Abstract
The aminosalicylates (5-ASA; also referred to as mesalamine-based agents) are considered as first-line in the maintenance of remission of mild to moderate ulcerative colitis (UC). Traditionally these agents have required a large pill burden and multiple daily dosing regimens which may account for the low adherence rates, especially in patients in remission. Extended-release mesalamine is the first once daily mesalamine product approved by the Food and Drug Administration for the maintenance of UC remission. This review will examine the pharmacokinetics, dosing, efficacy, and safety data of extended-release mesalamine, and discuss the potential role of improving medication compliance and decreasing costs in UC maintenance.
Keywords: ulcerative colitis, 5-ASA, mesalamine, adherence, compliance, quality of life, costs
Introduction
Ulcerative colitis (UC) is a chronic relapsing–remitting inflammatory condition that affects up to 238 per 100,000 persons in North America.1 The goals of treatment of UC include achieving and maintaining symptom-free remission with minimal side-effects. The aminosalicylates (5-ASA; also referred to as mesalamine-based agents) are the recommended first-line therapy for induction and maintenance of remission in mild to moderate UC.2 The mechanism of action of these agents is unknown but there is evidence to show that they have many direct anti-inflammatory effects on the colonic mucosa. One of the major mechanisms appears to be mediated by local inhibition of the activation of nuclear factor-κB, which is a central transcription regulatory factor involved in initiating and perpetuating inflammatory processes.3,4 These local anti-inflammatory effects are dependent on the ability of the active drug to reach the site of inflammation intact; therefore different 5-ASA formulations have been designed to ensure the drug is not degraded or absorbed before reaching the inflamed mucosa. These formulations include pH-dependent enteric coatings that release the active drug at a set pH, pro-drug formulations that depend on colonic bacteria for drug release, and moisture-dependent coatings that facilitate delayed release of mesalamine. Traditionally, 5-ASA formulations have required multiple daily dosing and a large pill burden, which may account for their low adherence rates of only 40% to 60%,5,6 especially challenging in patients who are in remission.7–10 Lack of adherence to 5-ASA agents has been shown to increase the probability of clinical relapse in UC11 and has been related to increased costs of disease.12–14 As a result, there has been much interest in the development of effective, once daily-dosed mesalamine products, with the expectation of greater compliance, and decreased disease burden and costs.
Extended-release mesalamine (Apriso™; Salix Pharmaceuticals, Morrisville, NC) is the first once daily-dosed mesalamine product indicated for the maintenance of UC remission released in the United States. This granulated mesalamine formulation is available elsewhere in the world under the trade name Salofalk® (Dr Falk Pharma GmbH, Freiburg, Germany), with data for induction of UC. This review will examine the pharmacokinetics, dosing, efficacy, and safety data of extended-release mesalamine, and discuss the potential role of improving medication compliance in UC maintenance.
Pharmacokinetics
Background
The therapeutic efficacy of 5-ASA agents in the treatment of UC depends on the ability of the mesalamine to reach the mucosal surface of the inflamed colon; systemic absorption is not known to have additional benefit and is generally considered undesirable due to concerns of mesalamine-induced nephrotoxicity. Since mesalamine would be rapidly absorbed from the small intestine if left unprotected, the challenge has been to transfer mesalamine unadulterated through the upper and middle intestinal tract, but then have effective release in the lower tract to allow luminal contact between the active 5-ASA and the colonic mucosa.
Some of the 5-ASA formulations use pH-dependent mechanisms to ensure delivery to the site of action. The two most commonly used pH-dependent delivery systems utilize a Eudragit®-S resin (designed to dissolve at pH ≥ 7), and a Eudragit®-L resin (Evonik Rohm GmbH, Darmstadt, Germany), which disintegrates at pH ≥ 6. Variability in pH throughout the gut can therefore have a marked effect on the location of the release of mesalamine that is coated or otherwise protected by these agents.
Studies of gut luminal pH levels and colonic transit time
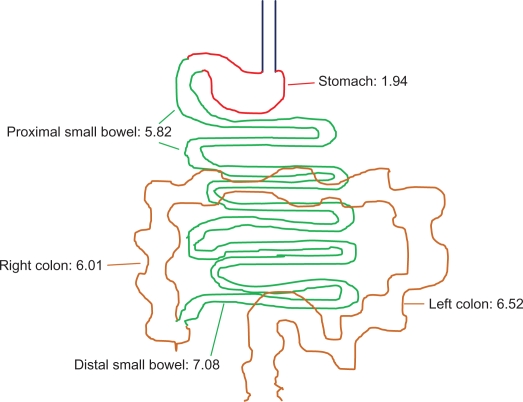
The pH throughout the gut of both healthy volunteers and patients with mild to moderate UC has been measured and compared in small studies. Using 13 healthy volunteers, Fallingborg et al15 determined the pH in the stomach to be <3.0 in all patients. Median pH was 6.6 in the proximal small bowel, 7.3 in the distal small bowel, 5.7 in the right colon, and 6.6 in the left colon. In 10 patients with mild to moderate UC, the results were similar. Rubin et al used the newly developed SmartPill pHp™ (the SmartPill Corporation, Buffalo, NY) to study 10 patients with active UC: 9 were male, 4 with pancolitis, 6 with left-sided colitis.16 Mean pH levels are shown in Figure 1. All 10 patients achieved a luminal pH of ≥6.0 for a minimum of 120 minutes; 1 patient never reached a pH of 7.0, and 6 patients maintained pH ≥ 7.0 for less than 120 minutes.
Figure 1.
Mean luminal pH measurement in 10 patients with active ulcerative colitis.16
Bosworth et al17 compared the pH and transit times between 5 otherwise healthy UC patients and 5 normal controls. There was no difference in the mean pH of the small bowel (mean ± SD: 6.89 ± 0.72 in the UC patients vs 6.83 ± 0.01 in the controls) between the two groups but the mean colonic pH in the UC patients was significantly lower than that of the normal controls (mean ± SD: 6.14 ± 0.37 vs 7.06 ± 0.41, P = 0.006). The number of hours that a pH of >7.0 or >6.0 was achieved in the colon was significantly less in the UC patients as compared to the controls (mean ± SD: 4.33 ± 2.32 hours vs 14.10 ± 6.31 hours, P = 0.01, for pH > 6.0; 0.30 ± 0.17 hours vs 9.33 ± 5.29 hours, P = 0.005, for pH > 7.0, respectively).
The implications of a more acidic colonic luminal pH in patients with active UC could include the modification of the most desirable pH release system for the delivery of a luminally active medication, such as mesalamine. The pH levels of a UC colon in remission have not been well studied but would be presumably less acidic that in active colitis.
Some of these same studies also measured colonic transit times.15–17 In normal volunteers the median colonic transit time was 17.5 hours15 and in patients with mild to moderate UC the mean colonic transit time was 15.41 hours (range 6.91–23.91).16 When mild UC patients were compared with normal controls, there was no significant difference in mean colonic transit time between the two groups (mean ± SD: 12.66 ± 5.37 vs 30.68 ± 21.47, P = 0.10).17
Extended-release mesalamine
Extended-release mesalamine consists of a gelatin capsule filled with hundreds of tiny mesalamine granules, each of which is encapsulated in an outer Eudragit®-L coating that is intended to protect the mesalamine from degradation in the stomach and begin breaking down at pH ≥ 6. The intestinal contents then activate an inner core of the mesalamine granules, which is made of a polymer matrix that allows for time-dependent release of mesalamine.18 Pharmacokinetic studies have shown that this mesalamine formulation arrives in the small intestine after 0.8 ± 0.5 (mean ± SD) hours and in the ileum after 4 ± 1 hours.19 The tablets start to disintegrate when they reach the colon after a mean of 5.8 ± 1.2 hours post-dose and the initial disintegration of the tablets start in the ascending or transverse colon after an average of 6.9 ± 1.1 hours post-dose. The tablets arrive in the transverse colon after a mean of 7.3 ± 1 hours and in the descending colon after a mean of 9.5 ± 2.1 hours.19,20
An estimated 32% ± 11% (mean ± SD) of the administered dose of extended-release mesalamine is absorbed systemically in the fasting state and peak plasma concentrations are observed at about 4 hours after administration.18
The pharmacokinetics, safety, and tolerability of once daily versus twice daily dosing of 1.6 g of extended-release mesalamine in 30 healthy volunteers showed that both dosing regimens were comparable in terms of systemic exposure (area under the curve, AUC), although the time to maximum concentration was shorter in the once daily group versus the twice daily group.21 Adverse events did not differ between the two groups. The effect of a high-fat meal on the pharmacokinetics of 1.6 g mesalamine granules in 30 healthy volunteers was also analyzed, with no difference shown in the maximum plasma concentration (Cmax) or the AUC between the fasting and high-fat states, although the time to Cmax was increased by a high-fat meal.22 Based on these results, the once daily extended-release granulated mesalamine may be dosed with or without food.
Dosing
The current Food and Drug Administration (FDA) approved dose for granulated extended-release mesalamine for maintenance of UC remission is 1.5 g (four 0.375 g capsules) once daily in the morning. Other doses have been tested in the induction and in the maintenance of remission of UC.
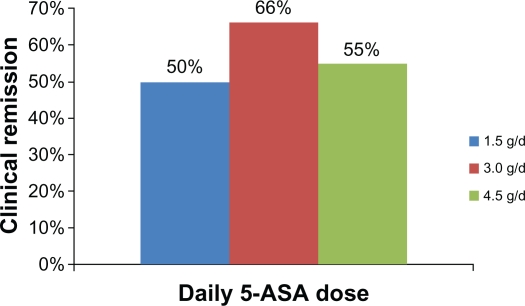
One randomized double-blind, dose-finding induction study compared 3 different daily doses of granulated extended-release mesalamine (1.5 g, 3.0 g, and 4.5 g) for 8 weeks in 321 patients with mild to moderate active UC.23 Clinical remission was measured by the Rachmilewitz clinical activity index (CAI; number of stools, percentage of bloody stools, abdominal pain, general well-being, fever, extraintestinal manifestations, erythrocyte sedimentation rate, and hemoglobin). Rates of clinical remission are shown in Figure 2. Endoscopic response rates, measured by a 5-point index assessing granularity, vascular pattern, vulnerability, and integrity of the mucosa, were 53% (1.5 g), 84% (3.0 g), and 70% (4.5 g), with significant differences seen between the 1.5 g and 3.0 g groups. Histological improvement rates were 42%, 63%, and 56%, respectively, as determined by the 4-point Riley score. Global tolerability was found to be very good or good in 82% of patients in the 1.5 g group, 88% in the 3.0 g/day group and 75% in the 4.5 g group.
Figure 2.
Clinical remission rates at 8 weeks in 321 patients with active mild-to-moderate ulcerative colitis to three different daily doses of granulated extended-release mesalamine.23
A different study of maintenance of UC remission randomized 647 patients with quiescent UC to receive granulated extended-release mesalamine 3.0 g once daily, 1.5 g once daily, or 0.5 g 3 times daily for a 1 year.24 Remission was defined as an endoscopic score of ≤3 within 3 months of baseline evaluation, and as a CAI ≤ 4. One-year remission rates were statistically similar at 75% (3.0 g/d), 61% (1.5 g/d), and 70% (0.5 g 3 times daily). All three dosing regimens were well tolerated; there were no medication-related serious adverse events reported.
Efficacy and safety in the maintenance of remission
Two randomized double-blind phase III studies compared the efficacy of granulated extended-release mesalamine (1.5 g once daily) with placebo in UC patients who were in remission for 1 to 12 months.25,26 Remission was defined, using the revised Sutherland Disease Activity Index (DAI) scale, as a score of 0 on the rectal bleeding component and ≤ 1 on the mucosal appearance component.
The first study randomized 305 patients with quiescent UC; 193 (63%) completed the study.25 A total of 112 patients (37%) withdrew from the study due to adverse events (54 patients) or lack of efficacy (36 patients). The primary efficacy end-point, defined as the proportion of patients who were relapse-free after 6 months of treatment, was achieved in a significantly higher number of mesalamine-treated patients (79% vs 58% for placebo, P < 0.001). Relapse risk over the 6-month course of the study was lower in the mesalamine group compared to placebo (hazard ratio = 0.42, P < 0.001). The adverse event rates were similar between the two groups, UC flares being twice as common in the placebo group than in the mesalamine group (27% vs 11%, respectively) and headaches being more common in the mesalamine group (11% vs 7%). Two serious adverse events were reported in each group but only 1 (placebo group), in which a patient experienced a severe UC recurrence, was considered study-drug related.
The second study showed very similar results. Of 257 patients with quiescent UC who were randomized, 176 (69%) completed the study.26 A total of 81 patients (32%) withdrew from the study due to adverse events (15 patients) or lack of efficacy (47 patients). The primary efficacy end-point, defined as the proportion of patients who were relapse-free after 6 months of treatment, was achieved in a significantly higher number of granulated extended-release mesalamine patients (80% vs 57% placebo, P = 0.03). Relapse risk over the 6-month course of the study was also lower in the mesalamine group compared with placebo (hazard ratio = 0.057, P = 0.02). The adverse event rates were similar between the two groups, UC flares being twice as common in the placebo group than in the mesalamine group (22% vs 11%, respectively) and headaches being more common in the mesalamine group (11% vs 8%). A total of five serious adverse events were reported in four patients (two patients per group) but none was considered to be related to the study drug.
A third placebo-controlled randomized study evaluated the efficacy of granulated extended-release mesalamine in the maintenance of remission in patients with quiescent UC who switched from other 5-ASA preparations.27 A total of 487 UC patients in remission were randomized to 1.5 g of granulated extended-release mesalamine once daily (N = 322) or placebo (N = 165) for 6 months. The 5-ASA preparations previously used by these patients included nongranulated mesalamine, sulfasalazine, balsalazide, suppositories/enemas, and olsalazine. Remission was defined using the revised Sutherland DAI scale as a score of 0 on the rectal bleeding component and <2 on the mucosal appearance component. The primary efficacy end-point, defined as the proportion of patients who were relapse-free during 6 months of treatment, was higher in the granulated extended-release mesalamine group (78%) than in the placebo group (59%; P < 0.001).
Compliance issues
The efficacy of the 5-ASA agents in maintaining remission depends to some extent on medication adherence. Noncompliance rates in UC have been shown to be high, especially in patients with quiescent UC. Multiple studies have examined the reasons behind the high rates of noncompliance in these patients. One study investigated the adherence rates and risk factors for nonadherence in 94 patients with quiescent UC and found the overall adherence rate to be only 40%.8 Risk factors for nonadherence included male gender, single marital status, and left-sided UC disease location. The most common reasons cited by the patients for their nonadherence were forgetfulness and high pill burden. Another separate study showed that patients with inactive disease were less likely to be compliant with their medications than symptomatic patients,9 and the high number of pills and multiple daily dosing were also associated with nonadherence. Other studies have reported similar findings.28,29
The barriers to patient compliance are important to understand because the consequences of nonadherence include increased relapse rates, which in turn impact morbidity, quality of life, and overall health care costs. In a prospective study, Kane et al11 found that nonadherence with mesalamine increases the risk of clinical relapse among patients with quiescent UC. Adherence to medication in this study was associated with an 89% chance of maintaining remission compared with only a 39% chance in patients who were nonadherent (P = 0.001). Overall, nonadherent patients had a five-fold higher risk of relapse than adherent patients (hazard ratio = 5.5; 95% confidence interval: 2.3–13.0; P < 0.001).
Other potential benefits of higher levels of adherence with 5-ASA medications include improved patient quality of life and lower incidence rates of colorectal dysplasia and cancer. UC relapses have also been shown to have a negative impact on quality of life. Han et al30 studied 111 patients with UC and found a strong association between patients’ UC symptoms and quality of life. Avoidance of relapse by improved adherence with effective medication may help maintain acceptable quality of life. The long-term use of 5-ASA agents has been shown to possibly reduce colorectal cancer risk.31 This benefit is thought to be related to decreased inflammation secondary to decreased long-term relapse rates, or perhaps from COX-2 inhibition of colonic neoplasia. As a result, nonadherence to therapy may also lead to increased rates of colorectal cancer and dysplasia.
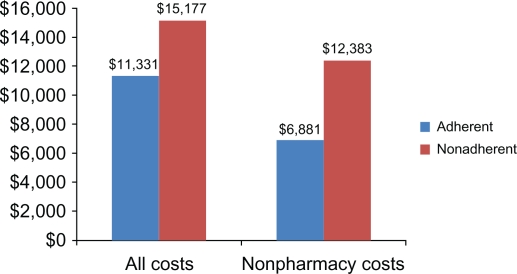
Nonadherence to medications have also been shown to lead to higher medical costs, due to increased rates of disease relapse. The average cost of a single case of treatment failure has been shown to exceed US$11,500 per patient.32 Compared to patients in remission, UC patients who relapse incur 2 to 3 times higher costs, with a 20-fold increase in costs if the relapses result in a hospitalization.13 The relationship between health care costs and 5-ASA adherence rates in patients with active UC was directly studied by Mitra et al33 in 1693 UC patients captured in a large health insurance claims database. Nonadherence, defined as a 5-ASA medication possession ratio of <0.8, was found in 72% of the patients; these patients had higher average per-patient 1-year health care costs (US$15,177) than patients who were adherent (US$11,331). Excluding pharmacy costs even further emphasized the difference, with nonadherent patients racking up average costs of US$12,383 versus only US$6,881 for patients who were adherent with their 5-ASA medications (Figure 3).
Figure 3.
Annual average per-patient 1-year health care costs (US$) in 1693 ulcerative colitis patients captured in a large health insurance claims database.33
Conclusion
Traditionally, 5-ASA formulations have required multiple daily dosing and a large pill burden, which may account for their low adherence rates, especially in patients who are in remission. Lack of adherence to 5-ASA agents has been shown to increase the probability of clinical relapse in UC and has been related to increased costs of disease. Extended-release mesalamine is the first once daily-dosed mesalamine product FDA-indicated for the maintenance of UC remission. The efficacy of this formulation, simplified dosing regimen, and low pill burden may improve adherence and, as a result, decrease the frequency of relapses, lower the incidence of UC complications such as colon cancer, and reduce UC-related health care costs.
Footnotes
Disclosure
Dr Lilliana Oliveira has no disclosures. Dr Russell Cohen declares the following: Consultant and lecturer for Abbott, Salix Pharmaceuticals, Shire Pharmaceuticals; Consultant for Advogent Group, Axcan Scandipharm, Centocor, Elan Pharmaceuticals, Guidepoint Global Consulting, Millennium Pharmaceuticals/Takeda, Prometheus Laboratories, sanofi-aventis, Tactical Advantage Group, Warner-Chilcott; Lecturer for Merck Sharp and Dohme Farmaceutica/Merck, Brazil, Schering-Plough/Merck, Mexico.
References
- 1.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5(12):1424–1429. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Carter MJ, Lobo AJ, Travis SP. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004 Sep;53(Suppl 5):V1–V16. doi: 10.1136/gut.2004.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bantel H, Berg C, Vieth M, Stolte M, Kruis W, Schulze-Osthoff K. Mesalazine inhibits activation of transcription factor NF-kappaB in inflamed mucosa of patients with ulcerative colitis. Am J Gastroenterol. 2000;95(12):3452–3457. doi: 10.1111/j.1572-0241.2000.03360.x. [DOI] [PubMed] [Google Scholar]
- 4.MacDermott RP. Progress in understanding the mechanisms of action of 5-aminosalicylic acid. Am J Gastroenterol. 2000;95(12):3343–3345. doi: 10.1111/j.1572-0241.2000.03342.x. [DOI] [PubMed] [Google Scholar]
- 5.Hanauer S, Schwartz J, Robinson M, et al. Mesalamine capsules for treatment of active ulcerative colitis: results of a controlled trial. Pentasa Study Group. Am J Gastroenterol. 1993;88(8):1188–1197. [PubMed] [Google Scholar]
- 6.Riley SA. What dose of 5-aminosalicylic acid (mesalazine) in ulcerative colitis? Gut. 1998;42(6):761–763. doi: 10.1136/gut.42.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerveny P, Bortlik M, Kubena A, Vlcek J, Lakatos PL, Lukas M. Non-adherence in inflammatory bowel disease: results of factor analysis. Inflamm Bowel Dis. 2007;13(10):1244–1249. doi: 10.1002/ibd.20189. [DOI] [PubMed] [Google Scholar]
- 8.Kane SV, Cohen RD, Aikens JE, Hanauer SB. Prevalence of nonadherence with maintenance mesalamine in quiescent ulcerative colitis. Am J Gastroenterol. 2001;96(10):2929–2933. doi: 10.1111/j.1572-0241.2001.04683.x. [DOI] [PubMed] [Google Scholar]
- 9.Shale MJ, Riley SA. Studies of compliance with delayed-release mesalazine therapy in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18(2):191–198. doi: 10.1046/j.1365-2036.2003.01648.x. [DOI] [PubMed] [Google Scholar]
- 10.Van Hees PA, van Tongeren JH. Compliance to therapy in patients on a maintenance dose of sulfasalazine. J Clin Gastroenterol. 1982;4(4):333–336. doi: 10.1097/00004836-198208000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Kane S, Huo D, Aikens J, Hanauer S. Medication nonadherence and the outcomes of patients with quiescent ulcerative colitis. Am J Med. 2003;114(1):39–43. doi: 10.1016/s0002-9343(02)01383-9. [DOI] [PubMed] [Google Scholar]
- 12.Tindall WN, Boltri JM, Wilhelm SM. Mild-to-moderate ulcerative colitis: your role in patient compliance and health care costs. J Manag Care Pharm. 2007;13(7 Suppl A):S2–S12. doi: 10.18553/jmcp.2007.13.s7-a.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bassi A, Dodd S, Williamson P, Bodger K. Cost of illness of inflammatory bowel disease in the UK: a single centre retrospective study. Gut. 2004;53(10):1471–1478. doi: 10.1136/gut.2004.041616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen RD, Yu AP, Wu EQ, Xie J, Mulani PM, Chao J. Systematic review: the costs of ulcerative colitis in Western countries. Aliment Pharmacol Ther. 2010;31(7):693–707. doi: 10.1111/j.1365-2036.2010.04234.x. [DOI] [PubMed] [Google Scholar]
- 15.Fallingborg J, Christensen LA, Ingeman-Nielsen M, Jacobsen BA, Abildgaard K, Rasmussen HH. pH-profile and regional transit times of the normal gut measured by a radiotelemetry device. Aliment Pharmacol Ther. 1989;3(6):605–613. doi: 10.1111/j.1365-2036.1989.tb00254.x. [DOI] [PubMed] [Google Scholar]
- 16.Rubin DT, Mikolajczyk A, Surma B. Measurement of luminal pH in patients with mildly to moderately active UC: a pilot study using SMARTpill pH. Gastroenterology. 2009;135(S1):A-349. [Google Scholar]
- 17.Bosworth BP, Cohen M, Weine D. Colonic pH is lower in patients with mild ulcerative colitis compared to normal controls. Gastroenterology. 2009;136(S1):A68683. [Google Scholar]
- 18.Salix Pharmaceuticals Inc . Product information for AprisoTM (mesalamine) extended-release capsules. Morrisville, NC: Salix Pharmaceuticals Inc.; 2009. [Google Scholar]
- 19.Brunner M, Assandri R, Kletter K, et al. Gastrointestinal transit and 5-ASA release from a new mesalazine extended-release formulation. Aliment Pharmacol Ther. 2003;17(3):395–402. doi: 10.1046/j.1365-2036.2003.01445.x. [DOI] [PubMed] [Google Scholar]
- 20.Brunner M, Greinwald R, Kletter K, et al. Gastrointestinal transit and release of 5-aminosalicylic acid from 153Sm-labelled mesalazine pellets vs tablets in male healthy volunteers. Aliment Pharmacol Ther. 2003;17(9):1163–1169. doi: 10.1046/j.1365-2036.2003.01564.x. [DOI] [PubMed] [Google Scholar]
- 21.Safdi A, Pieniaszek H, Grigston A, Forbes W. Multiple-dose pharmacokinetics of granulated mesalamine, a unique formulation providing delayed and extended release of 5-ASA. Am J Gastroenterol. 2008;103(S1):S439–S440. [Google Scholar]
- 22.Safdi A, Pieniaszek H, Grigston A, Forbes W. Minimal effect of a high-fat meal on the pharmacokinetics of once-daily granulated mesalamine. Am J Gastroenterol. 2008;103(S1):S440. [Google Scholar]
- 23.Kruis W, Bar-Meir S, Feher J, et al. The optimal dose of 5-aminosalicylic acid in active ulcerative colitis: a dose-finding study with newly developed mesalamine. Clin Gastroenterol Hepatol. 2003;1:36–43. doi: 10.1053/jcgh.2003.50006. [DOI] [PubMed] [Google Scholar]
- 24.Kruis W. Once daily 3 g mesalamine is the optimal dose for maintaining clinical remission in ulcerative colitis: a double-blind, double-dummy, randomized controlled, dose-ranging study. Gastroenterology. 2008;134:A489. [Google Scholar]
- 25.Gordon G, Pruitt RE, Ringold M, Sedghi S, Merchant K. Once-daily 1.5-g granulated mesalamine is effective and safe in maintenance of remission in mild-to-moderate UC [abstract] Am J Gastroenterol. 2008;103:1128. [Google Scholar]
- 26.Zakko SMU, Pruitt RE, Merchant K, Shaw A. Safety profile of once-daily 1.5-g granulated mesalamine as maintenance therapy for mild-to-moderate ulcerative colitis: results from 2 phase III trials [abstract] Am J Gastroenterol. 2008;103:1119. [Google Scholar]
- 27.Lichtenstein GR. Once-daily 1.5-g granulated mesalamine effectively maintains remission in patients with ulcerative colits who switch from different 5-ASA formulations. Am J Gastroenterol. 2008;103(S1):S429–S430. [Google Scholar]
- 28.Hawthorne AB, Rubin G, Ghosh S. Review article: medication non-adherence in ulcerative colitis – strategies to improve adherence with mesalazine and other maintenance therapies. Aliment Pharmacol Ther. 2008;27(12):1157–1166. doi: 10.1111/j.1365-2036.2008.03698.x. [DOI] [PubMed] [Google Scholar]
- 29.Kane SV. Systematic review: adherence issues in the treatment of ulcerative colitis. Aliment Pharmacol Ther. 2006;23(5):577–585. doi: 10.1111/j.1365-2036.2006.02809.x. [DOI] [PubMed] [Google Scholar]
- 30.Han SW, McColl E, Barton JR, James P, Steen IN, Welfare MR. Predictors of quality of life in ulcerative colitis: the importance of symptoms and illness representations. Inflamm Bowel Dis. 2005;11(1):24–34. doi: 10.1097/00054725-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Velayos FSTJ, Walsh JM. Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and metaanalysis of observational studies. Am J Gastroenterol. 2005;100:1345–1353. doi: 10.1111/j.1572-0241.2005.41442.x. [DOI] [PubMed] [Google Scholar]
- 32.Mackowiak JI. A two-stage decision analysis to assess the cost of 5-aminosalicylic acid failure and the economics of balsalazide versus mesalamine in the treatment of ulcerative colitis. Manag Care Interface. 2006;19(10):39–46. 56. [PubMed] [Google Scholar]
- 33.Mitra D, Hodgkins P, Yen L, Solomon D, Davis K, Cohen R. The impact of oral 5-aminosalicylic acid adherence on all-cause healthcare costs among ulcerative colitis patients [abstract] Inflamm Bowel Dis. 2009;15(2):S32. [Google Scholar]