SUMMARY
Lack of activity causes bone loss In most animals. Hibernating bears have physiological processes to prevent cortical and trabecular bone loss associated with reduced physical activity, but different mechanisms of torpor among hibernating species may lead to differences in skeletal responses to hibernation. There are conflicting reports regarding whether small mammals experience bone loss during hibernation. To investigate this phenomenon, we measured cortical and trabecular bone properties in physically active and hibernating juvenile and adult 13-lined ground squirrels (Ictidomys tridecemlineatus, previous genus name Spermophilus). Cortical bone geometry, strength and mineral content were similar in hibernating compared with active squirrels, suggesting that hibernation did not cause macrostructural cortical bone loss. Osteocyte lacunar size increased (linear regression, P=0.001) over the course of hibernation in juvenile squirrels, which may indicate an osteocytic role in mineral homeostasis during hibernation. Osteocyte lacunar density and porosity were greater (+44 and +59%, respectively; P<0.0001) in hibernating compared with active squirrels, which may reflect a decrease in osteoblastic activity (per cell) during hibernation. Trabecular bone volume fraction in the proximal tibia was decreased (–20%; P=0.028) in hibernating compared with physically active adult squirrels, but was not different between hibernating and active juvenile squirrels. Taken together, these data suggest that 13-lined ground squirrels may be unable to prevent microstructural losses of cortical and trabecular bone during hibernation, but importantly may possess a biological mechanism to preserve cortical bone macrostructure and strength during hibernation, thus preventing an increased risk of bone fracture during remobilization in the spring.
KEY WORDS: hibernation, bone, ground squirrel, disuse
INTRODUCTION
Bone loss is observed following many forms of reduced skeletal loading including limb immobilization, spaceflight and long-term bed rest (Leblanc et al., 1990; Vico et al., 2000; Li et al., 2005). Hibernation is a naturally occurring example of disuse, but bone loss in response to hibernation may vary between species. In particular, bears preserve both cortical and trabecular bone during hibernation (Floyd et al., 1990; Pardy et al., 2004; McGee-Lawrence et al., 2009a; McGee-Lawrence et al., 2009b), but there is conflicting evidence regarding whether small mammalian hibernators lose bone. Early studies suggested that small mammals experience bone loss (e.g. decreased bone mineral content and decreased cortical bone thickness) during cold stress and hibernation (Bruce and Wiebers, 1970; Haller and Zimny, 1977; Steinberg et al., 1981; Kwiecinski et al., 1987). However, a recent study on golden-mantled ground squirrels showed cortical bone strength was not affected by hibernation (Utz et al., 2009). Quantitative studies of bone geometry, microstructure and mineralization are needed to address these differing reports and to better understand the effects of physical inactivity on skeletal integrity in small mammalian hibernators. Understanding differences in the skeletal responses of hibernating and non-hibernating species to changes in mechanical loading may shed light on the physiological adaptability of the skeletal maintenance system.
If small mammals, unlike bears, do experience bone loss during hibernation, the mechanism could be linked to differences in their patterns of torpor. Bears are immobile and do not eat, drink or excrete waste for 5–7 months per year, and therefore are likely to have evolved the ability to maintain balanced bone remodeling (and prevent bone loss) so they can recycle catabolized calcium from the serum back into the skeleton and prevent hypercalcemia. In contrast, small hibernating mammals arouse, raise their body temperature and excrete waste every 3–25 days during hibernation (Hock, 1957; Lesser et al., 1970; Carey et al., 2003). Urination, for example, occurs during interbout arousal periods in Columbian ground squirrels (Moy, 1971), and urine produced during hibernation is known to contain calcium (Pengelley et al., 1971; Shackelford and Caire, 1993). Thus, small mammals have a mechanism by which calcium liberated from bone during hibernation could be excreted from the body, and therefore these animals may not have evolved the ability to maintain balanced bone remodeling activity and prevent bone loss as hibernating bears do (McGee et al., 2008). This is supported by the observation that bone formation is reduced in the metatarsals of hibernating hamsters, but resorption continues at a slow rate (Tempel et al., 1978). It has also been suggested that hibernating bats, hamsters and ground squirrels mobilize calcium from bone via osteocytic osteolysis to maintain homeostatic calcium levels by replacing calcium lost in the urine, and thus may also lose bone during inactivity because of increased osteocyte lacunar area (Haller and Zimny, 1977; Steinberg et al., 1981; Kwiecinski et al., 1987; Shackelford and Caire, 1993). This is evidenced by the fact that bone loss can occur in these animals during hibernation despite the fact that unbalanced osteoclastic activity may not be observed (Nunez et al., 1972; Steinberg et al., 1981; Doty and Nunez, 1985). Though the osteolytic capacity of osteocytes has been questioned in the past (Parfitt, 1977; Marotti, 1981; Boyde and Jones, 1987), recent literature suggests that osteocytes may use their resorptive capacities to modify their local microenvironment for mineral homeostasis (Cullinane, 2002; Tazawa et al., 2004; Lane et al., 2006).
However, knowledge of bone metabolism in small hibernating mammals is incomplete; there are limited quantitative data from previous studies, and it is unclear if hibernation leads to detrimental changes in bone properties such as porosity, cortical area, mineral content and moment of inertia (which all play important roles in bone mechanical properties). Furthermore, the effects of hibernation on trabecular bone, which typically shows greater losses than cortical bone during disuse (Grynpas et al., 1995; Lang et al., 2004), have not been studied. Therefore, the goal of this study was to determine the effects of hibernation on cortical and trabecular bone properties in a small, obligate hibernating mammal (13-lined ground squirrel) to determine if its mechanism of hibernation results in compromised bone structure and strength.
MATERIALS AND METHODS
Samples
All procedures were approved by the University of Wisconsin Institutional Animal Care and Use Committee. Thirteen-lined ground squirrels, Ictidomys tridecemlineatus (Mitchell 1821) (previous genus name Spermophilus), were collected in the Madison, WI, USA, area or were born in the animal facility to wild-caught females. Squirrels were housed in conventional rodent cages (30×18×18 cm) that contained shredded paper and a short (15 cm) tube for environmental stimulation. Animal rooms were maintained at 22°C with a 12 h:12 h light:dark cycle. Squirrels had free access to water and food (Teklad Rodent Chow 7001, supplemented with sunflower seeds) except for pups born in captivity, which were food-restricted (12 g chow per day) after weaning to prevent excessive weight gain (rate of weight gain and body mass at euthanasia of these squirrels were similar to those of wild-caught squirrels). Thirteen-lined ground squirrels are sexually mature as yearlings when they emerge from hibernation in spring, and lifespan may be as long as 3–4 years in the wild. For the purposes of this study, squirrels were designated as ‘juveniles’ if they were less than 1 year old; all others were designated as ‘adults’. Of the 60 juveniles studied, 35 were wild-caught (30 hibernating, five active) and 25 were born in the animal facility (13 hibernating, 12 active).
Physically active squirrels (the ‘active’ animals for group comparisons) were killed in the mid to late summer by decapitation after being anesthetized with isoflurane. Active juvenile squirrels were killed during their first summer, meaning they had never hibernated. To induce a hibernating state, squirrels were transferred in the mid to late fall to a room maintained at 4°C and kept dark except for brief periods (<20 min) of low lighting once per day. Juvenile squirrels began hibernating in the first winter after birth, at approximately 4–5 months of age; it was not possible to determine exact ages of the adult squirrels. Water and food were removed after squirrels entered their first torpor bout. When killed, the hibernators had been without food and water for 71±26 days (mean ± s.d.; range, 21–123 days). Torpor bouts were 3–25 days in length and were interspersed with arousals that lasted ∼11 h (Carey et al., 2003). For the purposes of this study, winter squirrels in various hibernation states (torpor, interbout euthermia, etc.) were pooled and referred to as ‘hibernating’. Torpid hibernators were decapitated without prior anesthetic. Femurs and tibias were collected at sacrifice from juvenile (<140 g body mass) and adult hibernating and summer-active squirrels. All bones were cleaned of soft tissue and stored in plastic bags at –20°C. Number of femurs sampled: 37 hibernating and eight active juveniles; 14 hibernating and 16 active adults. Number of tibiae sampled: 43 hibernating and 17 active juveniles; 14 hibernating and 19 active adults.
Whole bone bending
The right femur from each squirrel was fully rehydrated in 0.9% saline and loaded to failure in three-point bending, using a mechanical testing system (Instron, model no. 8872, Canton, MA, USA), about the mediolateral axis with the anterior surface in compression using a crosshead speed of 1 mm min–1. The lower supports were separated by a span of 15 mm, and all contact points were rounded (r=2 mm) to minimize local deformation of the bone (Turner and Burr, 2001).
Cortical bone geometrical properties
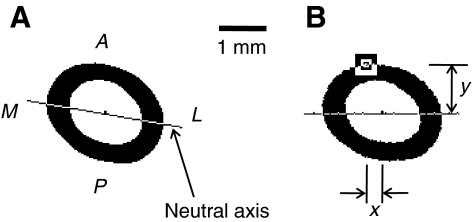
The left femur from each squirrel was prepared for histological examination. Diaphyseal segments of bone extending from the femoral midshaft to the patellar articular surface (approximately 8 mm long) were removed, cleaned of marrow, fixed in 70% ethanol and stained for 96 h in Villanueva osteochrome (Polysciences Inc., Warrington, PA, USA) bone stain. The stained bone sections were embedded in methyl methacrylate and sectioned with a diamond saw to expose the midshaft cross section. Images of the midshaft cross sections were captured using a digital camera (Spot Insight QE, Diagnostic Instruments Inc., Sterling Heights, MI, USA), and Scion Image analysis software (Scion Corporation, Frederick, MD, USA) was used to calculate the periosteal area (combined cross-sectional area of cortical bone and medullary canal), cortical area (cross-sectional bone area between periosteal and endocortical borders) and endocortical area (cross-sectional area of medullary canal inside endocortical border) for each sample. A custom macro in Scion Image was used to calculate the cross-sectional moments of inertia for the mediolateral (bending) axis (IML) and anteroposterior axis (IAP), the product of inertia (IPr), maximum moment of inertia (Imax), centroid of the cross section, neutral axis, and the x- and y-distances of the cortex location furthest from the neutral axis (Fig. 1).
Fig. 1.
(A) Anatomical orientations and neutral axis for a hibernating 13-lined ground squirrel femur. A, M, P and L are anterior, medial, posterior and lateral anatomical directions, respectively. (B) x- and y-distances for the cortex location that is furthest from the neutral axis in the same femoral cross section. The point furthest from the neutral axis, which is the location of maximum stress in three-point bending, is indicated inside the square.
Cortical bone mechanical properties
A typical load-deformation curve obtained from testing is shown in Fig. 2. Beam bending theory was used to calculate the whole bone mechanical properties of each femur. Load data were converted to stress using Eqn 1:
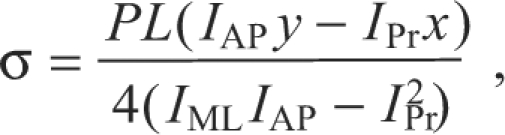 |
(1) |
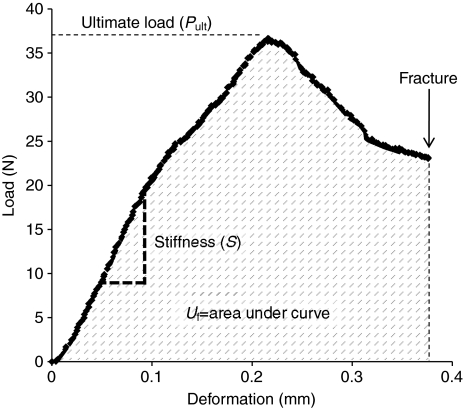
where P is the load, L is the span between the lower supports and x and y are the distances to the cortex location furthest from the neutral axis. Ultimate stress (ρUlt) was calculated from Eqn 1, where P was equal to the ultimate load (PUlt). Failure energy (Uf) was found by calculating the area under the load-deformation curve up to fracture. Stiffness (S) was calculated as the slope of the linear portion of the load-deformation curve. Elastic modulus was not quantified because this property is underestimated in whole bone three-point bending because of shear effects (Turner and Burr, 1993).
Fig. 2.
Representative load–deformation curve captured during the whole-bone bending test. Pult is the ultimate load. Stiffness (S) is calculated as the slope of the linear portion of the curve. Failure energy (Uf) is calculated as the area under the curve up to the point of fracture.
Cortical bone mineral content
Following the bending test, diaphyseal segments of bone extending from the femoral midshaft to the minor trochanter were removed from the right femurs and cleaned of marrow with a water jet. The bone segments were placed into a furnace at 100°C for 24 h to remove water, then weighed (dry mass). They were then heated to 600°C for 48 h to remove the organic matrix, cooled to 100°C and weighed again (ash mass). Mass measurements were made shortly after removal from the furnace to ensure that rehydration did not occur. The ash fraction was calculated as the ash mass divided by the dry mass.
Cortical bone microstructural properties
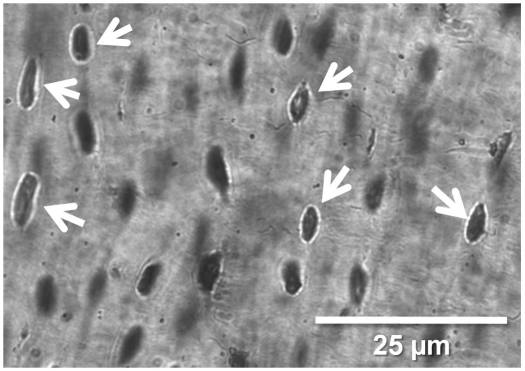
Thin sections from the left femoral midshaft were obtained from the embedded diaphyseal segments and ground to a thickness of 50–90 μm. All histomorphometric measurements were performed using a digital camera (SPOT Insight QE), microscope (Olympus CX41, Center Valley, PA, USA) and image analysis software (Bioquant Osteo, Nashville, TN, USA). One microscope field of view (×400 total magnification, approximately 0.07 mm2) located in the middle of the cortex along each anatomical direction was sampled for osteocyte lacunar measurements; measurements were averaged across all four sites in a cross section to obtain a total measure for the sample. Lacunae that were not in the plane of focus (i.e. lacunae without a sharp, defined border) were excluded from analyses (Fig. 3). The size of each lacuna and the total number of lacunae were quantified, and average osteocyte lacunar area was calculated. For each cross section, osteocyte lacunar density was calculated as the total number of lacunae divided by bone area, and osteocyte lacunar porosity was calculated as the total osteocyte lacunar area divided by total bone area.
Fig. 3.
Lacunar microstructure in the 13-lined ground squirrel femur. Lacunae in the plane of focus (white arrows) were used for quantification of lacunar properties including average osteocyte lacunar area [Ot.Lc.Ar, measured as the area (μm2) inside the lacunar border], osteocyte lacunar density (Ot.Lc.Dn, measured as the total number of lacunae per total bone area; μm2) and osteocyte lacunar porosity (Ot.Lc.Po, measured as the percentage total lacunar area divided by total bone area).
Trabecular bone architecture
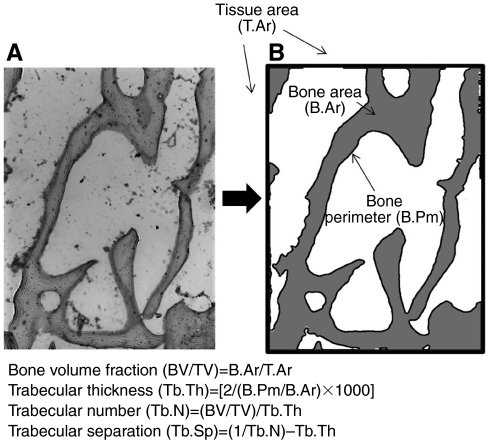
Left proximal tibias were fully decalcified in EDTA, embedded in TissueTek OCT cryomedium and sectioned on a cryostat. Frontal sections (4 μm thick) of each bone were obtained and stained with Basic Fuchsin to highlight microstructure. Trabecular tissue area (mm2), trabecular bone area (mm2) and trabecular perimeter (mm) were quantified for each section in the central region of the proximal tibia (300 μm away from cortical shell and growth plate); bone volume fraction (BV/TV; %) was calculated as trabecular bone area divided by trabecular tissue area (Fig. 4). Other architectural properties including trabecular thickness (mm), trabecular number (mm–1) and trabecular separation (mm) were calculated (Fig. 4) according to a parallel plate model (Parfitt et al., 1987; Accardo et al., 2005).
Fig. 4.
Trabecular bone microstructure in the 13-lined ground squirrel tibia. (A) Original view of trabecular architecture as seen with light microscopy. (B) Digitized image used for quantification of trabecular architecture. Tissue area, trabecular bone area and trabecular bone perimeter were quantified and used to calculate trabecular bone architectural properties according to a parallel plate model. BV, bone volume; TV, tissue volume.
Statistics
Bone properties were compared between active and hibernating squirrels and between juvenile and adult squirrels using two-factor ANOVA with interaction (factor 1: season, factor 2: age; interaction term: season × age). Tests with P<0.05 were considered to be statistically significant. Two-factor ANOVA tests that indicated a significant seasonal difference between hibernating and active animals were followed up with one-factor ANOVA within each age group (juveniles and adults) to determine if seasonal changes were specific to one age class. Males and females were combined for all analyses because sex and body weight distributions were similar between hibernating and active groups. Bone properties were regressed against hibernation length for the juvenile and adult hibernating squirrels to look for progressive changes in bone strength and structure over the course of hibernation. Regressions within each age class were performed separately to account for potential differences in regression slopes between immature and mature animals. Cook's distance was calculated for each data point to identify and eliminate potentially influential samples that would impact the regression model parameter estimates.
RESULTS
Geometrical properties
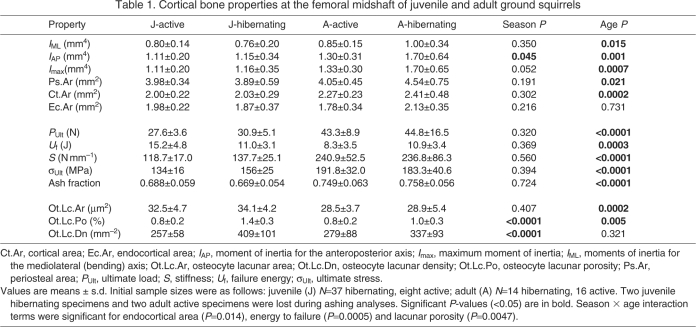
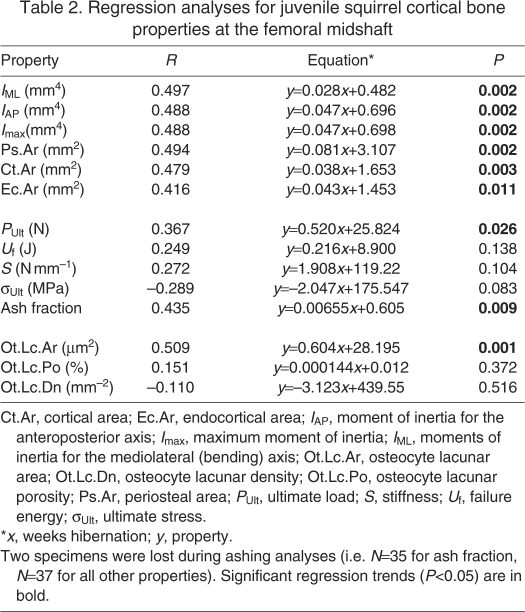
IAP was greater (P=0.045) in hibernating compared with active ground squirrels, when juvenile and adult were pooled (i.e. in the two-factor ANOVA). No other bone geometrical properties were significantly different between seasons, although the difference in Imax approached significance (P=0.052; Table 1). A follow-up one-factor ANOVA revealed that the difference in IAP between hibernating and active animals was significant for the adult squirrels (P=0.038), with hibernating squirrels having larger IAP, but that there was no difference between groups for the juvenile squirrels (P=0.727). All bone geometrical properties showed an increasing trend over the course of hibernation in the juvenile squirrels (Table 2), whereas no properties changed over the course of hibernation in the adult squirrels (P>0.346; data not shown).
Table 1.
Cortical bone properties at the femoral midshaft of juvenile and adult ground squirrels
Table 2.
Regression analyses for juvenile squirrel cortical bone properties at the femoral midshaft
All bone geometrical properties were significantly greater in adult than in juvenile ground squirrels (P<0.021), except endocortical area, which was statistically similar between groups (P=0.731) (Table 1). There was a significant season × age interaction term for endocortical area (P=0.014), but no other interactions were significant.
Whole bone mechanical properties
Whole bone mechanical properties (ultimate load, failure energy, stiffness, ultimate stress) were not different between hibernating and active squirrels (Table 1). Ultimate load of the femur increased over the course of hibernation in the juvenile squirrels (P=0.026), whereas ultimate stress tended to decrease during hibernation (P=0.083, r=–0.289; Table 2). Failure energy and stiffness did not change during hibernation for the juvenile squirrels (Table 2). No whole bone mechanical properties changed over the course of hibernation in the adult squirrels (Table 2).
Ultimate load, ultimate stress and stiffness were greater (P<0.0001), and failure energy was lower (P=0.0003) in the adult compared with the juvenile ground squirrels (Table 1). There was a significant season × age interaction term for failure energy (P=0.0005) but no other interactions were identified.
Ash fraction
There were no differences (P>0.360) in the ash fraction between active and hibernating squirrels (P=0.724; Table 1). The ash fraction increased over the course of hibernation for the juvenile squirrels, but did not change during hibernation for the adult squirrels (Table 2). Ash fraction was greater in adult than in juvenile squirrels (P<0.0001; Table 1).
Cortical bone microstructural properties
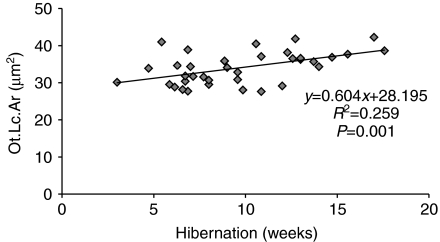
Average osteocyte lacunar area was similar between hibernating and active squirrels (Table 1). In contrast, osteocyte lacunar porosity and lacunar density were greater (P<0.0002) in the hibernating compared with the active squirrels (Table 1). The difference in osteocyte lacunar porosity between hibernating and active animals was significant for both juvenile (P=0.0001) and adult (P=0.045) squirrels. Similarly, the difference in osteocyte lacunar density between hibernating and active animals was significant for the juvenile squirrels (P=0.0002) and approached significance for the adult animals (P=0.091). Average osteocyte lacunar area increased over the course of hibernation for the juvenile squirrels (Fig. 5), but osteocyte lacunar porosity and osteocyte lacunar density did not change (Table 2). No osteocyte lacunar properties changed over the course of hibernation for the adult squirrels (Table 2). Average osteocyte lacunar area and lacunar porosity were greater in the juvenile than in the adult squirrels, but osteocyte lacunar density was not affected by age (Table 1). Evidence of rapid bone turnover (i.e. extensive osteoid surfaces, eroded surfaces, refilling or resorption cavities) was not observed in any of the juvenile or adult animals.
Fig. 5.
Osteocyte lacunar area (Ot.Lc.Ar) increased linearly over the course of hibernation for the juvenile squirrels.
Trabecular bone microstructural properties
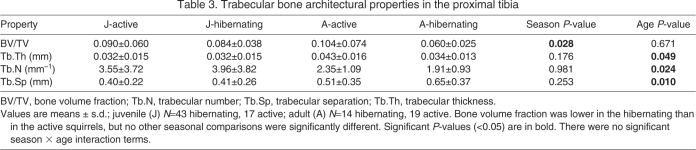
Trabecular bone volume fraction was lower in hibernating than active squirrels (P=0.028), but no other architectural properties showed seasonal differences (Table 3). The difference in BV/TV between hibernating and active animals was significant for the adult squirrels (P=0.040), but not for the juvenile squirrels (P=0.603). There were no linear changes in trabecular bone properties over the course of hibernation (data not shown). Trabecular thickness and trabecular separation were greater (P<0.049) and trabecular number was lower (P=0.024) in adult than in juvenile squirrels (Table 3).
Table 3.
Trabecular bone architectural properties in the proximal tibia
DISCUSSION
Hibernating species, which use metabolic depression to survive extreme environmental conditions and lack of food, provide natural animal models of organismal adaptation to stressful environments. Knowledge of the mechanisms used by these species to survive the hibernation season provides insight into both physiological regulation during extreme conditions and natural tolerance to pathophysiological states such as ischemia, hypothermia, malnutrition and musculoskeletal disuse. Regarding the musculoskeletal system, hibernating mammals are physically inactive for approximately 6 months per year, and this length of disuse would induce substantial bone loss (e.g. decreased bone structure and strength) in most (non-hibernating) animals. Early studies suggested that small hibernating mammals may lose bone (Haller and Zimny, 1977; Steinberg et al., 1981; Kwiecinski et al., 1987), but most of this research lacked rigorous quantification of a range of bone properties. The current study expanded on these earlier observations by investigating the effects of hibernation on cortical and trabecular bone structure, strength and mineralization in 13-lined ground squirrels. We saw no evidence of macrostructural cortical bone loss in hibernating ground squirrels, since cortical bone geometrical properties and whole bone mechanical properties were comparable between hibernating and active squirrels (Table 1). These results, which are in agreement with a recent finding on the preservation of bone strength in hibernating golden-mantled squirrels (Utz et al., 2009), suggest that 13-lined ground squirrels have adapted to prevent disuse-induced macrostructural cortical bone loss, and that hibernation does not lead to an increased risk of bone fracture upon remobilization.
It is, however, unclear whether all hibernating animals experience similar skeletal responses to disuse. Hibernating bears can prevent bone loss because they are able to maintain balanced bone remodeling during physical inactivity (Donahue et al., 2003; Donahue et al., 2006; McGee et al., 2008; McGee-Lawrence et al., 2009a), possibly because of a biological need to recycle calcium. Bears maintain constant serum calcium levels even though they are anuric and do not eat or drink during hibernation (Nelson, 1987; Floyd et al., 1990), and thus may have evolved the ability to maintain balanced bone remodeling to prevent hypercalcemia during hibernation. In contrast, other hibernators, such as ground squirrels, interrupt torpor bouts with periodic arousals to euthermia, during which they excrete calcium-containing waste (Hock, 1957; Bruce and Wiebers, 1970; Lesser et al., 1970; Pengelley et al., 1971). This provides a potential avenue for calcium and other products of bone catabolism to be excreted from the body, as occurs during other disuse situations such as human bedrest (LeBlanc et al., 1995; Inoue et al., 2000). Thus, hibernating ground squirrels may not have a biological need to maintain balanced bone turnover to prevent hypercalcemia, and it was not known whether they possess the ability to prevent bone loss during disuse associated with hibernation. The results of the current study suggest that 13-lined ground squirrels, like bears, also possess a mechanism to limit bone loss during hibernation since hibernating ground squirrels preserved cortical bone geometry and strength. However, important differences were noted: trabecular bone loss was observed in the adult hibernating squirrels (Table 3). Furthermore, cortical bone osteocyte lacunar porosity was greater in hibernating squirrels (Table 1), and osteocyte lacunar area increased over the course of hibernation in the juvenile squirrels (Fig. 5, Table 2), which may indicate that cortical bone loss occurred on a microstructural scale, as has been noted in small hibernators in previous studies (Steinberg et al., 1981; Kwiecinski et al., 1987). These changes had no effect on whole bone mechanical properties, but could be preliminary indications of hibernation-induced changes in calcium homeostasis. Thus, unlike bears, which maintain cortical bone geometry, cortical bone microstructure and trabecular bone properties during hibernation (McGee-Lawrence et al., 2008; McGee et al., 2008; McGee-Lawrence et al., 2009a), 13-lined ground squirrels might not be completely resistant to disuse-induced bone loss. Mechanistic investigations of the effects of hibernation on calcium regulatory mechanisms in this species will be the subject of future investigations.
It is important to consider that the juvenile ground squirrels used in this study could have been undergoing skeletal growth at the time of euthanasia. Although ground squirrels can reach adult size before their first hibernation period (Clark and Skryja, 1969; Zimmerman, 1972; Kiell and Millar, 1977), ash fraction and nearly all cortical bone geometrical and mechanical properties were greater in the adult than in the juvenile ground squirrels (Table 1), suggesting that skeletal growth was not completed in the first year of life. Regression trends for juvenile bone geometrical properties such as cortical area and ash fraction (Table 2) revealed that these properties increased over the course of hibernation, mimicking typical age-related increasing trends seen in these properties for many other species. This raises the possibility that these bone properties could have been influenced by age-related changes that may have masked the effects of hibernation. Bone geometrical properties increase with age in maturing animals (Keller et al., 1986) and skeletal growth can continue even under disuse conditions (Globus et al., 1986; Abram et al., 1988; Biewener and Bertram, 1994), therefore these increasing trends could have influenced the comparisons of hibernating and active juvenile squirrels, masking potential bone loss induced by disuse. However, cortical bone geometrical and mechanical properties did not change over the course of hibernation in the adult squirrels, and importantly, average bone geometrical properties were unexpectedly larger in the adult hibernating compared with adult active squirrels (Table 1). These data support the idea that both the juvenile and adult squirrels were able to prevent macrostructural cortical bone loss during hibernation.
Osteocyte lacunar density, and consequently lacunar porosity, increased in the hibernating compared with active squirrels (Table 1). At present, it is unclear why osteocyte lacunar density would be elevated as a consequence of hibernation, although it may be linked to changes in osteoblast function. Osteocyte lacunar density is also increased in osteoporotic humans relative to age-matched controls, and may reflect decreased bone formation activity per osteoblast [resulting in earlier cell entrapment in the bone matrix (Shih et al., 1993; Mullender et al., 1996)]. Consistent with this hypothesis, osteoblasts are less active during hibernation (Steinberg et al., 1986) and bone formation decreases in hibernating compared with active rodents (Tempel et al., 1978). Thus, the increased osteocyte lacunar density that we observed in hibernating squirrels in this study could be indicative of a hibernation-induced reduction in osteoblast activity and bone formation, where instead of making bone, osteoblasts terminally differentiate into osteocytes sooner during hibernation. Further dynamic and quantitative histomorphometric studies of bone resorption and formation are necessary to better define the mechanisms affecting osteocyte lacunar microstructure and skeletal turnover in small mammalian hibernators.
Average osteocyte lacunar area increased over the course of hibernation in the juvenile squirrels (Fig. 5), which could be indicative of osteocyte activity. Calcium-containing waste is periodically excreted by hibernating ground squirrels, but new calcium is not ingested during hibernation because the animals are fasting. Consequently, it is likely that these ground squirrels must employ a mechanism to maintain homeostatic calcium levels by using the skeleton as a calcium reservoir. To promote survival, hibernating mammals must find a balance between conserving energy by entering a state of disuse, maintaining calcium homeostasis and minimizing bone loss to maintain skeletal strength at or above a minimum threshold to avoid bone fracture upon emergence from hibernation. Bone remodeling by osteoblasts and osteoclasts, although necessary for calcium homeostasis, is costly in terms of metabolic energy and could lead to detrimental alterations in bone structure and strength; an alternative mechanism for calcium homeostasis is through osteocytic activity. Osteocytes have receptors for parathyroid hormone (PTH), the primary regulator of serum calcium levels (Noble and Reeve, 2000), and osteocytes can produce acid phosphatase, a bone demineralization agent, in response to resorptive signals from continuous PTH elevation, which may cause osteocyte lacunar enlargement (Tazawa et al., 2004). This suggests that osteocytes can detect and respond to signals aimed at serum calcium maintenance. If employed, this mechanism would probably lead to a small but steady increase in osteocyte lacunar size, as was observed in the juvenile squirrels during hibernation (Table 2). However, aside from changes in mechanical loading, it is possible that prolonged fasting could have caused average osteocyte lacunar area to increase in the juvenile squirrels during hibernation. Osteocyte lacunae formed during periods of low dietary calcium intake are larger and more irregularly shaped than those of animals on a normal diet because of abnormal development of bone matrix tissue (Sissons et al., 1984; Sissons et al., 1990). The squirrels in this study were not fluorochrome labeled, and consequently, pre-existing lacunae could not be distinguished from those that formed during hibernation. Therefore, we cannot conclusively determine the mechanism behind the increasing trend for average osteocyte lacunar size in the juvenile hibernating squirrels. These data are further confounded by the lack of hibernation-related regression trends in the adult squirrels, which is possibly the result of the lower sample size for this population. Despite these limitations, neither the juvenile nor adult hibernating ground squirrels showed evidence of rapid, unbalanced bone turnover (i.e. osteoblast and osteoclast activity) that is observed following lack of dietary calcium (Sissons et al., 1984), or disuse (Bain and Rubin, 1990; Li et al., 2005). This supports the idea that hibernating squirrels possess a biological mechanism to mitigate disuse and nutritionally induced imbalances in bone remodeling and subsequent loss of bone macrostructure and strength.
Regardless of the effects of disuse and starvation on osteocyte lacunar size in ground squirrels, hibernation did not have a negative effect on bone mineral content or cortical bone geometrical properties that relate to bone strength. Ultimate load, ultimate stress and stiffness of the femurs were not affected by hibernation, suggesting that overall, cortical bone seems to be resistant to disuse-induced bone loss in 13-lined ground squirrels, as was recently demonstrated in golden-mantled ground squirrels (Utz et al., 2009). Therefore, small hibernators, including ground squirrels, may be useful models for investigating biological mechanisms of cortical bone preservation during disuse. However, in contrast to the preservation of cortical bone, adult hibernating 13-lined ground squirrels had significantly lower trabecular bone volume than active animals, suggesting that trabecular bone may not be protected in adult hibernating squirrels. Future studies quantifying bone histomorphometry and serum concentrations of calcium regulatory hormones will be important for a more complete understanding of bone and calcium metabolism in small hibernators, and will enhance our understanding of how biological regulatory mechanisms vary between species that have adapted to the extreme environmental and physiological conditions of hibernation.
Footnotes
This research was supported by grant no. AR050420 from NIH. Additional funding was received from the National Science Foundation Graduate Research Fellowship Program, American Association of University Women, Michigan Space Grant Consortium, and the Michigan Technological University Department of Educational Opportunity. Deposited in PMC for release after 12 months.
REFERENCES
- Abram A. C., Keller T. S., Spengler D. M. (1988). The effects of simulated weightlessness on bone biomechanical and biochemical properties in the maturing rat. J. Biomech. 21, 755-767 [DOI] [PubMed] [Google Scholar]
- Accardo A. P., Strolka I., Toffanin R., Vittur F. (2005). Medical imaging analysis of the three dimensional (3D) architecture of trabecular bone: techniques and their applications. In Medical Imaging Systems Technology: Methods in General Anatomy (ed. Leondes C. T.), p. 1-41 Singapore: World Scientific Publishing Co. Pte. Ltd. [Google Scholar]
- Bain S. D., Rubin C. T. (1990). Metabolic modulation of disuse osteopenia: endocrine-dependent site specificity of bone remodeling. J. Bone Miner. Res. 5, 1069-1075 [DOI] [PubMed] [Google Scholar]
- Biewener A. A., Bertram J. E. (1994). Structural response of growing bone to exercise and disuse. J. Appl. Physiol. 76, 946-955 [DOI] [PubMed] [Google Scholar]
- Boyde A., Jones S. J. (1987). Early scanning electron microscopic studies of hard tissue resorption: their relation to current concepts reviewed. Scanning Microsc. 1, 369-381 [PubMed] [Google Scholar]
- Bruce D. S., Wiebers J. E. (1970). Calcium and phosphate levels in bats (Myotis lucifugus) as function of season and activity. Experientia 26, 625-627 [DOI] [PubMed] [Google Scholar]
- Carey H. V., Andrews M. T., Martin S. L. (2003). Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol. Rev. 83, 1153-1181 [DOI] [PubMed] [Google Scholar]
- Clark T. W., Skryja D. D. (1969). Postnatal development and growth of the golden-mantled ground squirrel, Spermophilus lateralis lateralis. J. Mamm. 50, 627-629 [Google Scholar]
- Cullinane D. M. (2002). The role of osteocytes in bone regulation: mineral homeostasis versus mechanoreception. J. Musculoskelet. Neuronal Interact. 2, 242-244 [PubMed] [Google Scholar]
- Donahue S. W., Vaughan M. R., Demers L. M., Donahue H. J. (2003). Bone formation is not impaired by hibernation (disuse) in black bears Ursus americanus. J. Exp. Biol. 206, 4233-4239 [DOI] [PubMed] [Google Scholar]
- Donahue S. W., Galley S. A., Vaughan M. R., Patterson-Buckendahl P., Demers L. M., Vance J. L., McGee M. E. (2006). Parathyroid hormone may maintain bone formation in hibernating black bears (Ursus americanus) to prevent disuse osteoporosis. J. Exp. Biol. 209, 1630-1638 [DOI] [PubMed] [Google Scholar]
- Doty S. B., Nunez E. A. (1985). Activation of osteoclasts and the repopulation of bone surfaces following hibernation in the bat, Myotis lucifugus. Anat. Rec. 213, 481-495 [DOI] [PubMed] [Google Scholar]
- Floyd T., Nelson R. A., Wynne G. F. (1990). Calcium and bone metabolic homeostasis in active and denning black bears (Ursus americanus). Clin. Orthop. 8, 301-309 [PubMed] [Google Scholar]
- Globus R. K., Bikle D. D., Morey-Holton E. (1986). The temporal response of bone to unloading. Endocrinology 118, 733-742 [DOI] [PubMed] [Google Scholar]
- Grynpas M. D., Kasra M., Renlund R., Pritzker K. P. (1995). The effect of pamidronate in a new model of immobilization in the dog. Bone 17, 225S-232S [DOI] [PubMed] [Google Scholar]
- Haller A. C., Zimny M. L. (1977). Effects of hibernation on interradicular alveolar bone. J. Dent. Res. 56, 1552-1557 [DOI] [PubMed] [Google Scholar]
- Hock R. J. (1957). Hibernation. In Cold Injury (ed. Ferrier M. I.), p. 61-133 New York: Josiah Macy; [Google Scholar]
- Inoue M., Tanaka H., Moriwake T., Oka M., Sekiguchi C., Seino Y. (2000). Altered biochemical markers of bone turnover in humans during 120 days of bed rest. Bone 26, 281-286 [DOI] [PubMed] [Google Scholar]
- Keller T. S., Spengler D. M., Carter D. R. (1986). Geometric, elastic, and structural properties of maturing rat femora. J. Orthop. Res. 4, 57-67 [DOI] [PubMed] [Google Scholar]
- Kiell D. J., Millar J. S. (1977). Growth of juvenile arctic ground squirrels (Spermophilus parryii) at McConnell River, N.W.T. Can. J. Zool. 56, 1475-1478 [Google Scholar]
- Kwiecinski G. G., Krook L., Wimsatt W. A. (1987). Annual skeletal changes in the little brown bat, Myotis lucifugus lucifugus, with particular reference to pregnancy and lactation. Am. J. Anat. 178, 410-420 [DOI] [PubMed] [Google Scholar]
- Lane N. E., Yao W., Balooch M., Nalla R. K., Balooch G., Habelitz S., Kinney J. H., Bonewald L. F. (2006). Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J. Bone Miner. Res. 21, 466-476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang T., LeBlanc A., Evans H., Lu Y., Genant H., Yu A. (2004). Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J. Bone Miner. Res. 19, 1006-1012 [DOI] [PubMed] [Google Scholar]
- Leblanc A. D., Schneider V. S., Evans H. J., Engelbretson D. A., Krebs J. M. (1990). Bone mineral loss and recovery after 17 weeks of bed rest. J. Bone Miner. Res. 5, 843-850 [DOI] [PubMed] [Google Scholar]
- LeBlanc A., Schneider V., Spector E., Evans H., Rowe R., Lane H., Demers L., Lipton A. (1995). Calcium absorption, endogenous excretion, and endocrine changes during and after long-term bed rest. Bone 16, 301S-304S [DOI] [PubMed] [Google Scholar]
- Lesser R. W., Moy R., Passmore J. C., Pfeiffer E. W. (1970). Renal regulation of urea excretion in arousing and homeothermic ground squirrels (Citellus columbianus). Comp. Biochem. Physiol. 36, 291-296 [DOI] [PubMed] [Google Scholar]
- Li C. Y., Price C., Delisser K., Nasser P., Laudier D., Clement M., Jepsen K. J., Schaffler M. B. (2005). Long-term disuse osteoporosis seems less sensitive to bisphosphonate treatment than other osteoporosis. J. Bone Miner. Res. 20, 117-124 [DOI] [PubMed] [Google Scholar]
- Marotti G. (1981). Three dimensional study of osteocyte lacunae. In Bone Histomorphometry (ed. Jee W. S. S., Parfitt A. M.), pp. 223-229 Paris: Armour Montagu; [Google Scholar]
- McGee M. E., Maki A. J., Johnson S. E., Nelson O. L., Robbins C. T., Donahue S. W. (2008). Decreased bone turnover with balanced resorption and formation prevent cortical bone loss during disuse (hibernation) in grizzly bears (Ursus arctos horribilis). Bone 42, 396-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee-Lawrence M. E., Carey H. V., Donahue S. W. (2008). Mammalian hibernation as a model of disuse osteoporosis: the effects of physical inactivity on bone metabolism, structure, and strength. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R1999-R2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee-Lawrence M. E., Wojda S. J., Barlow L. N., Drummer T. D., Castillo A. B., Kennedy O., Condon K. W., Auger J., Black H. L., Nelson O. L., et al. (2009a). Grizzly bears (Ursus arctos horribilis) and black bears (Ursus americanus) prevent trabecular bone loss during disuse (hibernation). Bone 45, 1186-1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee-Lawrence M. E., Wojda S. J., Barlow L. N., Drummer T. D., Bunnell K., Auger J., Black H. L., Donahue S. W. (2009b). Six months of disuse during hibernation does not increase intracortical porosity or decrease cortical bone geometry, strength, or mineralization in black bear (Ursus americanus) femurs. J. Biomech. 42, 1378-1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy R. M. (1971). Renal function in the hibernating ground squirrel Spermophilus columbianus. Am. J. Physiol. 220, 747-753 [DOI] [PubMed] [Google Scholar]
- Mullender M. G., van der Meer D. D., Huiskes R., Lips P. (1996). Osteocyte density changes in aging and osteoporosis. Bone 18, 109-113 [DOI] [PubMed] [Google Scholar]
- Nelson R. A. (1987). Black bears and polar bears-still metabolic marvels. Mayo Clin. Proc. 62, 850-853 [DOI] [PubMed] [Google Scholar]
- Noble B. S., Reeve J. (2000). Osteocyte function, osteocyte death and bone fracture resistance. Mol. Cell. Endocrinol. 159, 7-13 [DOI] [PubMed] [Google Scholar]
- Nunez E. A., Whalen J. P., Krook L. (1972). An ultrastructural study of the natural secretory cycle of the parathyroid gland of the bat. Am. J. Anat. 134, 459-480 [DOI] [PubMed] [Google Scholar]
- Pardy C. K., Wohl G. R., Ukrainetz P. J., Sawers A., Boyd S. K., Zernicke R. F. (2004). Maintenance of bone mass and architecture in denning black bears (Ursus americanus). J. Zool. Lond. 263, 359-364 [Google Scholar]
- Parfitt A. M. (1977). The cellular basis of bone turnover and bone loss: a rebuttal of the osteocytic resorption-bone flow theory. Clin. Orthop. Relat. Res. 127, 236-247 [PubMed] [Google Scholar]
- Parfitt A. M., Drezner M. K., Glorieux F. H., Kanis J. A., Malluche H., Meunier P. J., Ott S. M., Recker R. R. (1987). Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2, 595-610 [DOI] [PubMed] [Google Scholar]
- Pengelley E. T., Asmundson S. J., Uhlman C. (1971). Homeostasis during hibernation in the golden-mantled ground squirrel, Citellus lateralis. Comp. Biochem. Physiol. 38A, 645-653 [DOI] [PubMed] [Google Scholar]
- Shackelford J., Caire W. (1993). Variation in pH, volume, osmolality, and sodium and calcium levels of the urine of hibernating Myotis velifer from Western Oklahoma. Southwest. Nat. 38, 159-163 [Google Scholar]
- Shih M. S., Cook M. A., Spence C. A., Palnitkar S., McElroy H., Parfitt A. M. (1993). Relationship between bone formation rate and osteoblast surface on different subdivisions of the endosteal envelope in aging and osteoporosis. Bone 14, 519-521 [DOI] [PubMed] [Google Scholar]
- Sissons H. A., Kelman G. J., Marotti G. (1984). Mechanisms of bone resorption in calcium-deficient rats. Calcif. Tissue Int. 36, 711-721 [DOI] [PubMed] [Google Scholar]
- Sissons H. A., Kelman G. J., Ling L., Marotti G., Cane V., Muglia M. A. (1990). A light and scanning electron microscopic study of osteocyte activity in calcium-deficient rats. Calcif. Tissue Int. 46, 33-37 [DOI] [PubMed] [Google Scholar]
- Steinberg B., Singh I. J., Mitchell O. G. (1981). The effects of cold-stress, hibernation, and prolonged inactivity on bone dynamics in the golden hamster, Mesocricetus auratus. J. Morphol. 167, 43-51 [DOI] [PubMed] [Google Scholar]
- Steinberg B., Singh I. J., Mitchell O. G. (1986). An autoradiographic study of the uptake of tritiated proline by osteoblasts during hibernation. Histol. Histopathol. 1, 155-160 [PubMed] [Google Scholar]
- Tazawa K., Hoshi K., Kawamoto S., Tanaka M., Ejiri S., Ozawa H. (2004). Osteocytic osteolysis observed in rats to which parathyroid hormone was continuously administered. J. Bone Miner. Metab. 22, 524-529 [DOI] [PubMed] [Google Scholar]
- Tempel G. E., Wolinsky I., Musacchia X. J. (1978). Bone and serum calcium in normothermic, cold-acclimated and hibernating hamsters. Comp. Biochem. Physiol. 61A, 145-147 [Google Scholar]
- Turner C. H., Burr D. B. (1993). Basic biomechanical measurements of bone: a tutorial. Bone 14, 595-608 [DOI] [PubMed] [Google Scholar]
- Turner C. H., Burr D. B. (2001). Experimental techniques for bone mechanics. In Bone Mechanics Handbook (ed. Cowin S. C.), pp. 7-35 Boca Raton, FL: CRC Press; [Google Scholar]
- Utz J. C., Nelson S., O’Toole B. J., van Breukelen F. (2009). Bone strength is maintained after 8 months of inactivity in hibernating golden mantled ground squirrels, Spermophilus lateralis. J. Exp. Biol. 212, 2746-2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vico L., Collet P., Guignandon A., Lafage-Proust M. H., Thomas T., Rehaillia M., Alexandre C. (2000). Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet 355, 1607-1611 [DOI] [PubMed] [Google Scholar]
- Zimmerman E. G. (1972). Growth and age determination in the thirteen-lined ground squirrel, Spermophilus tridecemlineatus. Am. Midl. Nat. 87, 314-325 [Google Scholar]