Abstract
The mechanisms underpinning impaired defensive counterregulatory responses to hypoglycemia that develop in some people with diabetes who suffer recurrent episodes of hypoglycemia are unknown. Previous work examining whether this is a consequence of increased glucose delivery to the hypothalamus, postulated to be the major hypoglycemia-sensing region, has been inconclusive. Here, we hypothesized instead that increased hypothalamic glucose phosphorylation, the first committed intracellular step in glucose metabolism, might develop following exposure to hypoglycemia. We anticipated that this adaptation might tend to preserve glucose flux during hypoglycemia, thus reducing detection of a falling glucose. We first validated a model of recurrent hypoglycemia in chronically catheterized (right jugular vein) rats receiving daily injections of insulin. We confirmed that this model of recurrent insulin-induced hypoglycemia results in impaired counterregulation, with responses of the key counterregulatory hormone, epinephrine, being suppressed significantly and progressively from the first day to the fourth day of insulin-induced hypoglycemia. In another cohort, we investigated the changes in brain glucose phosphorylation activity over 4 days of recurrent insulin-induced hypoglycemia. In keeping with our hypothesis, we found that recurrent hypoglycemia markedly and significantly increased hypothalamic glucose phosphorylation activity in a day-dependent fashion, with day 4 values 2.8 ± 0.6-fold higher than day 1 (P < .05), whereas there was no change in glucose phosphorylation activity in brain stem and frontal cortex. These findings suggest that the hypothalamus may adapt to recurrent hypoglycemia by increasing glucose phosphorylation; and we speculate that this metabolic adaptation may contribute, at least partly, to hypoglycemia-induced counterregulatory failure.
1. Introduction
Intensified insulin therapy for diabetes is often limited in clinical practice by increased risk of hypoglycemia [1-3]. In health, blood glucose levels are tightly controlled within relatively narrow boundaries, with a falling blood glucose level being rapidly detected and a series of defensive counterregulatory responses triggered to prevent or limit hypoglycemia and restore euglycemia. Counterregulatory responses include the release of hormones such as glucagon and epinephrine and the generation of protective warning symptoms. Counterregulatory responses may become impaired after repeated exposure to hypoglycemia as experienced by diabetic patients undergoing intensive insulin therapy, placing them at significant risk of subsequent episodes of severe hypoglycemia—essentially a type of “stress desensitization” [1]. The mechanisms underlying the down-regulation of defensive counterregulatory responses following recurrent hypoglycemic exposure remain unclear.
To elicit counterregulatory responses, falling glucose levels need to be detected, probably in large part by sensors in specialized areas of the hypothalamus [4-8]. Although mechanisms used by specialized glucose sensors to detect changes in glucose availability are unclear, there is increasing evidence that these sensors may sense downstream products of glucose metabolism, for example, monitoring levels of adenosine triphosphate, analogous to the canonical pancreatic β-cell glucose-sensing mechanism [9-11].
Glucose metabolism in hypothalamus may therefore contribute to neuronal glucose sensing and potentially the control of hypoglycemia counterregulation. In keeping with this, several investigators have suggested that alterations in brain glucose uptake and/or metabolism may contribute to the impairment of hypoglycemia counterregulation following recurrent hypoglycemia [12-14]. In particular, recent human brain imaging studies have shown that recurrent hypoglycemia may selectively alter neuronal glucose utilization in hypothalamic [15] or thalamic [16] regions of the brain, although the biochemical adaptations underpinning these changes cannot easily be further defined in human studies.
Following glucose transport into neurons, the first committed step in glucose metabolism is phosphorylation, which is catalyzed by hexokinases (HK; EC 2.7.1.1), a group of related enzymes [17]. Given the potentially rate-limiting role of glucose phosphorylation in neuronal glucose sensing, we hypothesized that up-regulation of glucose phosphorylation activity may occur following recurrent hypoglycemia in parallel with impairment of glucose counterregulation. In this work, we aimed to examine whether brain glucose phosphorylation activity was up-regulated following recurrent hypoglycemia, reasoning that any adaptation related to glucose sensing (as opposed to a more general defensive adaptation to a decrease in glucose as the major brain fuel) would be restricted to hypothalamic and/or glucose-sensing areas rather than being a more global change in brain glucose phosphorylation. To address this issue directly, we used a rat model of impaired glucose counterregulation induced by exposing the animals to recurrent insulin-induced hypoglycemia and investigated glucose phosphorylation activity in brain protein preparations in vitro at a physiologic brain glucose concentration during hypoglycemia. Here, we demonstrate that recurrent hypoglycemia, with 4 sequential daily episodes of exposure to blood or plasma glucose concentration of less than 4 mmol/L [10,11], significantly increased glucose phosphorylation activity in hypothalamus preparations.
2. Materials and methods
2.1. Animals
We studied healthy adult male Sprague-Dawley rats (Charles River, UK) of approximately 300 g throughout. Rats were housed individually after surgery on a 12-hour on off light-dark cycle. The procedures were approved in advance by both a local University and UK Home Office ethics review. All chemicals were from Sigma-Aldrich (Gillingham, UK) except where otherwise stated.
2.2. Surgical preparation
Under inhaled anesthetic, rats underwent survival surgery for placement of vascular catheters into the right jugular vein [18]. Peri- and postoperative injectable analgesia and antibiotic cover were provided routinely, and only animals that had regained preoperative body weight with no signs of infection were studied.
2.3. Study design
We studied 2 separate cohorts of rats. In study 1, we first established that, in our hands, recurrent insulin-induced hypoglycemia resulted in impaired hormonal counterregulation, before examining brain glucose phosphorylation activity in study 2.
In study 1, we examined the sequential loss of hormonal counterregulation with recurrent hypoglycemia. Chronically catheterized rats were exposed to between 1 and 4 days of sequential insulin injections, with the daily insulin dose being reduced progressively from a starting dose of 10 U/kg down to 6 to 8 U/kg. We have found that this sequential daily reduction in insulin dose is necessary so as to avoid profound hypoglycemia as counterregulatory impairment develops. Food was removed for up to 180 minutes after insulin injections. On each day, plasma glucose concentration was measured via sampling from the jugular vein catheter at 0, 15, 30, 45, 60, and 90 minutes following insulin injection (data not shown) to confirm depth of hypoglycemic exposure. When glucose levels fell to 2 mmol/L or less, food was returned to the cage. To confirm that this paradigm resulted in impairment of hormonal counterregulation, we measured plasma levels of the key counterregulatory hormones, epinephrine and glucagon, on days 1 to 4 via the jugular vein catheter 60 minutes after insulin injection (Fig. 1).
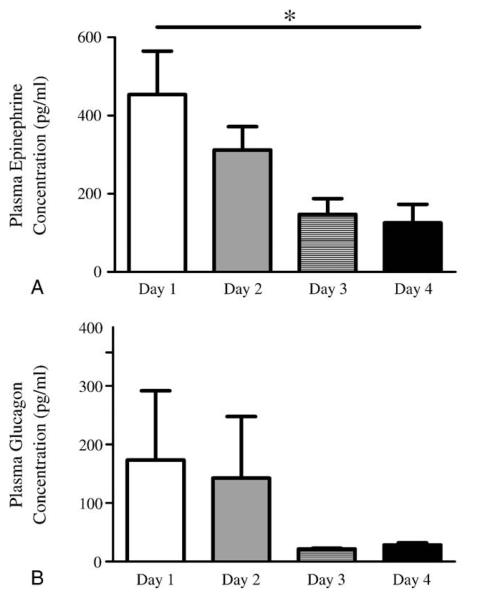
Fig. 1.
Plasma levels of the key counterregulatory hormones, epinephrine (A) and glucagon (B), in study 1 on days 1 to 4 with samples measured via the jugular vein catheter 60 minutes after insulin injection. *P < .05; 1-way analysis of variance.
Having validated that this model of recurrent hypoglycemia results in impaired hypoglycemic counterregulation, we then examined how brain glucose phosphorylation activity changed with recurrent hypoglycemia in study 2. A further cohort of chronically catheterized rats was exposed to between 1 and 4 days (designated H1 to 4) of sequential subcutaneous insulin injections, again with the daily insulin dose being reduced (10, 8, 6, and 5 U/kg on days 1 to 4, respectively). On each study day, a cohort of animals was designated for terminal study, whereas the remainder continued on to the next study day for subsequent hypoglycemic exposure, after which another cohort was euthanized, with the pattern repeating for up to 4 days. Plasma glucose concentrations were measured by repeated blood sampling via jugular vein catheters in animals designated for terminal studies on each study day, whereas whole blood glucose was assayed from tail vein puncture in animals designated for subsequent hypoglycemic studies on ensuing days. Importantly, food and glucose injections were not given to “rescue” the animals designated for terminal hypoglycemia studies. These rats were euthanized while still hypoglycemic, and brains were collected for brain glucose phosphorylation activity assays.
2.4. Glucose assays
We used 2 different methods for determining circulating glucose levels. In study 1, we assayed plasma glucose on 10-μL plasma samples drawn from jugular catheters using a bench top glucose analyzer (Analox GM9 [glucose oxidase method]; Analox Instruments, London, UK). For convenience, the testing to ensure that uncontrolled hypoglycemia did not develop following insulin injections on most days in study 2 was performed on whole blood glucose obtained by tail vein puncture using a standard clinical handheld reflectance meter (Therasense Freestyle; Abbot Laboratories, Maidenhead, UK). However, we used the Analox bench top analyzer to measure plasma glucose drawn from catheter samples on whichever of the days H1 to H4 was the terminal day for that animal in study 2 to determine more accurately the depth of hypoglycemic challenge. We have indicated clearly in text and figures where whole blood tail vein or catheter-sampled plasma glucose was assayed.
2.5. Brain glucose phosphorylation activity assay
Sections of hypothalamus, brain stem, and frontal cortex were dissected from the brain. Following this, tissue sections were homogenized in ice-cold buffer (50 mmol/L HEPES, 150 mmol/L KCl, 5 mmol/L MgCl2, and 1 mmol/L EDTA [pH 7.4], supplemented with 1 mmol/L dithiothreitol, 1 mmol/L phenylmethylsulfonyl fluoride, and 10 μmol/L leupeptin HCl) and centrifuged at 13 000 rpm for 20 minutes at 4°C. Supernatants were collected, and glucose phosphorylation activity was assayed spectrophotometrically (340 nm, 37°C) with a 96-well plate reader (Wallace 1420 Victor 3; Perkin Elmer, Cambridge, UK) by coupling glucose phosphorylation to a reporter assay, which oxidizes glucose-6-phosphate to 6-phosphogluco-δ-lactone with simultaneous reduction of NAD+ to NADH [19,20]. Total glucose phosphorylation activity in brain protein preparations was measured in vitro at 0.5 mmol/L, a physiologic brain glucose concentration during hypoglycemia, because brain glucose concentrations vary around 15% to 30% of plasma values [21]. The reaction mixture in 100-μL final volume contained 20 mmol/L HEPES (pH 7.1), 25 mmol/L KCl, 2 mmol/L MgCl2, 1 mmol/L dithiothreitol, 1 mmol/L NAD+, 1 mmol/L adenosine triphosphate, 10 units glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides, 1 mmol/L 3-O-methyl-N-acetyl glucosamine (Axxora, Bingham, UK), 25-μL tissue protein extract, and 0.5 mmol/L glucose (a physiologic brain glucose concentration during hypoglycemia). 3-O-methyl-N-acetyl glucosamine was incorporated in the assay to inhibit N-acetyl glucosamine kinase [22].
2.6. Epinephrine assay
Plasma epinephrine concentrations were assayed by enzyme-linked immunosorbent assay (ELISA) as previously described [23] with slight modifications. In brief, epinephrine assay was performed using a 2-day-procedure ELISA kit (IBL, Hamburg, Germany). Plasma samples underwent a solid phase extraction procedure on day 1, followed by enzymatic derivatization on day 2. The derivatized samples were then used in the ELISA assay. The small volume of blood obtained from rats necessitated reducing singleton plasma volume to 100 μL (instead of 500 μL that the kit manufacturer recommends). The extracts were analyzed in duplicate in the ELISA procedure. When the coefficient of variation of the results exceeded 15%, both results were reported and an average was counted.
2.7. Statistical analysis
Data were expressed as mean ± SEM. Statistical analyses were performed using the 2-tailed Students t test or 1-way analysis of variance. P < .05 was considered to be statistically significant.
3. Results
3.1. Study 1: validation of model of impaired counterregulation to hypoglycemia
In study 1, we investigated the pattern of impairment of epinephrine responses during recurrent hypoglycemia in another cohort of rats (Fig. 1A). Plasma epinephrine levels decreased significantly (P < .05) and day dependently with exposure to sequential hypoglycemia (Fig. 1A). Plasma glucagon levels also tended (P = not significant [NS]) to reduce following exposure to recurrent hypoglycemia (Fig. 1B). In summary, these data confirmed that epinephrine responses were suppressed over the course of 4 days of hypoglycemia. We then proceeded to examine changes in brain glucose phosphorylation in study 2.
3.2. Study 2: brain glucose phosphorylation activity with recurrent hypoglycemia
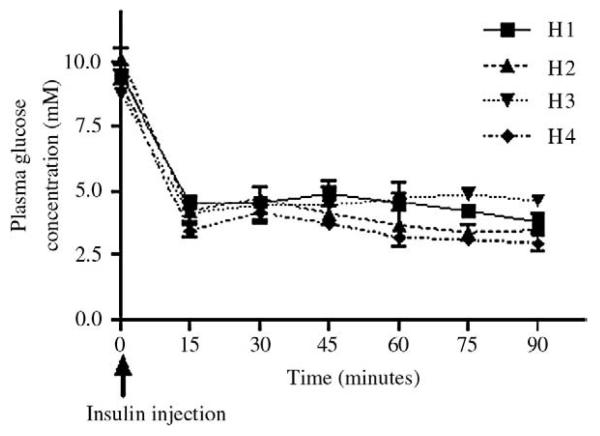
As expected, mean blood glucose levels on insulin injection days when checked using handheld reflectance meter were between 6 and 7 mmol/L, falling to nadir values less than 2.5 mmol/L following insulin injection (data not shown). On terminal study days, where we measured circulating glucose more accurately using plasma samples drawn from vascular catheters, baseline and nadir plasma glucose values were systematically higher than when measured on tail blood samples (Fig. 2). However, baseline values were similar on all 4 days. Of note, despite receiving a smaller dose of insulin, nadir plasma glucose levels were significantly lower (P < .05) in rats exposed to 4 days (H4) as opposed to 1 or 2 days (H1 or H2) of hypoglycemia. Although we did not measure hormonal counterregulation in study 2, these findings suggested that, as in study 1, rats exposed to 4 days (H4) of recurrent hypoglycemia had impaired glucose counterregulation relative to those exposed to 1 or 2 days of hypoglycemia (H1 or H2).
Fig. 2.
Plasma glucose concentrations in study 2 on terminal study days. Plasma glucose concentration was monitored by repeated blood sampling through implanted jugular vein catheters.
3.3. Effect of recurrent hypoglycemia on brain glucose phosphorylation activity during subsequent hypoglycemia
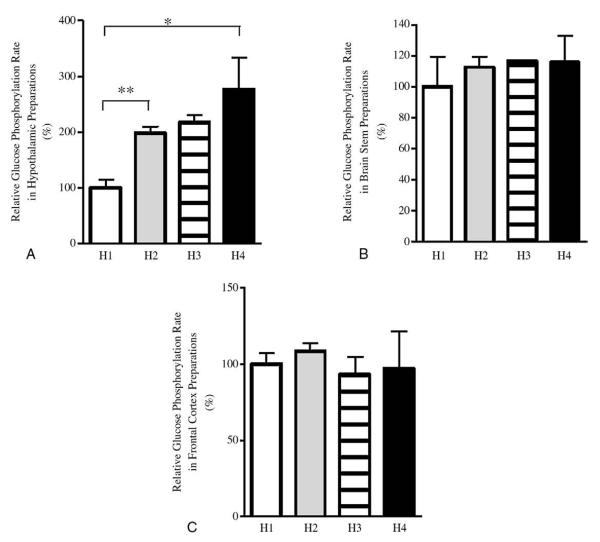
We examined in vitro glucose phosphorylation at a glucose level of 0.5 mmol/L, a level selected as being representative of that seen during hypoglycemia [21], and investigated glucose phosphorylation activity (total HK activity) in vitro at this glucose concentration in brain protein preparations from all groups in study 3. In parallel with impairment of hypoglycemia counterregulation, hypothalamic glucose phosphorylation activity measured at 0.5 mmol/L rose significantly and day dependently in animals with sequential exposure to hypoglycemia. In particular, hypothalamic glucose phosphorylation activity in H4 rats was 2.8 ± 0.6-fold (P < .05) higher than H1 rats (Fig. 3A). These findings suggest that recurrent insulin-induced hypoglycemia results in increased rate of glucose phosphorylation in hypothalamus during subsequent hypoglycemic episodes.
Fig. 3.
Glucose phosphorylation activity in brain protein preparations from rats exposed to 1 to 4 days of hypoglycemia (study 2) represented as H1 to H4, respectively, assayed at 0.5 mmol/L glucose. Final numbers of rats in groups H1 to H4 were 5, 3, 2, and 5, respectively. A, Glucose phosphorylation activity in hypothalamus preparations. B, Glucose phosphorylation activity in brain stem preparations. C, Glucose phosphorylation activity in frontal cortex preparations. *P < .05 and **P < .01; 2-tailed t test.
Next, we sought to examine if this alteration in brain glucose metabolism was restricted to the hypothalamus, consistent with a specific change in blood glucose–sensing regions, or was merely a global adaptive change in brain glucose metabolism. We measured total glucose phosphorylation activity in the presence 0.5 mmol/L glucose in vitro in protein preparations from other brain areas. There was no major change in glucose phosphorylation activity in either the brain stem or frontal cortex in animals with sequential exposure to hypoglycemia (Fig. 3B, C). These findings suggest that glucose phosphorylation activity in different brain regions is affected differently by recurrent hypoglycemia. In particular, increased hypothalamic glucose phosphorylation activity may be a specific adaptation to recurrent hypoglycemia by this nutrient-sensing brain region, where it has been shown previously that glucose-sensing neurons are located [7,8].
4. Discussion
The mechanisms underlying impaired glucose counter-regulation following repetitive hypoglycemia, as experienced by patients with diabetes undergoing aggressive insulin therapy, remain unclear. Several investigators have suggested that alterations in brain glucose metabolism may contribute to the impairment of glucose counterregulation following recurrent hypoglycemia [12-16]. Nonetheless, the precise nature of biochemical alterations in brain glucose metabolism that occurs following recurrent hypoglycemia is unclear.
Here we used a rat model of recurrent hypoglycemia-induced counterregulatory deficit and investigated glucose phosphorylation activity in brain protein preparations from these animals. We first validated our model demonstrating impaired epinephrine responses to hypoglycemia. Our findings are consistent with previous reports showing that recurrent hypoglycemia blunts epinephrine responses to hypoglycemia [24,25].
In study 2, we then measured brain glucose phosphorylation activity to compare progressive changes in hypothalamic glucose phosphorylation with the development of impaired counterregulation. Importantly, we demonstrate for the first time that recurrent hypoglycemia increases the capability for hypothalamus to phosphorylate glucose. One limitation of our work is that we have not directly measured hypothalamic glucose metabolism in vivo. Whether an increase in the potential for hypothalamus to phosphorylate glucose would, in itself, result in a relative preservation of glycolytic flux will depend in turn on whether glucose transport or phosphorylation is rate limiting under hypoglycemic conditions. Under normoglycemic conditions, brain glucose transport is almost certainly not rate limiting for glucose metabolism, although the situation is less clear at hypoglycemia [12-16,26]. It is possible that increased hypothalamic glucose phosphorylation following recurrent hypoglycemia might complement changes in glucose transport from blood into hypothalamus as an adaptation aimed at preserving local cerebral glucose metabolism. Assuming that glucose levels may be monitored by changes in downstream metabolites in hypothalamic sensing cells [10,11], this continued glycolytic flux might be predicted to mask the sensing of a fall in blood glucose [12], which might delay or even impair hypoglycemia counterregulation. Our findings lend credence to recent studies suggesting that the rate of glucose phosphorylation in the hypothalamus may play a critical role in the control of counterregulatory responses during hypoglycemia [27-29].
Although we found no change in glucose phosphorylation activity in the brain stem following recurrent hypoglycemia, there are undoubtedly hypoglycemia sensors in the brain stem that may well play a role in triggering responses to hypoglycemia [30]. Our observations may suggest that distinct glucose-sensing areas of brain respond differently to hypoglycemia. Alternatively, our brain collection in which we collected whole brain stem might have masked local changes in the relatively smaller mass of glucose-sensing cells in the brain stem.
Here, we have not distinguished between the different HK enzymes contributing to glucose phosphorylation activity. In particular, reports have suggested that glucokinase, the low-affinity HK implicated in pancreatic β-cell glucose sensing, might play a role in brain hypoglycemia sensing [30]. By design, we elected here to examine glucose phosphorylation at a physiologic brain glucose concentration of 0.5 mmol/L, a level likely to be seen in the brain during hypoglycemia, rather than use higher glucose levels to assay “maximal” glucose phosphorylation potential, reasoning that our approach would inform better likely about glucose flux during hypoglycemia. Although we did not try here to differentiate the likely contribution from glucokinase to total HK activity, we anticipate that this is likely to be relatively minor at this glucose level given the high Km of glucokinase. It is important to acknowledge that we have not excluded a change in glucokinase activity in a small number of sensing cells that would be undetected in our total hypothalamic assay contributing to impaired counterregulation. Such a change would not, however, explain our marked findings in this work.
In summary, our data show that the potential capability of hypothalamus to phosphorylate glucose activity increases in hypothalamus following repeated exposure to insulin-induced hypoglycemia. This novel finding suggests that an alteration in hypothalamic glucose metabolism at the level of glucose phosphorylation activity may contribute to the development of impaired counterregulation following exposure to recurrent hypoglycemia.
Acknowledgment
This work was supported by Juvenile Diabetes Research Foundation regular Grants 1-2003-78 and 1-2006-29 and Diabetes UK Grant RD05/003059 (to M.L.E.); the Diabetes Research Wellness Foundation (to M.A.O.); and the Cambridge Medical Research Council Centre for Study of Obesity and Related Disorders. L.K.H. was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK065171, American Diabetes Association, and the Wellcome Trust. PH was supported by the Sir Jules Thorne Trust, SH by the Evelyn Trust, S. Moore by a Medical Research Council studentship and S. Markkula by the Elmore Fund.
References
- 1.Cryer P, Davis S, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26:1902–12. doi: 10.2337/diacare.26.6.1902. [DOI] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study (UKPDS) Group Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 3.Diabetes Control and Complications Trial (DCCT) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The diabetes control and complications trial research group. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 4.Borg MA, Sherwin RS, Borg WP, et al. Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest. 1997;99:361–5. doi: 10.1172/JCI119165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borg W, Sherwin R, During M, et al. Local ventromedial hypothalamus glucopaenia triggers counterregulatory hormone release. Diabetes. 1995;44:180–4. doi: 10.2337/diab.44.2.180. [DOI] [PubMed] [Google Scholar]
- 6.Borg W, During M, Sherwin R, et al. Ventromedial hypothalamic lesions in rats suppress counterreguatory responses to hypoglycemia. J Clin Invest. 1994;93:1677–82. doi: 10.1172/JCI117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anand B, Chhina G, Sharma K, et al. Activity of single neurones in the hypothalamus feeding centers: effect of glucose. Am J Physiol. 1964;207:146–1154. doi: 10.1152/ajplegacy.1964.207.5.1146. [DOI] [PubMed] [Google Scholar]
- 8.Oomura Y, Kimura K, Ooyama H, et al. Reciprocal activities of the ventromedial and lateral hypothalamic area of cats. Science. 1964;143:484–5. doi: 10.1126/science.143.3605.484. [DOI] [PubMed] [Google Scholar]
- 9.Kang L, Dunn-Meynell A, Routh V, et al. Glucokinase is a critical regulator of ventromedial hypothalamic neuronal glucosensing. Diabetes. 2006;55:412–20. doi: 10.2337/diabetes.55.02.06.db05-1229. [DOI] [PubMed] [Google Scholar]
- 10.McCrimmon RJ, Evans ML, Fan X, et al. Activation of ATP-sensitive K+ channels in the ventromedial hypothalamus amplifies counter-regulatory hormone responses to hypoglycemia in normal and recurrently hypoglycemic rats. Diabetes. 2005;54:3169–74. doi: 10.2337/diabetes.54.11.3169. [DOI] [PubMed] [Google Scholar]
- 11.Evans ML, McCrimmon RJ, Flanagan DE, et al. Hypothalamic ATP-sensitive K + channels play a key role in sensing hypoglycemia and triggering counterregulatory epinephrine and glucagon responses. Diabetes. 2004;53:2542–51. doi: 10.2337/diabetes.53.10.2542. [DOI] [PubMed] [Google Scholar]
- 12.Lei H, Gruetter R. Effect of chronic hypoglycemia on glucose concentration and glycogen content in rat brain: a localized13 C NMR study. J Neurochem. 2006;99:260–8. doi: 10.1111/j.1471-4159.2006.04115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyle PJ, Kempers SF, O'Connor AM, et al. Brain glucose uptake and unawareness of hypoglycemia in patients with insulin dependent diabetes mellitus. N Engl J Med. 1995;333:1726–31. doi: 10.1056/NEJM199512283332602. [DOI] [PubMed] [Google Scholar]
- 14.Boyle PJ, Nagy RJ, O'Connor AM, et al. Adaptation in brain glucose uptake following recurrent hypoglycemia. Proc Natl Acad Sci. 1994;91:9352–6. doi: 10.1073/pnas.91.20.9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cranston I, Reed LJ, Marsden PK, et al. Changes in regional brain (18) F-fluorodeoxyglucose uptake at hypoglycemia in type 1 diabetic men associated with hypoglycemia unawareness and counter-regulatory failure. Diabetes. 2001;50:2329–36. doi: 10.2337/diabetes.50.10.2329. [DOI] [PubMed] [Google Scholar]
- 16.Arbelaez AM, Powers WJ, Videen TO, et al. Attenuation of counterregulatory responses to recurrent hypoglycemia by active thalamic inhibition: a mechanism for hypoglycemia-associated autonomic failure. Diabetes. 2008;57:470–5. doi: 10.2337/db07-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.González C, Ureta T, Sanchez R, et al. Multiple molecular forms of ATP: hexose 6-phosphotransferase from rat liver. Biochem Biophys Res Commun. 1964;16:347–52. doi: 10.1016/0006-291x(64)90038-5. [DOI] [PubMed] [Google Scholar]
- 18.Flanagan DE, Keshavarz T, Evans ML, et al. Role of corticotrophin-releasing hormone in the impairment of counterregulatory responses to hypoglycaemia. Diabetes. 2003;52:605–13. doi: 10.2337/diabetes.52.3.605. [DOI] [PubMed] [Google Scholar]
- 19.Niswender KD, Postic C, Jetton TL, et al. Cell-specific expression and regulation of a glucokinase gene locus transgene. J Biol Chem. 1997;272:22564–9. doi: 10.1074/jbc.272.36.22564. [DOI] [PubMed] [Google Scholar]
- 20.Jetton T, Liang Y, Pettepher C, et al. Analysis of upstream glucokinase promoter activity in transgenic mice and identification of glucokinase in rare neuroendocrine cells in the brain and gut. J Biol Chem. 1994;269:3641–54. [PubMed] [Google Scholar]
- 21.Abi-Saab WM, Maggs DG, Jones T, et al. Striking differences in glucose and lactate levels between brain extracellular fluid and plasma in conscious human subjects: effects of hyperglycemia and hypoglycemia. J Cereb Blood Flow Metab. 2002;22:271–9. doi: 10.1097/00004647-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Miwa I, Mita Y, Murata T, et al. Utility of 3-O-methyl-N-acetyl-D-glucosamine, an N-acetylglucosamine kinase inhibitor, for accurate assay of glucokinase in pancreatic islets and liver. Enzyme Protein. 1994;48:135–42. doi: 10.1159/000474980. [DOI] [PubMed] [Google Scholar]
- 23.Westermann J, Hubl W, Kaiser N, et al. Simple, rapid and sensitive determination of epinephrine and norepinephrine in urine and plasma by non-competitive enzyme immunoassay, compared with HPLC. Clinical Laboratory. 2002;48:61–71. [PubMed] [Google Scholar]
- 24.Heller SR, Cryer PE. Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia in non-diabetic humans. Diabetes. 1991;40:223–6. doi: 10.2337/diab.40.2.223. [DOI] [PubMed] [Google Scholar]
- 25.Powell AM, Sherwin RS, Shulman GI. Impaired hormonal responses to hypoglycemia in spontaneously diabetic and recurrently hypoglycemic rats: reversibility and stimulus specificity of the deficits. J Clin Invest. 1993;92:2667–74. doi: 10.1172/JCI116883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gruetter R, Novotny EJ, Boulware SD, et al. Direct measurement of brain glucose concentrations in humans by 13C NMR spectroscopy. Proc Natl Acad Sci USA. 1992;89:1109–12. doi: 10.1073/pnas.89.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang L, Sanders NM, Dunn-Meynell AA, et al. Prior hypoglycemia enhances glucose responsiveness in some ventromedial hypothalamic glucosensing neurons. Am J Physiol Regul Integr Comp Physiol. 2008;294:R784–792. doi: 10.1152/ajpregu.00645.2007. [DOI] [PubMed] [Google Scholar]
- 28.Levin BE, Becker TC, Eiki J, et al. Ventromedial hypothalamic glucokinase is an important mediator of the counterregulatory response to insulin-induced hypoglycemia. Diabetes. 2008;57:1371–9. doi: 10.2337/db07-1755. [DOI] [PubMed] [Google Scholar]
- 29.Song Z, Routh VH. Recurrent hypoglycemia reduces the glucose sensitivity of glucose-inhibited neurons in the ventromedial hypothalamus nucleus. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1283–787. doi: 10.1152/ajpregu.00148.2006. [DOI] [PubMed] [Google Scholar]
- 30.Andrew SF, Dinh TT, Ritter S. Localized glucoprivation of hindbrain sites elicits corticosterone and glucagon secretion. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1792–8. doi: 10.1152/ajpregu.00777.2006. [DOI] [PubMed] [Google Scholar]