Abstract
Phenolic compounds were extracted from Morton lentils using acidified aqueous acetone. The crude Morton extract (CME) was applied onto a macroresin column and desorbed by aqueous methanol to obtain a semi-purified Morton extract (SPME). The SPME was further fractionated over Sephadex LH-20 column into five main fractions (Fr I – Fr V). The phytochemical contents such as total phenolic content (TPC), total flavonoid content (TFC), and condensed tannin content (CTC) of the CME, SPME, and its fractions were examined by colorimetric methods. Antioxidant activity of extracts and fractions were screened by DPPH scavenging activity, trolox equivalent antioxidant capacity (TEAC), ferric reduced antioxidant power (FRAP), and oxygen radical absorbing capacity (ORAC) methods. In addition, the compositions of active fractions were determined by HPLC-DAD and HPLC-MS methods. Results showed that fraction enriched in condensed tannins (Fr V) exhibited significantly higher value of TPC, CTC and higher antioxidant activity as compared to the crude extract, SPME and low-molecular-weight fractions (Fr I – IV). Eighteen compounds existed in those fractions, and seventeen were tentatively identified by UV and MS spectra. HPLC-MS analysis revealed Fr II contained mainly kaempferol glycoside, Fr III and Fr IV mainly contained flavonoid glycosides, and Fr V was composed of condensed tannins. The results suggested that extract of Morton lentils is a promising source of antioxidant phenolics, and may be used as a dietary supplement for health promotion.
Keywords: Lens culinaris var. Morton, phenolics, antioxidant activity, fractionation
Introduction
Legumes, including lentils (Lens culinaris L.), peas (Pisum sativum L.), chickpeas (Cicer arietinum L.) and common beans (Phaseolus vulgaris L.), are among the oldest crops cultivated by humans, and are an important crops consumed in Europe, the Middle East, Africa and south Asia. Although the global consumption of pulse is in decline, lentils consumption is steadily increasing. The annual production of lentils is reported to be about 4 megatons (1). Lentils are not only an excellent source of macronutrients such as protein, fatty acids, fibers, and carbohydrates, but also contains phytochemicals (2), which can be categorized into phenolic acids, flavanols, flavonols, soyasaponins, phytic acid, and condensed tannins (3). Some phytochemicals were thought to be antinutrional factors in the past. However, the functions of phytochemicals in lentils have been reversed nowadays, and it works as a “double-edged sword” (4). Epidemiological studies suggest that lentils confer protection against chronic diseases through a multitude of biological activities including antioxidant, anticancer, angiotensin I-converting enzyme inhibition, reducing blood lipid, and reducing the risk of cardiovascular diseases (5, 6).
A recent study aiming at comparing the phenolic contents and antioxidant activity of legumes, including peas, lentils, chick peas, common beans, and soybeans in our lab showed that lentils possessed the highest concentrations of phenolic contents and antioxidant activity, and the higher antioxidant activity was strongly correlated with the phenolic contents (7). The antioxidant activity of the extract from legumes has been investigated. Amarowicz et al. studied the antioxidant activity of low-molecular-weight and tannin fractions of Adzuki bean using a β-carotene-linoleate model, DPPH scavenging activity, and reducing power (8). Troszyńska et al. determined the phenolic contents of seed coat of legumes and their Sephadex LH-20 fractions (9). Karamac et al. only extracted and fractionated tannin fractions from tannin-rich plant materials including red lentils and green lentils (10). However, systematic studies on the fractionation of lentils extract into different groups, and subsequently characterization their antioxidant activity and phenolic compositions are lacking.
The objectives of this study were: (1) to concentrate the phenolic substances in the crude lentils extract by adsorption-desorption and to fractionate substances using gel filtration column into different groups; (2) to determine the antioxidant potential of lentils extracts and its fractions; and (3) to characterize the phenolic compositions of fractions by HPLC and HPLC-MS.
Meterials and Methods
Materials and Chemicals
Lentils (Lens culinaris var. Morton) were purchased from Spokane Seed Co. (Spokane, WA). The lentils were ground to powder with an IKA® all basic mill (IKA Works Inc., Wilmington, NC) and passed through a 60-mesh sieve. The powders were stored at -20 °C before use. XAD-7 was purchased from Sigma-Aldrich (St. Louis, MO), and Sephadex LH-20 from Pharmacia LKB Biotech. (Uppsala, Sweden).
Sixteen phenolic acid standards (gallic, protocatechuic, 2, 3, 4-trihydroxybenzoic, p-hydroxybenzoic, gentistic, vanillic, caffeic, chlorogenic, syringic, p-coumaric, m-coumaric, o-coumaric, ferullic, salicylic, sinapic, and trans-cinnamic acids), three aldehydes (vanillin, syringealdehyde, and protocatechualdehyde), (+)-catechin, (-)-epicatechin, epigallocatechin, epicatechin gallate, myricetin, luteolin, quercetin, kaempferol, 2-diphenyl-1-picryhydrazyl radical (DPPH), fluorescein disodium (FL), 6-hydroxy-2,5,7,8-tetramethlchroman-2-carboxylic acid (Trolox), 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid (ABTS), potassium persulfate, trifluoroacetic acid (TFA), Folin-Ciocalteu reagent, sodium carbonate, and 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ) were purchased from Sigma-Aldrich Inc. (St. Louis, MO). Kaempferol-3-glucoside and quercetin-3-glucoside were purchased from Extrasynthese S. A. (Genay, France). The 2, 2′-azobis (2-amidino-propane) dihydrochloride (AAPH) was purchased from Wako Chemicals USA (Richmond, VA). HPLC-grade solvents (methanol, B & J Brand®), analytical grade acetic acid and other analytical grade solvent used for extraction were purchased from VWR international (West Chester, PA).
Extraction and Fractionation of Crude Morton Extract (CME)
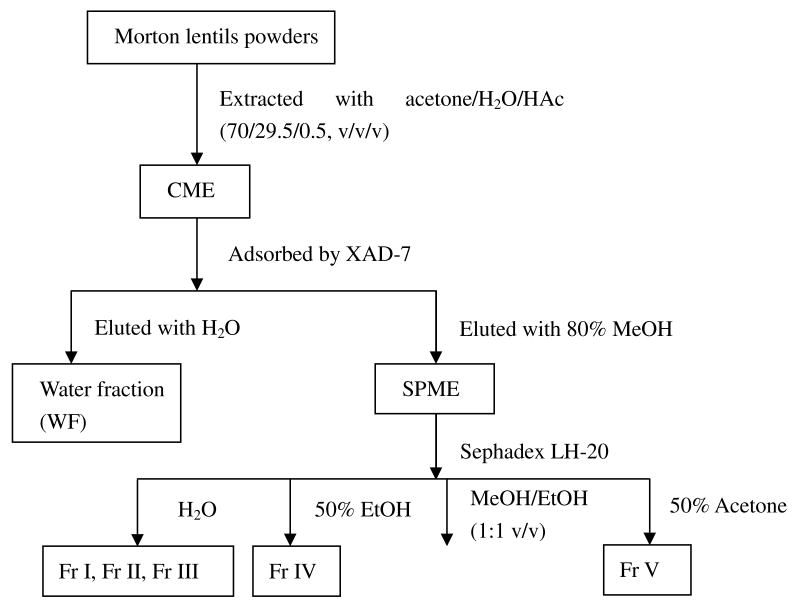
The experimental protocol used for the extraction and fractionation of CME is shown in Figure 1. Morton powders (200 g) were extracted with a solvent mixture (acetone/water/acetic acid 70:29.5:0.5, v/v/v) with a solid to solvent ratio of 1:10 (w/v), and subsequently placed on a magnetic stirrer (Thermolyne, Dubuque, IA) at room temperature for 12 h. The extract was filtered through Whatman No. 1 filter paper in a Buchner funnel. The residues were re-extracted twice under the same conditions, and all of the supernatants were combined and concentrated to a small volume at 40 °C using a rotary evaporator (Labconco Co., Kansas City, Mo) under vacuum. Then the CME was obtained by lyophilizing the concentrated extract and stored at -20 °C until use.
Figure 1.
The flow diagram of extraction and fractionation of Morton lentils.
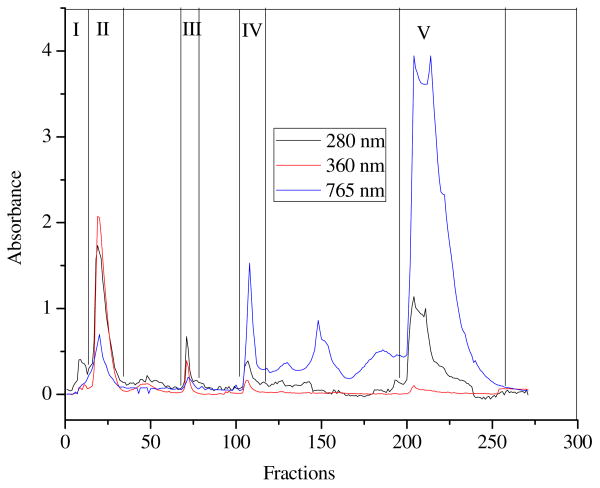
Two methods were used consecutively for the fractionation of phenolic compounds from CME: adsorption-desorption over a macroporous XAD-7 resin and Sephadax-LH 20 column chromatography (11). Four g of CME was suspended in 20 mL of water by vortexing vigorously. The suspension was centrifuged to remove the insoluble part and the supernatant was filtered to get a clear solution. The residue was suspended in water twice, and all the supernatants were combined. The clear solution was poured in a column previously packed with a macroporous resin XAD-7 (column of 20 × 1.6 cm, i. d., bed volume (BV) = 33.5 mL). The solution was pumped down through the column at a speed of 1.8 bed volumes/h (BV/h). The resin was washed with 2 BV of distilled water to remove the sugars, organic acids and other water-soluble compounds (water eluate). The 80% methanol was used to elute the phenolic compounds at a speed of 3.6 BV/h. The eluate was rotary-evaporated under vacuum to remove solvents, then freeze-dried to yield semi-purified Morton extract (SPME). A quantity of 0.3 g SPME was re-dissolved in water, and the obtained solution was further fractionated over a Sephadex LH-20 column (35 × 2.6 cm, i. d., BV = 185 mL). The column was eluted successively with H2O (900 mL), 50% aqueous ethanol (600 mL), ethanol/methanol (1:1, v/v, 400 mL), and 50% aqueous acetone (800 mL) at a flow rate of 2 mL/min, and 270 fractions of 10 mL each fraction were collected. The absorbance of each fraction was determined at 280 and 360 nm, using a Multiskan Spectrum microplate reader (Thermo Electron Corporation, Asheville, NC). Moreover, each fraction was reacted with Folin–Ciocalteau reagent, and the absorbance of the obtained solution was determined at 765 nm. The fractions were combined into five fractions (Fr I to V) according to the peaks as shown in Fig 2.
Figure 2.
Elution curve of fractionation of semi-purified Morton extract over Sephadex LH-20.
10 mL per fraction was collected. Each fraction was monitored at 280 and 360 nm, and each fraction was also detected at 765 nm after reacted with Folin-Ciocaltaeu reagent. Fractions were combined according to their absorbance.
Determination of phenolic substances
Determination of total phenolic content (TPC)
Total phenolic in all samples was determined with Folin–Ciocalteau assay (12) with minor modifications using gallic acid as a standard phenolic compound. Briefly, the freeze-dried samples (50 mg) were dissolved in 5 mL of 50% methanol, then 5 μL of appropriately diluted extracts or standard gallic acid solutions (31.25, 62.5, 125, 250, 500, and 1000 μg/mL) were mixed with 195 μL of distilled water in a well of 96-well plate, then 25 μL of Folin–Ciocalteau reagent solution was added. After 6 min, 75 μL of 7% Na2CO3 was added and mixed gently. The reaction mixture was kept in dark for 2 h and its absorbance was measured at 765 nm against blank solution, which was prepared by the same procedure described above except that extract solution was substituted by 5 μL of water, using the microplate reader. The TPC was expressed as mg gallic acid equivalents (mg GAE/g).
For determining the absorbance of each fraction at 765 nm after reacting with Folin–Ciocalteau reagent, the same procedure described above was employed except that 40 μL of fractions and 160 μL of H2O were mixed in a well, and the elution curve was obtained by plotting the absorbance at 765 nm against the numbers of fractions.
Determination of total flavonoid content (TFC)
Total flavonoids in the extracts were determined using a slightly modified colorimetric method described previously (13). A 30 μL aliquot of appropriately diluted sample solution was mixed with 180 μL of distilled water in a well of 96-well plate, and subsequently 10 μL of a 5% NaNO2 solution was added. After 6 min, 20 μL of a 10% AlCl3 solution was added and allowed to stand for 6 min, then 60 μL of 4% NaOH solution was added to the mixture and stood for another 15 min. Absorbance of the mixture was determined at 510 nm versus a prepared water blank using a Multiskan Spectrum microplate reader. (+)-Catechin was used as standard compound for the quantification of total flavonoids. All values were expressed as milligrams of catechin equivalents per 1 g sample (mg CE/g sample).
Determination of condensed tannin content (CTC)
The CTC in the extracts and its fractions was determined using the modified vanillin assay (14). Ten μL of appropriately diluted sample solution was mixed with 200 μL of 4% vanillin solution (in methanol) in a well of 96-well plate, and then 100 μL of concentrate HCl was added and mixed. After 15 min, the absorbance of the mixture was determined at 500 nm against a blank solution, which was prepared by the same procedure described above except that extract solution was substituted by 10 μL of water. Different concentrations of (+)-catechin ranged from 31.25 to 1000 μg/ml was used as standard compound for the quantification of total condensed tannins. All values were expressed as milligrams of catechin equivalents per 1 g sample (mg CE/g).
Determination of Antioxidant Activity
DPPH free radical scavenging activity
The radical scavenging activity of the extracts and fractions against DPPH free radical was measured using the method of Brand-Williams (15), slightly modified as follows: 10 μL of appropriately diluted samples or Trolox solutions (31.25, 62.5, 125, 250, 500, 750, and 1000 μM) were added to 190 μl of DPPH solution (final concentration was 0.1 mM in methanol) in a well of 96-well plate. The mixture was shaken gently and left to stand at room temperature in the dark for 30 min. Thereafter, the absorbance at 517nm was measured against methanol using a Multiskan Spectrum microplate reader. The DPPH radical scavenging activity of extracts was calculated from the standard curve of Trolox and expressed as micromoles of Trolox equivalents (TE) per gram of sample (μmol TE/g).
To determine the IC50 of samples on DPPH, four concentrations ranging from 0.00625 to 5 mg/mL (concentrations depended on the type of samples) were used. Controls containing methanol instead of samples were made. Ascorbic acid, vitamin E, and Trolox were used as positive controls. The inhibition of the DPPH radical by the samples was calculated according to the following formula: DPPH scavenging activity (%) = (1 - Abs. of sample/Abs. of control) × 100. The percentage of scavenging activity was plotted against the sample concentration to obtain the IC50, defined as the concentration of samples necessary to cause 50% inhibition.
Determination of Trolox Equivalent Antioxidant Capacity (TEAC)
This assay was performed as reported previously with slight modification (16). In brief, ABTS radical cations were prepared by mixing equal volume of ABTS (7 mM in H2O) and potassium persulfate (4.9 mM in H2O), and the solution was stood in the dark for 12 – 16 h at room temperature, then the above solution was filtered and diluted with 80% ethanol to an absorbance of about 0.7 at 734 nm. Ten μL of appropriately diluted samples were added to 190 μL of ABTS solution in a well of 96-well plate, and the absorbance were recorded at 734 nm after 30 min incubation at room temperature. Trolox was used as standard, and standard calibration curve was obtained for Trolox at concentrations of 15.63, 31.25, 62.5, 125, 250, and 500 μM. The TEAC of samples was calculated from the standard curve of Trolox and expressed as micromoles of Trolox equivalents (TE) per gram of sample (μmol TE/g). The scavenging activity of different concentrations of extracts and fractions against ABTS•+ radical were also measured to calculate the IC50, and the procedure was similar to the DPPH scavenging method described above.
FRAP assay
The ferric reducing antioxidant power assay was performed as previously described by Benzie and Strain (17). This method was developed to measure the ferric reduction ability of plasma at a low pH. When the ferric 2,4,6-tripyridyl-s-triazine complex (Fe3+-TPTZ) is reduced to the ferrous form (Fe2+-TPTZ), an intense blue color is developed. Briefly, The FRAP reagent was prepared by mixing 10 volumes of 250 mM acetate buffer (pH 3.6), with one volume of 10 mM TPTZ in 40 mM HCl and with one volume of 20 mM FeCl3·6 H2O. A total of 10μL of properly diluted samples and 30 μL of distilled water were added to 260 μL of freshly prepared FRAP reagent in a well of 96-well plate. The mixture was incubated at 37 °C throughout the reaction. After eight min, the absorbance was read using a Multiskan Spectrum microplate reader at 593 nm against reagent blank. The FRAP value was calculated and expressed as millimoles of Fe2+ equivalents per 100 g of sample (mmol Fe2+ equivalents/100 g) based on a calibration curve plotted using FeSO4·7H2O as standard at a concentration ranging from 0.125 to 2 mM.
ORAC assay
The determination of ORAC was carried out according to Prior and others (18) with slight modifications. A Fluostar Optima plate reader (BMG Labtech, Durham, NC) equipped with an incubator and two injector pumps, and fluorecsein as the probe and AAPH as the radical generator, were used. The analysis was performed using polystyrene 96-well microplates (flat bottom, Nalge Nunc International, Denmark), in which 20 μL of appropriately diluted extract, blank or Trolox were mixed with 200 μL of working fluorecsein solution (1.08 × 10-4 mM in phosphate buffer, pH 7.0) and incubated for 10 min at 37 °C in the built-in incubator. Subsequently, 20 μL of AAPH (43.2 mg/mL in PBS buffer) solution were pumped into the microplate to initiate the reaction. The plate was shaken for 30 s, and the fluorecsein was recorded for 60 cycles with 40 s per cycle with an excitation wavelength of 485 nm and an emission wavelength of 535 nm. All the samples were laid out in a “forward-then-reverse” manner and analyzed in duplicate. All fluorescence measurements were expressed relative to the initial reading. Final results were calculated based on the difference in the area under the fluorescence decay curve between the blank and each sample. The area under curve (AUC) was calculated as follows: AUC = [0.5 + (R2/R1 + R3/R1 + R3/R1 +……+ Rn/R1)] × CT, where R1 was the fluorescence reading at the initiation of the reaction, Rn was the reading of last measurement, and CT was cycle time in minutes. The net AUC was obtained by subtracting the AUC of the blank from that of a sample or standard. The final ORAC values were calculated as trolox equivalents per gram sample (μmol TE/g) using a standard curve prepared with 6.25 - 50 μM trolox.
HPLC-DAD and HPLC-MS Analysis of Phenolic Compounds
HPLC-DAD analysis was performed on an Agilent 1200 series HPLC systems equipped with a G13798 degasser, G1312A binary pump, G1329A autosampler, and G1315D diode array detector (Agilent Technologies, Santa Clara, CA). HPLC separation was achieved using a Zorbax Stablebond Analytical SB-C18 column (250 × 4.6 mm, 5 μm, Agilent Technologies, Rising Sun, MD) at 40 °C. Elution was performed using mobile phase A (0.1% TFA aqueous solution) and mobile phase B (methanol), and samples (20 μL) were eluted at a flow rate of 0.7 mL/min. The UV-vis spectra were scanned from 220 to 600 nm on a DAD with detection wavelengths of 270 nm. The solvent gradient in volumetric ratios was as follows: 5-30% B over 50 min. The solvent gradient was held at 30% B for an additional 15 min and increased to 100% B at 66 min. The solvent gradient was held at 100% B for an additional 10 min to clean up the column, followed by re-equilibration of the column for 5 min with 95% A and 5% B before the next run. Identification of phenolic compounds was made either by comparison of their retention time, UV and MS spectra with available standards or by LC/MS and MS/MS studies with the spectra consistent with published data [30-31].
LC/MS was performed on an Agilent 6100 ion trap mass spectrometer equipped with ESI interface (LC/MSD, Trap SL, Agilent Technologies, Santa Clara, CA). The HPLC separation conditions were almost the same as HPLC-DAD except that the TFA in mobile phase was substituted by acetic acid. The mass spectrometer was operated in both negative and positive ion mode under the following conditions: nebulizer pressure, 20 psi; dry gas (N2), 8 L/min; dry temperature, 325 °C; capillary voltage, 3500 v. The mass spectra were recorded in the scale from m/z 100 to 2500. The MS/MS spectra of interested ions were obtained using Smart Fragmentation mode: cut-off 15% of precursor mass, start amplitude 30%, end amplitude 200%, and other MS conditions were the same as used in LC-MS.
Statistical Analysis
Data were expressed as mean ± standard deviation of triplicate measurements. The data were statistically analyzed using statistical software, SAS Version 9.1, (SAS institute Inc. Cary, NC). One-way analysis of variance (ANOVA) and Pearson correlation coefficients were conducted, and significant difference was defined at p < 0.05.
Results and Discussions
Extraction, fractionation, and phytochemical contents
Our previous study confirmed that acidic aqueous acetone (acetone/H2O/HOAc, 70/29.5/0.5, v/v/v) was the best systems for extraction phenolics from lentils since the solvent systems gave rise to the highest TPC and antioxidant activity (19). The yield of CME was 10.1%, which is much higher than that of green lentils and red lentils (5.4 and 5.3%, respectively) reported by Karamac et al. (10). The difference might be attributed to the genotype of lentils and different extraction method. The TPC, TFC, and CTC of the Crude Morton extract were 70.0 mg GAE/g, 30.0 mg CE/g and 61.6 mg CE/g, respectively. Lentils possessed the highest concentrations of TP among apples, cherries, plums, broccoli, cabbages, grapes, dry beans, onions, and potatoes. The TPC in lentils in the present study was 6.93 mg GAE/g lentil powder, which was in the same range as the contents reported by Xu and Chang (7), and higher than that of other fruits and vegetables.
Adsorption on macroporous resin and then desorption by aqueous methanol or ethanol is a popular method for the concentration of polyphenols or recovery natural antioxidants, and are extensively used in food, pharmaceutical, and cosmetic industry. The purification is mainly through the adsorption capacity of resins for compounds with different molecular weight, polarity, or shape of the molecules in the solution, which leads to differences in affinity for the resins (20). After purification with resin, the yield of water eluate (WE) and 80% MeOH eluate (semipurified Morton extract, SPME) was 73.1% and 11.0%, respectively. Although the yield of WE was much higher than that of SPME, the TPC (4.9 mg GAE/g) in WE was almost 80 times lower than that of SPME (377.2 mg GAE/g). Therefore, the WE fraction was not taken into account in the following study. Almost all phenolic compounds was adsorbed on the macroporous resin and desorbed by 80% MeOH, which demonstrated that this method was effective in removing organic acids, sugars, and proteins from crude extracts. The TPC, TFC, and CTC in SPME was increased by 5.4, 6.8, 5.2 folds compared to those in the crude extract (Table 1).
Table 1.
The yield, TPC, TFC, and CTC of extracts and fractions of Morton lentils.
| Yield (%) |
TPC (mg GAE/g) |
TFC (mg CE/g) |
CTC (mg CE/g) |
|
|---|---|---|---|---|
| CME | 10.1 # | 70.0 ± 2.2 f | 30.0 ± 0.7 d | 61.6 ± 2.7 d |
| SPME | 11.0 $ | 377.2 ± 8.6 c | 202.7 ± 24.3 b | 319.5 ± 2.9 c |
| Fr I | 4.7 * | 9.0 ± 0.7 g | 0.1 ± 0.0 d | nd |
| Fr II | 10.0 * | 111.8 ± 7.4 e | 5.9 ± 1.1 d | nd |
| Fr III | 0.7 * | 262.6 ± 19.6 d | 88.7 ± 5.4 c | 96.5 ± 9.4 d |
| Fr IV | 3.3 * | 434.4 ± 25.9 b | 367.7 ± 21.3 a | 546.8 ± 22.1 b |
| Fr V | 41.0 * | 633.3 ± 25.8 a | 193.9 ± 5.2 b | 744.5 ± 62.2 a |
based on Morton lentils powders,
based on CME,
based on SPME. GAE, gallic acid equivalents; CE, catechin equivalents. nd: not detected. Results were expressed as means ± standard deviation (n = 3), values with different letters within a column were significantly different (p < 0.05).
Adsorption to Sephadex LH-20 in aqueous ethanol and selective debinding with aqueous acetone is an established method for separating tannins from non-tannin phenolics (11). The SPME was further fractionated using a Sephadex LH-20 column, which was eluted successively with H2O, 50% ethanol, ethanol/methanol (1:1, v/v), and 50% acetone (Figure 1). Five fractions were obtained from this column according to their absorbance at 280, 360, and 765 nm (Figure 2), which represented a total recovery of 59.7% of the SPME applied to the column. Fr V, eluted with 50% acetone, and Fr II, eluted with water, were the main fractions. The yield of fractions and their TPC, TFC, and CTC were shown in Table 1. Fr I contained almost no phenolic compounds. The highest TPC was found in Fr V, contained 633.3 mg GAE/g followed by Fr IV, Fr III, and Fr II, which contained 434.4, 262.6, and 111.8 mg GAE/g, respectively. In the case of flavonoids content, Fr IV contained the highest TFC (367.7 mg CE/g), which showed that this fraction, eluted by 50% EtOH, was mainly comprised of flavonoids other than phenolic acids or condensed tannins. The CTC, measured using the vanillin/HCl method, was determined among all fractions. Since condensed tannins are relatively high-molecular-weight compounds and can be eluted by acetone with an appropriate polarity. Fr I and Fr II contained no condensed tannins. The CTC in Fr V was the highest (744.5 mg CE/g), whereas Fr III and Fr IV showed positive color reaction with vanillin/HCl reagent, and it seemed to contain large amounts of condensed tannins. However, the color reaction might be caused by the reaction between catechin or other monomeric flavanols and vanillin/HCl reagent (21), thus overestimating the content of condensed tannins in Fr III and Fr IV.
Antioxidant activity of extracts and fractions
The antioxidant activity can not be evaluated by only a single method due to the complex nature of phytochemicals, and the antioxidant activity determination is reaction-mechanism dependent. Therefore, it is important to employ multiple assays to evaluate the antioxidant activity of plant extract or phytochemicals (22). Numerous antioxidant methods have been developed to evaluate antioxidant activity and to explain how antioxidants function. Of these, total antioxidant activity, reducing power, DPPH assay, and ORAC are most commonly accepted assays to evaluate antioxidant activity of food matrix. Therefore, a serials of assays including DPPH scavenging activity, ABTS•+ scavenging activity, FRAP, and ORAC were used for determination of antioxidant activity of extracts and fractions of Morton lentils.
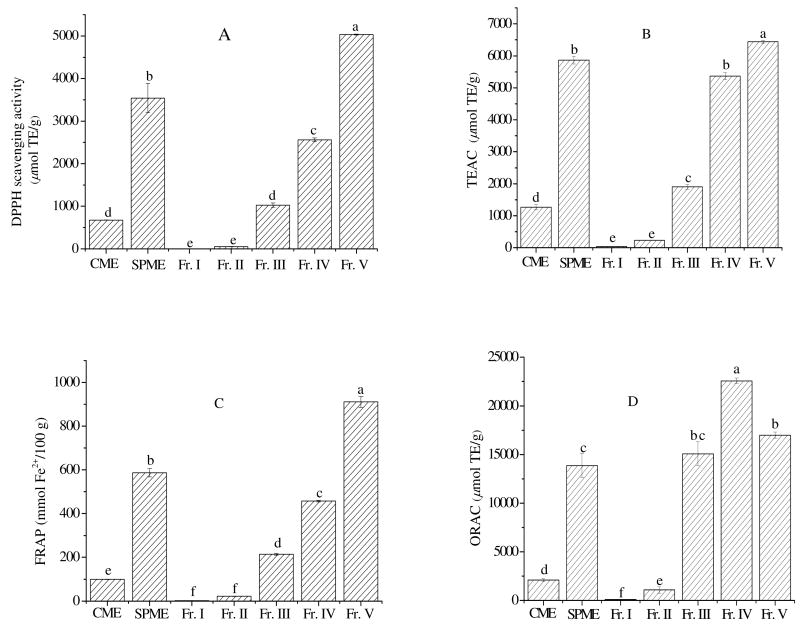
DPPH radical is a stable organic free radical. When accepting an electron or hydrogen in the presence of a hydrogen-donating antioxidant, it can be reduced to a non-radical form DPPH-H. Because it can accommodate many samples in a short period and is sensitive enough to detect active ingredients at low concentrations, it has been extensively used for screening antiradical activities of fruit and vegetable juices or extracts (22). The DPPH scavenging activity of all extracts and fractions was expressed as Trolox equivalents and IC50, which was defined as the necessary concentration at which the radicals generated by the reaction systems were scavenged by 50%. The results were shown in Figure 3A and Table 2. All extracts and fractions except for Fr I and Fr II possessed good DPPH radical scavenging activity. Fr V presented highest DPPH scavenging activity (5031.6 μmol TE/g), followed by SPME (3541.3 μmol TE/g), Fr IV (2562.8 μmol TE/g), Fr III (1027.0 μmol TE/g), and finally by CME (677.8 μmol TE/g). Regarding the IC50 values, it was not possible to determine the IC50 of Fr I and Fr II at the range of concentrations tested in the present study. At the highest concentration (5 mg/ml), only 10.4% and 26.8% of DPPH radicals were scavenged by Fr I and Fr II, respectively. The DPPH scavenging activity was in the following order: ascorbic acid > Fr V > SPME > Vitamin E > trolox > Fr IV > Fr III > CME. Ascorbic acid, a strong antioxidant, exhibited the highest DPPH scavenging activity as anticipated. In the case of fractions, Fr V, which had the highest levels of total phenolics, had the lowest IC50 value similar to that of ascorbic acid, and its radical scavenging activity overmatched α-tocopherol and trolox.
Figure 3.
Antioxidant activity of extracts and fractions of Morton lentils.
(A), DPPH scavenging activity; (B), TEAC; (C), FRAP; (D), ORAC. Results were expressed as means ± standard deviation (n = 3), bars sharing different letters were significantly different (p < 0.05).
Table 2.
IC50 (mg/ml) of extracts and fractions of Morton lentils against DPPH and ABTS free radicals.
| DPPH | ABTS | |
|---|---|---|
| CME | 0.781 ± 0.011 a | 0.282 ± 0.002 b |
| SPME | 0.144 ± 0.001 c d | 0.053 ± 0.001 c |
| Fr I | > 5 | > 5 |
| Fr II | > 5 | 1.171 ± 0.275 a |
| Fr III | 0.643 ± 0.183 b | 0.116 ± 0.011 b c |
| Fr IV | 0.213 ± 0.006 c | 0.044 ± 0.002 c |
| Fr V | 0.095 ± 0.002 d | 0.031 ± 0.001 c |
| Ascorbic acid | 0.086 ± 0.006 d | 0.062 ± 0.001 c |
| α-Tocopherol | 0.158 ± 0.003 c d | 0.124 ± 0.024 b c |
| Trolox | 0.164 ± 0.003 c d | 0.092 ± 0.002 c |
Results were expressed as means ± standard deviation (n = 3), values with different letters within a column were significantly different (p < 0.05).
The TEAC assay is one of the popular indirect methods of determining the antioxidant activity of compounds or extracts. ABTS•+, a cation free radical soluble in both water and organic media, is produced by reacting ABTS solution with potassium persulfate or metmyoglobin. In the absence of antioxidants, the ABTS•+ radical is rather stable, but it reacts energetically with an H atom donor and is converted into a non-colored form of ABTS (23). The TEAC, expressed as trolox equivalents, was illustrated in Figure 3B. Fr V had the highest TEAC value (6436.5 mg TE/g), while Fr I exhibited the lowest value (41.5 mg TE/g). Interestingly, the trolox equivalents of extract and fractions determined by TEAC were almost 2 times higher than that of corresponding samples determined by DPPH assay. Antioxidant compounds scavenging ABTS radical at higher level compared to DPPH radical were also reported by Sachindra et al. (23). In terms of IC50, Fr V had the lowest value, which demonstrated Fr V possessed the strongest radical scavenging activity against ABTS•+. Moreover, the ABTS •+ scavenging activity of Fr V was even higher than that of ascorbic acid. The IC50 calculated from regression equation showed the following order: Fr V > Fr IV > SPME > ascorbic acid > Fr III > Vitamin E > trolox > CME > Fr II. However, the differences in IC50 among Fr V, Fr IV, SPME, ascorbic acid, trolox, Vitamin E, and Fr III were not significant. The ABTS method was more sensitive than the DPPH assay when measuring the antioxidant activity of water-soluble proteins and peptides, partly due to the differences of radical's solubility and diffusivity in the reaction medium (24).
Concerning the ferric reducing capacity of the extracts and fractions, the trend was almost the same as those of the DPPH assay and TEAC assay. The FRAP value was presented in Figure 3C. Fr V possessed the highest reducing power (910.8 mmol Fe2+ equivalents/100 g), followed by SPME (586.7 mmol Fe2+ equivalents/100 g), Fr IV (457.5 mmol Fe2+ equivalents/100 g), Fr III (214.0 mmol Fe2+ equivalents/100 g), CME (99.6 mmol Fe2+ equivalents/100 g), and Fr II (21.8 mmol Fe2+ equivalents/100 g). Fr I almost did not exhibit any reducing power.
Finally, the ORAC value of the extracts and fractions were determined. ORAC is perhaps the most commonly used antioxidant activity assay, it depends on the free radical damage to a fluorescent probe to result in a downward change of fluorescent intensity. Antioxidants can compete with free radicals, thus leading to the inhibition of decay of fluorescent probe. The ORAC assay can provide information on a sample's ability to scavenge peroxyl radical through a hydrogen atom transfer mechanisms (18). In the case of ORAC, the trend was a little different from that of free radicals scavenging activity and ferric reducing power. Fr V, which possessed the highest free radicals scavenging activity and reducing power, had a little lower ORAC than that of Fr IV. This observation was in accordance with the conclusion that the correlation coefficient between CTC and ORAC was relatively lower than that between TFC, TPC and ORAC (12). The order of ORAC of the extracts and fractions was: Fr IV > Fr V > Fr III > SPME > Fr II > CME > Fr I (Figure 3D).
Fr V exhibited high antioxidant activity in all assays. Considering the relatively high yield of Fr V, the antioxidant activity of Morton lentils may be attributed largely to the condensed tannins. However, other phytochemicals such as catechin and flavonols also contributed to the total antioxidative capacity of Morton lentils. Our results are consistent with those of Amarowicz (8) and Alasalvar (25), who reported that high-molercular-weight or condensed tannin-rich fractions from adzuki bean and hazenut skin exhibited the highest radical scavenging activity and antioxidant activity. Phenolic hydroxyl groups attached to the flavanol skeleton (26), and the presence of an interflavonoid link (27) might play an important role in the higher radical scavenging activity and antioxidant activity of condensed tannins.
Phenolic compound profiles of fractions by HPLC-MS
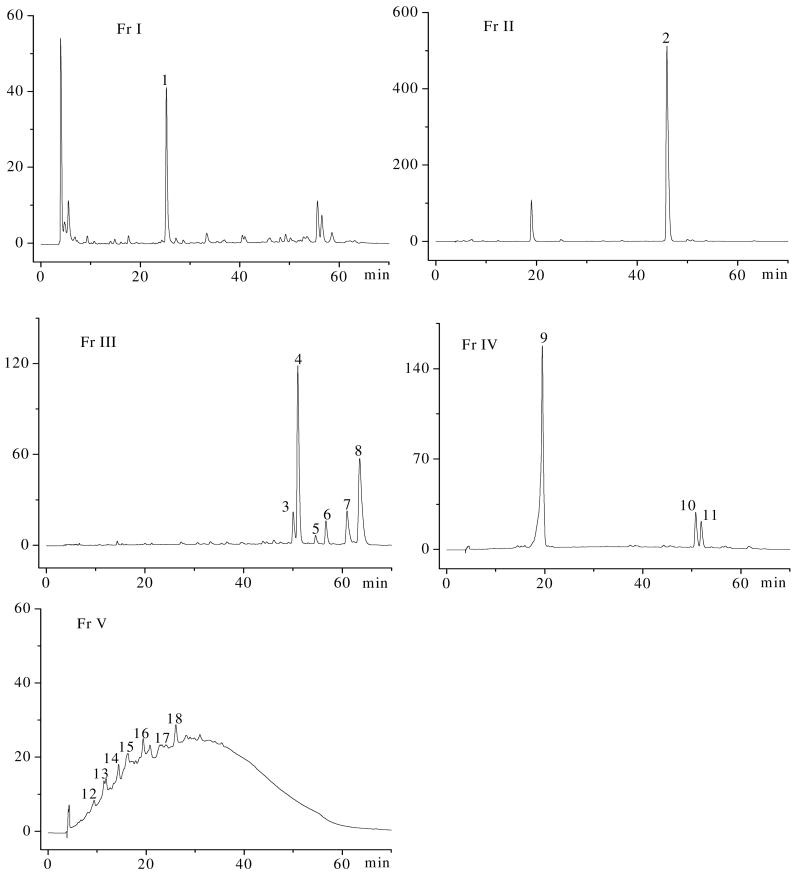
The SPME was separated over Sephadex LH-20 into five main fractions, which could be analyzed by HPLC-MS to identify the phenolic compound profiles through UV spectra and mass spectra. The HPLC chromatographic profiles of the fractions recorded at 270 nm are shown in Figure 4, and the UV spectra, mass spectra, as well as the tentative identification of phenolic compounds are summarized in Table 3.
Figure 4.
HPLC profiles of phenolic compounds in five fractions of Morton lentils recorded at 270 nm.
Table 3.
The HPLC retention times, UV and ESI/MS data, and tentative identification of phenolic compounds in fractions of Morton lentils.
| Fractions | Peak No. |
Rt (min) |
MS | UV (λmax nm) |
Tentative identification | |
|---|---|---|---|---|---|---|
| negative | positive | |||||
| Fr I | 1 | 25.2 | 446.9 | 159.3, 265.2, 467.2 | 267 | n.i. |
| Fr II | 2 | 45.9 | 901, 285.3 | 265, 348 | kaempferol tetraglycoside | |
| Fr III | 3 | 50.0 | 755.7 | 757.5, 287.9 | 269, 348 | kaempferol triglycoside |
| 4 | 51.0 | 755.5 | 757.5, 287.9 | 269, 348 | kaempferol triglycoside | |
| 5 | 54.6 | 725.3 | 727.4, 287.5 | 272, 347 | kaempferol triglycoside | |
| 6 | 56.7 | 597.4, (579, 561, 507, 489, 477, 447, 387, 357) | 599.4 | 230, 288, 329 sh | 3′,5′-di-C-β-glucopyranosyl phloretin | |
| 7 | 61.0 | 523.8, 1047.7 | 547.5, 1049.6 | 267, 321, 351 sh | flavonoid derivative | |
| 8 | 63.5 | 523.7, 1047.7 | 547.5, 1049.7 | 270, 319, 351 sh | flavonoid derivative | |
| Fr IV | 9 | 19.5 | 451.2, 903.6 | 291.2, 453.1 | 279 | catechin-3-O-glucoside |
| 10 | 50.8 | 609.8 | 611.1, 449.2, 287.2 | 268,349 | kaempferol-di-hexoside | |
| 11 | 51.9 | 609.3 | 611.1, 449.2, 287.2 | 268,349 | kaempferol-di-hexoside | |
| Fr V | 12 | 9.0 | 897.5 | 277 | prodelphinidin trimer | |
| 13 | 11.5 | 882.3 | 278 | digallate procyanidin dimer | ||
| 14 | 14.1 | 880.9, 1169.4 | 278 | digallate procyanidin trimer | ||
| 15 | 16.0 | 881.7, 1169.1 | 278 | digallate procyanidin trimer | ||
| 16 | 19.3 | 1153.4 | 278 | procyanidin tetramer | ||
| 17 | 24.3 | 593.8, 880.9, 1169.2 | 277 | prodelphinidin tetramer | ||
| 18 | 26.2 | 1153.3 | 278 | procyanidin tetramer | ||
n.i., not identified.
Data in parentheses was the fragment ions in MS/MS.
An unidentified compound was detected in the Fr I. It yielded [M+H]+ at m/z at 467, [M-H-H2O]- at m/z 447, and positive fragment ions at m/z 265, 159.
A predominant compound was presented in the Fr II. Peak 2 exhibited absorption maximum at 265 and 348 nm, which are considered as the typical UV spectra of flavone derivatives. It had the pseudo-molecular ions at m/z 901 [M-H]-, and fragment ions at m/z 285 [aglycone -H]-, which suggested the aglycone was kaempferol. This compound was tentatively identified as kaempferol tetraglycoside, which included two units of hexose and two units of deoxyhexose, as reported by Taylor et al. (28). Kaempferol glycosides existed in lentils very commonly, and many kinds of kaempferol glycosides were reported in the literatures (28, 29).
Six major peaks (3-8) were detected in the Fr III. All compounds showed similar UV spectra with λmax between 318 and 350 nm and at around 270 nm. Peaks 3 and 4 possessed the same UV and MS spectra. They had the pseudo-molecular ions at m/z 755 [M-H]- and 757 [M+H]+, which further yielded fragment ions at m/z 287 [aglycone +H]+. Compared with peak 2, the loss of 146 amu, which corresponded to the loss of a deoxyhexose sugar, was observed. Thus, both compounds were tentatively identified as the isomers of kaempferol triglycoside, which included two units of hexose and one unit of deoxyhexose. Peak 5 showed pseudo-molecular ion at m/z 725 [M-H]-and 727 [M+H]+, and fragment ions at m/z 287[aglycone +H]+. Compared with peaks 3 and 4, the loss of 30 amu was due to the substitution of one hexose by one pentose. The peak 5 was tentatively identified as kaempferol triglycoside including one hexose, one deoxyhexose, and one pentose units. The UV spectra of peak 6 exhibited absorption maximum at 230 and 288 nm, along with a shoulder at 329 nm, which is similar to the spectra of dihydroxyflavonoid. The negative and positive ESI-MS spectra showed pseudo-molecular ion at m/z 597 [M-H]- and 599 [M+H]+, respectively. MS/MS analysis provided the typical fragment ions of 6,8-di-C-hexosyl flavones, namely, 579 [M-H-18]-, 561 [M-H-18-18]-, 507 [M-H-90]-, 477 [M-H-120]-, 387 [M-H-90-120]-, and 357 [M-H-120-120]-. These ions are indicative for C-glycosylflavonoids (30). The ions at m/z 357 [aglycone + 83] and 387 [aglycone + 113] suggested the aglycone was tetrahydroxydihydrochalcone (MW = 274). The UV and mass spectra were consistent with those reported previously (31). Therefore, peak 6 was identified as 3′,5′-di-C-β-glucopyranosyl phloretin. To our best knowledge, this was the first time to report C-glycosylflavonoid from legumes. Peaks 7 and 8 were identified as isomers of flavonoid derivatives. It showed pseudo-molecular ion at m/z 523 [M-H]-, molecular complex at m/z 1047 [2M-H]-, sodium adducts at m/z 547 [M+Na]+, and molecular complex at m/z 1049 [2M+H]+.
The HPLC chromatogram of the Fr IV showed three main peaks. Peak 9 had the molecular weight of 452 as manifested by the pseudo-molecular ion at m/z 451 [M-H]- and 453 [M+H]+, as well as molecular complex at m/z 903 [2M-H]-. In addition, a fragment ion at m/z 291 [M+H-162]+ due to the loss of glucose was observed. On the basis of UV and MS spectra, this compound was tentatively identified as catechin-3-O-glucoside. This compound was also detected in the seed coat of lentils from Spain (32). Peaks 10 and 11 had almost the same UV and MS spectra. They had the pseudo-molecular ions at m/z 609 [M-H]- and 611 [M+H]+, which further yielded fragment ions at m/z 449 [M+H-162]+ and 287 [M+H-162-162]+. The loss of 162 amu corresponded to the loss of a hexose sugar, and both compounds were tentatively identified as the isomers of kaempferol-di-hexoside.
The HPLC chromatogram of the Fr V was completely different from the above fractions. All peaks had the λmax about 277 nm, and a broad-hump, characteristics for oligomers and polymers (33). In the mass spectrum of peak 12, a negative ion at m/z 897 [M-H]- was observed. It was tentatively identified as prodelphinidin trimer with two units of (epi)gallocatechin and one unit of (epi)catechin. Peak 13 was tentatively identified as digallate procyanidin dimer, which exhibited a pseudo-molecular ion at m/z 882 [M-H]-, corresponded to two units of (epi)catechin gallate. In the case of peaks 14 and 15, a pseudo-molecular ion at m/z 1169 [M-H]- appeared, and they were tentatively identified as isomers of digallate procyanidin trimer with two units of (epi)catechin gallate and one unit of (epi)catechin. A fragment ion at m/z 882 [M-H]- was due to the loss of one unit of (epi)catechin (288 amu). Peaks 16 and 18 were tentatively identified as isomers of procyanidin tetramer with four units of (epi)catechin, since a pseudo-molecular ion at m/z 1153 [M-H]- appeared in the MS spectra. Regarding peak 17, a pseudo-molecular ion at m/z 1169 [M-H]-, as well as fragment ions at m/z 881 [M-H]- and m/z 593 [M-H]- corresponded to the loss of (epi)catechin, were exhibited. Thus, peak 17 was tentatively identified as prodelphinidin tetramer with three units of (epi)catechin and one unit of (epi)gallocatechin.
Correlations among antioxidant activity and phytochemical contents
Many previous studies reported that the antioxidant capacity of plant extracts could be attributed to the total phenolic content (7, 19, 34). To analyze the correlative relationships among total antioxidant activity (DPPH, ABTS, FRAP, and ORAC) and TPC, as well as TFC and CTC, a Pearson correlation analysis was conducted, and the results were shown in Table 4.
Table 4. Pearson correlation coefficients (R2) among the antioxidant activity and phytochemical contents.
| TFC | CTC | DPPH | TEAC | FRAP | ORAC | |
|---|---|---|---|---|---|---|
| TPC | 0.6215* | 0.9094** | 0.8993* | 0.8768** | 0.9293** | 0.7605* |
| TFC | 0.6706* | 0.5055 | 0.7388* | 0.5016 | 0.8344** | |
| CTC | 0.8637** | 0.8496** | 0.8834** | 0.6440* | ||
| DPPH | 0.9273** | 0.9948** | 0.5348 | |||
| TEAC | 0.9103** | 0.6984* | ||||
| FRAP | 0.5616* |
Correlation is significant at the 0.05 level (two-tailed);
Correlation is significant at the 0.01 level (two-tailed) (n = 21).
Significant correlations were found among all phytochemical contents. The strongest correlations was found between CTC and TPC (R2 = 0.91, p < 0.01), which suggested that condensed tannins could contribute a lot to the total phenolic content in lentils.
By comparing coefficients among antioxidant activity and phytochemical contents, significant correlations (p < 0.05 or p < 0.01) existed between various parameters except for that between DPPH and TFC, and between FRAP and TFC. Namely, considering DPPH scavenging activity, a good correlation was found with TPC (R2 = 0.89) and an inferior one with CTC (R2 = 0.86). TEAC correlated well with TPC (R2 = 0.87), followed with CTC (R2 = 0.84) and TFC (R2 = 0.73). For FRAP, the correlation coefficient was 0.92, and 0.88 with TPC, and CTC, respectively. For ORAC, the highest correlation was found with TFC (R2 = 0.83), whereas the correlation between ORAC and CTC was the lowest (R2 = 0.64). The strongest free radical scavenging activity and reducing power was detected in Fr V, which contained the highest TPC and CTC. These results suggested that total phenolics, especially condensed tannins, were mostly responsible for the antioxidant activity and reducing power of Morton lentils.
Among antioxidant activity assays (DPPH, TEAC, FRAP, and ORAC), they all showed significant correlations, suggesting that all antioxidant activity assays were reliable and interchangeable. For example, DPPH correlated well with FRAP (R2 = 0.99, p < 0.01), TEAC (R2 = 0.92, p < 0.01), and ORAC (R2 = 0.53). Whereas TEAC correlated well with FRAP (R2 = 0.91, p < 0.01) and ORAC (R2 = 0.69, p < 0.05), and FRAP correlated significantly with ORAC (R2 = 0.56, p < 0.05). The correlation coefficients among the ORAC assay and other assays (lower than 0.7) were generally lower than that among DPPH, TEAC, and FRAP assays (greater than 0.9), which was in agreement with that by a regression analysis (47). The ORAC assay takes into account the kinetic action of antioxidants, which might explain the discrepancy between the results obtained with the ORAC assay and those obtained with other colorimetric assays.
In summary, a semi-purified Morton extract was obtained by adsorption-desorption on macroporous resin, and it was further fractionated into five fractions over Sephedax LH-20. Phenolic compounds identified in those fractions comprised a range of low-molecular-weight flavonol glycosides, flavanol glycosides, C-glycosyl dihydrochalcone, and high-molercular-weight condensed tannins. The Fr V, whose compositions were mainly condensed tannins, exhibited the highest antioxidant activities in different assays used for antioxidant efficacies, and possessed the highest total phenolic content. The present study also indicated that antioxidant activity of the Morton lentils extract and its fractions correlated well with their phenolic contents. On the basis of their antioxidant activity, the Moton lentils extract and its condensed tannins fraction might be developed for the food industry as dietary supplement for health promotion. Further researches are required for determining the antioxidant activity of Morton lentils extract in vivo.
Acknowledgments
This work was supported by grants No. 13600 and 15053 from USDA CSREES-University of Idaho CSFL Research Program.
Literatures Cited
- 1.Thavarajah D, Thavarajah P, Sarker A, Vandenberg A. Lentils (Lens culinaris Medikus subspecies culinaris): A whole food for increased iron and zinc intake. J Agric Food Chem. 2009;57:5413–5419. doi: 10.1021/jf900786e. [DOI] [PubMed] [Google Scholar]
- 2.Rochfort S, Panozzo J. Phytochemicals for Health, the Role of Pulses. J Agric Food Chem. 2007;55:798–7994. doi: 10.1021/jf071704w. [DOI] [PubMed] [Google Scholar]
- 3.Xu BJ, Chang SKC. Phenolic Substance Characterization and Chemical and Cell-Based Antioxidant Activities of 11 Lentils Grown in the Northern United States. J Agric Food Chem. 2010;58:1509–1517. doi: 10.1021/jf903532y. [DOI] [PubMed] [Google Scholar]
- 4.Champ MM. Non-nutrient bioactive substances of pulses. Brit J Nutr. 2002;88(Suppl. 3):S307–S319. doi: 10.1079/BJN2002721. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd J, Codde SM, Ford I, Isles CG, Lorimer AR, McFarlane PW, Mckillop JH, Packhard CJ. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 6.Duane WC. Effects of legume consumption on serum cholesterol, biliary lipids, and sterol metabolism in humans. J Lipid Res. 1997;38:1120–1128. [PubMed] [Google Scholar]
- 7.Xu BJ, Chang SKC. Comparative analysis of phenolic composition, antioxidant capacity, and color of cool season legumes and other selected food legumes. J Food Sci. 2007;72:S167–S177. doi: 10.1111/j.1750-3841.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- 8.Amarowicz R, Estrella I, Hernández T, Troszyńska A. Antioxidant activity of extract of adzuki bean and its fractions. J Food Lip. 2008;15:119–136. [Google Scholar]
- 9.Troszynska A, Bednarska A, Latosz A, Kozlowska H. Polyphenolic compounds in the seed coat of legume seeds. Pol J Food Nutr Sci. 1997;47:37–45. [Google Scholar]
- 10.Karamac M, Kosinska A, Rybarczyk A, Amarowicz R. Extraction and chromatographic separation of tannin fractions from tannin-rich plant material. Pol J Food Nutr Sci. 2007;57:471–474. [Google Scholar]
- 11.Lee CH, Krueger CG, Reed JD, Richards MP. Inhibition of hemoglobin-mediated lipid oxidation in washed fish muscle by cranberry components. Food Chem. 2006;99:591–599. [Google Scholar]
- 12.Singleton VL, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- 13.Shin Y, Ryu JA, Liu RH, Nock JF, Polar-Cabrera K, Watkins CB. Fruit quality, antioxidant contents and activity, and antiproliferative activity of strawberry fruit stored in elevated CO2 atmospheres. J Food Sci. 2008;73:S339–S344. doi: 10.1111/j.1750-3841.2008.00857.x. [DOI] [PubMed] [Google Scholar]
- 14.Price ML, Van Scoyoc S, Butler LG. A critical evaluation of the vanillin reaction as an assay for tannin in sorghum-grain. J Agric Food Chem. 1978;26:1214–1218. [Google Scholar]
- 15.Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol. 1995;28:25–30. [Google Scholar]
- 16.Zhu F, Cai YZ, Sun M, Ke J, Lu D, Corke H. Comparison of major phenolic constituents and in vitro antioxidant activity of diverse Kudingcha genotypes from Ilex kudingcha, Ilex cornuta, and Ligustrum robustum. J Agric Food Chem. 2009;57:6082–6089. doi: 10.1021/jf901020h. [DOI] [PubMed] [Google Scholar]
- 17.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 18.Prior RL, Hoang H, Gu LW, Wu XL, Bacchiocca M, Howard L, Hampsch-Woodill M, Huang DJ, Ou BX, Jacob R. Assay for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL) of plasma and other biological and food samples. J Agric Food Chem. 2003;51:3273–3279. doi: 10.1021/jf0262256. [DOI] [PubMed] [Google Scholar]
- 19.Xu BJ, Chang SKC. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J Food Sci. 2007;72:S159–S166. doi: 10.1111/j.1750-3841.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- 20.Silva EM, Pompeu DR, Larondelle Y, Rogez H. Optimisation of the adsorption of polyphenols from Inga edulis leaves on macroporous resins using an experimental design methodology. Sep Purif Tech. 2007;53:274–280. [Google Scholar]
- 21.Schofield P, Mbugu DM, Pell AN. Analysis of condensed tannins: a review. Anim Feed Sci Technol. 2001;91:21–40. [Google Scholar]
- 22.Sánchez-Moreno C. Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci Technol Int. 2002;8:121–137. [Google Scholar]
- 23.Sachindra NM, Sato E, Maeda H, Hosokawa M, Niwano Y, Kohno M, Miyashita K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J Agric Food Chem. 2007;55:8516–8522. doi: 10.1021/jf071848a. [DOI] [PubMed] [Google Scholar]
- 24.Tang X, He Z, Dai Y, Xiong YL, Xie M, Chen J. Peptide fractionation and free radical scavenging activity of zein hydrolysate. J Agric Food Chem. 2010;58:587–593. doi: 10.1021/jf9028656. [DOI] [PubMed] [Google Scholar]
- 25.Alasalvar C, Karamać M, Kosińska A, Rybarczyk Anna, Shahidi F, Amarowicz R. Antioxidant Activity of Hazelnut Skin Phenolics. J Agric Food Chem. 2009;57:4645–4650. doi: 10.1021/jf900489d. [DOI] [PubMed] [Google Scholar]
- 26.Yokozawa T, Chen CP, Dong E, Tanaka T, Nonaka GI, Nishioka I. Study on the inhibitory effect of tannins and flavonoids against the 1,1-diphenyl-2-picrylhydrazyl radical. Biochem Pharmacol. 1998;56:213–222. doi: 10.1016/s0006-2952(98)00128-2. [DOI] [PubMed] [Google Scholar]
- 27.Ursini F, Rapuzzi I, Toniolo R, Tubaro F, Bontempelli G. Methods in enzymology. Vol. 335. Academic Press; New York: Characterization of antioxidant effect of procyanidins; pp. 338–350. [DOI] [PubMed] [Google Scholar]
- 28.Taylor WG, Fields PG, Sutherland DH. Fractionation of lentil seeds (Lens culinaris Medik.) for insecticidal and flavonol tetraglycoside components. J Agric Food Chem. 2007;55:5491–5498. doi: 10.1021/jf0705062. [DOI] [PubMed] [Google Scholar]
- 29.Pivec V, Lachman J, Rehakova V. Flavonoids and saccharides in the seeds of lentil (Lens esculenta Moench.) J UniV Agric, Prague, Fac Agron, Ser A. 1993;55:65–72. [Google Scholar]
- 30.Waridel P, Wolfender JL, Ndjoko K, Hobby KR, Major HJ, Hostettmann K. Evaluation of quadrupole time-of-flight tandem mass spectrometry and ion-trap multiple-stage mass spectrometry for the differentiation of C-glycosidic flavonoid isomers. J Chromatogr A. 2001;926:29–41. doi: 10.1016/s0021-9673(01)00806-8. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa K, Kawasaki A, Omura M, Yoshida T, Ikoma Y, Yano M. 3′,5′-Di-C-β-glucopyranosylphloretin, a flavonoid characteristic of the genus. Fortunella Phytochemistry. 2001;57:737–742. doi: 10.1016/s0031-9422(01)00132-7. [DOI] [PubMed] [Google Scholar]
- 32.Dueñas M, Sun B, Hernández T, Estrella I, Spranger MI. Proanthocyanidin Composition in the Seed Coat of Lentils (Lens culinaris L.) J Agric Food Chem. 2003;51:7999–8004. doi: 10.1021/jf0303215. [DOI] [PubMed] [Google Scholar]
- 33.Jerez M, Sineiro J, Nuñez MJ. Fractionation of pine bark extracts: selecting procyanidins. Eur Food Res Technol. 2009;229:651–659. [Google Scholar]
- 34.Dudonné S, Vitrac X, Coutiére P, Woillez M, Mérillon JM. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J Agric Food Chem. 2009;57:1768–1774. doi: 10.1021/jf803011r. [DOI] [PubMed] [Google Scholar]