SUMMARY
The neuropeptide Fs (NPFs) are an invertebrate subgroup of the FMRFamide-like peptides, and are proposed by some to be the homologs of vertebrate neuropeptide Y. Although there is some information about the identity, tissue distribution and function of NPFs in insects, essentially nothing is known about them in crustaceans. We have identified and characterized NPF-encoding transcripts from the penaeid shrimp Litopenaeus vannamei and Melicertus marginatus. Two transcripts were identified from each species. For each shrimp species, the two transcripts differed from one another by the presence or absence of an insert in the portion of the open reading frame that encodes the NPF peptide. The two NPF isoforms are identical in L. vannamei and M. marginatus, with their predicted structures being KPDPSQLANMAEALKYLQELDKYYSQVSRPRFamide and KPDPSQLANMAEALKYLQELDKYYSQVSRPSPRSAPGPASQIQALENTLKFLQLQELGKLYSLRARPRFamide. RT-PCR tissue profiling showed both transcripts are broadly distributed within the nervous system of each species. The transcript encoding the shorter NPF was detected in some, but not all, midgut samples. The transcript encoding the longer NPF was absent in the midgut of both species, and neither transcript was detected in their skeletal muscle. Juvenile L. vannamei fed on a diet supplemented with the shorter NPF exhibited a marked increase in food intake relative to control individuals that did not receive the supplement; the NPF-fed shrimp also showed a significant increase in growth relative to the control group. Our data suggest that NPF is present in both the nervous system and midgut of penaeid shrimp, functioning, at least in part, as a powerful orexigenic agent.
KEY WORDS: Litopenaeus vannamei, Marsupenaeus japonicus, Melicertus marginatus, FMRFamide-like peptide (FLP), neuropeptide F, neuropeptide Y
INTRODUCTION
Circulating peptides act as modulators of physiology and behavior in all multicellular animals (Kastin, 2006). One group of peptides that has been shown to be highly pleiotropic is the neuropeptide Y (NPY) superfamily, which includes NPY itself, peptide tyrosine–tyrosine and pancreatic polypeptide (for reviews, see Larhammar, 1996; Larhammar et al., 1998; Conlon and Larhammar, 2005; Sundström et al., 2008; Larhammar et al., 2009). In vertebrates, these peptides are known to play crucial roles in the control of feeding-related behaviors, stress responses and aggression (e.g. Pedrazzini et al., 2003; Emeson and Morabito, 2005; Karl and Herzog, 2007; Chee and Colmers, 2008; Thorsell, 2008; Benarroch, 2009).
Although no authentic NPYs have been identified in invertebrates, a broadly conserved family of peptides, commonly referred to as the neuropeptide Fs (NPFs), have been proposed as their homologs; the NPFs are also commonly placed as a subgroup of the FMRFamide-like peptide superfamily (e.g. Walker et al., 2010). These peptides, first identified from the platyhelminth Moniezia expansa (Maule et al., 1991), are typified by GRPRFamide carboxyl (C)-termini, Tyr residues located 10 and 17 amino acids from their carboxyl ends, and an overall length of 36 amino acids (Christie et al., 2010a). Since the initial description (Maule et al., 1991), additional isoforms of NPF have been identified from members of many taxa, including the Rotifera, Platyhelminthes, Mollusca, Annelida and Arthropoda (e.g. Rajapara et al., 1992; Brown et al., 1999; Dougan et al., 2002; Stanek et al., 2002; Humphries et al., 2004; Roller et al., 2008; Nuss et al., 2010).
Despite the large number of species in which NPFs have been identified, little is known about the functional roles they play in invertebrates. What data there are come largely from insects. Interestingly, the physiological roles that have thus far been ascribed to the insect NPFs mirror those played by NPYs in vertebrates. For example, insect NPFs are well-known regulators of food intake (e.g. Gonzalez and Orchard, 2009; Krashes et al., 2009), have been implicated in modulating behavioral and sensory responses to stressful stimuli (e.g. Xu et al., 2010), and act as modulators of aggressive behavior (e.g. Dierich and Greenspan, 2007).
Although a myriad of FMRFamide-like peptides have been identified from crustacean species (e.g. Trimmer et al., 1987; Mercier et al., 1993; Torfs et al., 2002; Fu et al., 2005; Dickinson et al., 2007; Stemmler et al., 2007; Ma et al., 2008; Ma et al., 2009a; Ma et al., 2009b; Ma et al., 2010), essentially nothing is known about the identity, tissue distribution or function(s) of members of the NPF subgroup in these animals (Christie et al., 2010a). In fact, data on crustacean NPFs are currently limited to two transcriptome-mining studies in which isoforms of NPF were predicted from expressed sequence tags (ESTs) from the penaeid shrimp Marsupenaeus japonicus and the cladocerans Daphnia magna and D. pulex (Christie et al., 2008; Gard et al., 2009). In M. japonicus, the prediction was considered highly speculative, as only a putative C-terminal portion of the pro-hormone could be deduced (Christie et al., 2008). There is no information on the tissue distribution of NPF in these or any other crustacean. Likewise, the functional roles served by NPFs in crustaceans are currently unknown, although injection or ingestion of porcine NPY has been shown to increase the rates of food intake and growth in the penaeids M. japonicus and Penaeus semisulcatus (Kiris et al., 2004).
In the study presented here, we undertook a combined molecular and physiological investigation directed at assessing the presence, distribution and potential orexigenic actions of NPFs in penaeid shrimp.
MATERIALS AND METHODS
Animals and tissue collection
Animals
Adult Pacific white shrimp, Litopenaeus vannamei (Boone 1931), were purchased from Island Aquaculture (Kaneohe, HI, USA), and juvenile animals were purchased from Paradise Shrimp Farm Corporation (Waialua, HI, USA). Aloha prawns, Melicertus marginatus (Randall 1840), were collected by hand from the reef flats off of Kuli'ou'ou Beach Park, Maunalua Bay, O'ahu (Hawaii Kai, HI, USA). Regardless of species, animals were maintained in aerated aquaria containing natural, full-strength (30–35 p.p.t.) seawater at ambient (∼22°C) temperature.
Tissue collection
Regardless of species, animals were anesthetized by packing on ice for 15–30 min, after which the eyestalk ganglia, supraesophageal ganglion (brain), thoracic ganglia, abdominal ganglia, midgut and mixed thoracic and abdominal skeletal muscle were isolated by manual dissection. Dissected tissues were immediately placed into RNAlater (Sigma-Aldrich, St Louis, MO, USA; catalog no. R0901) and then stored at –80°C for later RNA isolation (see below).
Molecular cloning and peptide prediction
Cloning
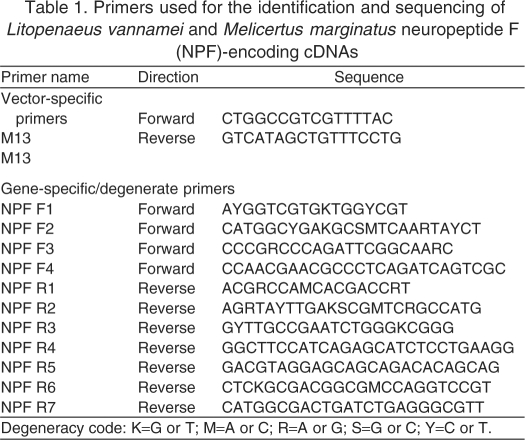
Putative NPF-encoding cDNAs were identified and characterized using a strategy combining reverse transcriptase-polymerase chain reaction (RT-PCR) using gene-specific primers and 3′ and 5′ rapid amplification of cDNA ends (RACE); the initial ‘gene-specific’ primers were designed using sequence data derived from a M. japonicus EST [accession no. CI998017 (Yamano and Unuma, 2006; Christie et al., 2008)]. All primers were custom synthesized by Integrated DNA Technologies, Inc. (Coralville, IA, USA; Table 1).
Table 1.
Primers used for the identification and sequencing of Litopenaeus vannamei and Melicertus marginatus neuropeptide F (NPF)-encoding cDNAs
For isolation of RNA, brains and eyestalk ganglia (from two individuals) were removed from RNAlater and blotted dry to remove excess liquid. Total RNA was then isolated using a SV Total RNA Isolation System (Promega, Madison, WI, USA; catalog no. Z3100). Each of the tissue types was processed individually, and prior to proceeding with RNA isolation, was first manually minced in lysis buffer with spring scissors and then further homogenized using a QIAshredder spin-column homogenizer (Qiagen Inc., Valencia, CA, USA; catalog no. 79654). RNA concentration was determined using a Nanodrop ND1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA); RNA quality was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). cDNA was synthesized from mixed brain and eyestalk mRNA (initially isolated individually, as described above, but pooled at this step) using a SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA, USA; catalog no. 18080051). All RACE experiments were done using a SMART RACE cDNA Amplification Kit (Clontech/Takeda, Palo Alto, CA, USA). PCR was carried out on a DNA Engine thermocycler (Bio-Rad Laboratories, Hercules, CA, USA) using GoTaq Master Mix (Promega; catalog no. M7138). The PCR products obtained were cloned into a pCR2.1 TOPO vector using a TOPO TA cloning kit (Invitrogen; catalog no. K4510-20) and sequenced with an ABI 3100 16-capillary sequencer (Applied Biosystems Inc., Foster City, CA, USA) using both vector- and gene-specific forward and reverse sequencing primers (Integrated DNA Technologies Inc.; Table 1). The sequence trace files resulting from each round of sequencing were analyzed using 4Peaks software (mekentosj.com), and the high quality nucleotide sequences were aligned using Lasergene software (DNASTAR Inc., Madison, WI, USA) or the online program MAFFT version 6 [http://mafft.cbrc.jp/alignment/software/ (Katoh et al., 2002; Katoh and Toh, 2008)].
Peptide prediction
Peptide predictions were made using a previously established protocol (e.g. Christie, 2008a; Christie, 2008b; Christie et al., 2008; Dickinson et al., 2009; Gard et al., 2009; Ma et al., 2009a; Christie et al., 2010b; Christie et al., 2010c; Ma et al., 2010; Stemmler et al., 2010). Specifically, nucleotide sequences were translated using the Translate tool of ExPASy [Swiss Institute of Bioinformatics, Basel, Switzerland; http://www.expasy.ch/tools/dna.html (Gasteiger et al., 2003)]. Signal peptide predictions were made with the online program SignalP 3.0, using both the Neural Networks and the Hidden Markov Models algorithms [Center for Biological Sequence Analysis, Technical University of Denmark, Lyngby, Denmark; http://www.cbs.dtu.dk/services/SignalP/ (Bendtsen et al., 2004)]. Pro-hormone cleavage sites were predicted on the basis of the information presented by Veenstra and/or homology to known pre/prepro-hormone processing schemes (Veenstra, 2000). Prediction of the sulfation state of tyrosine (Tyr) residues was done using the online program Sulfinator [Swiss Institute of Bioinformatics; http://www.expasy.org/tools/sulfinator/ (Monigatti et al., 2002)]. Other post-translational modifications, e.g. C-terminal amidation at glycine (Gly) residues, were predicted by homology to known NPF isoforms.
RT-PCR tissue profiling
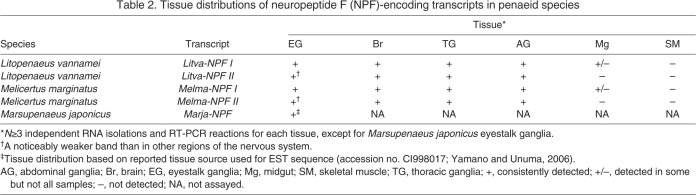
The tissue distributions of NPF-encoding transcripts were determined using RT-PCR profiling in both L. vannamei and M. marginatus. Specifically, primers were designed to amplify products from each of the two transcripts in each species and were used to probe eyestalk ganglia, brain, thoracic ganglia, abdominal ganglia, midgut and skeletal muscle. Total RNA was isolated from individual tissue samples using an SV Total RNA Isolation System as described earlier. Likewise, cDNA synthesis and PCR were accomplished as described above. To confirm the identity of the RT-PCR products, one sample of each product from the brain of each species was cloned into a pCR 2.1 TOPO vector using a TOPO TA cloning kit (Invitrogen) and sequenced using vector-specific sequencing primers (Table 2) as described earlier. One midgut sample from L. vannamei was also sequenced to confirm its identity.
Table 2.
Tissue distributions of neuropeptide F (NPF)-encoding transcripts in penaeid species
Assessment of orexigenic activity
The biological effect of L. vannamei and M. marginatus KPDPSQLANMAEALKYLQELDKYYSQVSRPRFamide (hereafter referred to as Litva- and Melma-NPF I, respectively) on food intake and growth was tested in a 15-day feeding trial using juvenile L. vannamei. The experimental protocol included a 5-day pre-trial period (days 1–5), a 5-day trial period (days 6–10) and 5-day post-trial period (days 11–15). During the pre- and post-trial periods, the experimental and control groups were treated equally; during the trial period each shrimp was given an initial food pellet that had been treated with either 1 μl of distilled water (control) or with 1 μl of a 10–3 mol l–1 Litva-NPF (custom synthesized by GenScript Corporation, Piscataway, NJ, USA) dissolved in distilled water (NPF treated). The feeding trial was conducted as a blind experiment such that the experimenters did not know which treatment contained the control or the peptide-laced pellets.
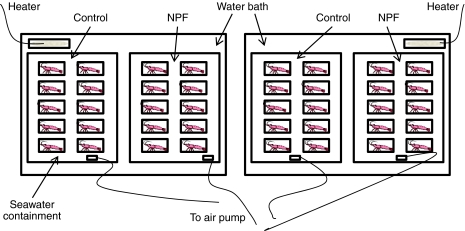
The experimental set-up is shown in Fig. 1. Two water baths were maintained at a temperature of 26±1°C. Two containers filled with seawater were placed into each bath. Ten shrimp were kept in each of these secondary containers in individual baskets as shown in Fig. 1. The experimental design consisted of two groups (water baths), with each group containing 10 control and 10 NPF-treated shrimp. The containers were aerated continuously and the salinity was kept between 33 and 35 p.p.t. This experimental design allowed us to track each shrimp for pellet consumption, molting and mass. Shrimp were fed twice per day (at 11:00 and 19:00 h) using a commercial marine shrimp feed (Rangen 40/5 no. 4 crumble pellets; Rangen Inc., Buhl, ID, USA; mean mass per pellet: 16.5±1.9 mg; ±s.d.). These small pellets were chosen because the shrimp consumed each pellet completely before feeding on the next.
Fig. 1.
Schematic drawing of experimental set up for the assessment of the effect of neuropeptide F (NPF) on food intake and growth. View from above shows two temperature-controlled water baths each with a container with a set of control and NPF-treated shrimp. The containers were kept aerated with bubbling stones and housed 10 shrimp, with each individual maintained in a basket (7.5×11.5×6.5 cm; width × length × height). Drawing not to scale.
At each feeding, eight pellets were added to each basket as follows. During the pre- and post-trial periods (days 1–5 and 11–15, respectively), a single initial unlaced pellet was added to each basket and the shrimp was allowed 15 min to consume it. During the trial period (days 6–10) the initial pellet was either a control or an NPF-treated pellet. Consumption of the initial pellet was assessed at each feeding during the trial period (days 6–10). In the NPF treatment group, the initial laced pellet was consumed nearly every time, and only on six occasions (out of 200 individual feedings) did a shrimp not consume the laced pellet. In the control shrimp, failure to consume the initial pellet occurred more frequently: 25 out of 200 feedings. The initial 15 min consumption period was followed by the addition of seven more pellets to each basket, and the shrimp were allowed to feed for 1 h. Following each feeding (1.25 h in total), pellet consumption was determined for each individual and all uneaten pellets were removed. This design assured that each individual received the same ‘dose’ of Litva-NPF I during the trial period (2 μl of 10–3 mol l–1 daily); control animals received no NPF but the same complement of feed. It should be noted that an individual shrimp typically consumed fewer than eight pellets per feeding (only on seven of 600 feedings were all eight pellets consumed). Thus, the shrimp always had access to an excess of feed.
In addition to consumption, individual baskets were checked at each feeding for molted exuvia. When exuvia were found, they were removed from the basket to prevent them from being consumed by the animals. At the end of the post-trial period (day 16), each individual was removed and weighed again. Statistical analyses of pellet consumption and mass change were performed with SPSS software (IBM, Chicago, IL, USA) using a univariate analysis of variance.
RESULTS
Nucleotide sequences of neuropeptide-F-encoding transcripts
L. vannamei
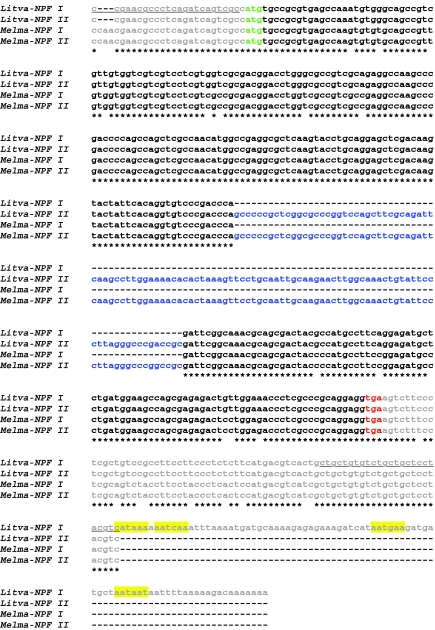
Two NPF-encoding transcripts, Litva-NPF I (accession no. HQ428126) and Litva-NPF II (accession no. HQ428127), were identified and characterized from mixed eyestalk ganglia and brain cDNA. As is shown in Fig. 2, Litva-NPF I is a 457 base-pair (bp), putative full-length transcript consisting of a 24 bp 5′ untranslated region (UTR), a 273 bp open reading frame (ORF; which includes the stop codon) and a 160 bp 3′ UTR, which contains four variant polyadenylation signal sequences located 14, 29, 67 and 74 bp upstream of a 7 bp poly(A)+ tail (very likely truncated because of its identification using 3′ RACE). Litva-NPF II is a partial cDNA, missing a portion of its 3′ UTR (Fig. 2). The sequence that is known is 482 bp in length, and consists of a 24 bp 5′ UTR, a 384 bp ORF and a 74 bp portion of the 3′ UTR (Fig. 2); this partial transcript is identical to that of Litva-NPF I, with the exception of a 111 bp insert in the ORF (Fig. 2).
Fig. 2.
Alignment of the nucleotide sequences of Litopenaeus vannamei and Melicertus marginatus neuropeptide F-encoding transcripts. Litva-NPF I, Litopenaeus vannamei NPF I (accession no. HQ428126); Litva-NPF II; Litopenaeus vannamei NPF II (accession no. HQ428127); Melma-NPF I, Melicertus marginatus NPF I (accession no. HQ428128); Melma-NPF II, Melicertus marginatus NPF II (accession no. HQ428129). Dashes in the nucleotide sequences indicate gaps (if internal) or missing nucleotides (if at the 3′ end). Nucleotides that are shared between all four transcripts are denoted by a star. In this alignment, the 5′ and 3′ untranslated regions (UTRs) of the cDNAs are shown in gray, the start codon of each open reading frame (ORF) is shown in green and the stop codon of each transcript is shown in red (the intervening ORF sequences are shown in black, with the exception of inserts in Litva-NPF II and Melma-NPF II, which are shown in blue). Four variant polyadenylation signal sequences in the 3′ UTR of Litva-NPF I are highlighted yellow. The sequence used to design primers for RT-PCR tissue profiling is underlined.
M. marginatus
As was the case in L. vannamei, two NPF-encoding transcripts, Melma-NPF I (accession no. HQ428128) and Melma-NPF II (accession no. HQ428129), were identified and characterized from M. marginatus mixed eyestalk ganglia and brain cDNA, each a partial sequence, missing a portion of its 3′ UTR (Fig. 2). The known sequence of Melma-NPF I is 374 bp in length, consisting of a 27 bp 5′ UTR, a 273 bp ORF and a 74 bp portion of the 3′ UTR (Fig. 2). The known sequence of Melma-NPF II is 485 bp in length and consists of a 27 bp 5′ UTR, a 384 bp ORF and a 74 bp portion of the 3′ UTR (Fig. 2); this partial transcript is identical to that of Melma-NPF I, with the exception of a 111 bp insert in its ORF (Fig. 2).
Structural analysis of deduced prepro-NPFs and prediction of mature NPF and precursor-related peptide isoforms
L. vannamei
The predicted peptide of the ORF of Litva-NPF I is a 90 amino acid, putative full-length prepro-hormone, here named Litva-prepro-NPF I (Fig. 3 and Fig. 4A). SignalP analysis of this protein suggests that the first 29 amino acids form a signal peptide, with putative cleavage between Ala29 and Lys30 (Fig. 3 and Fig. 4A). Within the pro-hormone sequence, one dibasic processing site is present (Fig. 3 and Fig. 4A). Prohormone convertase processing at the dibasic locus is predicted to liberate two peptides, which, following carboxypeptidase processing and subsequent α-amidation of one peptide and sulfation of Tyr in the other, have the predicted mature structures KPDPSQLANMAEALKYLQELDKYYSQVSRPRFamide (Fig. 4A,Bi) and SDY(SO3H)AMPSGDALMEASERLLETLA (Fig. 4A,Biii; sulfinator E-value for the sulfation state of the position 3 Tyr=42). The former peptide possesses the structural motifs characteristic of a NPF isoform (for a review, see Christie et al., 2010a) and was named Litva-NPF I. The latter peptide is not an NPF, nor does it posses any structural motif that would place it into any known peptide family (Christie et al., 2010a); for convenience of later discussion, this peptide was named Litva-NPF precursor-related peptide (Litva-NPRP).
Fig. 3.
Alignment of the deduced amino acid sequences of L. vannamei and M. marginatus NPF-containing prepro-hormones. Litva-PP-NPF I, L. vannamei prepro-NPF I; Litva-PP-NPF II, L. vannamei prepro-prepro-NPF II; Melma-PP-NPF I, M. marginatus prepro-NPF I; Melma-PP-NPF II, M. marginatus prepro-prepro-NPF II. Dashes in the sequences indicate gaps, a star indicates amino acids that are identical in all sequences, a colon indicates amino acids that are highly conserved, and a single dot indicates amino acids that are conserved. In each protein, the predicted signal peptide is shown in gray, with a predicted prohormone convertase cleavage site shown in black. The isoform of NPF is shown in red, with a precursor-related peptide shown in blue.
Fig. 4.
Predicted mature peptides encoded by penaeid NPF precursor proteins. (A) Schematic diagram showing the putative post-translational processing of the L. vannamei prepro-NPF I protein. The predicted protein of the nucleotide sequence of Litva-prepro-NPF I is a 90-amino-acid prepro-hormone (top sequence), the first 29 amino acids of which are predicted to function as a signal peptide. Signal peptidase processing between Ala29 and Lys30 would produce a 61-amino-acid pro-hormone (second sequence). On the basis of homology to known insect pro-hormone cleavage sites, a single Lys–Arg processing site was identified. Prohormone convertase processing at this locus would liberate two peptides (third line of sequences); the dibasic and tribasic residues, respectively, of which are predicted to be removed through the action of carboxypeptidase. In one of these peptides, carboxypeptidase action would expose a glycine residue (fourth line of sequences), which probably serves as a target for α-amidation by peptidyl-amidating monooxygenase. Action by this enzyme would result in the amidation of the C-terminus this peptide (fifth line of sequence). In addition, post-translational processing of a tyrosine residue by tyrosylprotein sulfotransferase in the other peptide is predicted to result in the addition of a sulfate group to it (fifth line of sequence). The putative, mature peptide KPDPSQLANMAEALKYLQELDKYYSQVSRPRFamide possesses all of the hallmarks of a NPF and was named Litopenaeus vannamei NPF I (Litva-NPF I). The second peptide produced from the precursor, SDY(SO3H) AMPSGDALMEASERLLETLA, is termed here Litopenaeus vannamei NPF precursor-related peptide (Litva-NPRP). (B) Alignments of peptides produced from known penaeid NPF precursors: (Bi) standard-length NPF isoforms; (Bii) internally extended NPFs; (Biii) NPRPs. Comparison of the sequences in Bi shows that the standard-length NPFs from Marsupenaeus japonicus (Marja-NPF) (Christie et al., 2008), L. vannamei and M. marginatus are identical in structure, as are the extended NPFs of L. vannamei and M. marginatus. Variation at the positions two and three amino acids (highlighted in gray in Biii) are present in NPRPs of the three shrimp. † indicates that the position 3 Tyr residue is sulfated.
The predicted peptide of the ORF of Litva-NPF II is a 127 amino acid, putative full-length prepro-hormone, here named Litva-prepro-NPF II (Fig. 3). This prepro-hormone is identical to that deduced from Litva-NPF I with the exception of a 37-amino-acid insert in the middle of the NPF isoform (Fig. 3). Like Litva-prepro-NPF I, processing of Litva-prepro-NPF II is predicted to produce two peptides, the internally extended NPF isoform KPDPSQLANMAEALKYLQELDKYYSQVSRPSPRSAPGPASQIQALENTLK FLQLQELGKLYSLRARPRFamide (named Litva-NPF II; Fig. 3 and Fig. 4Bii) and SDY(SO3H)AMPSGDALMEASERLLETLA, which is identical to the precursor-related peptide derived from Litva-prepro-NPF I (Fig. 3 and Fig. 4Biii).
M. marginatus
The predicted peptide of the ORF of Melma-NPF I is a 90 amino acid, putative full-length prepro-hormone; the deduced protein was named Melma-prepro-NPF I (Fig. 3). As can be seen in Fig. 3, Melma-prepro-NPF I is identical to Litva-prepro-NPF I with the exception of four amino acid substitutions in the predicted signal peptide and one in the precursor-related peptide (Fig. 3). Thus, the putative mature NPFs derived from the two proteins are identical, i.e. KPDPSQLANMAEALKYLQELDKYYSQVSRPRFamide (Fig. 3Bi), whereas the putative mature NPRPs differ by a single amino acid, i.e. SDY(SO3H)PMPSGDALMEASERLLETLA in Melma-prepro-NPF I (Sulfinator E-value for the sulfation state of the position 3 Tyr=33) vs SDY(SO3H)AMPSGDALMEASERLLETLA in Litva-prepro-NPF I (Fig. 4Biii).
Similar to the situation just described, the deduced protein from Melma-prepro-NPF II, named here Melma-prepro-NPF II, is identical to Litva-prepro-NPF II, with the exception of the amino acid substitutions noted between Melma-prepro-NPF I and Litva-prepro-NPF I (Fig. 3). Thus, both Melma-prepro-NPF II and Litva-prepro-NPF II contain the same putative mature, internally extended NPF, i.e. KPDPSQLANMAEALKYLQELDKYYSQVSRPSPRSAPGPASQIQALENTLKFLQLQELGKLYSLRARPRFamide (Fig. 4Bii); the NPRP contained within Melma-prepro-NPF II is identical to that described earlier from Melma-prepro-NPF I, i.e. SDY(SO3H)PMPSGDALMEASERLLETLA (Fig. 4Biii).
RT-PCR tissue profiling of prepro-NPF transcripts
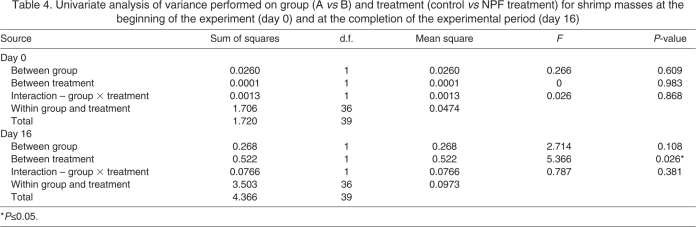
In insects, NPFs are broadly distributed within the nervous system and are present in midgut epithelial endocrine cells (e.g. Brown et al., 1999; Stanek et al., 2002; Nuss et al., 2008; Veenstra et al., 2008; Veenstra, 2009; Nuss et al., 2010). Using a primer set designed to detect both NPF transcripts (underlined in Fig. 2), we conducted RT-PCR tissue profiling to assess the distributions of the messages in neural and other tissues in L. vannamei and M. marginatus (N≥3 independent runs for each tissue; Figs 5, 6 and Table 2). Fig. 5 shows a representative RT-PCR run for a full set of L. vannamei tissues taken from a single individual. In both species, robust bands corresponding to the predicted prepro-NPF I PCR product (374 bp in length) were consistently detected in eyestalk ganglia, brain, thoracic ganglia and abdominal ganglia. Similarly, weaker bands corresponding to the predicted prepro-NPF II PCR product (485 bp in length) were consistently detected in these regions of the nervous system in both species. In the eyestalk ganglia samples of both L. vannamei and M. marginatus, the band for prepro-NPF II, although always present, was extremely weak. In fact, in some samples, such as the L. vannamei sample shown in Fig. 5, the prepro-NPF II product was barely detectible in the eyestalk ganglia. In the midgut samples of both L. vannamei and M. marginatus, a weak band corresponding to prepro-NPF I was detected in some, but not all, samples (two of five L. vannamei midgut samples and one of four M. marginatus midgut samples; Figs 5 and 6); no band corresponding to prepro-NPF II was detected in the midgut of either species. Neither prepro-NPF I nor prepro-NPF II were detected in mixed thoracic and abdominal skeletal muscle samples taken from either shrimp species and no products were detected in negative controls (data not shown).
Fig. 5.
RT-PCR profiling of L. vannamei prepro-NPFs in nervous system, midgut and skeletal muscle. Using a gene-specific primer that amplifies products from both Litva-PP-NPF I and II, RT-PCR tissue profiling was conducted to assess the distributions of the two transcripts. A strong band of the predicted Litva-PP-NPF I product (374 bp in length) was consistently detected in eyestalk ganglia (EG; lane 1), brain (Br; lane 2), thoracic ganglia (TG; lane 3) and abdominal ganglia (AG; lane 4). Weaker bands corresponding to the predicted Litva-PP-NPF II product (485 bp in length) were consistently detected in these tissues as well. A weak band corresponding to the Litva-PP-NPF I product was detected in some, but not all midgut samples (Mg; lane 5); no detection of the Litva-PP-NPF II product was seen in this tissue. Neither Litva-PP-NPF I nor Litva-PP-NPF II was detected in samples of skeletal muscle (SM; lane 6). A base pair ladder (L) is shown in lane 7.
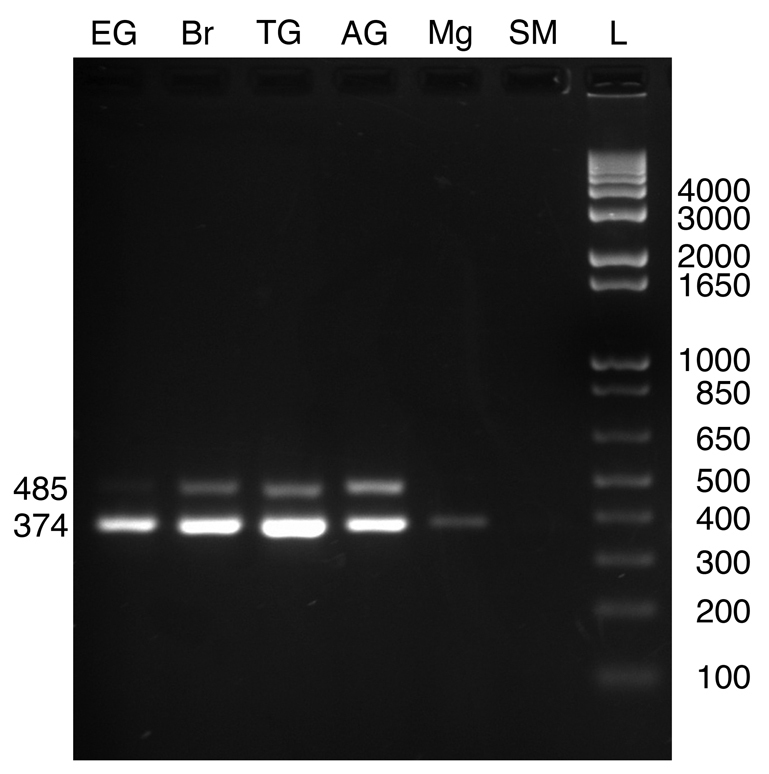
Fig. 6.
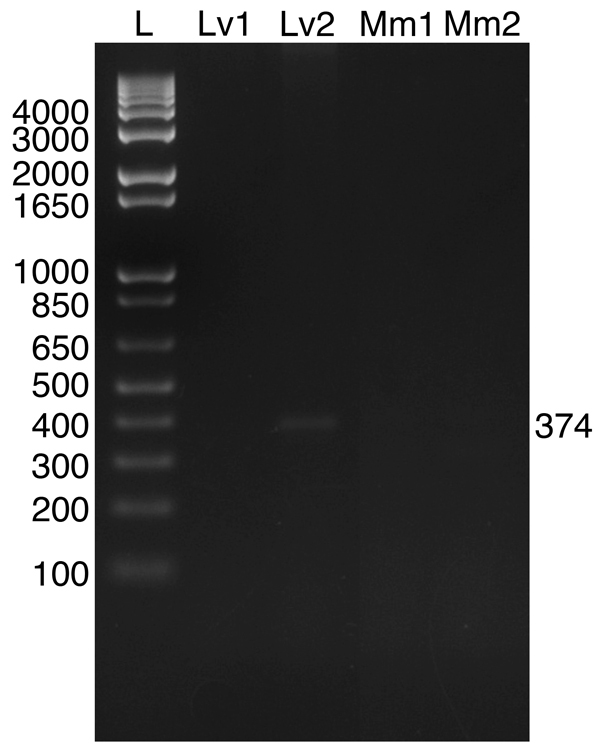
RT-PCR profiling of prepro-NPFs in midgut tissues of L. vannamei and M. marginatus. The presence or absence of Litva-prepro-NPF I and II or Melma-prepro-NPF I and II were assessed in L. vannamei and M. marginatus midgut samples, respectively, using RT-PCR with a gene-specific primer set that amplifies products from both transcripts in each species [a 374 base pair (bp) product predicted for Litva- and Melma-prepro-NPF I and a 485 bp product predicted for Litva- and/Melma-prepro-NPF II]. The results of PCR amplifications from two L. vannamei midguts (Lv1 and Lv2) and two M. marginatus midguts (Mm1 and Mm2) are shown. In only Lv2 was a weak band detected, corresponding to that predicted for Litva-prepro-NPF I. Sequence analysis of this band showed it to be identical to the amino acid sequence of the predicted Litva-prepro-NPF I product (data not shown). A base pair ladder (L) is shown in the first lane.
To confirm the identity of the detected PCR products, one sample of each from the brain of L. vannamei and the brain of M. marginatus was subcloned and sequenced; the four PCR products were identical to the expected sequences predicted from their respective cDNAs (data not shown). In addition, the prepro-NPF I product in one midgut sample from L. vannamei was sequenced, which confirmed it to be identical to the predicted portion of the Litva-prepro-NPF I transcript.
Assessment of the effects of NPF on food intake and growth
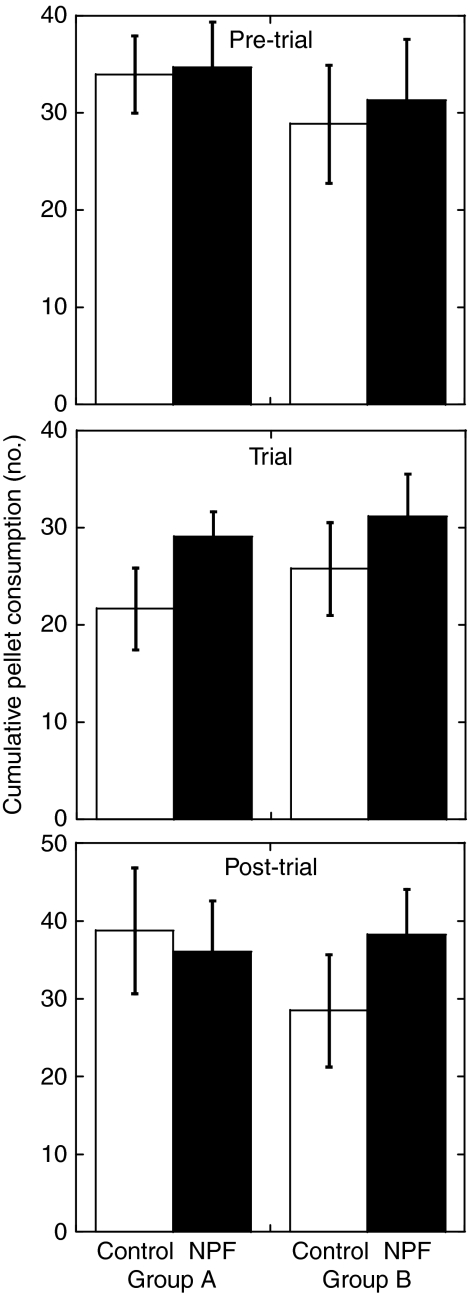
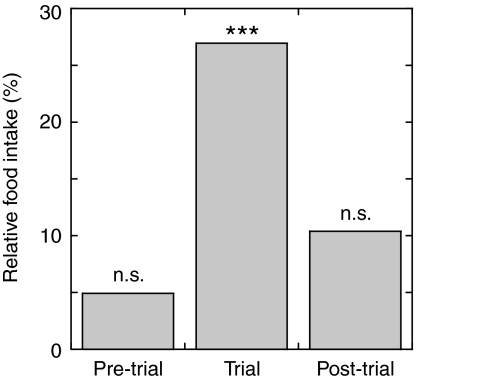
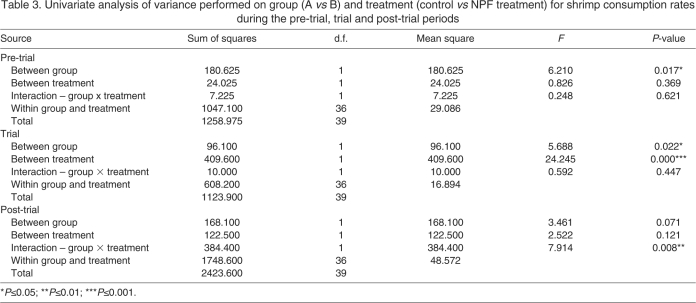
To assess the influence of oral administration of the NPF I peptide on food intake and growth in juvenile L. vannamei, a feeding trial was undertaken utilizing custom synthesized peptide. As can be seen in Figs 7 and 8, the rate of food ingestion was markedly higher in animals receiving NPF-treated food than in control animals during the trial period, but not during the pre- and post-trial periods. During the trial period, food intake in the NPF-treated group was nearly 30% higher than in the control group (Figs 7 and 8). Comparison of these data using a univariate analysis of variance revealed the difference in food intake between the treatment groups to be highly significant (P<0.001; Table 3 and Fig. 7). In contrast, consumption rates during the pre- and post-trial periods did not differ significantly between treatment groups (Table 3 and Fig. 7). Thus, the effect of the peptide supplement was only observed during the trial period. Interestingly, we did observe group (A vs B in Fig. 7) effects during the pre-trial and the trial period (P≤0.05; Table 3 and Fig. 7), and a significant interaction between group and treatment during the post-trial period (P≤0.01; Table 3 and Fig. 7), the origins of which remain unknown.
Fig. 7.
Cumulative consumption of food pellets during the 15 day NPF feeding experiment, separated into pre-trial (top), trial (middle) and post-trial (bottom) periods, for the control and NPF-treated shrimp. A and B refer to the two containment groups. NPF-treated individuals had their diet enhanced with a daily addition of 2 μl of 10–3 mol l–1 synthetic Litva-NPF I peptide. Each treatment and group combination contained 10 individuals (see Table 2 for statistical results). Error bars show the standard deviation.
Fig. 8.
Relative food intake, as a percentage difference between the NPF-treated and control shrimp during the pre-trial, trial and post-trial periods. NPF-treated individuals had their diet enhanced with a daily addition of 2 μl of 10–3 mol l–1 synthetic Litva-NPF I peptide during the trial period only. Significance: ***P<0.001; n.s., not significant.
Table 3.
Univariate analysis of variance performed on group (A vs B) and treatment (control vs NPF treatment) for shrimp consumption rates during the pre-trial, trial and post-trial periods
During the 15-day feeding trial, a total of 52 molts were collected. Of the 40 shrimp, 21 molted once, 14 molted twice and 1 molted three times. Only four individuals (10%) did not molt during the experiment. Although the rates of molting between groups and treatments were similar during the 15-day trial, molting was highest during the NPF trial; three molts per day were recorded during both the pre- and post-trial periods, but five molts per day during the trial itself. As the rate of food intake is greatly reduced just prior to and after molting (J.K.D. and S.R.M., unpublished observations), the feeding rates of shrimp during the trial period were lower in both the control and NPF-treated groups than they were during both the pre- and post-trial periods.
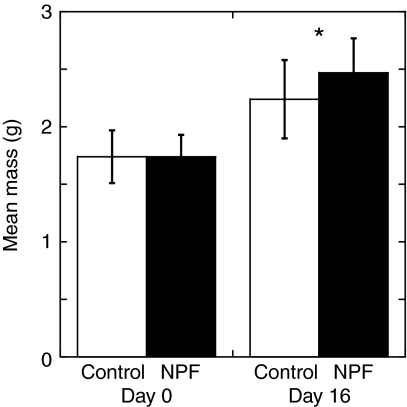
In terms of growth, at the beginning of the experiment, mean shrimp masses were similar in all four treatment groups (Fig. 9, Table 4). The mean initial mass of the 40 juvenile shrimp was 1.74±0.21 g (±s.d.; Fig. 9). In spite of the very short dosing period (5 days), we observed a statistically significant difference in final mass between the two treatments (∼10%; univariate ANOVA, P≤0.05; Table 4, Fig. 9). Specifically, the mean final masses of the control and NPF-treated shrimp were 2.24±0.34 g and 2.47±0.30 g, respectively, for an overall mass difference of 0.23 g. No group effect was noted with respect to growth rate, nor was there a significant interaction between group and treatment for this parameter (Table 4).
Fig. 9.
Mean mass of control and NPF-treated shrimp at the beginning of the experiment (day 0) and at the end (day 16). NPF-treated individuals had their diet enhanced with a daily addition of 2 μl of 10–3 mol l–1 synthetic Litva-NPF I peptide during the 5-day trial period (but not during the pre- and post-trial periods). Initial mean masses of control and NPF-treated shrimp were the same. An approximately 10% difference in mean masses was seen between the NPF-treated animals and the control shrimp at day 16 (see Table 3 for statistical results). Number of shrimp in each treatment: 20. Error bars show the standard deviation. Significance: *P≤0.05.
Table 4.
Univariate analysis of variance performed on group (A vs B) and treatment (control vs NPF treatment) for shrimp masses at the beginning of the experiment (day 0) and at the completion of the experimental period (day 16)
DISCUSSION
The peptides encoded by NPF precursors appear highly conserved in penaeid species
Although bioinformatic studies of ESTs have suggested the presence of NPFs in crustaceans (Christie et al., 2008; Gard et al., 2009), the data presented here is the first full characterization of NPF-encoding transcripts from members of this arthropod subphylum. In total, four transcripts were identified and characterized, two from L. vannamei and two from M. marginatus. In each species, the two transcripts differed from one another by a 111 bp insert present in the portion of the ORF that encodes the NPF peptide (Fig. 2). The predicted short and long prepro-hormones from L. vannamei and M. marginatus are essentially identical, with only five amino acid substitutions noted between the two sets of proteins, four of which are present in the putative signal sequences (Fig. 3). For each species, two NPF isoforms and one precursor-related peptide were predicted (Figs 3 and 4). Interestingly, the shorter predicted L. vannamei and M. marginatus NPFs, Litva- and Melma-NPF I, are identical to that identified by transcriptome mining from a M. japonicus EST (Christie et al., 2008) (Fig. 4Bi); the precursor-related peptide deduced from M. japonicus is also very similar to those predicted for L. vannamei and M. marginatus (Christie et al., 2008) (Fig. 4Biii). Thus, from the current data, it would appear that the prepro-NPFs, and the peptides putatively liberated from them, are highly conserved among penaeid species. Likewise, although slightly different in overall length, comparisons of the shorter of the penaeid NPFs with those of the cladoceran crustaceans D. magna and D. pulex (Christie et al., 2008; Gard et al., 2009), reveal extensive amino acid identity and similarity between the isoforms, particularly in their C-termini (data not shown); a similar situation exists when the sequence of the shorter penaeid NPF is compared with those of insects [e.g. the termite Reticulitermes flavipes (Nuss et al., 2010); data not shown].
Comparison with insect transcripts suggest that the two NPF-encoding cDNAs identified from both L. vannamei and M. marginatus are splice variants
A feature shared by the genes encoding vertebrate NPYs and those encoding most invertebrate NPFs is the presence of an intron positioned in the gene adjacent to the second to last arginine (Arg) residue in the coding sequence (Mair et al., 2000). In arthropods, this structural feature is present in the NPF genes of the mosquitoes Anopheles gambiae (Garczynski et al., 2005) and the silkworm Bombyx mori (Roller et al., 2008), but is absent in the NPF gene of the fruit fly Drosophila melanogaster (Brown et al., 1999). In the termite R. flavipes, it is suggested that this intron is involved in the production of a short and a long NPF (Nuss et al., 2010). At present, it is unclear if the insert seen in the R. flavipes long NPF is an incompletely processed intron or a true alternatively spliced variant (Nuss et al., 2010).
Here, we have identified transcripts encoding both short and long isoforms of NPF in the shrimp L. vannamei and M. marginatus. Interestingly, in addition to being identical in length and position within the NPF peptides, comparison of the insert with that from the termite shows that both are highly similar in sequence (46% amino acid identity and 81% amino acid similarity between the inserts). Thus, the data from insects suggest that the shrimp short and long transcripts may be similar and result from an incompletely processed intron or alternative splicing, though additional experiments will be needed to confirm, and differentiate between, these hypotheses.
RT-PCR tissue profiling suggests NPFs are brain and gut peptides in penaeid shrimp
In insects, there is a large literature describing the distribution of NPFs in neural and other tissues. Immunohistochemical, molecular and mass spectral studies suggest that NPFs are broadly distributed within insect nervous systems (Brown et al., 1999; Stanek et al., 2002; Gonzalez and Orchard, 2008; Nuss et al., 2008; Nuss et al., 2010). Similarly, they have been detected, using a variety of methods, from insect gut epithelial endocrine cells (e.g. Brown et al., 1999; Stanek et al., 2002; Nuss et al., 2008; Veenstra et al., 2008; Veenstra, 2009; Nuss et al., 2010). This dual brain and gut distribution suggests that NPFs may function as locally released autocrines or paracrines, as well as circulating hormones in members of the Insecta. Here, we have shown, using RT-PCR tissue profiling, that the transcripts encoding the penaeid NPFs are also present in both neural and midgut tissues of L. vannamei and M. marginatus, suggesting dual autocrine or paracrine and hormonal signaling by NPFs in both shrimp species.
RT-PCR showed NPF I and NPF II transcripts were present in all neural tissues, with the former being the major product and the latter a minor one. Interestingly, in eyestalk ganglia, the NPF I product was always robust and similar in intensity to that seen in the other neural tissues, but the NPF II band was significantly weaker in relative intensity than those present in brain, thoracic ganglia or abdominal ganglia. In fact, in some individuals, the NPF II product band was so faint in the samples of eyestalk ganglia that it suggests the possibility of tissue-specific regulation of NPF II expression in this region of the nervous system. This said, the presence of at least some NPF II expression in the eyestalk predicts that both NPF isoforms are present in all neural tissues, but that their relative proportions are skewed toward a greater abundance of the shorter isoform, particularly in the eyestalk. A similar pattern of relative expression of the short vs long transcripts is present in the mosquito A. gambiae [i.e. fig. 2 in Garczynski et al. (Garczynski et al., 2005)]. However, given the limited number of species from which comparisons of the abundance of two transcripts can be made, it is unclear if there is any rule of relative expression of short vs long NPF transcripts in members of the Arthropoda, though as additional data are generated it will be interesting to see if one develops.
In the midgut samples of L. vannamei and M. marginatus examined in our study, the shorter, but not the longer, of the NPF transcripts was detected in some but not all individuals. These results suggest a differential regulation of expression for the two transcripts (and peptides) within the midgut, and that NPF I, but not NPF II, probably functions as a gut-derived paracrine and/or endocrine in both species. At present, it is unclear why the expression of NPF I varied among individuals, though it is possible that this was due to the feeding status of the animals, a physiological state known to influence the expression of tachykinin-related peptides in the midgut of some crustaceans and insects (Winther and Nässel, 2001; Christie et al., 2007) (A.E.C., unpublished data), and a factor not controlled for in our study. Alternatively, expression of NPF may be related to the molt cycle, as is the expression of crustacean hyperglycemic hormone in gut epithelial endocrine cells of the crab Carcinus maenas (Chung et al., 1999; Webster et al., 2000), and individuals in this study may have been at different stages of the cycle. As we expected, no expression of NPF transcripts was seen in muscle tissues assayed from either shrimp species (or in negative controls), strengthening our identifications in the tissues where one or both transcripts were detected.
An orexigenic role for NPF in penaeid shrimp
Members of the NPY/NPF peptide families are well-known regulators of food intake. In crustaceans, although no authentic NPY has been identified, injection or ingestion of porcine NPY has been shown to induce modest increases in food intake and growth in the penaeid shrimp M. marginatus and P. semisulcatus (Kiris et al., 2004). In our study we assessed the effects of orally administered Litva-NPF I on these parameters using juvenile L. vannamei. In this feeding trial, the native NPF peptide had a significant effect on food consumption, increasing the rate of food intake by approximately 30% in the peptide-fed animals relative to controls. This increase in consumption was only observed during the peptide application period, suggesting a direct and short-lived effect on appetite. In penaeid shrimp, food intake is directly related to growth (Kiris et al., 2004). However, because of the short period of peptide application (5 days), we had not expected to see any measurable effect on growth (here measured as final mass gain). Nevertheless, the final masses of the NPF-supplemented individuals were approximately 10% higher than those in the control group, a difference that was statistically significant. Interestingly, in the earlier study using the porcine NPY, enhanced growth was only observed after a continued dosing period of 30 days (Kiris et al., 2004), strongly suggesting that the native penaeid NPF is a more potent orexigenic agent in these animals than the non-native NPY.
At present it is unclear where and how NPF is exerting its orexigenic influence in penaeids. Because the peptide was orally administered, action on or through the digestive tract seems likely. Given its presence in the midgut, it is possible that the peptide acts as a local paracrine agent in this tissue, possibly modulating nutrient/ion/water absorption by the tissue directly, or by modulating the release of other peptide paracrines or hormones from this tissue. Orally administered NPF would have direct access to receptors on the luminal surface of the gut epithelium to affect these processes. NPF receptors on the luminal surface of the gut may also act to modulate the frequency and amplitude of spontaneous contractions of the digestive tract, changing the rate of food passage or processing through it. Alternatively, it is possible that NPF is transported across the surface of the gut, acting on targets to which it would be delivered via the circulatory system. Similarly, if NPF affects the release of other hormones from gut epithelial endocrine cells, these signaling agents too could exert their actions on a variety of other tissues. Potential targets for hormonally delivered NPF (or other molecules whose release is triggered by it) include the stomatogastric neuromuscular system, which controls the ingestion, chewing and filtering of ingested food within the foregut, and the cardiac neuromuscular system, which is the major player in the control of hemolymph circulation, and hence movement of nutrients from the digestive system to other tissues within the shrimp. Given the interactive nature of physiological control systems in all animals, it is quite likely that the actions of NPF on food intake and growth in penaeids will ultimately be shown to be a multi-step effect, involving multiple signaling pathways and tissues. Clearly, future experiments directed at assessing the influence NPF on other target tissues need to be conducted to address this issue. Moreover, as such studies are conducted, it will be interesting to see how extensive the bioactivity of this peptide is in penaeid shrimp and other crustacean species, and how the identified targets compare with those of NPF in insects and other invertebrates, as well as with those of NPY in vertebrate species.
ACKNOWLEDGEMENTS
We thank Ms Heather Hillard and Mr Garret Lynch for their help in collecting the M. marginatus used in this study. Drs Daniel Hartline and Ian Cooke are thanked for their input on experimental design and for reading and commenting on early drafts of this study.
Footnotes
Financial support for this work was provided by NIH grant number P20 RR-016463 from the INBRE Program of the National Center for Research Resources (to Patricia Hand, MDIBL), National Science Foundation OCE0923692 (to Daniel Hartline, University of Hawaii at Manoa), through institutional funds provided by MDIBL (to A.E.C.), and by the Cades Foundation of Honolulu, Hawaii (to A.E.C.). Deposited in PMC for release after 12 months.
REFERENCES
- Benarroch E. E. (2009). Neuropeptide Y: its multiple effects in the CNS and potential clinical significance. Neurology 72, 1016-1020 [DOI] [PubMed] [Google Scholar]
- Bendtsen J. D., Nielsen H., von Heijne G., Brunak S. (2004). Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340, 783-795 [DOI] [PubMed] [Google Scholar]
- Brown M. R., Crim J. W., Arata R. C., Cai H. N., Chun C., Shen P. (1999). Identification of a Drosophila brain-gut peptide related to the neuropeptide Y family. Peptides 20, 1035-1042 [DOI] [PubMed] [Google Scholar]
- Chee M. J., Colmers W. F. (2008). Y eat? Nutrition 24, 869-877 [DOI] [PubMed] [Google Scholar]
- Christie A. E. (2008a). Neuropeptide discovery in Ixodoidea: an in silico investigation using publicly accessible expressed sequence tags. Gen. Comp. Endocrinol. 157, 174-185 [DOI] [PubMed] [Google Scholar]
- Christie A. E. (2008b). In silico analyses of peptide paracrines/hormones in Aphidoidea. Gen. Comp. Endocrinol. 159, 67-79 [DOI] [PubMed] [Google Scholar]
- Christie A. E., Kutz-Naber K. K., Stemmler E. A., Klein A., Messinger D. I., Goiney C. C., Conterato A. J., Bruns E. A., Hsu Y. W., Li L., et al. (2007). Midgut epithelial endocrine cells are a rich source of the neuropeptides APSGFLGMRamide (Cancer borealis tachykinin-related peptide Ia) and GYRKPPFNGSIFamide (Gly1-SIFamide) in the crabs Cancer borealis, Cancer magister and Cancer productus. J. Exp. Biol. 210, 699-714 [DOI] [PubMed] [Google Scholar]
- Christie A. E., Cashman C. R., Brennan H. R., Ma M., Sousa G. L., Li L., Stemmler E. A., Dickinson P. S. (2008). Identification of putative crustacean neuropeptides using in silico analyses of publicly accessible expressed sequence tags. Gen. Comp. Endocrinol. 156, 246-264 [DOI] [PubMed] [Google Scholar]
- Christie A. E., Stemmler E. A., Dickinson P. S. (2010a). Crustacean neuropeptides. Cell. Mol. Life. Sci. 67, 4135-4169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie A. E., Durkin C. S., Hartline N., Ohno P., Lenz P. H. (2010b). Bioinformatic analyses of the publicly accessible crustacean expressed sequence tags (ESTs) reveal numerous novel neuropeptide-encoding precursor proteins, including ones from members of several little studied taxa. Gen. Comp. Endocrinol. 167, 164-178 [DOI] [PubMed] [Google Scholar]
- Christie A. E., Stevens J. S., Bowers M. R., Chapline M. C., Jensen D. A., Schegg K. M., Goldwaser J., Kwiatkowski M. A., Pleasant T. K., Shoenfeld L., et al. (2010c). Identification of a calcitonin-like diuretic hormone that functions as an intrinsic modulator of the American lobster, Homarus americanus, cardiac neuromuscular system. J. Exp. Biol. 213, 118-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J. S., Dircksen H., Webster S. G. (1999). A remarkable, precisely timed release of hyperglycemic hormone from endocrine cells in the gut is associated with ecdysis in the crab Carcinus maenas. Proc. Natl. Acad. Sci. USA 96, 13103-13107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon J. M., Larhammar D. (2005). The evolution of neuroendocrine peptides. Gen. Comp. Endocrinol. 142, 53-59 [DOI] [PubMed] [Google Scholar]
- Dickinson P. S., Stevens J. S., Rus S., Brennan H. R., Goiney C. C., Smith C. M., Li L., Towle D. W., Christie A. E. (2007). Identification and cardiotropic actions of sulfakinin peptides in the American lobster Homarus americanus. J. Exp. Biol. 210, 2278-2289 [DOI] [PubMed] [Google Scholar]
- Dickinson P. S., Wiwatpanit T., Gabranski E. R., Ackerman R. J., Stevens J. S., Cashman C. R., Stemmler E. A., Christie A. E. (2009). Identification of SYWKQCAFNAVSCFamide: a broadly conserved crustacean C-type allatostatin-like peptide with both neuromodulatory and cardioactive properties. J. Exp. Biol. 212, 1140-1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierick H. A., Greenspan R. J. (2007). Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat. Genet. 39, 678-682 [DOI] [PubMed] [Google Scholar]
- Dougan P. M., Mair G. R., Halton D. W., Curry W. J., Day T. A., Maule A. G. (2002). Gene organization and expression of a neuropeptide Y homolog from the land planarian Arthurdendyus triangulatus. J. Comp. Neurol. 454, 58-64 [DOI] [PubMed] [Google Scholar]
- Emeson R. B., Morabito M. V. (2005). Food fight: the NPY-serotonin link between aggression and feeding behavior. Sci. STKE 277, pe12 [DOI] [PubMed] [Google Scholar]
- Fu Q., Kutz K. K., Schmidt J. J., Hsu Y. W., Messinger D. I., Cain S. D., de la Iglesia H. O., Christie A. E., Li L. (2005). Hormone complement of the Cancer productus sinus gland and pericardial organ: an anatomical and mass spectrometric investigation. J. Comp. Neurol. 493, 607-626 [DOI] [PubMed] [Google Scholar]
- Garczynski S. F., Crim J. W., Brown M. R. (2005). Characterization of neuropeptide F and its receptor from the African malaria mosquito, Anopheles gambiae. Peptides 26, 99-107 [DOI] [PubMed] [Google Scholar]
- Gard A. L., Lenz P. H., Shaw J. R., Christie A. E. (2009). Identification of putative peptide paracrines/hormones in the water flea Daphnia pulex (Crustacea; Branchiopoda; Cladocera) using transcriptomics and immunohistochemistry. Gen. Comp. Endocrinol. 160, 271-287 [DOI] [PubMed] [Google Scholar]
- Gasteiger E., Gattiker A., Hoogland C., Ivanyi I., Appel R. D., Bairoch A. (2003). ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 31, 3784-3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R., Orchard I. (2008). Characterization of neuropeptide F-like immunoreactivity in the blood-feeding hemipteran, Rhodnius prolixus. Peptides 29, 545-558 [DOI] [PubMed] [Google Scholar]
- Gonzalez R., Orchard I. (2009). Physiological activity of neuropeptide f on the hindgut of the blood-feeding hemipteran, Rhodnius prolixus. J. Insect Sci. 9, 1-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries J. E., Kimber M. J., Barton Y. W., Hsu W., Marks N. J., Greer B., Harriott P., Maule A. G., Day T. A. (2004). Structure and bioactivity of neuropeptide F from the human parasites Schistosoma mansoni and Schistosoma japonicum. J. Biol. Chem. 279, 39880-39885 [DOI] [PubMed] [Google Scholar]
- Karl T., Herzog H. (2007). Behavioral profiling of NPY in aggression and neuropsychiatric diseases. Peptides 28, 326-333 [DOI] [PubMed] [Google Scholar]
- Kastin A. J. (2006). Handbook of Biologically Active Peptides. First Edition Burlington, MA: Academic Press; [Google Scholar]
- Katoh K., Toh H. (2008). Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform. 9, 286-298 [DOI] [PubMed] [Google Scholar]
- Katoh K., Misawa K., Kuma K., Miyata T. (2002). MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30, 3059-3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiris I. G., Eroldogan O. T., Kir M., Kumlu M. (2004). Influence of neuropeptide Y (NPY) on food intake and growth of penaeid shrimps Marsupenaeus japonicus and Penaeus semisulcatus (Decapoda: Penaeidae). Comp. Biochem. Physiol. 139A, 239-244 [DOI] [PubMed] [Google Scholar]
- Krashes M. J., DasGupta S., Vreede A., White B., Armstrong J. D., Waddell S. (2009). A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell 139, 416-427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larhammar D. (1996). Evolution of neuropeptide Y, peptide YY and pancreatic polypeptide. Regul. Pept. 62, 1-11 [DOI] [PubMed] [Google Scholar]
- Larhammar D., Söderberg C., Lundell I. (1998). Evolution of the neuropeptide Y family and its receptors. Ann. NY Acad. Sci. 839, 35-40 [DOI] [PubMed] [Google Scholar]
- Larhammar D., Sundström G., Dreborg S., Daza D. O., Larsson T. A. (2009). Major genomic events and their consequences for vertebrate evolution and endocrinology. Ann. NY Acad. Sci. 1163, 201-208 [DOI] [PubMed] [Google Scholar]
- Ma M., Chen R., Sousa G. L., Bors E. K., Kwiatkowski M. A., Goiney C. C., Goy M. F., Christie A. E., Li L. (2008). Mass spectral characterization of peptide transmitters/hormones in the nervous system and neuroendocrine organs of the American lobster Homarus americanus. Gen. Comp. Endocrinol. 156, 395-409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M., Bors E. K., Dickinson E. S., Kwiatkowski M. A., Sousa G. L., Henry R. P., Smith C. M., Towle D. W., Christie A. E., Li L. (2009a). Characterization of the Carcinus maenas neuropeptidome by mass spectrometry and functional genomics. Gen. Comp. Endocrinol. 161, 320-334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M., Wang J., Chen R., Li L. (2009b). Expanding the Crustacean neuropeptidome using a multifaceted mass spectrometric approach. J. Proteome Res. 8, 2426-2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M., Gard A. L., Xiang F., Wang J., Davoodian N., Lenz P. H., Malecha S. R., Christie A. E., Li L. (2010). Combining in silico transcriptome mining and biological mass spectrometry for neuropeptide discovery in the Pacific white shrimp Litopenaeus vannamei. Peptides 31, 27-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair G. R., Halton D. W., Shaw C., Maule A. G. (2000). The neuropeptide F (NPF) encoding gene from the cestode, Moniezia expansa. Parasitology 120, 71-77 [DOI] [PubMed] [Google Scholar]
- Maule A. G., Shaw C., Halton D. W., Thim L., Johnston C. F., Fairweather I., Buchanan K. D. (1991). Neuropeptide F: a novel parasitic flatworm regulatory peptide from Moniezia expansa (Cestoda:Cyclophyllidea). Parasitology 102, 309-316 [Google Scholar]
- Mercier A. J., Orchard I., TeBrugge V., Skerrett M. (1993). Isolation of two FMRFamide-related peptides from crayfish pericardial organs. Peptides 14, 137-143 [DOI] [PubMed] [Google Scholar]
- Monigatti F., Gasteiger E., Bairoch A., Jung E. (2002). The Sulfinator: predicting tyrosine sulfation sites in protein sequences. Bioinformatics 18, 769-770 [DOI] [PubMed] [Google Scholar]
- Nuss A. B., Forschler B. T., Crim J. W., Brown M. R. (2008). Distribution of neuropeptide F-like immunoreactivity in the Eastern Subterranean termite, Reticulitermes flavipes. J. Insect Sci. 8, 1-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss A. B., Forschler B. T., Crim J. W., TeBrugge V., Pohl J., Brown M. R. (2010). Molecular characterization of neuropeptide F from the eastern subterranean termite Reticulitermes flavipes (Kollar) (Isoptera: Rhinotermitidae). Peptides 31, 419-428 [DOI] [PubMed] [Google Scholar]
- Pedrazzini T., Pralong F., Grouzmann E. (2003). Neuropeptide Y: the universal soldier. Cell. Mol. Life. Sci. 60, 350-377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajpara S. M., Garcia P. D., Roberts R., Eliassen J. C., Owens D. F., Maltby D., Myers R. M., Mayeri E. (1992). Identification and molecular cloning of a neuropeptide Y homolog that produces prolonged inhibition in Aplysia neurons. Neuron 9, 505-513 [DOI] [PubMed] [Google Scholar]
- Roller L., Yamanaka N., Watanabe K., Daubnerová I., Zitnan D., Kataoka H., Tanaka Y. (2008). The unique evolution of neuropeptide genes in the silkworm Bombyx mori. Insect Biochem. Mol. Biol. 38, 1147-1157 [DOI] [PubMed] [Google Scholar]
- Stanek D. M., Pohl J., Crim J. W., Brown M. R. (2002). Neuropeptide F and its expression in the yellow fever mosquito, Aedes aegypti. Peptides 23, 1367-1378 [DOI] [PubMed] [Google Scholar]
- Stemmler E. A., Cashman C. R., Messinger D. I., Gardner N. P., Dickinson P. S., Christie A. E. (2007). High-mass-resolution direct-tissue MALDI-FTMS reveals broad conservation of three neuropeptides (APSGFLGMRamide, GYRKPPFNGSIFamide and pQDLDHVFLRFamide) across members of seven decapod crustaean infraorders. Peptides 28, 2104-2115 [DOI] [PubMed] [Google Scholar]
- Stemmler E. A., Bruns E. A., Cashman C. R., Dickinson P. S., Christie A. E. (2010). Molecular and mass spectral identification of the broadly conserved decapod crustacean neuropeptide pQIRYHQCYFNPISCF: the first PISCF-allatostatin (Manduca sexta- or C-type allatostatin) from a non-insect. Gen. Comp. Endocrinol. 165, 1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström G., Larsson T. A., Brenner S., Venkatesh B., Larhammar D. (2008). Evolution of the neuropeptide Y family: new genes by chromosome duplications in early vertebrates and in teleost fishes. Gen. Comp. Endocrinol. 155, 705-716 [DOI] [PubMed] [Google Scholar]
- Thorsell A. (2008). Central neuropeptide Y in anxiety- and stress-related behavior and in ethanol intake. Ann. NY Acad. Sci. 1148, 136-140 [DOI] [PubMed] [Google Scholar]
- Torfs P., Baggerman G., Meeusen T., Nieto J., Nachman R. J., Calderon J., De Loof A., Schoofs L. (2002). Isolation, identification, and synthesis of a disulfated sulfakinin from the central nervous system of an arthropods the white shrimp Litopenaeus vannamei. Biochem. Biophys. Res. Commun. 299, 312-320 [DOI] [PubMed] [Google Scholar]
- Trimmer B. A., Kobierski L. A., Kravitz E. A. (1987). Purification and characterization of FMRFamidelike immunoreactive substances from the lobster nervous system: isolation and sequence analysis of two closely related peptides. J. Comp. Neurol. 266, 16-26 [DOI] [PubMed] [Google Scholar]
- Veenstra J. A. (2000). Mono- and dibasic proteolytic cleavage sites in insect neuroendocrine peptide precursors. Arch. Insect Biochem. Physiol. 43, 49-63 [DOI] [PubMed] [Google Scholar]
- Veenstra J. A. (2009). Peptidergic paracrine and endocrine cells in the midgut of the fruit fly maggot. Cell Tissue Res. 336, 309-323 [DOI] [PubMed] [Google Scholar]
- Veenstra J. A., Agricola H. J., Sellami A. (2008). Regulatory peptides in fruit fly midgut. Cell Tissue Res. 334, 499-516 [DOI] [PubMed] [Google Scholar]
- Walker R. J., Papaioannou S., Holden-Dye L. (2010). A review of FMRFamide- and RFamide-like peptides in metazoa. Invert. Neurosci. 9, 111-153 [DOI] [PubMed] [Google Scholar]
- Webster S. G., Dircksen H., Chung J. S. (2000). Endocrine cells in the gut of the shore crab Carcinus maenas immunoreactive to crustacean hyperglycaemic hormone and its precursor-related peptide. Cell Tissue Res. 300, 193-205 [DOI] [PubMed] [Google Scholar]
- Winther A. M., Nässel D. R. (2001). Intestinal peptides as circulating hormones: release of tachykinin-related peptide from the locust and cockroach midgut. J. Exp. Biol. 204, 1269-1280 [DOI] [PubMed] [Google Scholar]
- Xu J., Li M., Shen P. (2010). A G-protein-coupled neuropeptide Y-like receptor suppresses behavioral and sensory response to multiple stressful stimuli in Drosophila. J. Neurosci. 30, 2504-2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano K., Unuma T. (2006). Expressed sequence tags from eyestalk of kuruma prawn, Marsupenaeus japonicus. Comp. Biochem. Physiol. 143A, 155-161 [DOI] [PubMed] [Google Scholar]