclei and microtubules in live muscle fibers of young, middle-aged, and old mice. J. Appl. Physiol. 100, 2024-2030 [DOI] [PubMed] [Google Scholar]
- Bugnard E., Zaal K. J. M., Ralston E. (2005). Reorganization of microtubule nucleation during muscle differentiation. Cell Motil. Cytoskeleton 60, 1-13 [DOI] [PubMed] [Google Scholar]
- Conlon I., Raff M. (1999). Size control in animal development. Cell 96, 235-244 [DOI] [PubMed] [Google Scholar]
- Dearolf J. L., McLellan W. A., Dillaman R. M., Frierson D., Jr, Pabst D. A. (2000). Precocial development of axial locomotor muscle in bottlenose dolphins (Tursiops truncatus). J. Morphol. 244, 203-215 [DOI] [PubMed] [Google Scholar]
- Edgerton V. R., Roy R. R. (1991). Regulation of skeletal muscle fiber size, shape and function. J. Biomech. 24, 123-133 [DOI] [PubMed] [Google Scholar]
- Enesco M., Puddy D. (1964). Increase in the number of nuclei and weight in skeletal muscle of rats of various ages. Am. J. Anat. 114, 235-244 [DOI] [PubMed] [Google Scholar]
- Etnier S. A., Dearolf J. L., McLellan W. A., Pabst D. A. (2004). Postural role of lateral axial muscles in developing bottlenose dolphins (Tursiops truncatus). Proc. Biol. Sci. 271, 909-918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galavazi G., Szirmai J. A. (1971). Cytomorphometry of skeletal muscle: the influence of age and testosterone on the rat m. levator ani. Z. Zellforsch. 121, 507-530 [DOI] [PubMed] [Google Scholar]
- George J. C., Berger A. J. (1966). The pectoralis: histology and histochemistry. In Avian Myology (ed. George J. C., Berger A. J.), pp. 25-37 New York: Academic Press Inc. [Google Scholar]
- Goldspink G. (1964). The combined effects of muscle exercise and reduced food intake on skeletal muscle fibers. J. Cell. Comp. Physiol. 63, 209-216 [DOI] [PubMed] [Google Scholar]
- Goldspink G., Wilkes D., Ennion S. (2001). Myosin expression during ontogeny, post-hatching growth, and adaptation. In Muscle Development and Growth (ed. Johnston I. A.), pp. 43-72 San Diego, CA: Academic Press; [Google Scholar]
- Grady R. M., Starr D. A., Ackerman G. L., Sanes J. R., Han M. (2005). Syne proteins anchor muscle nuclei at the neuromuscular junction. Proc. Natl. Acad. Sci. USA 102, 4359-4364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory T. R. (2001). Coincidence, coevolution, or causation? DNA content, cell size, and the C-value enigma. Biol. Rev. Camb. Philos. Soc. 76, 65-101 [DOI] [PubMed] [Google Scholar]
- Gundersen K., Bruusgaard J. C. (2008). Nuclear domains during muscle atrophy: nuclei lost or paradigm lost? J. Physiol. 586, 2675-2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall Z. W., Ralston E. (1989). Nuclear domains in muscle cells. Cell 59, 771-772 [DOI] [PubMed] [Google Scholar]
- Hardy K. M., Dillaman R. M., Locke B. R., Kinsey S. T. (2009). A skeletal muscle model of extreme hypertrophic growth reveals the influence of diffusion on cellular design. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R1855-R1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy K. M., Lema S. C., Kinsey S. T. (2010). The metabolic demands of swimming behavior influence the evolution of skeletal muscle fiber design in the brachyuran crab family Portunidae. Mar. Biol. 157, 221-236 [Google Scholar]
- Hermanson J. W., Hurley K. J. (1990). Architectural and histochemical analysis of the Biceps brachii muscle of the horse. Acta Anat. 137, 146-156 [DOI] [PubMed] [Google Scholar]
- Howard C. V., Reed M. G. (1998). Unbiased Stereology: Three-dimensional Measurement in Microscopy. Oxford: BIOS Scientific Publishers Limited; [Google Scholar]
- Johnston I. A. (2006). Environment and plasticity of myogenesis in teleost fish. J. Exp. Biol. 209, 2249-2264 [DOI] [PubMed] [Google Scholar]
- Johnston I. A., Abercromby M., Vieira V. L. A., Sigursteindóttir R. J., Kristjánsson B. K., Sibthorpe D., Skúlason S. (2004). Rapid evolution of mucle fibre number in post-glacial populations of Artic charr Salvelinus alpinus. J. Exp. Biol. 207, 4343-4360 [DOI] [PubMed] [Google Scholar]
- Kadi F., Eriksson A., Holmner S., Butler-Browne B. S., Thornell L.-E. (1999). Cellular adaptation of the trapezius muscle in strength-trained athletes. Histochem. Cell Biol. 111, 189-195 [DOI] [PubMed] [Google Scholar]
- Kinsey S. T., Hardy K. M., Locke B. R. (2007). The long and winding road: influences on intracellular metabolite diffusion on cellular organization and metabolism in skeletal muscle. J. Exp. Biol. 210, 3505-3512 [DOI] [PubMed] [Google Scholar]
- Kinsey S. T., Locke B. R., Dillaman R. M. (2011). Molecules in motion: influences of diffusion on metabolic structure and function in skeletal muscle. J. Exp. Biol. 214, 263-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumans J. T. M., Akster H. A. (1995). Myogenic cells in development and growth of fish. Comp. Biochem. Physiol. 110A, 3-20 [Google Scholar]
- Liu J., Hoglund A., Karlsson P., Lindblad J., Qaisar R., Aare S., Bengtsson E., Larsson L. (2008). Myonuclear domain size and myosin isoform expression in muscle fibres from mammals representing 100 000-fold difference in body size. Exp. Physiol. 94, 117-129 [DOI] [PubMed] [Google Scholar]
- Locke B. R., Kinsey S. T. (2008). Diffusional constraints on energy metabolism in skeletal muscle. J. Theor. Biol. 254, 417-429 [DOI] [PubMed] [Google Scholar]
- Luby-Phelps K. (2000). Cytoarchitecture and physical properties of cytoplasm: volume, viscosity, diffusion, intracellular surface area. Int. Rev. Cytol. 192, 189-221 [DOI] [PubMed] [Google Scholar]
- Manta P., Vassilopoulos D., Spengos M. (1987). Nucleo-cytoplasmic ratio in ageing skeletal muscle. Eur. Arch. Psychiatr. Neurol. Sci. 236, 235-236 [DOI] [PubMed] [Google Scholar]
- Matthew C. A., Moore M. J. (1987). Numbers of myonuclei and satellite cell nuclei in latissimus dorsi muscles of the chicken. Cell Tissue Res. 248, 235-238 [DOI] [PubMed] [Google Scholar]
- Mauro A. (1961). Satellite cell of skeletal muscle fibers. J. Cell Biol. 9, 493-496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall G. E., Allen D. L., Linderman J. K., Grindeland R. E., Roy R. R., Mukku V. R., Edgerton V. R. (1998). Maintenance of myonuclear domain size in rat soleus after overload and growth hormone/IGF-I treatment. J. Appl. Physiol. 84, 1407-1412 [DOI] [PubMed] [Google Scholar]
- Moss F. P. (1968). The relationship between the dimensions of the fibers and the number of nuclei during normal growth of skeletal muscle in the domestic fowl. Am. J. Anat. 122, 555-564 [DOI] [PubMed] [Google Scholar]
- Moss F. P., Leblond C. P. (1970). Nature of dividing nuclei in the skeletal muscle of growing rats. J. Cell Biol. 44, 459-462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyack A. C., Locke B. R., Valencia A., Dillaman R. M., Kinsey S. T. (2007). Scaling of postcontractile phosphocreatine recovery in fish white muscle: effect of intracellular diffusion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R2077-R2088 [DOI] [PubMed] [Google Scholar]
- Ohira Y., Yoshinaga T., Ohara M., Nonaka I., Yoshioka T., Yamashita-Goto K., Shenkman B. S., Kozlovskaya I. B., Roy R. R., Edgerton V. R. (1999). Myonuclear domain and myosin phenotype in human soleus after bed rest with or without loading. J. Appl. Physiol. 87, 1776-1785 [DOI] [PubMed] [Google Scholar]
- Ohira Y., Tanaka T., Yoshinaga T., Kawano F., Nomura T., Nonaka I., Allen D. L., Roy R. R., Edgerton V. R. (2001). Ontogenetic, gravity-dependent development of rat soleus muscle. Am. J. Physiol. Cell Physiol. 280, C1008-C1016 [DOI] [PubMed] [Google Scholar]
- Pavlath G. K., Rich K., Wevster S. B., Blau H. (1989). Localization of muscle gene products in nuclear domains. Nature 337, 570-573 [DOI] [PubMed] [Google Scholar]
- Pizon V., Gerbal F., Diaz C. C., Karsenti E. (2005). Microtubule-dependent transport and organization of sarcomeric myosin during skeletal muscle differentiation. EMBO J. 24, 3781-3792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott D. M. (1956). Relation between cell growth and cell division. II. The effect of cell size on cell growth rate and generation time in Amoeba proteus. Exp. Cell Res. 11, 86-94 [DOI] [PubMed] [Google Scholar]
- Presnell J. K., Schreibman M. P. (ed.) (1997). Solution preparation. In Humason’s Animal Tissue Techniques, pp. 473-474 Baltimore, MD: Johns Hopkins University Press; [Google Scholar]
- Rahkila P., Kalervo V., Saraste J., Metsikko K. (1997). Endoplasmic reticulum to Golgi trafficking in multinucleated skeletal muscle fibers. Exp. Cell Res. 234, 452-464 [DOI] [PubMed] [Google Scholar]
- Ralston E., Lu Z., Biscocho N., Soumaka E., Mavroidis M., Prats C., Lomo R., Capetanaki Y., Ploug T. (2006). Blood vessel and desmin control the positioning of nuclei in skeletal muscle fibers. J. Cell. Physiol. 209, 874-882 [DOI] [PubMed] [Google Scholar]
- Rosser B. W. C., Dean M. S., Bandman E. (2002). Myonuclear domain size varies along the lengths of maturing skeletal muscle fibers. Int. J. Dev. Biol. 46, 747-754 [PubMed] [Google Scholar]
- Rowlerson A., Veggetti A. (2001). Cellular mechanisms of post-embryonic muscle growth. In Muscle Development and Growth (ed. Johnston I. A.), pp. 103-140 San Diego, CA: Academic Press; [Google Scholar]
- Roy R. R., Monke S. R., Allen D. L., Edgerton V. R. (1999). Modulation of myonuclear number in functionally overloaded and exercised rat plantaris fibers. J. Appl. Physiol. 87, 634-642 [DOI] [PubMed] [Google Scholar]
- Russell B., Dix D. (1992). Mechanisms for intracellular distribution of mRNA: in situ hybridization studies in muscle. J. Appl. Physiol. Cell Physiol. 262, C1-C8 [DOI] [PubMed] [Google Scholar]
- Sänger A. M., Stoiber W. (2001). Muscle fiber diversity and plasticity. In Muscle Development and Growth (ed. Johnston I. A.), pp. 187-250 San Diego, CA: Academic Press; [Google Scholar]
- Schmalbruch H., Hellhammer U. (1977). The number of nuclei in adult rat muscles with special reference to satellite cells. Anat. Rec. 189, 169-176 [DOI] [PubMed] [Google Scholar]
- Scholz D., Baicu C. F., Tuxworth W. J., Xu L., Kasiganesan H., Menick D. R., Cooper G., IV (2008). Microtubule-dependent distribution of mRNA in adult cardiocytes. Am. J. Physiol. Heart Circ. Physiol. 294, H1135-H1144 [DOI] [PubMed] [Google Scholar]
- Starr D. A. (2007). Communication between the cytoskeleton and the nuclear envelope to position the nucleus. Mol. BioSyst. 3, 583-589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr D. A. (2009). A nuclear-envelope bridge positions nuclei and moves chromosomes. J. Cell Sci. 122, 577-586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickland N. C., Widdowson E. M., Goldspink G. (1975). Effects of severe energy and protein deficiencies on the fibres and nuclei in skeletal muscle of pigs. Br. J. Nutr. 34, 421-428 [DOI] [PubMed] [Google Scholar]
- Swatland H. J. (1994). Structure and Development of Meat Animals and Poultry. Lancaster: Technomic; [Google Scholar]
- Wada K. I., Katsuta S., Soya H. (2003). Natural occurrence of myofiber cytoplasmic enlargement accompanied by decrease in myonuclear number. Jpn. J. Physiol. 53, 145-150 [DOI] [PubMed] [Google Scholar]
- Weatherley A. H. (1990). Approaches to understanding fish growth. Trans. Am. Fish. Soc. 119, 662-672 [Google Scholar]
- Weatherley A. H., Gill H. S. (1981). Characteristics of mosaic muscle growth in rainbow trout Salmo gairdneri. Experientia 37, 1102-1103 [Google Scholar]
- Weatherley A. H., Gill H. S. (1985). Dynamics of increase in muscle fibers in teleosts in relation to size and growth. Experientia 41, 353-354 [Google Scholar]
- Weatherley A. H., Gill H. S. (ed.) (1987). Tissues and growth. In The Biology of Fish Growth, pp. 147-175 New York: Academic Press; [Google Scholar]
- Weatherley A. H., Gill H. S., Rogers S. C. (1979). Growth dynamics of muscle fibres, dry weight, and condition in relation to somatic growth rate in yearling rainbow trout (Salmo gairdneri). Can. J. Zool. 57, 2385-2392 [Google Scholar]
- Zar J. H. (1999). Biostatistical Analysis. Upper Saddle River, NJ: Prentice Hall; [Google Scholar]
- Zhang M., McLennan I. S. (1995). During secondary myotube formation, primary myotubes preferentially absorb new nuclei at their ends. Dev. Dyn. 204, 168-177 [DOI] [PubMed] [Google Scholar]
- Zimmerman A. M., Lowery M. S. (1999). Hyperplastic development and hypertrophic growth of muscle fibers in the white seabass (Atractoscion nobilis). J. Exp. Zool. 284, 299-308 [DOI] [PubMed] [Google Scholar]