Like the nuclear pore complex, FG repeat–containing P-granule proteins interact to help establish a size-exclusion barrier.
Abstract
The immortal and totipotent properties of the germ line depend on determinants within the germ plasm. A common characteristic of germ plasm across phyla is the presence of germ granules, including P granules in Caenorhabditis elegans, which are typically associated with the nuclear periphery. In C. elegans, nuclear pore complex (NPC)–like FG repeat domains are found in the VASA-related P-granule proteins GLH-1, GLH-2, and GLH-4 and other P-granule components. We demonstrate that P granules, like NPCs, are held together by weak hydrophobic interactions and establish a size-exclusion barrier. Our analysis of intestine-expressed proteins revealed that GLH-1 and its FG domain are not sufficient to form granules, but require factors like PGL-1 to nucleate the localized concentration of GLH proteins. GLH-1 is necessary but not sufficient for the perinuclear location of granules in the intestine. Our results suggest that P granules extend the NPC environment in the germ line and provide insights into the roles of the PGL and GLH family proteins.
Introduction
During animal development, somatic cells display ever more restricted developmental potential as they terminally differentiate into mature tissues, whereas germ cells must retain the ability to produce generation after generation of an organism and all of the cell types of each generation. Loss of these attributes of immortality and totipotency in the germ line results in sterility; conversely, acquisition of these attributes by somatic cells are hallmarks of both cancer and regenerative stem cells. A key to understanding how germ cells are regulated resides in the germ plasm, which contains germline determinants (Illmensee and Mahowald, 1974; Ikenishi et al., 1986). Ribonucleoprotein (RNP) aggregates called germ granules are found in the germ plasm of many species (Eddy, 1975; Extavour and Akam, 2003). Although depletion of many germ-granule components results in infertility (Chuma et al., 2009), the importance of the assembly of those components into granules within germ plasm has yet to be determined.
Caenorhabditis elegans germ granules (P granules) are dynamic, moving within the germline cytoplasm much like liquid droplets (Brangwynne et al., 2009). In somatic cells, P granules dissolve in part through protein degradation and autophagy (DeRenzo et al., 2003; Zhang et al., 2009). It was recently proposed that the primary dissolution of P granules that remain in the somatic blastomere after the first embryonic cell division is the result of under-saturation of P-granule components. In contrast, in the germline blastomere the concentration at which soluble P-granule components are saturated decreases, likely through nucleating factors specific to the germ line, resulting in super-saturation and aggregation of P-granule components (Brangwynne et al., 2009). In Drosophila, the fly-specific protein Oskar nucleates Vasa recruitment to germ granules (Ephrussi and Lehmann, 1992). In zebrafish, germ granules are nucleated by the vertebrate-specific Bucky Ball protein (Bontems et al., 2009). This theme is continued in C. elegans, where we show that the worm-specific protein PGL-1 nucleates worm Vasa homologues to form granules.
The DEAD-box helicase Vasa is a germ-granule component that that is conserved across phyla. In C. elegans, the Vasa-related proteins GLH-1, GLH-2, and GLH-4 contain a phenylalanine-glycine (FG) repeat domain. Multiple FG residues separated by 10–15 amino acids are a hallmark of many nuclear pore complex (NPC) proteins or Nups. These FG-rich domains form a cohesive meshwork of filaments through hydrophobic interactions involving the phenylalanines in FG motifs (Ribbeck and Görlich, 2002; Patel et al., 2007). Within NPCs, these interactions create a size-exclusion barrier that prevents diffusion of molecules larger than ∼45 kD between the nucleus and the cytoplasm (Weis, 2007). Several observations suggest that P granules share many characteristics with NPCs. First, the N-terminal FG domains of the GLH proteins contain numerous FG and GFGG residues spaced ∼10 amino acids apart (Kuznicki et al., 2000; Schisa et al., 2001). Second, P granules exhibit a perinuclear distribution and overlie nuclear pore clusters in the germ line (Pitt et al., 2000). Third, RNAi disruption of multiple C. elegans NPC components results in detachment of P granules from the nuclear periphery (Updike and Strome, 2009). Fourth, FG-containing Nups in C. elegans, such as the mRNA export factor DDX-19 and the peripheral Nups NPP-8 (CeNup155) and NPP-10 (CeNup98) colocalize with P granules (Sheth et al., 2010; Voronina and Seydoux, 2010). Fifth, P granules are sites of mRNA export (Sheth et al., 2010).
In this paper we investigate the NPC-like properties of P granules, including the ability of P granules to establish a size-exclusion barrier in the cytoplasm, the sensitivity of P granules to disruption of hydrophobic interactions, and the contribution of individual P-granule components to the nucleation and biogenesis of granules. Our results provide insights into how germ granules form and what function they may serve in the germ line.
Results and discussion
P granules create a size-exclusion barrier
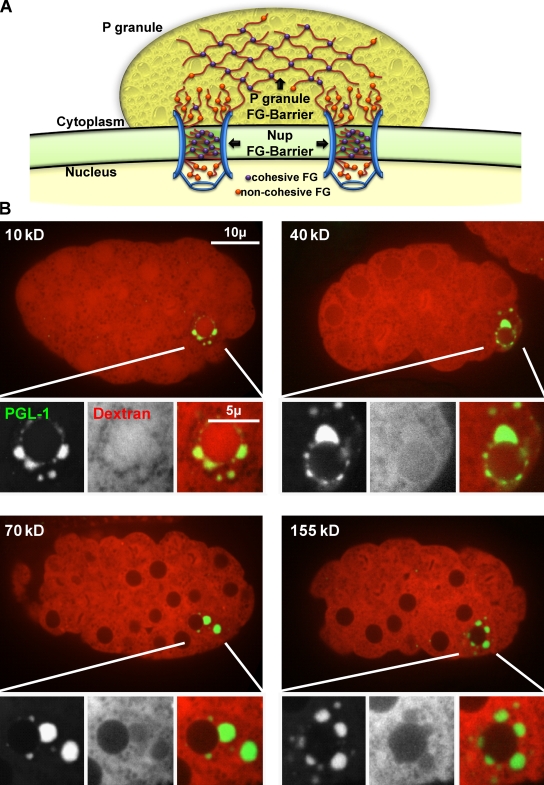
Hydrophobic FG-repeat domains within the NPC act both to prevent passive diffusion of proteins >45 kD into the nucleus and to facilitate transport of karyopherins and their cargo. Fluorescently labeled dextrans of varying sizes are commonly used to determine size-exclusion barriers in living cells (Peters, 1986). In C. elegans, fluorescent dextrans can be injected into the gonad syncytium and imaged in developing embryos 4–5 h later (Galy et al., 2003). At the end of each cell cycle, the nuclear envelope reforms around chromatin and excludes large dextrans (70 and 155 kD).
Because the constitutive P-granule components GLH-1, GLH-2, and GLH-4 contain Nup-like FG domains, we tested if P granules could also establish a size-exclusion barrier (Fig. 1 A). TRITC-labeled dextrans were injected into worms, and dextrans and GFP-tagged P granules (GFP::PGL-1) were simultaneously imaged in early embryos. As reported previously (Galy et al., 2003), we observed that dextrans of 70 and 155 kD are excluded from nuclei but dextrans of 10 and 40 kD are not (Fig. 1 B). We found that P granules also exclude 70- and 155-kD dextrans, but not 10- and 40-kD dextrans. These results demonstrate that, like NPCs, P granules also establish a size-exclusion barrier somewhere between 40 and 70 kD, a barrier that may be somewhat less stringent than NPCs, as 40-kD dextran is at least partially excluded from the nucleus but not from P granules. The size-exclusion barrier created by P granules may be the result of interactions between the FG repeats of GLH-1, GLH-2, and GLH-4 and other P-granule proteins.
Figure 1.
P granules create a size-exclusion barrier. (A) Model illustrating how P granules could extend the NPC environment through FG interactions. (B) C. elegans embryos from GFP::PGL-1 (green) worms injected with TRITC-labeled dextrans of different molecular weights (red).
Hydrophobic interactions are required for P-granule integrity
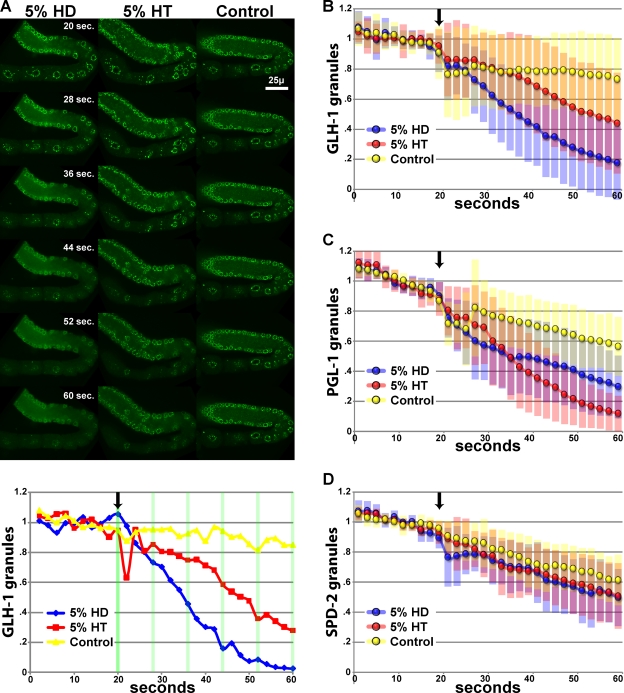
Treatment of cells with low concentrations of aliphatic alcohols such as 1,6-hexanediol (HD) disrupts hydrophobic interactions between FG residues and compromises the nucleocytoplasmic size-exclusion barrier; less hydrophobic alcohols such as 1,2,3-hexanetriol (HT) are less effective (Ribbeck and Görlich, 2002; Shulga and Goldfarb, 2003). To determine if hydrophobic interactions are important for P-granule structure, as would be expected for interacting FG domains, we exposed dissected gonads from a GLH-1::GFP–expressing strain to a 5% final volume of HD or HT in buffer, or buffer alone (control) (Fig. 2, A and B; Video 1). We observed rapid dispersal of GLH-1::GFP granules when exposed to HD compared with the control (50% of granules had dispersed by 18 s after the addition of HD, P < 0.0005 compared with control). We observed less rapid dispersal of GLH-1::GFP granules when exposed to HT (50% of granules had dispersed by 34 s after HT, P = 0.046 compared with control). Three observations make it unlikely that the dispersal of GLH-1::GFP granules resulted from the effect of HD on NPCs. First, both cytoplasmic and perinuclear GLH-1::GFP granules dispersed. Although cytoplasmic P granules in maturing oocytes are associated with NPC components, cytoplasmic P granules in the rachis are not; both classes of cytoplasmic P granules dissolved. Second, perinuclear GLH-1::GFP granules began to disperse before the influx of GFP from the cytoplasm into the nucleus (Video 2). Third, P granules dissolved after exposure to HD instead of detaching from the nuclear periphery as they do after Nup RNAi (Updike and Strome, 2009). Our results suggest that the integrity of P granules depends on hydrophobic interactions.
Figure 2.
Hydrophobic interactions are required for P-granule integrity. (A) Top, dissected GLH-1::GFP gonads exposed to hexanediol (HD), hexanetriol (HT), or egg buffer (Control). Bottom, normalized P-granule counts from the image series in A. Arrow indicates the time HD, HT, or control buffer was added. See Video 1 for complete time series. (B–D) GLH-1::GFP granule averages (B), GFP::PGL-1 granule averages (C), and GFP::SPD-2 granule averages (D) for HD, HT, and control. Standard deviations for each condition are shown in blue, red, and yellow shaded bars. 20 dissected gonads were analyzed for each treatment of each GFP line.
The above results could be explained by alcohol disruption of GLH-1 interactions within granules and dispersal specifically of GLH-1, or by disruption of hydrophobic interactions that hold P granules together. To distinguish between these possibilities, we examined a different P-granule protein. PGL-1 is a constitutive P-granule protein that lacks an FG domain. We exposed dissected gonads from a GFP::PGL-1–expressing strain to low concentrations of HD and HT (Fig. 2 C). Both HD and HT dispersed PGL-1 granules (P < 0.0005 compared with control). Our results suggest that hydrophobic interactions within P granules contribute to the integrity of P granules as a whole.
To investigate the specificity of granule disruption by aliphatic alcohols, we also exposed dissected gonads from GFP::SPD-2 worms to HD and HT (Fig. 2 D; Video 3). SPD-2 is a component of pericentriolar material and is also found in cytoplasmic granules in the germ line. GFP::SPD-2 granules were only subtly affected by HD or HT, suggesting that the much more substantial P-granule disruption by HD and HT is a consequence of disrupting hydrophobic interactions between P-granule components.
GLH proteins must be nucleated to form granules
Our results indicate that P granules, like NPCs, establish a size-exclusion barrier and depend on hydrophobic interactions for their structural integrity. We hypothesize that these granule properties are attributable to interactions between the FG domains of P-granule proteins. There are two nonexclusive models for the role of FG repeats within NPCs: (1) noncohesive FG domains create hydrophobic, entropic barriers at pores, and/or (2) cohesive FG domains interact to form a size-selective hydrogel (Weis, 2007). The ability of Nup FG domains to form granules has been assayed by expression of fluorescent FG fusions in yeast. This has been used to distinguish between cohesive (granule-forming) and noncohesive (non-granule forming) FG domains (Patel et al., 2007).
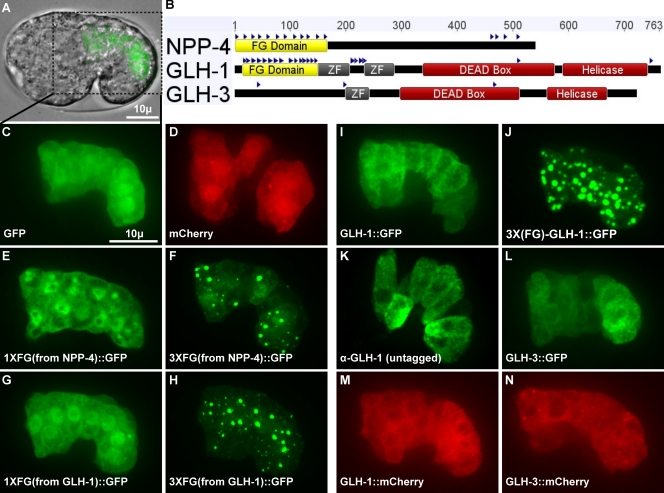
To test the sufficiency of P-granule proteins and domains to form granules in vivo but outside the context of the germ line and therefore independently of other germline-specific factors, we drove ectopic expression of those proteins and domains in intestinal cells of C. elegans embryos (Fig. 3). For comparison of GLH-1 to a bona fide NPC component, we tested NPP-4. After expression of the NPP-4 FG domain (Fig. 3 B) behind the intestine-specific elt-2 promoter, most NPP-4(FG)::GFP localized to the nucleus, but we also observed NPP-4(FG)::GFP in the cytoplasm, some of it in granular form (Fig. 3 E). Weak FG interactions are facilitated by anchoring domains within the NPC, which increases the local FG concentration. An increase in local FG concentration can also be accomplished by multimerizing FG domains. Indeed, intestinal expression of three fused NPP-4 FG domains caused GFP granules to form more readily than a single FG domain in both the nucleus and the cytoplasm (Fig. 3 F).
Figure 3.
Ectopic expression of P-granule components in the intestine. (A) Comma-stage embryo expressing GFP behind the elt-2 intestinal promoter. (B) FG repeats (blue triangles), FG domains (yellow), zinc fingers (gray), and DEAD-box helicase domains (red) in NPP-4, GLH-1, and GLH-3. (C–N) 10-µm projections of different ectopic expression constructs in the intestine of comma-stage embryos.
To determine if the FG domain of GLH-1 is sufficient for granule formation, we tagged the domain with GFP. The GLH-1 FG domain was dispersed throughout the nucleus and cytoplasm (Fig. 3 G). However, like the multimerized NPP-4 FG domain, three multimerized GLH-1 FG domains readily formed granules in the intestine (Fig. 3 H). We also tested the granule-forming capacity of the FG domain in the context of full-length GLH-1 and tested full-length GLH-3, which naturally lacks an FG domain (Fig. 3, B, I, and K–N). These full-length proteins remained dispersed throughout the cytoplasm and did not form granules. However, by inserting two additional FG domains in the N terminus of full-length GLH-1, granules readily formed (Fig. 3 J). These results show that GLH-1 on its own does not form granules outside of the germ line. The FG repeats must reach a critical concentration threshold before granules can form (Pappu et al., 2008). This suggests a requirement for GLH nucleation factors in the germ line.
PGL-1 promotes granule formation and nucleates the formation of GLH-1 and GLH-3 granules
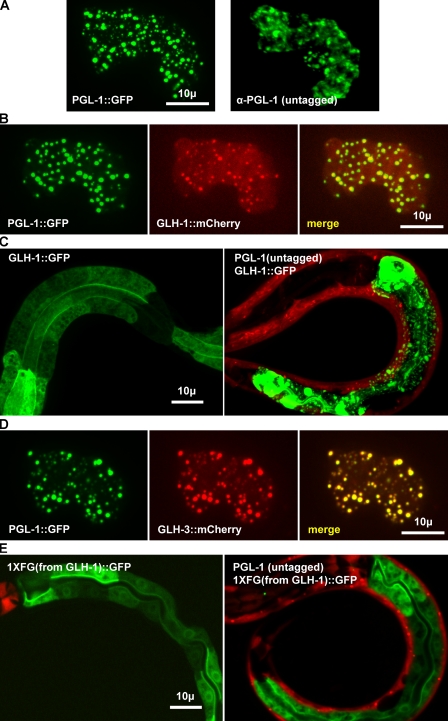
Because the PGL proteins have been shown to interact with each other by yeast two-hybrid and in vitro pull-down assays (Kawasaki et al., 2004), we tested if PGL-1 can self-aggregate into granules when expressed outside of the germ line. PGL-1::GFP and untagged PGL-1 were ectopically expressed in the intestine (Fig. 4 A). Both untagged and GFP-tagged PGL-1 formed granules. Because PGL-1 is sufficient for granule formation outside of the germ line, we reasoned that PGL-1 may act as a nucleating factor for the aggregation of other P-granule components like GLH-1. To test this, lines expressing PGL-1::GFP in the intestine were crossed with lines expressing GLH-1::mCherry in the intestine. PGL-1::GFP recruited GLH-1::mCherry to granules in the intestine, implicating PGL-1 as a P-granule–nucleating component (Fig. 4 B). Untagged PGL-1 also recruited GLH-1::GFP to granules (Fig. 4 C; 21/22 worms expressing PGL-1 had granular GLH-1::GFP, 3/24 worms lacking expression of PGL-1 had granular GLH-1::GFP). To determine if the recruitment of GLH protein to PGL-1 granules requires an FG domain, lines expressing PGL-1::GFP in the intestine were crossed with lines expressing GLH-3::mCherry in the intestine. Despite the absence of an FG domain in GLH-3 (Fig. 3 B), GLH-3 was also recruited to PGL-1::GFP granules (Fig. 4 D), revealing that PGL-1 recruitment does not require an FG domain. Consistent with recruitment of GLH-1 to PGL-1 granules independently of GLH-1’s FG domain, untagged PGL-1 did not recruit the 1XFG domain from GLH-1 to granules (Fig. 4 E, n = 30).
Figure 4.
PGL-1 nucleates granule formation. (A) GFP-tagged and untagged PGL-1 expressed in the intestine. (B and D) GLH-1::mCherry (B) or GLH-3::mCherry (D) coexpressed with PGL-1::GFP in intestinal cells. (C and E) Young larvae expressing GLH-1::GFP (C) or the FG domain of GLH-1 tagged with GFP (E) in intestinal cells. The larvae In the right panels additionally express untagged PGL-1 in intestinal cells (transgenic PGL-1–expressing worms identified by myo-3::mCherry expression in body muscle).
GLH-1 is necessary but not sufficient for the perinuclear association of PGL granules in intestinal cells
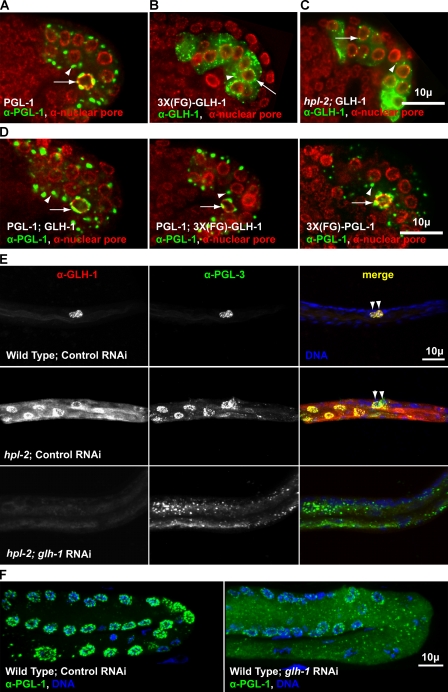
We noticed that PGL-1 granules were not concentrated around the nuclear periphery in the intestine (Fig. 5 A), whereas granules of GLH-1 with 3X multimerized FG domains were often perinuclear (Fig. 5 B). To test if GLH-1 is sufficient to recruit PGL-1 granules to a perinuclear location, we generated worms that coexpress PGL-1 and GLH-1, or coexpress PGL-1 and 3XFG-GLH-1, or express PGL-1 fused to the 3XFG domain of GLH-1 in their intestines (Fig. 5 D). In all of the lines examined, fewer than 10% of PGL-1 granules were in contact with the nuclear envelope (n = 10 embryos per line). Thus, association or attachment of a single or multimerized FG domain was not sufficient to drive PGL-1 granules to perinuclear locations.
Figure 5.
GLH-1 is necessary but not sufficient for the perinuclear association of PGL granules. (A–D) Single XY plane of comma-stage embryos expressing tagged PGL-1 or GLH-1 constructs, immunostained for nuclear pores (red) and either PGL-1 or GLH-1 (green), as noted. (A) Embryo expressing PGL-1 in the intestine. Ectopic PGL-1 granules in the intestine (arrowhead), endogenous PGL-1 granules in the germ cells (arrow). (B) Embryo expressing 3X(FG)-GLH-1 in the intestine. Cytoplasmic granules (arrowhead), perinuclear granules (arrow). (C) hpl-2(tm1489) embryo expressing GLH-1 in the intestine. Cytoplasmic granules (arrowhead), perinuclear granules (arrow). (D) GLH-1 (left) and 3X(FG)-GLH-1 (middle) coexpressed with PGL-1 in intestinal cells. 3XFG from GLH-1 fused to PGL-1 (right). Ectopic PGL-1 granules in the intestine (arrowhead), endogenous PGL-1 granules in the germ cells (arrow). (E) Wild-type and hpl-2(tm1489) L1s grown at 26°C on control bacteria and glh-1(RNAi) bacteria and immunostained for GLH-1 and PGL-3. Germ cells (arrowheads). (F) Germ lines from wild-type worms grown from L1 to adulthood at 26°C on control bacteria and glh-1(RNAi) bacteria.
hpl-2 mutants offer another avenue to investigate P granules in intestinal cells. In hpl-2 mutants, germline genes are ectopically expressed in the intestine and aggregates of P-granule components like PGL-1, PGL-3, and GLH-1 form around intestinal nuclei (Fig. 5 E, middle; Wang et al., 2005; Petrella et al., 2011). Likewise, full-length GLH-1::GFP assembles into cytoplasmic and perinuclear granules in hpl-2 mutant embryos (Fig. 5 C, 44/50 embryos with perinuclear GLH-1 granules). When GLH-1 was depleted from hpl-2 mutants by RNAi, PGL-3–containing granules were no longer concentrated around the nuclear periphery but instead were found throughout the intestinal cytoplasm (Fig. 5 E, bottom; dispersal of PGL-3 granules in 39/52 hpl-2; glh-1(RNAi) worms). Thus, GLH-1 is required for the perinuclear localization of PGL granules. These findings in the intestine are consistent with findings in the germ line: loss of GLH-1 by RNAi or mutation causes PGL granules to lose their nuclear association. Although long-term loss results in variable degrees of dispersal of PGL proteins from granules (Kawasaki et al., 1998; Spike et al., 2008), a more immediate effect of glh-1 RNAi is the presence of small PGL granules that no longer localize to the nuclear periphery in germ cells (Fig. 5 F; Updike and Strome, 2009).
Concluding remarks
The P-granule assembly pathway, worked out by molecular epistasis tests over the past decade, placed GLH-1 upstream of PGL proteins: in the absence of GLH-1, small PGL granules disperse throughout the cytoplasm, while the combined absence of the PGL proteins has no obvious effect on the perinuclear location of GLH-1 granules in the adult germ line (Kawasaki et al., 1998, 2004; Spike et al., 2008). Our studies of the properties of individual P-granule proteins expressed in the intestine, as well as the studies of Hanazawa et al. (2011), refine this pathway by showing that PGL-1 and PGL-3 are capable of self assembling into granules in the absence of other germline-specific proteins and that GLH-1 requires nucleating factors, like PGL-1, for de novo granule formation. These insights required examining the roles of P-granule proteins under conditions of de novo granule assembly (in early embryos and in the intestine) instead of under conditions of granule maintenance (the adult germ line). One role of GLH-1 is to retain PGL aggregates in large assemblies at the nuclear periphery; however, GLH-1 cannot do this on its own. We speculate that in the germ line, GLH-1 aggregation seeded by the PGL proteins facilitates interaction with other FG-containing proteins to retain P granules at the nuclear periphery, potentially explaining why P granules detach from the periphery when certain Nups are depleted (Updike and Strome, 2009). It was recently reported that in mouse testes, Vasa immunoprecipitates with the peripheral FG-rich Nup98, but this interaction was not observed in tissue culture cells (Voronina and Seydoux, 2010), suggesting that Vasa, like GLH-1, requires nucleation by additional germline components to localize to the nuclear periphery.
Similar to FG repeats, hydrophobic GW/WG repeats are found in the Argonaute-interacting protein GW182, a component of P bodies (Eulalio et al., 2009). P granules share many properties with P bodies and also contain Argonautes like PRG-1, WAGO-1, ALG-3, and CSR-1 (Batista et al., 2008; Claycomb et al., 2009; Gu et al., 2009; Conine et al., 2010). The Argonautes in P granules may interact with FG repeats like those found in GLH-1, GLH-2, and GLH-4. Another interesting protein that may share roles with the GLHs is the embryo implantation factor Trophinin, whose C terminus contains 45 FGs spaced every 10–15 aa. Trophinin is expressed in the cytoplasm of mammalian germ cells and localizes to the nuclear periphery (Aoyama et al., 2005).
More than 40 proteins associated with P granules either possess RNA-binding domains or are thought to regulate translation (Updike and Strome, 2010). We propose that the NPC-like properties of P granules provide a specialized hydrophobic microenvironment in germ cells that may facilitate post-transcriptional processing events while selectively excluding large protein complexes from gaining access to mRNAs and endogenous siRNAs. The aggregation tendencies of P granules may help stabilize or sequester regulatory proteins, such as translation initiation factors and Argonautes (Amiri et al., 2001; Updike and Strome, 2009), within the granules. Reconstituting germ granules in the intestine offers a way to further investigate germ granule assembly and function.
Materials and methods
Microscopy
A Volocity acquisition and spinning disk confocal system (PerkinElmer) fitted on an inverted microscope (Eclipse TE2000-E; Nikon) with an EM-CCD camera (Hamamatsu Photonics) was used to acquire all images. A Nikon 60x oil objective (NA 1.4) was used to acquire Figs. 1, 3, 4 (A, B, and D), and 5 (A–D and F). A Nikon 40x oil objective (NA 1.3) was used to acquire Figs. 2, 4 (C and E), and 5 E, and Videos 1–3. All images were acquired at room temperature.
Injection of TRITC-labeled dextrans
A final concentration of 4 mg/ml of TRITC-labeled dextran (10, 40, 70, and 155 kD; Sigma-Aldrich) in injection buffer (20 mM KPO4, pH 7.5, 3 mM K citrate, and 2% polyethylene glycol-6000) was injected into the syncytial gonad of worms as described previously (Galy et al., 2003). Injected bnIs1(pie-1p::GFP::PGL-1) worms were incubated 4–4.5 h at 24°C, then dissected to harvest embryos. 28–50 cell stage embryos were mounted on an agar pad in egg buffer (25 mM Hepes, 120 mM NaCl2, 2 mM MgCl2, 2 mM CaCl2, and 48 mM KCl2) and single planes of green and red channels were acquired every 10 s for 2 min.
Dispersal of GFP granules with aliphatic alcohols
Gonads expressing GLH-1::GFP, GFP::PGL-1, GFP::SPD-2, or both GFP::SPD-2 and PGL-1::RFP were dissected in 15 µl egg buffer on polylysine-coated coverslips and mounted drop up on an inverted microscope. Fluorescence images were acquired every 2 s for 1 min. At 20 s, an equal 15-µl volume of egg buffer alone (control) or egg buffer containing 10% (final 5%) 1,6-hexandiol (Acros Organics) in egg buffer or 10% (final 5%) 1,2,3 hexanetriol (Acros Organics) was added. 20 dissected gonads were imaged for each transgene in each of the 3 solutions (coded A, B, or C to allow the experiment to be scored blind). Volocity was used to find and count GFP granules larger than 200 nm2 with an intensity that was at least 40% of saturation. Typically, the first time point contained ∼100–200 granules defined by these parameters. P-granule quantity was then normalized using the average of the first nine time points. Image sequences were not corrected for photobleaching, accounting for the slopes before and after the addition of each solution at t = 20 s. An iterative permutation test was used to calculate the significance between the plotted lines.
Strain construction
C. elegans strains were maintained as described previously (Brenner, 1974). Unless otherwise stated, transgenic lines were created by injecting 20 ng/µl of the plasmid and generating simple extrachromosomal arrays. For each transgene, at least 10 transgenic lines were isolated and examined for expression consistency. The line carrying the most stable array was used for this study. These lines are listed in Table S1.
Intestinal expression experiments
For consistency in comparing transgenic expression at the same developmental time point, images were acquired in comma to early 1.5-fold embryos, except for the early larval stages shown in Figs. 4, C and E, and 5 E. 10-µm projections are shown in Figs. 3–5 with the exception of Fig. 5, A–D, which required single planes to show the subcellular location of granules.
Immunocytochemistry
Embryos and worms were fixed using methanol/acetone (Strome and Wood, 1983). Antibody dilutions were 1:30,000 rabbit anti–PGL-1 (Kawasaki et al., 1998), 1:5,000 mouse anti-NPC mAb414 (Covance; Blobel, 1985), 1:5,000 rat anti–PGL-3 (Kawasaki et al., 2004), 1:1,000 rabbit anti–GLH-1 (Gruidl et al., 1996; Kawasaki et al., 2004), and 1:400 Alexa Fluor 488 goat anti–rabbit IgG, Alexa Fluor 594 goat anti–mouse IgG, Alexa Fluor 488 goat anti–rat IgG, and Alexa Fluor 594 goat anti–rabbit IgG (Invitrogen).
glh-1 RNAi
glh-1 RNAi by feeding (Spike et al., 2008) was performed on wild-type and hpl-2(tm1489) worms grown at 26°C.
Online supplemental material
Video 1 is a time-lapse movie of Fig. 2 A showing GLH-1::GFP granules (green) in dissected C. elegans gonads exposed to HD, HT, and egg buffer alone. Video 2 is a time-lapse movie showing cytoplasmic and perinuclear GLH-1::GFP granules (green) in a dissected C. elegans gonad exposed to HD. Video 3 is a time-lapse movie showing GFP::SPD-2 (green) and PGL-1::RFP (red) granules in a dissected C. elegans gonad exposed to HD. Table S1 is a list of all C. elegans strains used in this study. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201010104/DC1.
Acknowledgments
We thank M. Rexach for advice on NPC biology, O. Bossinger for elt-2 promoter constructs, J. Nance for PGL-1::RFP, M. Sarov for recombineering reagents, A. Rechtsteiner for statistics advice, and M. Hanazawa and A. Sugimoto for helpful discussions and sharing unpublished results.
This research was supported by National Institutes of Health NRSA award GM84673 to D.L. Updike and National Institutes of Health grant GM34059 to S. Strome.
Footnotes
Abbreviations used in this paper:
- FG
- phenylalanine-glycine
- HD
- 1,6-hexanediol
- HT
- 1,2,3-hexanetriol
- NPC
- nuclear pore complex
- Nup
- NPC protein
References
- Amiri A., Keiper B.D., Kawasaki I., Fan Y., Kohara Y., Rhoads R.E., Strome S. 2001. An isoform of eIF4E is a component of germ granules and is required for spermatogenesis in C. elegans. Development. 128:3899–3912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama J., Nakayama Y., Sugiyama D., Saburi S., Nadano D., Fukuda M.N., Yamaguchi N. 2005. Apical cell adhesion molecule, trophinin, localizes to the nuclear envelope. FEBS Lett. 579:6326–6332 10.1016/j.febslet.2005.10.012 [DOI] [PubMed] [Google Scholar]
- Batista P.J., Ruby J.G., Claycomb J.M., Chiang R., Fahlgren N., Kasschau K.D., Chaves D.A., Gu W., Vasale J.J., Duan S., et al. 2008. PRG-1 and 21U-RNAs interact to form the piRNA complex required for fertility in C. elegans. Mol. Cell. 31:67–78 10.1016/j.molcel.2008.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G. 1985. Gene gating: a hypothesis. Proc. Natl. Acad. Sci. USA. 82:8527–8529 10.1073/pnas.82.24.8527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontems F., Stein A., Marlow F., Lyautey J., Gupta T., Mullins M.C., Dosch R. 2009. Bucky ball organizes germ plasm assembly in zebrafish. Curr. Biol. 19:414–422 10.1016/j.cub.2009.01.038 [DOI] [PubMed] [Google Scholar]
- Brangwynne C.P., Eckmann C.R., Courson D.S., Rybarska A., Hoege C., Gharakhani J., Jülicher F., Hyman A.A. 2009. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 324:1729–1732 10.1126/science.1172046 [DOI] [PubMed] [Google Scholar]
- Brenner S. 1974. The genetics of Caenorhabditis elegans. Genetics. 77:71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeks R.J., Canman J.C., Gabriel W.N., Meyer N., Strome S., Goldstein B. 2004. C. elegans PAR proteins function by mobilizing and stabilizing asymmetrically localized protein complexes. Curr. Biol. 14:851–862 10.1016/j.cub.2004.05.022 [DOI] [PubMed] [Google Scholar]
- Chuma S., Hosokawa M., Tanaka T., Nakatsuji N. 2009. Ultrastructural characterization of spermatogenesis and its evolutionary conservation in the germline: germinal granules in mammals. Mol. Cell. Endocrinol. 306:17–23 10.1016/j.mce.2008.11.009 [DOI] [PubMed] [Google Scholar]
- Claycomb J.M., Batista P.J., Pang K.M., Gu W., Vasale J.J., van Wolfswinkel J.C., Chaves D.A., Shirayama M., Mitani S., Ketting R.F., et al. 2009. The Argonaute CSR-1 and its 22G-RNA cofactors are required for holocentric chromosome segregation. Cell. 139:123–134 10.1016/j.cell.2009.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conine C.C., Batista P.J., Gu W., Claycomb J.M., Chaves D.A., Shirayama M., Mello C.C. 2010. Argonautes ALG-3 and ALG-4 are required for spermatogenesis-specific 26G-RNAs and thermotolerant sperm in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 107:3588–3593 10.1073/pnas.0911685107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coustham V., Bedet C., Monier K., Schott S., Karali M., Palladino F. 2006. The C. elegans HP1 homologue HPL-2 and the LIN-13 zinc finger protein form a complex implicated in vulval development. Dev. Biol. 297:308–322 10.1016/j.ydbio.2006.04.474 [DOI] [PubMed] [Google Scholar]
- DeRenzo C., Reese K.J., Seydoux G. 2003. Exclusion of germ plasm proteins from somatic lineages by cullin-dependent degradation. Nature. 424:685–689 10.1038/nature01887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy E.M. 1975. Germ plasm and the differentiation of the germ cell line. Int. Rev. Cytol. 43:229–280 10.1016/S0074-7696(08)60070-4 [DOI] [PubMed] [Google Scholar]
- Ephrussi A., Lehmann R. 1992. Induction of germ cell formation by oskar. Nature. 358:387–392 10.1038/358387a0 [DOI] [PubMed] [Google Scholar]
- Eulalio A., Tritschler F., Izaurralde E. 2009. The GW182 protein family in animal cells: new insights into domains required for miRNA-mediated gene silencing. RNA. 15:1433–1442 10.1261/rna.1703809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Extavour C.G., Akam M. 2003. Mechanisms of germ cell specification across the metazoans: epigenesis and preformation. Development. 130:5869–5884 10.1242/dev.00804 [DOI] [PubMed] [Google Scholar]
- Galy V., Mattaj I.W., Askjaer P. 2003. Caenorhabditis elegans nucleoporins Nup93 and Nup205 determine the limit of nuclear pore complex size exclusion in vivo. Mol. Biol. Cell. 14:5104–5115 10.1091/mbc.E03-04-0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruidl M.E., Smith P.A., Kuznicki K.A., McCrone J.S., Kirchner J., Roussell D.L., Strome S., Bennett K.L. 1996. Multiple potential germ-line helicases are components of the germ-line-specific P granules of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 93:13837–13842 10.1073/pnas.93.24.13837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W., Shirayama M., Conte D., Jr, Vasale J., Batista P.J., Claycomb J.M., Moresco J.J., Youngman E.M., Keys J., Stoltz M.J., et al. 2009. Distinct argonaute-mediated 22G-RNA pathways direct genome surveillance in the C. elegans germline. Mol. Cell. 36:231–244 10.1016/j.molcel.2009.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanazawa M., Masafumi Y., Sugimoto A. 2011. PGL proteins self-associate and bind RNPs to mediate germ granule assembly in C. elegans. J. Cell Biol. 192:929–937 10.1083/jcb.201010106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenishi K., Nakazato S., Okuda T. 1986. Direct evidence for the presence of germ cell determinant in vegetal pole cytoplasm of Xenopus laevis and in a subcellular fraction of it. Dev. Growth Differ. 28:563–568 10.1111/j.1440-169X.1986.00563.x [DOI] [PubMed] [Google Scholar]
- Illmensee K., Mahowald A.P. 1974. Transplantation of posterior polar plasm in Drosophila. Induction of germ cells at the anterior pole of the egg. Proc. Natl. Acad. Sci. USA. 71:1016–1020 10.1073/pnas.71.4.1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki I., Amiri A., Fan Y., Meyer N., Dunkelbarger S., Motohashi T., Karashima T., Bossinger O., Strome S. 2004. The PGL family proteins associate with germ granules and function redundantly in Caenorhabditis elegans germline development. Genetics. 167:645–661 10.1534/genetics.103.023093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki I., Shim Y.H., Kirchner J., Kaminker J., Wood W.B., Strome S. 1998. PGL-1, a predicted RNA-binding component of germ granules, is essential for fertility in C. elegans. Cell. 94:635–645 10.1016/S0092-8674(00)81605-0 [DOI] [PubMed] [Google Scholar]
- Kuznicki K.A., Smith P.A., Leung-Chiu W.M., Estevez A.O., Scott H.C., Bennett K.L. 2000. Combinatorial RNA interference indicates GLH-4 can compensate for GLH-1; these two P granule components are critical for fertility in C. elegans. Development. 127:2907–2916 [DOI] [PubMed] [Google Scholar]
- Pappu R.V., Wang X., Vitalis A., Crick S.L. 2008. A polymer physics perspective on driving forces and mechanisms for protein aggregation. Arch. Biochem. Biophys. 469:132–141 10.1016/j.abb.2007.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S.S., Belmont B.J., Sante J.M., Rexach M.F. 2007. Natively unfolded nucleoporins gate protein diffusion across the nuclear pore complex. Cell. 129:83–96 10.1016/j.cell.2007.01.044 [DOI] [PubMed] [Google Scholar]
- Peters R. 1986. Fluorescence microphotolysis to measure nucleocytoplasmic transport and intracellular mobility. Biochim. Biophys. Acta. 864:305–359 [DOI] [PubMed] [Google Scholar]
- Petrella L., Wang W., Spike C.A., Rechsteiner A., Reinke V., Strome S. 2011. synMuvB proteins antagonize germline fate in the intestine and ensure C. elegans survival. Development. 138:1069–1079 10.1242/dev.059501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt J.N., Schisa J.A., Priess J.R. 2000. P granules in the germ cells of Caenorhabditis elegans adults are associated with clusters of nuclear pores and contain RNA. Dev. Biol. 219:315–333 10.1006/dbio.2000.9607 [DOI] [PubMed] [Google Scholar]
- Ribbeck K., Görlich D. 2002. The permeability barrier of nuclear pore complexes appears to operate via hydrophobic exclusion. EMBO J. 21:2664–2671 10.1093/emboj/21.11.2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarov M., Schneider S., Pozniakovski A., Roguev A., Ernst S., Zhang Y., Hyman A.A., Stewart A.F. 2006. A recombineering pipeline for functional genomics applied to Caenorhabditis elegans. Nat. Methods. 3:839–844 10.1038/nmeth933 [DOI] [PubMed] [Google Scholar]
- Schisa J.A., Pitt J.N., Priess J.R. 2001. Analysis of RNA associated with P granules in germ cells of C. elegans adults. Development. 128:1287–1298 [DOI] [PubMed] [Google Scholar]
- Sheth U., Pitt J., Dennis S., Priess J.R. 2010. Perinuclear P granules are the principal sites of mRNA export in adult C. elegans germ cells. Development. 137:1305–1314 10.1242/dev.044255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulga N., Goldfarb D.S. 2003. Binding dynamics of structural nucleoporins govern nuclear pore complex permeability and may mediate channel gating. Mol. Cell. Biol. 23:534–542 10.1128/MCB.23.2.534-542.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spike C., Meyer N., Racen E., Orsborn A., Kirchner J., Kuznicki K., Yee C., Bennett K., Strome S. 2008. Genetic analysis of the Caenorhabditis elegans GLH family of P-granule proteins. Genetics. 178:1973–1987 10.1534/genetics.107.083469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome S., Wood W.B. 1983. Generation of asymmetry and segregation of germ-line granules in early C. elegans embryos. Cell. 35:15–25 10.1016/0092-8674(83)90203-9 [DOI] [PubMed] [Google Scholar]
- Updike D.L., Strome S. 2009. A genomewide RNAi screen for genes that affect the stability, distribution and function of P granules in Caenorhabditis elegans. Genetics. 183:1397–1419 10.1534/genetics.109.110171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updike D.L., Strome S. 2010. P granule assembly and function in Caenorhabditis elegans germ cells. J. Androl. 31:53–60 10.2164/jandrol.109.008292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronina E., Seydoux G. 2010. The C. elegans homolog of nucleoporin Nup98 is required for the integrity and function of germline P granules. Development. 137:1441–1450 10.1242/dev.047654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Kennedy S., Conte D., Jr, Kim J.K., Gabel H.W., Kamath R.S., Mello C.C., Ruvkun G. 2005. Somatic misexpression of germline P granules and enhanced RNA interference in retinoblastoma pathway mutants. Nature. 436:593–597 10.1038/nature04010 [DOI] [PubMed] [Google Scholar]
- Weis K. 2007. The nuclear pore complex: oily spaghetti or gummy bear? Cell. 130:405–407 10.1016/j.cell.2007.07.029 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yan L., Zhou Z., Yang P., Tian E., Zhang K., Zhao Y., Li Z., Song B., Han J., et al. 2009. SEPA-1 mediates the specific recognition and degradation of P granule components by autophagy in C. elegans. Cell. 136:308–321 10.1016/j.cell.2008.12.022 [DOI] [PubMed] [Google Scholar]