Abstract
Surgical interventional strategies for the treatment of obesity are being implemented at an increasing rate. The safety and feasibility of these procedures are questionable for most overweight or obese individuals. The use of long-term pharmacotherapy options, on the other hand, can target a greater portion of the obese population and provide early intervention to help individuals maintain a healthy lifestyle to promote weight loss. Medications that act on the central serotonergic pathways have been a relative mainstay for the treatment of obesity for the last 35 years. The clinical efficacy of these drugs, however, has been encumbered by the potential for drug-associated complications. Two drugs that act, albeit by different mechanisms, on the central serotonergic system to reduce food intake and decrease body weight are sibutramine and lorcaserin. Sibutramine is a serotonin and norepinephrine reuptake inhibitor, whereas lorcaserin is a selective 5HT2C receptor agonist. The recent worldwide withdrawal of sibutramine and FDA rejection of lorcaserin has changed the landscape not only for serotonin-based therapeutics specifically, but for obesity pharmacotherapy in general. The purpose of this review is to focus on the importance of the serotonergic system in the control of feeding and its potential as a target for obesity pharmacotherapy. Advances in refining and screening more selective receptor agonists and a better understanding of the potential off-target effects of serotonergic drugs are needed to produce beneficial pharmacotherapy.
Keywords: 5-hydroxytryptamine, serotonin 1B, fenfluramine, dexfenfluramine, satiety, dorsal raphe
Introduction
Obesity in the past several decades has become increasingly prevalent in the adult population of several countries, such as the US, Mexico, and the UK.1–3 The alarming rate of childhood obesity in these and other countries only exaggerates the health concern worldwide.4–6 In fact, the World Health Organization projects 700 million adults will be clinically obese (BMI ≥ 30, body mass index; kg/m2) by 2015.7,8 Individuals who are obese or even overweight (BMI 25–29.9) are at an increased risk of developing one or more chronic diseases, including diabetes mellitus type II, coronary heart disease, hypertension, and various cancers.9–11 Several studies have indicated that weight loss, even a 5% to 10% weight reduction, either lowers the risk of developing these comorbidities or helps in their therapeutic management.12–16 The overall societal healthcare burden and the individual risk to a person’s health and wellbeing caused by excessive weight gain, therefore, can be dramatically reduced by effective interventional strategies aimed at reducing body weight.
The type and degree of interventional strategies recommended by a clinician to treat obesity can vary depending on the severity of weight gain and obesity-related complications. The initial approach involves having patients reduce their total caloric intake by eating more nutritious foods while increasing their physical activity. Other behavioral approaches may involve self-monitoring of eating behaviors, cognitive therapies, and lifestyle modification support.12 In addition, pharmacotherapy can be initiated if moderately overweight individuals (BMI 27–29.9) have difficulty losing weight or are at a high risk for developing an obesity-related comorbidity. Pharmacotherapy is typically initiated in obese individuals with a BMI of ≥30 as part of a comprehensive treatment approach.12,17 In more severe obese individuals with a BMI of >40 (also known as class III obese) or a BMI > 35 with one or more obesity-related comorbidities, bariatric surgical options are considered as well.12,13,18,19 Bariatric surgery, in particular gastric bypass, compared with other interventional strategies is the most clinically effective at decreasing body weight and reducing the incidence and risk of most obesity-related comorbidities.19–21 Bariatric surgery, however, poses a mortality risk (as high as 10%) to patients undergoing the procedure and the immediate and the long-term cost-effectiveness is only significantly apparent in individuals with a BMI ≥ 40.22–24 In addition, there is a suggested higher risk of suicide in patients undergoing bariatric surgery. A recent survey of 16,683 bariatric operations found there were 31 suicides in the 3-year post-surgery period (13.7 per 10,000 among men and 5.2 per 10,000 among women), which is a much higher rate than general age and sex-matched population in the US (2.4 per 10,000 among men and 0.7 per 10,000 among women).25 Nonetheless, obese individuals with a BMI ≥ 40 represent only 10% to 12% of the adult overweight or obese population in the US, suggesting that bariatric surgery is not a feasible weight loss interventional strategy for most of the afflicted population.26 Hence, overweight or moderately obese individuals would benefit tremendously from effective pharmacotherapy before their weight gain becomes unmanageable or they develop associated comorbidities. When combined with other interventional strategies, in this sense, pharmacotherapy can help achieve or maintain ideal weight loss and possibly avoid more invasive life-threatening surgical interventions.
The development of effective long-term pharmacotherapy for obesity is a difficult task because mechanisms that promote sustained weight loss are often accompanied by mild to severe adverse events. The purpose of this review is to primarily focus on two obesity medications, sibutramine and lorcaserin, which act on the central serotonergic systems to reduce body weight. While the relevance of pharmacotherapy for obesity is certain to change in the next few years, particularly as a consequence of the withdrawal of sibutramine and an FDA advisory panel’s concerns over lorcaserin, this review will highlight the importance of serotonergic systems and the pharmacotherapy potential for serotonergic drugs in the long-term treatment of obesity.
Pharmacotherapy for the long-term treatment of obesity
The clinical endpoint for effective pharmacotherapy, as stipulated by the Food and Drug Administration (FDA, USA) and National Institute for Health and Clinical Excellence (NICE, UK), is based on mean efficacy and categorical efficacy. Mean efficacy is defined as a medication-associated (ie, greater than placebo) weight reduction of 5%. Categorical efficacy is defined as a significantly greater proportion (at least 35%) of those individuals receiving the medication compared with placebo controls maintaining a 5% weight loss from their initial weight.13,27 A mean efficacy of a 5% medication-associated weight reduction has been a difficult criterion to achieve in most large scale clinical trials and an overall efficacy of a medication is generally assessed more by a risk-benefit approach. Until fall 2010, the only two prescribed medications for the long-term treatment of obesity were orlistat and sibutramine. These drugs have completely different mechanisms of action, but each has shown to produce a varying degree of clinical efficacy at reducing body weight when administered along with interventional strategies aimed at changing a patient’s diet, physical activity, and eating behaviors.
Orlistat or tetrahydrolipstatin is a derivative of lipstatin, an inhibitor of lipases isolated from the Gram-positive bacterium Streptomyces toxytricini.28 Orlistat reduces the absorption of dietary fat by selectively and irreversibly binding to pancreatic and gastric lipases in the intestinal lumen. Inhibition of these lipases prevents the breakdown of triglycerides and diacylglycerides into free fatty acids for epithelial absorption and subsequent utilization.29 Orlistat (120 mg 3 times daily) reduces the absorption of dietary fat by approximately 30% and has been demonstrated to result in an approximately 3% greater orlistat-associated reduction in body weight in long-term (52-week) randomized double-blinded studies.30–32 The most commonly reported adverse events with orlistat treatment in a large number of subjects (as high as 30% above the control group) were related to mild to moderate gastrointestinal disturbances.30 Aside from rare cases of acute liver injury, orlistat is a moderately well-tolerated, relatively safe drug producing modest long-term weight loss.33 A similar favorable assessment of orlistat’s risk-benefit analysis is shared by drug regulatory agencies in the US, European Union, and Australia, which have all approved over-the-counter dosages of the drug.33–35
Sibutramine, on the other hand, was indefinitely suspended in August 2010 for the treatment of obesity by the European Union drug regulatory agency based on several reports of cardiovascular complications; the agency cited the “drug’s benefits do not outweigh the risks”.36 In October 2010, following the findings of a comprehensive study examining the potential cardiovascular risks and a split decision of an FDA advisory panel, Abbott Laboratories, the manufacturers of sibutramine, withdrew sibutramine from the US, Australia, and other countries.37 Sibutramine is in a class of drugs known as monoamine reuptake inhibitors and most drugs of this class are prescribed for the treatment of depression.38–40 The effectiveness of monoamine reuptake inhibitors is achieved by augmenting central nervous system (CNS) concentrations of monoamine neurotransmitters, such as dopamine (DA), norepinephrine (NE), and serotonin (5-hydroxytryptamine; 5HT). Sibutramine was ineffective in vivo as an antidepressant, but produced sustained weight loss by reducing food intake and increasing energy expenditure.38,41 Sibutramine and its active amine metabolites alter serotonergic and noradrenergic, but not dopaminergic, activity in brain areas that are involved in the control of appetite.38,42,43 Long-term treatment (∼52 weeks) with sibutramine (10 or 15 mg once daily) in randomized placebo-controlled studies has been shown to reduce body weight by 5% to 10% more than placebo control subjects.44,45 A meta-analysis examining 10 long-term weight-loss studies with sibutramine (15 mg once daily), however, found more modest weight loss, with a 4.3% sibutramine-associated reduction in body weight.17 Since its FDA approval in 1997, the widespread use of sibutramine to treat obesity was limited because the drug increased heart rate and blood pressure and was not indicated in obese or overweight patients with a history of cardiovascular disease.46,47 Prompting the withdrawal of sibutramine was a recently completed multicenter trial examining sibutramine on cardiovascular OUTcomes (SCOUT) in subjects with either a history of at least one risk factor for cardiovascular disease. The findings from this study demonstrated an overall 16% increase in the relative risk of cardiovascular events (ie, nonfatal myocardial infarction and stroke) with sibutramine treatment. Moreover, the sibutramine-associated weight loss at the end of the trial was modest, with approximately 3% greater weight loss than the placebo group.48 The small weight-loss benefit and increased risk of cardiovascular events in at risk obese patients in such a large study led to an unfavorable assessment by an FDA advisory panel and subsequent withdrawal of the medication.
Lorcaserin (ADP356) is another potential treatment for obesity that acts on the central serotonergic system to reduce food intake and body weight. Lorcaserin is a selective serotonin receptor (5HT2C) agonist and is believed to reduce food intake predominantly by influencing hypothalamic pathways involved in appetite.49 In a phase III clinical trial with 3182 overweight or obese subjects (known as BLOOM; Behavioral modification and Lorcaserin for Overweight and Obesity Management), there was a 4% lorcaserin (10 mg twice daily)-associated weight reduction at 52 weeks. Despite this modest weight loss, lorcaserin was associated with few subject-reported and no cardiovascular-related adverse events.50 The toxicology data in rodents presented to the FDA advisory panel, however, demonstrated a significant number of neoplasms in mammary and brain tissue of rats treated with lorcaserin (10 mg/kg, 30 mg/kg, 100 mg/kg per day) for 2 years.51 Based on the modest weight loss and the problematic carcinogenicity findings, the FDA advisory panel recommended that lorcaserin not be approved for the long-term treatment of obesity, and that decision was supported by the FDA’s complete response letter to the New Drug Application (NDA) filed for lorcaserin, which requested more data addressing these issues.52
Serotonin in the control of feeding behavior and metabolism
Serotonin (5HT) was initially isolated from beef serum in 1948 during the process of determining an active substance involved in vasoconstriction.53,54 Although 5HT has extensive biological actions in peripheral tissue and as a vasoactive amine, its role as a neurotransmitter in the CNS as a modulator of behavior and mood has received considerable attention.55 The 5HT-containing neurons are organized into nine nuclei (B1–B9) and are located in the midbrain and hindbrain areas. The dorsal raphe (B7), in particular, is a midbrain nucleus that contains a substantial portion of the total brain 5HT and has distinct projections to hypothalamic nuclei and other feeding-related forebrain areas.56,57 Obesity, either by genetic or diet-induced means, has been demonstrated to alter 5HT dorsal raphe neurons and 5HT terminal regions. For instance, the genetic obese fatty Zucker rats were shown to have hyperexcitable dorsal raphe neurons and greater feeding-induced hypothalamic 5HT levels compared with lean Zucker rats.58,59 Continuous infusions (14-day) of 5HT into a target hypothalamic region, ventromedial nucleus, has been shown to reduce food intake and body weight of lean Zucker rats, but not obese Zucker rats.60 Related to this, increases in 5HT transporter binding have been reported in the dorsal raphe of rats made obese by feeding a high-energy diet (68% carbohydrate and 13% fat) for 7 weeks. Taken together, these data suggest a dysregulation of central serotonergic pathways as a consequence of obesity.61
The direct functional involvement of 5HT in the modulation of feeding behavior was suggested by early experiments examining the anorectic potency of fenfluramine.62,63 Fenfluramine (3-trifluoromethyl-N-ethylamphetamine) is structurally similar to d-amphetamine, but fenfluramine is more potent as an anorectic agent without an abuse potential.64–66 Fenfluramine is a racemic compound with its active enantiomer being the d-isomer or dexfenfluramine.67 The mechanism of action for dexfenfluramine is the release of 5HT (and to a much lesser extent NE), whereas amphetamine is less selective and releases NE and DA from nerve terminals. Fenfluramine, dexfenfluramine, and d-amphetamine are classified as monoamine releasing agents, but specifically are transporter substrates causing the displacement of monoamines from intracellular storage independent of neuronal activity.40,62,68 In addition, active metabolites of fenfluramine and dexfenfluramine (eg, nor-fenfluramines) act as agonists at postsynaptic serotonin receptors to potentiate the serotonergic actions of the parent drugs.66,69 Dexfenfluramine also has actions to enhance energy expenditure since the body weight produced by the drug is greater than that achieved by pair-feeding animals.65,70,71 This enhanced energy expenditure partly occurs via increased fat oxidation.72 Fenfluramine was approved in 1973 and dexfenfluramine was approved in 1996 as medications for treatment of obesity in the US. Fenfluramine and later dexfenfluramine were part of an “off-label” combinational drug therapy with phentermine, an amphetamine analog stimulant FDA-approved for the short-term (up to 3 months) treatment of obesity, and the drug combination was known as “fen-phen” or “dexfen-phen”.66,73 Although the combinational therapies were effective in the long-term management (up to 12 months) of obesity and were widely prescribed, the therapies were associated with a significant increased risk of developing primary pulmonary hypertension and valvular heart disease.74–80 These adverse events were discovered to be caused by fenfluramine and dexfenfluramine and the two drugs were subsequently withdrawn from the market in 1997 at the FDA’s recommendation.81
Not only did experimental findings with fenfluramine and dexfenfluramine suggest that targeting the serotonergic systems produced clinically significant body weight reductions, these drugs also implicated 5HT’s involvement in the inhibitory control of eating. Acute peripheral injections of fenfluramine and dexfenfluramine have been demonstrated to increase hypothalamic concentrations 5HT.82,83 Also, when dexfenfluramine is chronically administered to rodents it reduces meal sizes and meal duration, and progresses the behavioral sequence of satiety, suggesting that the drug acts on the physiological functions involved in the normal cessation of a meal.84–86 That is, when rats are allowed to eat until satiety they display a temporal sequence of behaviors as meal consumption is terminating that begins with a reduction in eating followed by increases in grooming and other activities, and then a period of rest.84,85,87 This behavioral sequence is disrupted with amphetamine and food adulterated with bitter-tasting quinine, suggesting the anorectic responses produced by these agents are mediated differently from those involved with satiety.85,88 Generally speaking, it has been demonstrated that serotonergic compounds, which specifically and dose-dependently increase 5HT signaling to reduce food intake, maintain the integrity of the behavioral satiety sequence at a related range of doses.84,85,89 Similar reductions in eating rate and increased subjective satiety ratings have been demonstrated in human subjects administered fenfluramine and other serotonergic drugs, confirming the behavior interpretations made in rodents.70,90–92 Correspondingly, certain pharmacological conditions that decrease CNS levels of 5-HT promote overeating.57 Centrally injected selective serotonin depleting agents, such as p-chlorophenylalanine or 5,7-dihydroxytryptamine pretreatment with desmethlyimipramine, resulted in pronounced hyperphagia and increased body weight in rats.93,94
Sibutramine was approved for the long-term treatment of obesity by the FDA in November 1997 (coincidentally, dexfenfluramine was withdrawn in September, 1997). Sibutramine also increases the extracellular 5HT to reduce food intake, but does so by a different mechanism of action than dexfenfluramine. Acting as a NE and 5HT reuptake inhibitor, sibutramine (and its active metabolites) prevents the extracellular removal of monoamines and their effectiveness, and therefore is dependent on neuronal activity of 5HT neurons. Using anorectic doses of fenfluramine or sibutramine in rats, the magnitude of release of 5HT in the hypothalamus was shown to be 10- to 15-fold higher with fenfluramine (3 mg/kg) than with sibutramine (10 mg/kg).82 The reduced magnitude of 5HT release with sibutramine is mediated, in part, by indirect activation of somatodendritic autoreceptors, which modulate the intrinsic activity of 5HT neurons.82,95 This autoreceptor inhibition is not evident with monoamine releasers (eg, fenfluramine or dexfenfluramine) because their mechanism of action is not dependent on neuronal activation.82 Sibutramine, similar to serotonin-selective reuptake inhibitors (SSRI), was believed to mediate its actions by increases in basal or tonic levels of 5HT with repeated treatment. It appears that autoreceptor desensitization in the dorsal raphe rather than increased 5HT actions in the terminal is more likely involved in the sustained actions of SSRIs, but this needs further delineation for the anorectic actions of sibutramine.38,96,97 It is worth noting that SSRIs do not have a sustained effect on weight loss, hence it has been concluded that the combined actions on the 5HT and NE transporters are needed for the weight loss efficacy of sibutramine.39,98,99
Sibutramine has been reported to improve glucose regulation and lipid profiles in obese subjects, but the enhancement of these factors appears to be a consequence of weight loss rather than the direct actions of sibutramine on metabolism.38,100 Sibutramine has been demonstrated, however, to improve obesity-related energy expenditure. Dietary weight loss is accompanied by a decrease in energy expenditure, which tends to be an obstacle for optimal weight loss.101 Several studies have indicated that sibutramine attenuates the decline in energy expenditure that follows weight loss.102,103 The increased energy expenditure has been shown to be mediated by central modulation of sympathetic outflow, the thermogenic effects being blocked by antagonism of β3 adrenoceptor in rats.104 On the other hand, the effect of sibutramine on heart rate and blood pressure appears to be a paradoxical interplay between reduced central and increased peripheral sympathetic regulation, but this needs further clarification.56,105–108
The assortment of central and peripheral biological actions of 5HT is mediated by at least 14 different classified receptors that are differentiated based on structure, function, and intracellular signaling.109,110 The two receptors most critically involved in the control of feeding behavior and body weight homeostasis are the 5HT1B and 5HT2C receptors.111 Recent findings have also implicated 5HT2B receptors in the anorectic properties of dexfenfluramine; however, this receptor has been strongly implicated in the cardiopulmonary adverse effects of dexfenfluramine.112–114 The role of the 5HT2B receptor is likely to be a mechanism involved in potentiating 5HT release from serotonergic neurons, but its feasibility as a target for obesity certainly requires further examination.112
The 5HT1B receptor (classified at one time as the human 5HT1Dβ receptor115,116) is a G-protein couple receptor (GPCR) that negatively couples to adenylyl cyclase to inhibit cAMP formation. Like receptors of the 5HT1 class, the 5HT1B receptor has a high affinity for 5HT and is encoded by a gene sequence without introns.110 Located on nerve terminals, the 5HT1B receptor functions as an autoreceptor to inhibit the release of 5HT from serotonergic neurons or as heteroreceptors to inhibit the release of other (nonserotonin) neurotransmitters.109,110,117 The involvement of the 5HT1B receptor in feeding behavior was initially implicated using preferential antagonists with affinity for the receptor, such as (+/−) cyanopindolol and methiothepin, to block the anorectic actions of dexfenfluramine, whereas the selective agonist, CPP94235 (3-(1,2,5,6-tetrahydro-4-pyridyl)-5-propoxypyrrolo[3, 2-b] pyridine), reduced meal size and decreased feeding duration.118–121 Further evidence for a role in feeding behavior was demonstrated with the generation of the 5HT1B receptor knockout mouse. Following administration of fenfluramine (3 or 10 mg/kg), for instance, 5HT1B receptor knockout mice were unresponsive to its anorectic actions and demonstrated reduced or no neuronal activation in feeding related brain areas compared with wild types.122 It also has been reported that 5HT1B receptor knockout mice, compared with age-matched wild type mice, have higher body weights and consumed more food and water, but these findings are not consistently reported with 5HT1B knockout mice.122,123
The actions of the 5HT1B receptor in the CNS have been demonstrated to directly influence pathways involved in food intake and body weight homeostasis. Separate populations of neurons in arcuate nucleus (ARC) of the hypothalamus express the orexigenic peptides neuropeptide Y/agouti-related peptide (NPY/AgRP) or the precursor pro-opiomelanocortin (POMC) to the anorectic peptide alpha-melanocyte stimulating hormone (α-MSH). These two groups of neurons are activated or inhibited by other CNS pathways or peripheral circulating factors to control feeding behavior and energy expenditure. For example, the POMC-containing neurons release α-MSH to acts on melanocortin (MC) MC3/MC4 receptors lead to downstream effects that reduce food intake and increase energy expenditure. Reciprocally, increases in food intake and reduction in energy expenditures are demonstrated following activation of adjacent NPY/AgRP neurons. While NPY acts on a distinct set of receptors, AgRP is an endogenous antagonist for MC3/MC4 receptors.124–127 In a series of experiments it was determined 5 HT1B receptors are expressed on AgRP neurons and 5HT terminals are in close proximity to ARC neurons that contain AgRP. Furthermore, it was demonstrated that CPP 94235, a selective 5HT1B receptor agonist, reduces the membrane potential (ie, hyperpolarizes) of ARC neurons that express NPY/AgRP and reduces the inhibitory inputs on POMC neurons.128 Moreover, mice with disruptions in the MC4 receptor were unresponsive to the anorectic effects of dexfenfluramine or CPP 94253.128 Such data strongly suggest that 5HT actions are mediated by the 5HT1B receptor signaling to increase the MC4 receptor-mediated melanocortin pathways.
In contrast to the 5HT1B receptor, the 5HT2C receptor is a GPCR with cellular excitatory activation that leads to the accumulation of inositol phosphates and downstream activation of phospholipase C. Originally classified as the 5HT1C receptor based on its binding affinities for 5HT, the receptor was re-classified as 5HT2C after gene sequencing revealed it had introns in various coding regions and was structurally and functionally similar to receptors in the 5HT2 class.110 Similar to 5HT1B, the role of the 5HT2C receptor was implicated in feeding by several experiments using preferential and selective agonist and antagonists.120,129,130 Experiments utilizing 5HT2C receptor knockout mice have demonstrated hyperphagia and an obese phenotype.131–133 These mice also are less responsive to the anorectic properties of dexfenfluramine (3 mg/kg) and preferentially selective agonists.132,134 It has been shown that the 5HT2C receptor mediates the activity of ARC neurons, in a mechanism that is complementary to that of the 5HT1B receptor. For instance in the ARC, the 5HT2C receptor was also found in up to 80% of POMC neurons and activation of the 5HT2C receptor increased the firing rate of POMC neurons. Upstream activation of MC receptors was also needed because dexfenfluramine action can be blocked by antagonism of MC3/MC4 receptors, while the anorectic responses of MTII, an MC3/MC4 receptor agonist, were indistinguishable between wild type and 5HT2C receptors knockout mice.135 Together with the findings for the 5HT1B receptor, this suggests that 5HT activates POMC neuron and inhibits NPY/AGRP to reduce the expression of feeding behaviors. Such findings support the pharmacological studies that suggest that the 5HT1B and 5HT2C receptors reduce food intake by independent mechanisms.136
Serotonin has also been implicated in glucose regulation by action at the 5HT2C receptor. Early findings with 5HT2C receptor knockout mice indicated that these animals had elevated fasted blood glucose and insulin levels and impaired glucose tolerance compared with wild types.133 Using the preferential, mCPP (m-chlorophenyl-piperazine), or the selective, BVT.X, 5HT2C ligands, it was demonstrated that 5HT2C receptor compounds improved glucose homeostasis in diet-induced or mutant obese animals. This was accomplished by using low doses of the compounds, which did not affect food intake. The mechanisms were shown to be mediated by activation of POMC neurons and of downstream activation of MC4 receptors located in the intermediolateral cell nucleus of the spinal cord, suggesting 5HT2C receptor agonist alters sympathetic outflow to improve glucose homeostasis.137 A similar mechanisms of MC4 activation to increase sympathetic outflow is speculated to account for the hyperthermic effects noted with several selective 5HT2C receptor agonists, but this has not been experimentally determined.138,139 Lorcaserin (APD-356), a 5HT2C agonist, has not only completed a phase III study to determine effectiveness for the treatment of obesity (BLOOM), but is also undergoing a phase III study for the management of obesity and glucose regulation outcomes in subjects with diabetes mellitus type II (BLOOM-DM).51 Preliminary findings indicate improvements in fasting blood glucose levels and HbA1c levels in obese diabetic subjects treated with lorcaserin (10 mg twice daily).140
Chemistry and related pharmacology of sibutramine and lorcaserin
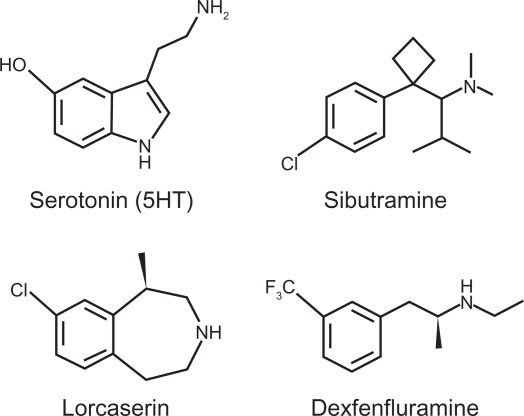
Sibutramine hydrochloride (BTS 54 524; N-1-(1-[4-chlorophenyl]cyclobutyl)-3-methylbutyl-N,N-dimethylamine hydrochloride monohydrate) is a racemic compound that is structurally similar to other β-phenylethylamine drugs, such as metamphetamine, phentermine, and dexfenfluramine (see Figure 1).41,141 It is suggested that the chlorine on the 4 position of the phenyl ring imparts affinity for the serotonin transporter, since it has been demonstrated that a halogenated-substituted phenyl rings is needed for SSRI specificity.142 In vitro and in vivo data have demonstrated that sibutramine weakly inhibits monoamine uptake in human and rat tissue.40,143,144 For instance, in vitro inhibition (half maximal inhibitory concentration, IC50) in rat brain tissue of sibutramine is 2.17 μM for NE, 477 μM for 5HT, and 10.8 μM for DA.143 Sibutramine, though, is metabolized by the liver primarily by the P450 isozyme CYP2B6 into two active metabolites that are more potent monoamine inhibitors than the parent compound.40,143–145 Sibutramine metabolism takes place by a series of demethylations, first to the primary (M1) metabolite (BTS 54 354; N-1-(1-[4-chlorophenyl] cyclobutyl]-3-methylbutyl-N-methylamine hydrochloride monohydrate) and then to secondary (M2) metabolite (BTS 54 505; N-1-(1-[4-chlorophenyl] cyclobutyl]-3-methylbutylamine hydrochloride monohydrate). The M1 metabolite demonstrated an in vitro inhibition of 0.14 μM for NE, 3.9 μM for 5HT, and 0.16 μM for DA, whereas the M2 metabolite in vitro inhibition was 0.06 μM for NE, 5.1 μM for 5HT, and 0.31 μM for DA.143 In vivo data indicate the two active metabolites of sibutramine are approximately equipotent for NE and 5HT inhibition with inactive inhibition for DA.40,143 The active metabolites display a similar degree of inhibition for 5HT as SSRI compounds.40,83 In humans the elimination half-life of oral administration of sibutramine is 1.1 hours while the half-lives of the active metabolites are much longer, at 14 hours and 16 hours for M1 and M2, respectively.146 In a recent positron emission tomography study in human subjects (n = 11), it was determined that brain serotonin transporter occupancy was significantly positively correlated (r2 = 0.59, P = 0.003) with plasma M2 levels, suggesting that 5HT inhibition is mediated predominately by M2.147
Figure 1.
Chemical structure of serotonin (5-hydroxytryptamine; 5HT) and serotonergic compounds that have been used for, or have the potential to treat, obesity.
Lorcaserin hydrochloride [(1R)-8-chloro-2,3,4,5 tetrahydro-1-methyl-1H-3-benzazepine] is a substituted 3-benzazepine, structurally resembling serotonin and nor-dexfenfluramine (dexfenfluramine metabolite). The compound is synthesized from a substituted phenylethylamine precursor with the chlorine substitution at the 8-position yielding high affinity and selectivity for the 5HT2C receptor.148 In fact, lorcaserin demonstrated ∼10-fold higher affinity for the human 5HT2C receptor compared with the 5HT2A and 5HT2B receptors (Ki values of 15 nM, 112 nM, and 174 nM, respectively) and >40-fold higher affinity compared with other 5HT receptors.149 Using in vitro functional assays examining inositol phosphate accumulation in HEK cells transfected with specific human 5HT receptors, lorcaserin was 18-fold and 104-fold more potent at the 5HT2C than at the 5HT2A and 5HT2B receptors, respectively.149 Lorcaserin is metabolized by the liver and the M1 metabolite is a sulfamate, which is inactive with no apparent affinity for any of the 5HT2 receptors.149,150 The elimination half-life of orally administered lorcaserin in humans is 11.1 hours and the half-life of the M1 metabolite is 41.3 hours.51
Clinical efficacy studies for sibutramine and lorcaserin
The efficacy of sibutramine for weight loss with diet intervention was tested in several clinical trials in obese subjects. In one double-blind randomized trial, sibutramine at 5 mg or 20 mg once daily was compared with placebo in 3 groups of subjects. Notably, medications were administered over an 8-week period during the holiday season with Thanksgiving falling between week 2 and 3, Christmas between week 6 and 7, and New Year’s falling on weeks 7 and 8. Weight loss in the 5 mg sibutramine group (n = 18) was approximately 2% greater than in the placebo group (n = 19), while weight loss in the 20 mg sibutramine group (n = 18) was approximately 4% greater than in placebo.151 In another multi-center double-blind randomized study using a wider dose range of sibutramine (6 doses between 1 and 30 mg once daily) in a large sample size (n = 1043) for a longer duration (24 weeks) the efficacy of sibutramine was further assessed. There was a dose-dependent relationship for weight loss with sibutramine over the trial. Doses of 1 mg or 5 mg produced an approximately 2% greater weight loss than in those individuals receiving placebo, while doses of 10 mg, 15 mg, 20 mg, and 30 mg produced an approximately 4% to 7% greater weight loss than in placebo. The categorical efficacy with 10 to 30 mg of sibutramine demonstrated that >58% of the population maintained 5% weight loss from baseline and >17% of the population maintained 10% weight loss from baseline over the 24 weeks. After the 24-week treatments there was a single-blind phase “washout” period of 6 weeks during which placebo was administered to all subjects. At the end of this period subjects regained weight, those losing the most during treatment regaining the most weight during the washout period.152 In a smaller study (n = 173) conducted in the same fashion, after a 6-week washout period from 24 weeks of sibutramine treatment ≥40% of the subjects from the 10- to 30-mg sibutramine group maintained a 5% weight loss from baseline and ≥12% maintained a 10% weight loss from baseline.153 The greatest amount of weight loss with sibutramine appears to occur during the first 12 weeks of treatment, weight stabilizing or increasing slightly with longer treatment.17,45,48,152 In a meta-analysis that examined the data from 10 double-blind randomized control studies (n = 2623) it was revealed that long-term treatment (1 year or more) with sibutramine (10–20 mg once daily) lost 3.7% to 5.0% more weight than individuals receiving placebo. In addition, from the same data set, approximately 35% more individuals receiving sibutramine than subjects receiving placebo maintained a ≥5% weight loss and 18% more than placebo maintained a ≥10% weight loss from baseline. In comparison, data from 13 studies (n = 4948) found that the FDA-approved gastrointestinal lipase inhibitor orlistat produced a weight loss 2.9% to 3.4% greater than in individuals receiving placebo. Proportionally, only approximately 21% more individuals taking orlistat maintained a ≥5% weight loss and approximately 12% maintained a ≥10% weight loss from baseline.17 Sibutramine was also more effective at reducing body weight directly compared with another serotonergic drug used to treat obesity, dexfenfluramine. This comparison was only with a single dose of both drugs, rather than a range of doses. For this study, 2 groups of obese subjects received either sibutramine (10 mg once daily; n = 112) or dexfenfluramine (15 mg twice daily; n = 114) for 12 weeks. Because there was no placebo control group, weight loss was compared with baseline values within groups. Sibutramine treatment produced a 5.4% weight loss, dexfenfluramine a 4.2% weight loss. Sibutramine was also associated with a greater proportion of subjects maintaining a ≥5% weight loss for the study duration, (46% compared with 34% for dexfenfluramine).154
The first double-blind placebo controlled randomized clinical trial for lorcaserin examined the efficacy of three doses (10 mg or 15 mg once daily, 10 mg twice daily) in obese individuals (n = 469) for 12 weeks. For this study no diet or lifestyle intervention strategy was endorsed by the experimenters for the study participants. Lorcaserin produced a dose-dependent reduction in weight, with a 1.4%, 2.3%, and 3.1% greater weight loss than placebo for the 10 mg (once daily), 15 mg (once daily), and 10 mg (twice daily) doses, respectively. The proportion of subjects losing ≥5% body weight from baseline was 12.8% (10 mg once daily), 19.5% (15 mg once daily), and 31.2% (10 mg twice daily).150 In a multicenter double-blind placebo-controlled randomized clinical trial in a larger number of obese subjects (n = 3182), the efficacy of lorcaserin (10 mg twice daily) was compared with placebo for 1 to 2 years. In addition to including diet and lifestyle interventional strategies, the efficacy of lorcaserin was further assessed by having a randomized portion of subjects in lorcaserin groups reassigned to placebo at the end of year 1 and followed for an additional 1 year. Efficacy, therefore, was assessed in 2 groups, lorcaserin compared with placebo, in year 1 and 3 groups at the end of year 2, lorcaserin compared with placebo compared with lorcaserin–placebo. At the end of year 1, lorcaserin resulted in 4.0% greater weight loss than placebo. A ≥5% weight loss from baseline body weight was achieved in 47.5% of subjects receiving lorcaserin compared with 20.3% receiving placebo. Only 22.6% of the lorcaserin group compared with 7.7% of the placebo maintained ≥10% weight loss from baseline. At the end of year 2, the degree of weight loss was maintained in the group continuing to receive lorcaserin, but weight gain was apparent in the group that was switched to placebo. The group that received lorcaserin–placebo had an identical weight loss to the placebo group by 28 weeks after the reassignment.50 There are no reported studies, either experimentally or from a meta-analysis, comparing the weight loss efficacy of lorcaserin with other medications for the treatment of obesity.
Safety and tolerability
Medications acting on serotonergic systems have received considerable scrutiny for cardiopulmonary complications because of the increased incidence of primary pulmonary hypertension and valvulopathies associated with earlier obesity medications, fenfluramine and dexfenfluramine. Sibutramine or lorcaserin has not been associated with any reported cases of confirmed pulmonary hypertension. In addition, pulmonary artery pressure has not been demonstrated to change with long-term treatment with either medication.50,155 Likewise, sibutramine has not been demonstrated to influence cardiac valve function or result in any evident valvulopathies.156 In a multicenter study examining weight loss efficacy of lorcaserin (see above), the occurrence of valvulopathy at 52 weeks was 2.7% in the subjects receiving lorcaserin (10 mg twice daily) and 2.3% in the placebo group.50 Preliminary findings at 52 weeks in the BLOOM-DM (n = 604) study for lorcaserin, however, have shown that the incidence of valvulopathies was 2.9% in subjects receiving lorcaserin (10 mg twice daily) and 0.5% in the placebo group.140 Although the study was not powered to determine a statistical difference in valvulopathies between groups, this almost 6-fold difference in incidence rates certainly raises concerns that likely need to be addressed in future lorcaserin studies. Notwithstanding, other documented safety issues and potential health risks have resulted in the withdrawal of sibutramine and the FDA rejection of lorcaserin.
At the time of FDA approval for sibutramine it was well demonstrated that the drug was associated with increased heart rate and blood pressure. Initially, the NDA for sibutramine contained 5 doses (5 mg, 10 mg, 15 mg, 20 mg, and 30 mg once daily). Because of the potential dose-dependent cardiovascular risk balanced against the potential benefit for the treatment of obesity, only 3 doses (5 mg, 10 mg, and 15 mg once daily) were approved by the FDA in 1997.152,157 Sibutramine, though, carries a bold-type warning that blood pressure and heart rate should be monitored in patients receiving the drug and it is contraindicated in individuals with a history of coronary artery disease, congestive heart failure, arrhythmias, or stroke.47 In a meta-analysis of 7 double-blind randomized control studies examining sibutramine treatment (≥1 year) in obese individuals, the drug was found to increase systolic blood pressure by 1.7 mm Hg, diastolic pressure by 2.4 mm Hg, and resting heart rate by 4.5 bpm compared with placebo.17 The increase in heart rate and blood pressure is likely to influence cardiovascular function. The recently completed SCOUT trial was conducted in 16 countries, initially enrolling >10,000 subjects, to examine the long-term effects (mean treatment was 3.4 years) of sibutramine on cardiovascular outcomes in overweight and obese subjects. Unlike other clinical studies with sibutramine, eligible subjects had to have a history of cardiovascular disease and/or diabetes mellitus type II with one risk factor for cardiovascular disease. As previously demonstrated, sibutramine was associated with an increase in blood pressure and heart rate; the drug also resulted in an increased rate of nonfatal myocardial infarction (+0.9%) and stroke (+0.7%).48 The most frequently reported adverse events with sibutramine are dry mouth, insomnia, and constipation. Typically, these events are dose-related, the 15 mg dose having a placebo-subtracted reported frequency of ∼20%, ∼5%, and ∼5%, respectively.47,152
The major safety concerns for the lorcaserin (10 mg twice daily) NDA are findings from the carcinogenicity studies in rodents. In particular, 2-year treatment with lorcaserin resulted in a higher combined incidence rate of mammary fibroadenomas and adenocarcinomas in female rats at the 10 mg/kg, 30 mg/kg, and 100 mg/kg compared with vehicle-treated animals. In the male rats at the 30 mg/kg and 100 mg/kg doses, there was also a higher incidence rate of combined mammary tumors and skin benign fibromas. In addition, male rats receiving the 100 mg/kg dose showed an increased incidence rate of astrocytomas, squamous carcinomas, Schwannomas, combined hepatocullular neoplasms, and follicular cell adenomas. Over the 2-year study, the survival rate for both genders of rats was strongly negatively influenced by lorcaserin. In fact, the female survival rate was 18.4% for 10 mg/kg, 7.7% for 30 mg/kg, and 0% for 100 mg/kg while the vehicle survival rate was 35%. Only the high dose appeared to influence the survival rate of males, which was 5.3% compared with 33.8% of the vehicle group. Necropsy findings confirmed that lorcaserin-induced tumors were associated with the excessive mortality observed with both genders of rats.51 In a multicenter phase III clinical trial with lorcaserin (10 mg twice daily) treatment (≥1 year) the most frequently subject-reported adverse event was headache (7.2% in lorcaserin compared with 4.3% in placebo). The frequency of headaches in the treated population, as well as other less frequent subject-reported adverse events, had a tendency to decrease with lorcaserin treatment length.50
Both sibutramine and lorcaserin appear to be well tolerated by obese subjects. The reported 1-year attrition rate appears to be about 35% for sibutramine and about 45% for lorcaserin.17,50 Certainly more long-term studies are needed to determine accurate drop-out and nonresponder rates with lorcaserin.
Patient-focused perspectives
Improvements in the quality of life, attitudes, and mood have been demonstrated to occur in overweight or obese patients with weight loss.158–160 Long-term pharmacotherapy for obesity, including sibutramine and lorcaserin, at doses that are effective at reducing body weight are also associated with improvements in the quality of life and subjective attitude scores.17,50,161 What is unclear is whether the medications per se or the medication-associated additional weight loss is responsible for the improvement in quality of life and patient attitude. Experiments addressing this issue with sibutramine have had mixed findings. In one study (n = 376), 6-month treatment with sibutramine (10 mg) combined with high-frequency contact with a dietician resulted in greater weight loss than the group receiving sibutramine only. Although quality of life assessment was significantly improved from baseline (ie, before intervention and/or sibutramine), there was no difference in scores between groups.161 This implies that sibutramine treatment alone had an impact on quality of life not directly related to the degree of weight loss. In contrast, a 12-month study (n = 236) in obese subject with diabetes mellitus type II, treatment with sibutramine (15 mg) produced significantly greater weight loss (7.3%) than the placebo group (2.4%), but did not change health-related quality of life ratings.162 Sibutramine (15 mg) treatment compared with placebo for 6 months also failed to improve quality of life scores in a population of bulimic patients (n = 304), but effectively reduced body weight and reduced the severity and frequency of binge eating episodes.163
Conclusion
Pharmacotherapy would greatly benefit effective interventional nonsurgical strategies for the management of obesity. As a result of the US and European drug regulatory agencies scrutinizing more closely the approval and the continued use of these medications, the approved drug market for the treatment of obesity has been reduced to one medication, orlistat. Aside from the frequently reported gastrointestinal disturbances and the well-documented modest long-term drug-associated weight loss (∼3%), orlistat is relatively safe. The continued approval of orlistat as a prescribed and over-the-counter treatment for obesity demonstrates that the weight-loss efficacy standards of the FDA and European regulatory agencies, 5% drug-associated weight loss and >35% of the treated population losing 5% of body weight, are not a contingent criteria for drug approval. The rationale in orlistat’s case is that any safe weight loss is beneficial. In order for drug manufacturers to develop and screen potential medications that receive approval by these agencies, the efficacy guidelines should be clearly revised to include the acceptable margin of drug-associated weight loss. In doing so and under the “any weight loss is beneficial” premise, this is likely to produce pharmacotherapy with reduced efficacy for weight loss, but with fewer and less severe adverse effects.
While drug regulatory agencies examine the risk–benefit analysis of each approved and proposed medication for the treatment of obesity, the unapproved herbal and “natural” dietary supplements consumer market is driven almost entirely by demand. In a study of 35,000 US adults approximately 34% had used dietary supplements for weight loss and approximately 50% of the entire sampled population overestimated the safety and efficacy of these unapproved weight loss supplements.164 Herbal and dietary supplements reduce appetite and promote weight loss by various mechanisms, some of which are not clearly defined or systematically examined.165 A number of these supplements (eg, “bitter orange” and other stimulants) promote weight loss by increasing autonomic activity (including increases in resting heart rate and blood pressure).166,167 Unlike patients that received sibutramine, however, individuals taking dietary supplements are likely not being observed regularly by a health care provider to monitor heart rate and blood pressure. Indeed, the risk of nonfatal myocardial infarction and stroke has not been assessed with herbal remedies and dietary supplements used to suppress appetite. In the past, the FDA has been reactive, rather than proactive, in regulating diet supplements (eg, ephedra). Taken in the broader context of a demonstrated need and willingness of the consumer market for substances that promote weight loss and the availability of the unregulated alternatives, an argument can be made that withdrawal of sibutramine is likely to be more detrimental to the overweight and obese population than the risks associated with sibutramine treatment. Another option not exercised by the FDA and other regulatory agencies is to include a “black box” warning on sibutramine, which appears to be more than reasonable considering the medication has been on the US market for 13 years. The data from the SCOUT clinical trial should be considered “worst case scenario”, since the primary outcome event was the incidence of nonfatal myocardial infarction, nonfatal stroke resuscitation after cardiac arrest, and cardiovascular death in subjects with a history of and/or risk factors for cardiovascular disease. What is also unclear is whether the cardiovascular risks associated with sibutramine are long lasting or permanent after cessation of sibutramine treatment. Overall, the rationale of the FDA for the withdrawal of sibutramine seems unwarranted and unnecessary considering the medication, certainly since its approval in 1997, has always been associated with increases in cardiovascular-related adverse effects.
In contrast, the decision of the FDA not to approve lorcaserin pending further data is entirely reasonable. The carcinogenicity findings in rats are too difficult to rectify without a defined mechanism of action, as is the related low survival rate. The low survival rate with lorcaserin is rather surprising considering that calorie restriction with weight loss decreases mortality and leads to a longer life span.168,169 A recent study re-examining the toxicology data from a 2-year study in rodents (n = 520 mice and n = 520 rats) found that sibutramine had no effect (either positive or negative) on longevity or mortality rate.170 In the toxicology studies with lorcaserin, on the other hand, the reduced survival rate trends exactly with the dose-dependent drug-induced tumor load, suggesting the cause of the increased mortality is likely a result of lorcaserin carcinogenicity. Further testing is undoubtedly needed to clearly determine whether the tumor burden was caused by lorcaserin or was related to toxicity as a result of the maximum tolerated dose being exceeded.
Therapeutically targeting the serotonergic systems will continue to be a viable approach for the treatment of obesity. This does not mean that other neurotransmitter systems or even combination therapies may prove to be more efficacious in combating obesity with fewer side effects. Even so, serotonin actions in producing subjective feelings of satiety by modulation of the neural mechanisms of appetite are beneficial not only for reducing and maintaining body weight, but also for controlling eating behavior. Because of the adverse effects associated with nonspecific serotonin drugs, such as dexfenfluramine and sibutramine, the recent direction of drug development has focused on specific receptor agonists. Recent findings with lorcaserin, the 5HT2C agonist, need further clarification while a specific 5HT1B receptor agonist has yet to be clinically developed. Although the immediate hope for a serotonin-based pharmacotherapy seems remote, given what is known about the serotonin systems and the control of appetite, the long-term probability is that a new serotonin-based therapeutic will emerge. In the short-term, the landscape of obesity pharmacotherapy would be better served by a clarification of reasonable efficacy standards by regulatory agencies.
Acknowledgments
The authors would like to thank Kathy Manger for her editorial assistance in preparing the manuscript.
Footnotes
Disclosure
The authors have no conflict of interests to disclose.
References
- 1.Marcason W. What are the latest figures for state-specific prevalence of obesity in the United States? J Am Diet Assoc. 2007;107(1):168. doi: 10.1016/j.jada.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 2.Neufeld LM, Hernandez-Cordero S, Fernald LC, Ramakrishnan U. Overweight and obesity doubled over a 6-year period in young women living in poverty in Mexico. Obesity (Silver Spring) 2008;16(3):714–717. doi: 10.1038/oby.2007.119. [DOI] [PubMed] [Google Scholar]
- 3.Zaninotto P, Head J, Stamatakis E, Wardle H, Mindell J. Trends in obesity among adults in England from 1993 to 2004 by age and social class and projections of prevalence to 2012. J Epidemiol Community Health. 2009;63(2):140–146. doi: 10.1136/jech.2008.077305. [DOI] [PubMed] [Google Scholar]
- 4.Han JC, Lawlor DA, Kimm SY. Childhood obesity. Lancet. 2010;15(9727):1737–1748. doi: 10.1016/S0140-6736(10)60171-7. 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jafar TH, Qadri Z, Islam M, Hatcher J, Bhutta ZA, Chaturvedi N. Rise in childhood obesity with persistently high rates of undernutrition among urban school-aged Indo-Asian children. Arch Dis Child. 2008;93(5):373–378. doi: 10.1136/adc.2007.125641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Popkin BM. Recent dynamics suggest selected countries catching up to US obesity. Am J Clin Nutr. 2010;91(1):284S–288S. doi: 10.3945/ajcn.2009.28473C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization Obesity and overweight fact sheet no. 311. 2006. WHO website. http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed February 4, 2011.
- 8.James WP. WHO recognition of the global obesity epidemic. Int J Obes (Lond) 2008;32(Suppl 7):S120–S126. doi: 10.1038/ijo.2008.247. [DOI] [PubMed] [Google Scholar]
- 9.Ford ES, Mokdad AH, Giles WH, Galuska DA, Serdula MK. Geographic variation in the prevalence of obesity, diabetes, and obesity-related behaviors. Obes Res. 2005;13(1):118–122. doi: 10.1038/oby.2005.15. [DOI] [PubMed] [Google Scholar]
- 10.Hursting SD, Berger NA. Energy balance, host-related factors, and cancer progression. J Clin Oncol. 2010;28(26):4058–4065. doi: 10.1200/JCO.2010.27.9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li F, Fisher KJ, Harmer P. Prevalence of overweight and obesity in older US adults: estimates from the 2003 Behavioral Risk Factor Surveillance System survey. J Am Geriatr Soc. 2005;53(4):737–739. doi: 10.1111/j.1532-5415.2005.53228_10.x. [DOI] [PubMed] [Google Scholar]
- 12.NHLBI Obesity Education Initiative The Practical Guide Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. NIH Publication No 00-4084. 2000 [Google Scholar]
- 13.Mayor S. NICE requires primary care trusts to tackle prevention and management of obesity. BMJ. 2006;333(7581):1239. doi: 10.1136/bmj.39062.338079.DB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Look AHEAD Research Group. Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170(17):1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersson C, Weeke P, Fosbol EL, et al. Acute effect of weight loss on levels of total bilirubin in obese, cardiovascular high-risk patients: an analysis from the lead-in period of the Sibutramine Cardiovascular Outcome trial. Metabolism. 2009;58(8):1109–1115. doi: 10.1016/j.metabol.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Mertens IL, van Gaal LF. Overweight, obesity, and blood pressure: the effects of modest weight reduction. Obes Res. 2000;8(3):270–278. doi: 10.1038/oby.2000.32. [DOI] [PubMed] [Google Scholar]
- 17.Rucker D, Padwal R, Li SK, Curioni C, Lau DC. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ. 2007;335(7631):1194–1199. doi: 10.1136/bmj.39385.413113.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mechanick JI, Kushner RF, Sugerman HJ, et al. American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic and Bariatric Surgery Medical Guidelines for Clinical Practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Surg Obes Relat Dis. 2008;4(5 Suppl):S109–S184. doi: 10.1016/j.soard.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Fontana MA, Wohlgemuth SD. The surgical treatment of metabolic disease and morbid obesity. Gastroenterol Clin North Am. 2010;39(1):125–133. doi: 10.1016/j.gtc.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Guller U, Klein LV, Hagen JA. Safety and effectiveness of bariatric surgery: Roux-en-Y gastric bypass is superior to gastric banding in the management of morbidly obese patients. Patient Saf Surg. 2009;3(1):10. doi: 10.1186/1754-9493-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sjostrom L, Gummesson A, Sjostrom CD, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 2009;10(7):653–662. doi: 10.1016/S1470-2045(09)70159-7. [DOI] [PubMed] [Google Scholar]
- 22.Picot J, Jones J, Colquitt JL, et al. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess. 2009;13(41):1–190. 215–357, iii–iv. doi: 10.3310/hta13410. [DOI] [PubMed] [Google Scholar]
- 23.Jensen C, Flum DR. The costs of nonsurgical and surgical weight loss interventions: is an ounce of prevention really worth a pound of cure? Surg Obes Relat Dis. 2005;1(3):353–357. doi: 10.1016/j.soard.2005.03.215. [DOI] [PubMed] [Google Scholar]
- 24.Salem L, Jensen CC, Flum DR. Are bariatric surgical outcomes worth their cost? A systematic review. J Am Coll Surg. 2005;200(2):270–278. doi: 10.1016/j.jamcollsurg.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 25.Tindle HA, Omalu B, Courcoulas A, Marcus M, Hammers J, Kuller LH. Risk of suicide after long-term follow-up from bariatric surgery. Am J Med. 2010;123(11):1036–1042. doi: 10.1016/j.amjmed.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basu A. Forecasting distribution of body mass index in the United States: is there more room for growth? Med Decis Making. 2010;30(3):E1–E11. doi: 10.1177/0272989X09351749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guidance for the clinical evaluation of weight-control drugs. Crit Rev Food Sci Nutr. 2001;41(1):91–94. doi: 10.1080/20014091091760. [DOI] [PubMed] [Google Scholar]
- 28.Hadvary P, Lengsfeld H, Wolfer H. Inhibition of pancreatic lipase in vitro by the covalent inhibitor tetrahydrolipstatin. Biochem J. 1988;256(2):357–361. doi: 10.1042/bj2560357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ko J, Small DM. Behavior of tetrahydrolipstatin in biological model membranes and emulsions. J Lipid Res. 1997;38(8):1544–1552. [PubMed] [Google Scholar]
- 30.Davidson MH, Hauptman J, DiGirolamo M, et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. JAMA. 1999;281(3):235–242. doi: 10.1001/jama.281.3.235. [DOI] [PubMed] [Google Scholar]
- 31.Zhi J, Melia AT, Guerciolini R, et al. Retrospective population-based analysis of the dose-response (fecal fat excretion) relationship of orlistat in normal and obese volunteers. Clin Pharmacol Ther. 1994;56(1):82–85. doi: 10.1038/clpt.1994.104. [DOI] [PubMed] [Google Scholar]
- 32.Hill JO, Hauptman J, Anderson JW, et al. Orlistat, a lipase inhibitor, for weight maintenance after conventional dieting: a 1-y study. Am J Clin Nutr. 1999;69(6):1108–1116. doi: 10.1093/ajcn/69.6.1108. [DOI] [PubMed] [Google Scholar]
- 33.Filippatos TD, Derdemezis CS, Gazi IF, Nakou ES, Mikhailidis DP, Elisaf MS. Orlistat-associated adverse effects and drug interactions: a critical review. Drug Saf. 2008;31(1):53–65. doi: 10.2165/00002018-200831010-00005. [DOI] [PubMed] [Google Scholar]
- 34.McClendon KS, Riche DM, Uwaifo GI. Orlistat: current status in clinical therapeutics. Expert Opin Drug Saf. 2009;8(6):727–744. doi: 10.1517/14740330903321485. [DOI] [PubMed] [Google Scholar]
- 35.Connery TP. Orlistat over the counter: Is it worth it? BMJ. 2008;336(7634):7. doi: 10.1136/bmj.39434.694421.3A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.EMA. European Medicines Agency Questions and answers on the suspension of medicines containing sibutramine. 2010. EMA/H/A-107/1256. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Sibutramine_107/WC500094238.pdf. Accessed February 4, 2011.
- 37.Abbott to Voluntarily Withdraw Meridia® (Sibutramine) in the US. Press Release 2010. Oct 8, http://www.abbott.com/global/url/pressRelease/en_US/Press_Release_0908.htm. Accessed February 4, 2011.
- 38.McNeely W, Goa KL. Sibutramine. A review of its contribution to the management of obesity. Drugs. 1998;56(6):1093–1124. doi: 10.2165/00003495-199856060-00019. [DOI] [PubMed] [Google Scholar]
- 39.Nelson DL, Gehlert DR. Central nervous system biogenic amine targets for control of appetite and energy expenditure. Endocrine. 2006;29(1):49–60. doi: 10.1385/endo:29:1:49. [DOI] [PubMed] [Google Scholar]
- 40.Heal DJ, Aspley S, Prow MR, Jackson HC, Martin KF, Cheetham SC. Sibutramine: a novel anti-obesity drug. A review of the pharmacological evidence to differentiate it from d-amphetamine and d-fenfluramine. Int J Obes Relat Metab Disord. 1998;22(Suppl 1):S18–S28. S29. discussion. [PubMed] [Google Scholar]
- 41.Buckett WR, Thomas PC, Luscombe GP. The pharmacology of sibutramine hydrochloride (BTS 54 524), a new antidepressant which induces rapid noradrenergic down-regulation. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12(5):575–584. doi: 10.1016/0278-5846(88)90003-6. [DOI] [PubMed] [Google Scholar]
- 42.Rowley HL, Butler SA, Prow MR, et al. Comparison of the effects of sibutramine and other weight-modifying drugs on extracellular dopamine in the nucleus accumbens of freely moving rats. Synapse. 2000;38(2):167–176. doi: 10.1002/1098-2396(200011)38:2<167::AID-SYN8>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 43.Wortley KE, Heal DJ, Stanford SC. Modulation of sibutramine-induced increases in extracellular noradrenaline concentration in rat frontal cortex and hypothalamus by alpha2-adrenoceptors. Br J Pharmacol. 1999;128(3):659–666. doi: 10.1038/sj.bjp.0702859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hauner H, Meier M, Wendland G, Kurscheid T, Lauterbach K. Weight reduction by sibutramine in obese subjects in primary care medicine: the SAT Study. Exp Clin Endocrinol Diabetes. 2004;112(4):201–207. doi: 10.1055/s-2004-817934. [DOI] [PubMed] [Google Scholar]
- 45.Smith IG, Goulder MA. Randomized placebo-controlled trial of long-term treatment with sibutramine in mild to moderate obesity. J Fam Pract. 2001;50(6):505–512. [PubMed] [Google Scholar]
- 46.Wooltorton E. Obesity drug sibutramine (Meridia): hypertension and cardiac arrhythmias. CMAJ. 2002;166(10):1307–1308. [PMC free article] [PubMed] [Google Scholar]
- 47.Meridia (sibutramine hydrochloride monohydrate) capsules. 2010. [Package insert] Document 600-452107.
- 48.James WP, Caterson ID, Coutinho W, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363(10):905–917. doi: 10.1056/NEJMoa1003114. [DOI] [PubMed] [Google Scholar]
- 49.Lam DD, Przydzial MJ, Ridley SH, et al. Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors. Endocrinology. 2008;149(3):1323–1328. doi: 10.1210/en.2007-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363(3):245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- 51.Arena Pharmaceuticals FDA Briefing Document; NDA 22529 Lorqess (lorcaserin hydrocloride) Tablets, 10 mg. 2010. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM225631.pdf. Accessed February 4, 2011.
- 52.FDA Issues Complete Response Letter for Lorcaserin New Drug Application . Press release from Arena Pharmaceuticals, Inc.; 2010. Oct 22, http://www.eisai.com/presskits/LorcaserinPDUFA.FINAL.pdf. Accessed February 4, 2011. [Google Scholar]
- 53.Rapport MM, Green AA, Page IH. Serum vasoconstrictor, serotonin; isolation and characterization. J Biol Chem. 1948;176(3):1243–1251. [PubMed] [Google Scholar]
- 54.Rapport MM, Green AA, Page IH. Crystalline Serotonin. Science. 1948;108(2804):329–330. doi: 10.1126/science.108.2804.329. [DOI] [PubMed] [Google Scholar]
- 55.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lechin F, van der Dijs B, Hernandez-Adrian G. Dorsal raphe vs median raphe serotonergic antagonism. Anatomical, physiological, behavioral, neuroendocrinological, neuropharmacological and clinical evidences: relevance for neuropharmacological therapy. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(4):565–585. doi: 10.1016/j.pnpbp.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 57.Medeiros MA, Costa-e-Sousa RH, Olivares EL, Cortes WS, Reis LC. A reassessment of the role of serotonergic system in the control of feeding behavior. An Acad Bras Cienc. 2005;77(1):103–111. doi: 10.1590/s0001-37652005000100008. [DOI] [PubMed] [Google Scholar]
- 58.De Fanti BA, Hamilton JS, Horwitz BA. Meal-induced changes in extracellular 5-HT in medial hypothalamus of lean (Fa/Fa) and obese (fa/fa) Zucker rats. Brain Res. 2001;902(2):164–170. doi: 10.1016/s0006-8993(01)02371-x. [DOI] [PubMed] [Google Scholar]
- 59.Ohliger-Frerking P, Horwitz BA, Horowitz JM. Serotonergic dorsal raphe neurons from obese zucker rats are hyperexcitable. Neuroscience. 2003;120(3):627–634. doi: 10.1016/s0306-4522(03)00360-9. [DOI] [PubMed] [Google Scholar]
- 60.Fetissov SO, Meguid MM. Serotonin delivery into the ventromedial nucleus of the hypothalamus affects differently feeding pattern and body weight in obese and lean Zucker rats. Appetite. 2010;54(2):346–353. doi: 10.1016/j.appet.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 61.Park S, Harrold JA, Widdowson PS, Williams G. Increased binding at 5-HT(1A), 5-HT(1B), and 5-HT(2A) receptors and 5-HT transporters in diet-induced obese rats. Brain Res. 1999;847(1):90–97. doi: 10.1016/s0006-8993(99)02055-7. [DOI] [PubMed] [Google Scholar]
- 62.Costa E, Groppetti A, Revuelta A. Action of fenfluramine on monoamine stores of rat tissues. Br J Pharmacol. 1971;41(1):57–64. doi: 10.1111/j.1476-5381.1971.tb09935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blundell JE, Leshem MB. Central action of anorexic agents: effects of amphetamine and fenfluramine in rats with lateral hypothalamic lesions. Eur J Pharmacol. 1974;28(1):81–88. doi: 10.1016/0014-2999(74)90115-0. [DOI] [PubMed] [Google Scholar]
- 64.Gotestam KG, Andersson BE. Self-administration of amphetamine analogues in rats. Pharmacol Biochem Behav. 1975;3(2):229–233. doi: 10.1016/0091-3057(75)90152-5. [DOI] [PubMed] [Google Scholar]
- 65.Ghosh MN, Parvathy S. Tolerance pattern of the anorexigenic action of amphetamines, fenfluramine, phenmetrazine and diethylpropion in rats. Br J Pharmacol. 1976;57(4):479–485. doi: 10.1111/j.1476-5381.1976.tb10374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garattini S, Mennini T, Samanin R. From fenfluramine racemate to d-fenfluramine. Specificity and potency of the effects on the serotoninergic system and food intake. Ann N Y Acad Sci. 1987;499:156–166. doi: 10.1111/j.1749-6632.1987.tb36207.x. [DOI] [PubMed] [Google Scholar]
- 67.Vivero LE, Anderson PO, Clark RF. A close look at fenfluramine and dexfenfluramine. J Emerg Med. 1998;16(2):197–205. doi: 10.1016/S0736-4679(97)00289-8. [DOI] [PubMed] [Google Scholar]
- 68.Rothman RB, Baumann MH. Serotonin releasing agents. Neurochemical, therapeutic and adverse effects. Pharmacol Biochem Behav. 2002;71(4):825–836. doi: 10.1016/s0091-3057(01)00669-4. [DOI] [PubMed] [Google Scholar]
- 69.Mennini T, Fracasso C, Cagnotto A, et al. In vitro and in vivo effects of the anorectic agent dexfenfluramine on the central serotoninergic neuronal systems of non-human primates. A comparison with the rat. Naunyn Schmiedebergs Arch Pharmacol. 1996;353(6):641–647. doi: 10.1007/BF00167183. [DOI] [PubMed] [Google Scholar]
- 70.Blundell JE, Tombros E, Rogers PJ, Latham CJ. Behavioural analysis of feeding: implications for the pharmacological manipulation of food intake in animals and man. Prog Neuropsychopharmacol. 1980;4(4–5):319–326. doi: 10.1016/0364-7722(80)90002-8. [DOI] [PubMed] [Google Scholar]
- 71.Halford JC, Harrold JA, Boyland EJ, Lawton CL, Blundell JE. Serotonergic drugs:effects on appetite expression and use for the treatment of obesity. Drugs. 2007;67(1):27–55. doi: 10.2165/00003495-200767010-00004. [DOI] [PubMed] [Google Scholar]
- 72.Boschmann M, Frenz U, Murphy CM, Noack R. Changes in energy metabolism and metabolite patterns of obese rats after application of dexfenfluramine. Pharmacol Biochem Behav. 1996;53(3):549–558. doi: 10.1016/0091-3057(95)02049-7. [DOI] [PubMed] [Google Scholar]
- 73.Bray GA. A concise review on the therapeutics of obesity. Nutrition. 2000;16(10):953–960. doi: 10.1016/s0899-9007(00)00424-x. [DOI] [PubMed] [Google Scholar]
- 74.Connolly HM, Crary JL, McGoon MD, et al. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337(9):581–588. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- 75.Teramae CY, Connolly HM, Grogan M, Miller FA., Jr Diet drug-related cardiac valve disease: the Mayo Clinic echocardiographic laboratory experience. Mayo Clin Proc. 2000;75(5):456–461. doi: 10.4065/75.5.456. [DOI] [PubMed] [Google Scholar]
- 76.Abenhaim L, Moride Y, Brenot F, et al. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. International Primary Pulmonary Hypertension Study Group. N Engl J Med. 1996;335(9):609–616. doi: 10.1056/NEJM199608293350901. [DOI] [PubMed] [Google Scholar]
- 77.Weintraub M, Sundaresan PR, Madan M, et al. Long-term weight control study. I (weeks 0 to 34). The enhancement of behavior modification, caloric restriction, and exercise by fenfluramine plus phentermine versus placebo. Clin Pharmacol Ther. 1992;51(5):586–594. doi: 10.1038/clpt.1992.69. [DOI] [PubMed] [Google Scholar]
- 78.Weintraub M, Sundaresan PR, Schuster B, et al. Long-term weight control study. IV (weeks 156 to 190). The second double-blind phase. Clin Pharmacol Ther. 1992;51(5):608–614. doi: 10.1038/clpt.1992.72. [DOI] [PubMed] [Google Scholar]
- 79.Weintraub M, Sundaresan PR, Schuster B, et al. Long-term weight control study. II (weeks 34 to 104). An open-label study of continuous fenfluramine plus phentermine versus targeted intermittent medication as adjuncts to behavior modification, caloric restriction, and exercise. Clin Pharmacol Ther. 1992;51(5):595–601. doi: 10.1038/clpt.1992.70. [DOI] [PubMed] [Google Scholar]
- 80.Weintraub M, Sundaresan PR, Schuster B, Moscucci M, Stein EC. Long-term weight control study. III (weeks 104 to 156). An open-label study of dose adjustment of fenfluramine and phentermine. Clin Pharmacol Ther. 1992;51(5):602–607. doi: 10.1038/clpt.1992.71. [DOI] [PubMed] [Google Scholar]
- 81.FDA Announces Withdrawal Fenfluramine and Dexfenfluramine (Fen-Phen) 1997. pp. P97–32.
- 82.Gundlah C, Martin KF, Heal DJ, Auerbach SB. In vivo criteria to differentiate monoamine reuptake inhibitors from releasing agents: sibutramine is a reuptake inhibitor. J Pharmacol Exp Ther. 1997;283(2):581–591. [PubMed] [Google Scholar]
- 83.Tao R, Fray A, Aspley S, Brammer R, Heal D, Auerbach S. Effects on serotonin in rat hypothalamus of D-fenfluramine, aminorex, phentermine and fluoxetine. Eur J Pharmacol. 2002;445(1–2):69–81. doi: 10.1016/s0014-2999(02)01751-x. [DOI] [PubMed] [Google Scholar]
- 84.McGuirk J, Muscat R, Willner P. Effects of chronically administered fluoxetine and fenfluramine on food intake, body weight and the behavioural satiety sequence. Psychopharmacology (Berl) 1992;106(3):401–407. doi: 10.1007/BF02245426. [DOI] [PubMed] [Google Scholar]
- 85.Halford JC, Wanninayake SC, Blundell JE. Behavioral satiety sequence (BSS) for the diagnosis of drug action on food intake. Pharmacol Biochem Behav. 1998;61(2):159–168. doi: 10.1016/s0091-3057(98)00032-x. [DOI] [PubMed] [Google Scholar]
- 86.Burton MJ, Cooper SJ, Popplewell DA. The effect of fenfluramine on the microstructure of feeding and drinking in the rat. Br J Pharmacol. 1981;72(4):621–633. doi: 10.1111/j.1476-5381.1981.tb09142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Willner P, McGuirk J, Phillips G, Muscat R. Behavioural analysis of the anorectic effects of fluoxetine and fenfluramine. Psychopharmacology (Berl) 1990;102(2):273–277. doi: 10.1007/BF02245933. [DOI] [PubMed] [Google Scholar]
- 88.Blundell JE, Rogers PJ, Hill AJ. Behavioural structure and mechanisms of anorexia: calibration of natural and abnormal inhibition of eating. Brain Res Bull. 1985;15(4):371–376. doi: 10.1016/0361-9230(85)90004-8. [DOI] [PubMed] [Google Scholar]
- 89.Tallett AJ, Blundell JE, Rodgers RJ. Sibutramine-induced anorexia: potent, dose-dependent and behaviourally-selective profile in male rats. Behav Brain Res. 2009;198(2):359–365. doi: 10.1016/j.bbr.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 90.Rogers PJ, Blundell JE. Effect of anorexic drugs on food intake and the micro-structure of eating in human subjects. Psychopharmacology (Berl) 1979;66(2):159–165. doi: 10.1007/BF00427624. [DOI] [PubMed] [Google Scholar]
- 91.Hill AJ, Blundell JE. Sensitivity of the appetite control system in obese subjects to nutritional and serotoninergic challenges. Int J Obes. 1990;14(3):219–233. [PubMed] [Google Scholar]
- 92.Lawton CL, Wales JK, Hill AJ, Blundell JE. Serotoninergic manipulation, meal-induced satiety and eating pattern: effect of fluoxetine in obese female subjects. Obes Res. 1995;3(4):345–356. doi: 10.1002/j.1550-8528.1995.tb00160.x. [DOI] [PubMed] [Google Scholar]
- 93.Breisch ST, Zemlan FP, Hoebel BG. Hyperphagia and obesity following serotonin depletion by intraventricular p-chlorophenylalanine. Science. 1976;192(4237):382–385. doi: 10.1126/science.130678. [DOI] [PubMed] [Google Scholar]
- 94.Saller CF, Stricker EM. Hyperphagia and increased growth in rats after intraventricular injection of 5,7-dihydroxytryptamine. Science. 1976;192(4237):385–387. doi: 10.1126/science.1257774. [DOI] [PubMed] [Google Scholar]
- 95.Auerbach SB, Lundberg JF, Hjorth S. Differential inhibition of serotonin release by 5-HT and NA reuptake blockers after systemic administration. Neuropharmacology. 1995;34(1):89–96. doi: 10.1016/0028-3908(94)00137-h. [DOI] [PubMed] [Google Scholar]
- 96.Gundlah C, Hjorth S, Auerbach SB. Autoreceptor antagonists enhance the effect of the reuptake inhibitor citalopram on extracellular 5-HT: this effect persists after repeated citalopram treatment. Neuropharmacology. 1997;36(4–5):475–482. doi: 10.1016/s0028-3908(97)00052-x. [DOI] [PubMed] [Google Scholar]
- 97.Popa D, Cerdan J, Reperant C, et al. A longitudinal study of 5-HT outflow during chronic fluoxetine treatment using a new technique of chronic microdialysis in a highly emotional mouse strain. Eur J Pharmacol. 2010;25(1–3):628. 83–90. doi: 10.1016/j.ejphar.2009.11.037. [DOI] [PubMed] [Google Scholar]
- 98.Jackson HC, Bearham MC, Hutchins LJ, Mazurkiewicz SE, Needham AM, Heal DJ. Investigation of the mechanisms underlying the hypophagic effects of the 5-HT and noradrenaline reuptake inhibitor, sibutramine, in the rat. Br J Pharmacol. 1997;121(8):1613–1618. doi: 10.1038/sj.bjp.0701311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jackson HC, Needham AM, Hutchins LJ, Mazurkiewicz SE, Heal DJ. Comparison of the effects of sibutramine and other monoamine reuptake inhibitors on food intake in the rat. Br J Pharmacol. 1997;121(8):1758–1762. doi: 10.1038/sj.bjp.0701312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Faria AN, Ribeiro Filho FF, Kohlmann NE, Gouvea Ferreira SR, Zanella MT. Effects of sibutramine on abdominal fat mass, insulin resistance and blood pressure in obese hypertensive patients. Diabetes Obes Metab. 2005;7(3):246–253. doi: 10.1111/j.1463-1326.2005.00465.x. [DOI] [PubMed] [Google Scholar]
- 101.Astrup A. Macronutrient balances and obesity: the role of diet and physical activity. Public Health Nutr. 1999;2(3A):341–347. doi: 10.1017/s1368980099000464. [DOI] [PubMed] [Google Scholar]
- 102.Hansen DL, Toubro S, Stock MJ, Macdonald IA, Astrup A. The effect of sibutramine on energy expenditure and appetite during chronic treatment without dietary restriction. Int J Obes Relat Metab Disord. 1999;23(10):1016–1024. doi: 10.1038/sj.ijo.0801059. [DOI] [PubMed] [Google Scholar]
- 103.Walsh KM, Leen E, Lean ME. The effect of sibutramine on resting energy expenditure and adrenaline-induced thermogenesis in obese females. Int J Obes Relat Metab Disord. 1999;23(10):1009–1015. doi: 10.1038/sj.ijo.0801045. [DOI] [PubMed] [Google Scholar]
- 104.Connoley IP, Liu YL, Frost I, Reckless IP, Heal DJ, Stock MJ. Thermogenic effects of sibutramine and its metabolites. Br J Pharmacol. 1999;126(6):1487–1495. doi: 10.1038/sj.bjp.0702446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Heusser K, Engeli S, Tank J, et al. Sympathetic vasomotor tone determines blood pressure response to long-term sibutramine treatment. J Clin Endocrinol Metab. 2007;92(4):1560–1563. doi: 10.1210/jc.2006-2499. [DOI] [PubMed] [Google Scholar]
- 106.Heusser K, Tank J, Diedrich A, et al. Influence of sibutramine treatment on sympathetic vasomotor tone in obese subjects. Clin Pharmacol Ther. 2006;79(5):500–508. doi: 10.1016/j.clpt.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 107.Lechin F, van der Dijs B. Acute effects of sibutramine administration on the autonomic nervous system in obese subjects. Clin Pharmacol Ther. 2007;81(3):326. 326–327. doi: 10.1038/sj.clpt.6100065. author reply. [DOI] [PubMed] [Google Scholar]
- 108.Birkenfeld AL, Schroeder C, Boschmann M, et al. Paradoxical effect of sibutramine on autonomic cardiovascular regulation. Circulation. 2002;106(19):2459–2465. doi: 10.1161/01.cir.0000036370.31856.73. [DOI] [PubMed] [Google Scholar]
- 109.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71(4):533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 110.Hoyer D, Clarke DE, Fozard JR, et al. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol Rev. 1994;46(2):157–203. [PubMed] [Google Scholar]
- 111.Garfield AS, Heisler LK. Pharmacological targeting of the serotonergic system for the treatment of obesity. J Physiol. 2009;587(Pt 1):49–60. doi: 10.1113/jphysiol.2008.164152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Banas SM, Doly S, Boutourlinsky K, et al. Deconstructing antiobesity compound action: requirement of serotonin 5-HT2B receptors for dexfenfluramine anorectic effects. Neuropsychopharmacology. 2011;36(2):423–433. doi: 10.1038/npp.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Launay JM, Herve P, Peoc’h K, et al. Function of the serotonin 5-hydroxytryptamine 2B receptor in pulmonary hypertension. Nat Med. 2002;8(10):1129–1135. doi: 10.1038/nm764. [DOI] [PubMed] [Google Scholar]
- 114.Nebigil CG, Hickel P, Messaddeq N, et al. Ablation of serotonin 5-HT(2B) receptors in mice leads to abnormal cardiac structure and function. Circulation. 2001;103(24):2973–2979. doi: 10.1161/01.cir.103.24.2973. [DOI] [PubMed] [Google Scholar]
- 115.Mochizuki D, Yuyama Y, Tsujita R, Komaki H, Sagai H. Cloning and expression of the human 5-HT1B-type receptor gene. Biochem Biophys Res Commun. 1992;185(2):517–523. doi: 10.1016/0006-291x(92)91655-a. [DOI] [PubMed] [Google Scholar]
- 116.Nothen MM, Erdmann J, Shimron-Abarbanell D, Propping P. Identification of genetic variation in the human serotonin 1D beta receptor gene. Biochem Biophys Res Commun. 1994;205(2):1194–1200. doi: 10.1006/bbrc.1994.2792. [DOI] [PubMed] [Google Scholar]
- 117.Bruinvels AT, Landwehrmeyer B, Gustafson EL, et al. Localization of 5-HT1B, 5-HT1D alpha, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology. 1994;33(3–4):367–386. doi: 10.1016/0028-3908(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 118.Grignaschi G, Sironi F, Samanin R. The 5-HT1B receptor mediates the effect of d-fenfluramine on eating caused by intra-hypothalamic injection of neuropeptide Y. Eur J Pharmacol. 1995;274(1–3):221–224. doi: 10.1016/0014-2999(94)00766-z. [DOI] [PubMed] [Google Scholar]
- 119.Neill JC, Cooper SJ. Selective reduction by serotonergic agents of hypertonic saline consumption in rats: evidence for possible 5-HT1C receptor mediation. Psychopharmacology (Berl) 1989;99(2):196–201. doi: 10.1007/BF00442807. [DOI] [PubMed] [Google Scholar]
- 120.Simansky KJ. Serotonergic control of the organization of feeding and satiety. Behav Brain Res. 1996;73(1–2):37–42. doi: 10.1016/0166-4328(96)00066-6. [DOI] [PubMed] [Google Scholar]
- 121.Lee MD, Simansky KJ. CP-94, 253: a selective serotonin1B (5-HT1B) agonist that promotes satiety. Psychopharmacology (Berl) 1997;131(3):264–270. doi: 10.1007/s002130050292. [DOI] [PubMed] [Google Scholar]
- 122.Lucas JJ, Yamamoto A, Scearce-Levie K, Saudou F, Hen R. Absence of fenfluramine-induced anorexia and reduced c-Fos induction in the hypothalamus and central amygdaloid complex of serotonin 1B receptor knock-out mice. J Neurosci. 1998;18(14):5537–5544. doi: 10.1523/JNEUROSCI.18-14-05537.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bouwknecht JA, van der Gugten J, Hijzen TH, Maes RA, Hen R, Olivier B. Male and female 5-HT(1B) receptor knockout mice have higher body weights than wildtypes. Physiol Behav. 2001;74(4–5):507–516. doi: 10.1016/s0031-9384(01)00589-3. [DOI] [PubMed] [Google Scholar]
- 124.Elmquist JK, Ahima RS, Maratos-Flier E, Flier JS, Saper CB. Leptin activates neurons in ventrobasal hypothalamus and brainstem. Endocrinology. 1997;138(2):839–842. doi: 10.1210/endo.138.2.5033. [DOI] [PubMed] [Google Scholar]
- 125.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22(2):221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 126.Schwartz MW, Baskin DG, Kaiyala KJ, Woods SC. Model for the regulation of energy balance and adiposity by the central nervous system. Am J Clin Nutr. 1999;69(4):584–596. doi: 10.1093/ajcn/69.4.584. [DOI] [PubMed] [Google Scholar]
- 127.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8(5):571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 128.Heisler LK, Jobst EE, Sutton GM, et al. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51(2):239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 129.Martin JR, Bos M, Jenck F, et al. 5-HT2C receptor agonists: pharmacological characteristics and therapeutic potential. J Pharmacol Exp Ther. 1998;286(2):913–924. [PubMed] [Google Scholar]
- 130.Kennett GA, Curzon G. Potencies of antagonists indicate that 5-HT1C receptors mediate 1–3 (chlorophenyl)piperazine-induced hypophagia. Br J Pharmacol. 1991;103(4):2016–2020. doi: 10.1111/j.1476-5381.1991.tb12369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tecott LH, Sun LM, Akana SF, et al. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374(6522):542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- 132.Vickers SP, Clifton PG, Dourish CT, Tecott LH. Reduced satiating effect of d-fenfluramine in serotonin 5-HT(2C) receptor mutant mice. Psychopharmacology (Berl) 1999;143(3):309–314. doi: 10.1007/s002130050952. [DOI] [PubMed] [Google Scholar]
- 133.Nonogaki K, Strack AM, Dallman MF, Tecott LH. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat Med. 1998;4(10):1152–1156. doi: 10.1038/2647. [DOI] [PubMed] [Google Scholar]
- 134.Fletcher PJ, Tampakeras M, Sinyard J, Slassi A, Isaac M, Higgins GA. Characterizing the effects of 5-HT(2C) receptor ligands on motor activity and feeding behaviour in 5-HT(2C) receptor knockout mice. Neuropharmacology. 2009;57(3):259–267. doi: 10.1016/j.neuropharm.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 135.Heisler LK, Cowley MA, Tecott LH, et al. Activation of central melanocortin pathways by fenfluramine. Science. 2002;297(5581):609–611. doi: 10.1126/science.1072327. [DOI] [PubMed] [Google Scholar]
- 136.Dalton GL, Lee MD, Kennett GA, Dourish CT, Clifton PG. Serotonin 1B and 2C receptor interactions in the modulation of feeding behaviour in the mouse. Psychopharmacology (Berl) 2006;185(1):45–57. doi: 10.1007/s00213-005-0212-3. [DOI] [PubMed] [Google Scholar]
- 137.Zhou L, Sutton GM, Rochford JJ, et al. Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin-4 receptor signaling pathways. Cell Metab. 2007;6(5):398–405. doi: 10.1016/j.cmet.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hayashi A, Suzuki M, Sasamata M, Miyata K. Thermogenic effect of YM348, a novel 5-HT2C-receptor agonist, in rats. J Pharm Pharmacol. 2004;56(12):1551–1556. doi: 10.1211/0022357044841. [DOI] [PubMed] [Google Scholar]
- 139.Hayashi A, Suzuki M, Sasamata M, Miyata K. Agonist diversity in 5-HT(2C) receptor-mediated weight control in rats. Psychopharmacology (Berl) 2005;178(2–3):241–249. doi: 10.1007/s00213-004-2019-z. [DOI] [PubMed] [Google Scholar]
- 140.Lorcaserin Phase 3 clinical trial in patients with type 2 diabetes shows statistically significant weight loss. Press Release Arena Pharmaceuticals. 2010 Nov 9; [Google Scholar]
- 141.Mancini MC, Halpern A. Pharmacological treatment of obesity. Arq Bras Endocrinol Metabol. 2006;50(2):377–389. doi: 10.1590/s0004-27302006000200024. [DOI] [PubMed] [Google Scholar]
- 142.Zhou Z, Zhen J, Karpowich NK, Law CJ, Reith ME, Wang DN. Antidepressant specificity of serotonin transporter suggested by three LeuT-SSRI structures. Nat Struct Mol Biol. 2009;16(6):652–657. doi: 10.1038/nsmb.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Luscombe GP, Hopcroft RH, Thomas PC, Buckett WR. The contribution of metabolites to the rapid and potent down-regulation of rat cortical beta-adrenoceptors by the putative antidepressant sibutramine hydrochloride. Neuropharmacology. 1989;28(2):129–134. doi: 10.1016/0028-3908(89)90048-8. [DOI] [PubMed] [Google Scholar]
- 144.Luscombe GP, Slater NA, Lyons MB, Wynne RD, Scheinbaum ML, Buckett WR. Effect on radiolabelled-monoamine uptake in vitro of plasma taken from healthy volunteers administered the antidepressant sibutramine HCl. Psychopharmacology (Berl) 1990;100(3):345–349. doi: 10.1007/BF02244604. [DOI] [PubMed] [Google Scholar]
- 145.Bae SK, Cao S, Seo KA, et al. Cytochrome P450 2B6 catalyzes the formation of pharmacologically active sibutramine (N-{1-[1-(4-chlorophenyl)cyclobutyl]-3-methylbutyl}-N,N-dimethylamine) metabolites in human liver microsomes. Drug Metab Dispos. 2008;36(8):1679–1688. doi: 10.1124/dmd.108.020727. [DOI] [PubMed] [Google Scholar]
- 146.Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet. 2007;369(9555):71–77. doi: 10.1016/S0140-6736(07)60033-6. [DOI] [PubMed] [Google Scholar]
- 147.Talbot PS, Bradley S, Clarke CP, et al. Brain serotonin transporter occupancy by oral sibutramine dosed to steady state: a PET study using (11) C-DASB in healthy humans. Neuropsychopharmacology. 2010;35(3):741–751. doi: 10.1038/npp.2009.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Smith BM, Smith JM, Tsai JH, et al. Discovery and structure-activity relationship of (1R)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1H-3-benzazepine (Lorcaserin), a selective serotonin 5-HT2C receptor agonist for the treatment of obesity. J Med Chem. 2008;51(2):305–313. doi: 10.1021/jm0709034. [DOI] [PubMed] [Google Scholar]
- 149.Thomsen WJ, Grottick AJ, Menzaghi F, et al. Lorcaserin, a novel selective human 5-hydroxytryptamine 2C agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther. 2008;325(2):577–587. doi: 10.1124/jpet.107.133348. [DOI] [PubMed] [Google Scholar]
- 150.Smith SR, Prosser WA, Donahue DJ, Morgan ME, Anderson CM, Shanahan WR. Lorcaserin (APD356), a selective 5-HT(2C) agonist, reduces body weight in obese men and women. Obesity (Silver Spring) 2009;17(3):494–503. doi: 10.1038/oby.2008.537. [DOI] [PubMed] [Google Scholar]
- 151.Weintraub M, Rubio A, Golik A, Byrne L, Scheinbaum ML. Sibutramine in weight control: a dose-ranging, efficacy study. Clin Pharmacol Ther. 1991;50(3):330–337. doi: 10.1038/clpt.1991.144. [DOI] [PubMed] [Google Scholar]
- 152.Bray GA, Blackburn GL, Ferguson JM, et al. Sibutramine produces dose-related weight loss. Obes Res. 1999;7(2):189–198. doi: 10.1002/j.1550-8528.1999.tb00701.x. [DOI] [PubMed] [Google Scholar]
- 153.Bray GA, Ryan DH, Gordon D, Heidingsfelder S, Cerise F, Wilson K. A double-blind randomized placebo-controlled trial of sibutramine. Obes Res. 1996;4(3):263–270. doi: 10.1002/j.1550-8528.1996.tb00544.x. [DOI] [PubMed] [Google Scholar]
- 154.Hanotin C, Thomas F, Jones SP, Leutenegger E, Drouin P. A comparison of sibutramine and dexfenfluramine in the treatment of obesity. Obes Res. 1998;6(4):285–291. doi: 10.1002/j.1550-8528.1998.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 155.Guven A, Koksal N, Cetinkaya A, Sokmen G, Ozdemir R. Effects of the sibutramine therapy on pulmonary artery pressure in obese patients. Diabetes Obes Metab. 2004;6(1):50–55. doi: 10.1111/j.1463-1326.2004.00314.x. [DOI] [PubMed] [Google Scholar]
- 156.Bach DS, Rissanen AM, Mendel CM, et al. Absence of cardiac valve dysfunction in obese patients treated with sibutramine. Obes Res. 1999;7(4):363–369. doi: 10.1002/j.1550-8528.1999.tb00419.x. [DOI] [PubMed] [Google Scholar]
- 157.FDA Recommends Against the Continued Use of Meridia (sibutramine) Questions and Answers. FDA press release. 2010 Oct 8; [Google Scholar]
- 158.Bond DS, Phelan S, Wolfe LG, et al. Becoming physically active after bariatric surgery is associated with improved weight loss and health-related quality of life. Obesity (Silver Spring) 2009;17(1):78–83. doi: 10.1038/oby.2008.501. [DOI] [PubMed] [Google Scholar]
- 159.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82(1 Suppl):222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 160.Fine JT, Colditz GA, Coakley EH, et al. A prospective study of weight change and health-related quality of life in women. JAMA. 1999;282(22):2136–2142. doi: 10.1001/jama.282.22.2136. [DOI] [PubMed] [Google Scholar]
- 161.Digenio AG, Mancuso JP, Gerber RA, Dvorak RV. Comparison of methods for delivering a lifestyle modification program for obese patients: a randomized trial. Ann Intern Med. 2009;150(4):255–262. doi: 10.7326/0003-4819-150-4-200902170-00006. [DOI] [PubMed] [Google Scholar]
- 162.Kaukua JK, Pekkarinen TA, Rissanen AM. Health-related quality of life in a randomised placebo-controlled trial of sibutramine in obese patients with type II diabetes. Int J Obes Relat Metab Disord. 2004;28(4):600–605. doi: 10.1038/sj.ijo.0802591. [DOI] [PubMed] [Google Scholar]
- 163.Wilfley DE, Crow SJ, Hudson JI, et al. Efficacy of sibutramine for the treatment of binge eating disorder: a randomized multicenter placebo-controlled double-blind study. Am J Psychiatry. 2008;165(1):51–58. doi: 10.1176/appi.ajp.2007.06121970. [DOI] [PubMed] [Google Scholar]
- 164.Pillitteri JL, Shiffman S, Rohay JM, Harkins AM, Burton SL, Wadden TA. Use of dietary supplements for weight loss in the United States: results of a national survey. Obesity (Silver Spring) 2008;16(4):790–796. doi: 10.1038/oby.2007.136. [DOI] [PubMed] [Google Scholar]
- 165.Lenz TL, Hamilton WR. Supplemental products used for weight loss. J Am Pharm Assoc. 2003;44(1):59–67. 67–58. doi: 10.1331/154434504322713246. 2004; quiz. [DOI] [PubMed] [Google Scholar]
- 166.Dwyer JT, Allison DB, Coates PM. Dietary supplements in weight reduction. J Am Diet Assoc. 2005;105(5 Suppl 1):S80–S86. doi: 10.1016/j.jada.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 167.Bui LT, Nguyen DT, Ambrose PJ. Blood pressure and heart rate effects following a single dose of bitter orange. Ann Pharmacother. 2006;40(1):53–57. doi: 10.1345/aph.1G488. [DOI] [PubMed] [Google Scholar]
- 168.Carlson JC, Riley JC. A consideration of some notable aging theories. Exp Gerontol. 1998;33(1–2):127–134. doi: 10.1016/s0531-5565(97)00068-5. [DOI] [PubMed] [Google Scholar]
- 169.Merry BJ. Dietary restriction in rodents–delayed or retarded ageing? Mech Ageing Dev. 2005;126(9):951–959. doi: 10.1016/j.mad.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 170.Smith DL, Jr, Robertson HT, Desmond RA, Nagy TR, Allison DB. No compelling evidence that sibutramine prolongs life in rodents despite providing a dose-dependent reduction in body weight. Int J Obes (Lond) 2010 Nov 16; doi: 10.1038/ijo.2010.247. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]