Abstract
The peptide corticotropin-releasing factor (CRF) was initially identified as a critical component of the stress response. CRF exerts its cellular effects by binding to one of two cognate G-protein coupled receptors (GPCRs), CRF receptor 1 (CRFR1) or 2 (CRFR2). While these GPCRs were originally characterized as being coupled to Gαs, leading to downstream activation of adenylyl cyclase (AC) and subsequent increases in cAMP, it has since become clear that CRFRs couple to and activate numerous other downstream signaling cascades. In addition, CRF signaling influences the activity of many diverse brain regions, affecting a variety of behaviors. One of these regions is the striatum, including the nucleus accumbens (NAc). CRF exerts profound effects on striatal-dependent behaviors such as drug addiction, pair-bonding, and natural reward. Recent data indicate that at least some of these behaviors regulated by CRF are mediated through CRF activation of the transcription factor CREB. Thus, we aimed to elucidate the signaling pathway by which CRF activates CREB in striatal neurons. Here we describe a novel neuronal signaling pathway whereby CRF leads to a rapid Gβγ- and MEK-dependent increase in CREB phosphorylation. These data are the first descriptions of CRF leading to activation of a Gβγ-dependent signaling pathway in neurons, as well as the first description of Gβγ activation leading to downstream CREB phosphorylation in any cellular system. Additionally, these data provide additional insight into the mechanisms by which CRF can regulate neuronal function.
Introduction
Stress is any actual or perceived disturbance of an organism's environment. An acute stress response, which includes the release of corticotropin-releasing factor (CRF), is often necessary for the preservation of organismal integrity during brief anxiety situations. CRF binding to its cognate G-protein coupled receptors (GPCRs), CRF receptor 1 (CRFR1) and 2 (CRFR2), mediates the influence of CRF on brain cells. However, in addition to their beneficial role in an acute stress response, both stress and CRF have been implicated in pathological disease states, including drug addiction.
Several lines of evidence suggest that stress, and CRF in particular, influence addiction and addictive behaviors. Drugs of abuse have been shown to activate the hypothalamic-pituitary-adrenal (HPA) axis [1], initiating the stress response. Human individuals with a history of chronic stress are more likely to become addicts, and previously abstinent addicts are more likely to relapse following exposure to an acute stressor [1]–[3]. A stress event both increases drug-seeking behavior as well as facilitates conditioned-place preference to drugs of abuse in animal models of addiction [4]–[10]. Furthermore, stress and CRF potentiate the rewarding effects of drugs of abuse [11].
Recent findings suggest that CRF-induced CREB phosphorylation within the nucleus accumbens (NAc) underlies at least some of these effects of stress on addictive behaviors [11]. As CREB signaling in NAc is critical for the rewarding actions of drugs of abuse [12]–[16], CRF activation of CREB represents a putative molecular mechanism by which stress could manipulate the neural circuitry underlying drug addiction.
Not surprisingly, the effects of CRF on NAc functioning are not limited to actions related to drug abuse, but also affect the rewarding actions of more physiological stimuli. For example, CRF acting in NAc plays an essential role in prairie-vole pair bonding [17], as well as enhances the incentive salience of a sucrose reward [18]. And while research thus far has focused on the NAc, CRF neurotransmission is also an essential component to connectivity in the dorsal striatum [19]–[22].
Although the influence of CRF on striatal functioning has been established, the molecular mechanisms by which this occurs remain unclear. Given the known relevance for CRF activation of CREB, we characterized the intracellular signaling pathway by which this occurs. While CRFRs are classically thought of as Gαs-coupled GPCRs that exert their effects via activation of adenylyl cyclase (AC) and subsequent increases in the second messenger cAMP [23], [24], these receptors have since been shown to couple to multiple G-protein signaling cascades in neurons [25]. Through use of pharmacological and genetic approaches, we report a novel signaling pathway whereby CRF leads to a rapid Gβγ-dependent increase in CREB phosphorylation: an effect mediated by MAPK signaling. In addition to illuminating the pathways by which CRF affects striatal neurons, this is the first example of CRF leading to downstream Gβγ-dependent signaling, as well as the first example of Gβγ activation leading to CREB phosphorylation.
Results
CRF Induces Rapid CREB Phosphorylation via Activation of CRFR1
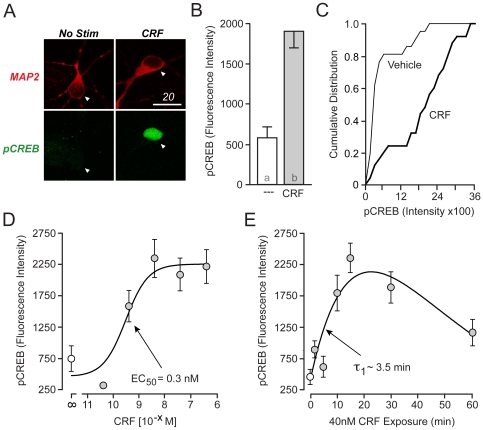
Stress-induced activation of CREB in the striatum requires CRFR1 activation [11]. Thus, we attempted to determine whether CRF can lead to CREB phosphorylation (pCREB) in cultured striatal neurons from 1- to 2-day old rat pups, and if so, through which molecular pathway(s). Indeed, a 15-min application of CRF (40 nM) significantly increased CREB phosphorylation relative to vehicle-stimulated control neurons (Figure 1), but had no effect on total CREB staining (Figure S1). When measuring pCREB fluorescence, CRF produced an observable shift in the population response of striatal neurons (Figure 1C). Specifically, plotting the pCREB data as a cumulative histogram revealed that, CRF produced a large shift in pCREB fluorescence intensity in approximately 80% of the striatal neurons (Figure 1C). Furthermore, CRF increased CREB phosphorylation in a concentration-dependent manner with an EC50 = 0.3 nM (Figure 1D), consistent with a CRFR-mediated event (binding affinity of CRF for CRFR1 ∼ 5–10 nM [26]). CRF-induced increases in pCREB occurred in a rapid and time-dependent manner (τ∼3.5 minutes), with extended exposure (1 hr) producing apparent desensitization (Figure 1E).
Figure 1. CRF rapidly stimulates CREB phosphorylation.
(A) Immunolabeled confocal images of cultured striatal neurons from 1- to 2-day old rat pups with MAP2 (red) and pCREB (green). Neurons stimulated with CRF (40 nM) for 15 min exhibited increased CREB phosphorylation (Scale Bar = 20 µm). (B) Quantification of immunostaining, revealing CRF-mediated CREB phosphorylation (p<0.0001). (C) CRF induced a rightward shift in the plot of pCREB fluorescence intensity of approximately 80% of striatal neurons (D) CRF increased CREB phosphorylation in a concentration-dependent manner, with EC50 = 0.3 nM. Concentrations ≥ 4 nM induced a signal that differed with statistical significance from vehicle-stimulated (NS) neurons. (E) Time course of 40 nM CRF-induced CREB phosphorylation, with τ = 3.5 min. Statistically different groups are denoted by different alphabetical characters in corresponding bar graphs in this and subsequent figures. P-values <0.05 were considered a priori as significant.
The striatum is composed of two major sub-regions, the dorsal striatum (i.e. caudate/putamen) and nucleus accumbens (NAc). To determine whether CRF-induced CREB phosphorylation is restricted to neurons from one of these two anatomical regions, we created dorsal striatum “enriched” and NAc “enriched” cultures. Both cultures displayed similarly robust increases in CREB phosphorylation upon CRF application (data not shown), suggesting that there are no overt differences in CRF-responsiveness between these two brain regions. Thus, we performed our remaining experiments in whole striatal cultures.
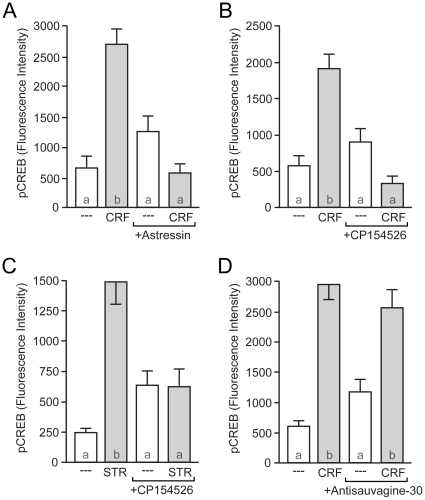
The striatum expresses both CRFRs, although CRFR1 to a greater extent than CRFR2 [27]–[30]. We confirmed expression of both receptors in our cultured neuronal system using real-time PCR (data not shown). To determine whether CRF-induced CREB phosphorylation occurs via activation of these classic CRFRs, we pre-incubated our striatal neurons with the non-specific CRFR peptide antagonist astressin (50 nM). Indicative of a CRFR-mediated event, this treatment completely abolished CRF-induced CREB phosphorylation (Figure 2A). Next, we sought to determine which receptor mediates CRF-induced CREB phosphorylation. Pre-incubation with the specific CRFR1 antagonist CP154526 (100 nM) eliminated CRF-induced CREB phosphorylation (Figure 2B). In addition, a 15 min application of the CRFR1-specific agonist stressin-1 (STR; 70 nM) mimicked the effect of CRF, and this effect was also blocked with CP154526 (Figure 2C). To determine any putative role of CRFR2 in CRF-induced CREB phosphorylation, we utilized two CRFR2-preferring peptide antagonists. Both antisauvagine-30 (100 nM; Figure 2D) and K41498 (10 nM) (not shown) failed to block CRF-induced CREB phosphorylation. Together, these data strongly demonstrate that CRF induced CREB phosphorylation in striatal neurons is mediated exclusively through CRFR1, with CRFR2 playing no discernable role.
Figure 2. CRFR1 mediates CRF-induced CREB phosphorylation.
(A) CRF-mediated CREB phosphorylation was blocked by the non-specific CRFR antagonist astressin (50 nM; F = 20.19), (B) and the CRFR1-specfic antagonist CP154526 (100 nM; F = 17.40). (C) CRF-induced CREB phosphorylation was mimicked by the CRFR1 agonist stressin-1 (STR; 70 nM). STR-induced CREB phosphorylation was also blocked by CP154526 (F = 15.99). (D) The CRFR2-specific antagonist antisauvagine-30 (100 nM) had no effect on CRF-induced CREB phosphorylation (F = 17.10).
CRF Does Not Induce CREB Phosphorylation via the AC/cAMP/PKA Pathway
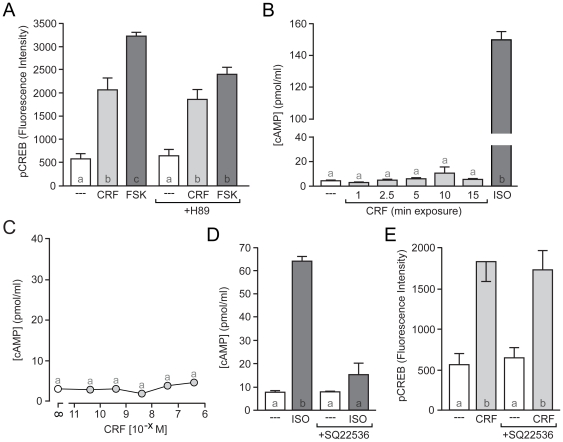
CRFR1 is primarily thought of as a GPCR that signals through Gαs to produce subsequent increases in cAMP. Hence, we decided first to test the hypothesis that CRF activation of CRFR1 leads to an AC/cAMP/PKA-dependent increase in CREB phosphorylation. To assay for the involvement of this pathway, we began with inhibition of PKA. A PKA-specific concentration [31], [32] of the protein kinase inhibitor H-89 (2 µM) failed to block CRF-induced CREB phosphorylation (Figure 3A). Importantly, this concentration of H89 attenuated CREB phosphorylation stimulated by the AC agonist forskolin (FSK; 25 µM), which signals to CREB through multiple cAMP-responsive proteins including PKA (Figure 3A). Given this unexpected result, we attempted to block CRF-induced CREB phosphorylation with three additional PKA antagonists: the cell permeable peptide PKA inhibitor PKI, 14–22 amide (1 µM), KT5720 (1 µM), and cAMPS-Rp, triethylammonium salt (10 µM). All three compounds also failed to eliminate CRF-induced CREB phosphorylation (data not shown). Together, these data demonstrate that PKA is not involved in CRF-induced CREB phosphorylation in striatal neurons.
Figure 3. CRF-induced CREB phosphorylation occurs independently of the AC/cAMP/PKA pathway.
(A) A PKA-specific concentration of the protein kinase inhibitor H89 (2 µM) failed to block CRF-induced CREB phosphorylation, but attenuated forskolin (FSK)-induced CREB phosphorylation (F = 35.78). (B) In the presence of the phosphodiesterase inhibitor IBMX (75 µM), CRF (40 nM) failed to increase cAMP during the time-course in which it increases pCREB. The β-adrenergic receptor agonist isoproterenol (ISO; 10 µM) was used as a positive control (F = 3226). (C) Several concentrations of CRF up to and including 400 nM failed to increase cAMP accumulation (in the presence of IBMX). (D) Inhibiting AC activity with SQ22536 (90 µM) completely blocked ISO-induced cAMP accumulation (F = 101.4). (E) Inhibition of AC with SQ22536 had no effect on CRF-induced CREB phosphorylation (F = 17.10).
We next determined whether CRF was in fact eliciting increases in cAMP under conditions in which CRF was producing CREB phosphorylation. Hence, we quantified cAMP concentrations following the CRF stimulation paradigm used to elicit increases in CREB phosphorylation. To prevent potential loss of the cAMP signal during the agonist stimulations, the phosphodiesterase inhibitor IBMX (75 µM) was included both prior to and during stimulation. Exposure of striatal cultures to CRF (40 nM) for durations that elicited CREB phosphorylation failed to induce any measurable increase in cAMP concentration (Figure 3B). In fact, stimulation of striatal cultures for 15 min with various concentrations of CRF up to and including 400 nM (Figure 3C) failed to induce a significant accumulation of cAMP. As a positive control for our assay [33], a 15 min application of the β-adrenergic receptor agonist isoproterenol (ISO; 10 µM) resulted in a significant increase in cAMP (Figure 3B).
To directly test whether cAMP signaling played a role in CRF-induced CREB phosphorylation, we treated striatal cultures with the AC antagonist SQ22536 (90 µM). While SQ22536 completely blocked ISO-induced cAMP formation (Figure 3D), it failed to affect CRF-induced CREB phosphorylation (Figure 3E). Together, these data demonstrate that rapid CRF-induced CREB phosphorylation in striatal neurons operates independently of AC, cAMP, and PKA signaling.
CRF Induces CREB Phosphorylation via Calcium-Independent MAPK Signaling
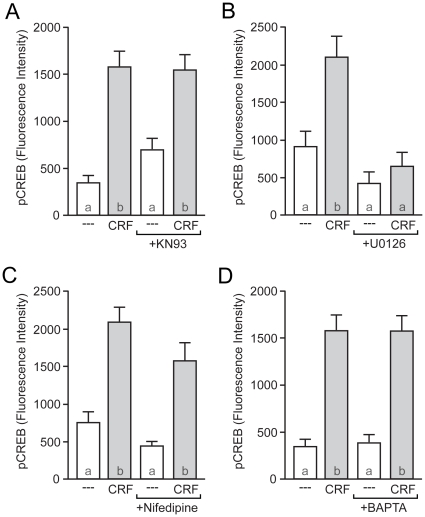
Independent of PKA, several downstream kinase pathways have been shown to regulate CREB phosphorylation [34], [35]. One such kinase is CaMKIV. However, blocking CaMK activation with KN-93 (2 µM) had no effect on CRF-induced CREB phosphorylation (Figure 4A), although this concentration of KN-93 blocks depolarization-induced CREB phosphorylation [36].
Figure 4. CRF-induced CREB phosphorylation occurs via a MEK/MAPK-dependent mechanism.
(A) The CaMK inhibitor KN-93 (2 µM) did not affect CRF-induced CREB phosphorylation (F = 17.65). (B) CRF-induced CREB phosphorylation was blocked by the MEK antagonist U0126 (10 µM; F = 11.97). (C) The L-type Ca2+ channel blocker nifedipine (5 µM) had no effect on CRF-induced CREB phosphorylation (F = 18.50). (D) Chelating intracellular calcium with BAPTA-AM (30 min incubation with 5 µM) failed to block CRF-induced CREB phosphorylation (F = 12.72).
MAPK signaling is another pathway that has been shown capable of CREB activation in a variety of neuronal preparations. In contrast to previous experiments, the MEK (MAPK kinase) inhibitor U0126 (10 µM) blocked CRF-induced CREB phosphorylation (Figure 4B). A second MEK antagonist, PD98059 (25 µM), yielded a similar result (data not shown). MAPK-dependent CREB phosphorylation is often calcium dependent, following calcium entry through L-type calcium channels, or release of calcium from intracellular stores. However, CRF-mediated CREB phosphorylation was not affected by either the L-type calcium channel blocker nifedipine (5 µM; Figure 4C) or by chelating intracellular calcium through pre-incubation with BAPTA-AM (Figure 4D). Removing extracellular calcium during CRF stimulation also had no effect on CRF-mediated CREB phosphorylation (data not shown). Thus, CRF activation of MAPK signaling appears to be calcium independent.
CRF Induces CREB Phosphorylation via a Gβγ-Dependent Mechanism
We next decided to test the involvement of several upstream signalers that could lead to downstream MAPK activation, including Akt, receptor tyrosine kinases (RTKs), Ras, Raf, ROCK, and PI3K. We utilized pharmacological blockers for each of these molecules to test for any contribution in CRF-induced CREB phosphorylation. Remarkably, inhibitors of Akt (10-DEBC; 5 µM), RTKs (K252A; 100 nM), Ras (XRP44X; 2 µM), Raf (GW5074; 1 µM) ROCK (H-1152; 500 nM), and PI3K (wortmannin; 500 nM) all failed to affect CRF-induced CREB phosphorylation (data not shown). Unable to identify an intermediary signaling molecule upstream of MEK but downstream of CRFR1, we decided to test for the involvement of different G-protein subunits in CRF-induced CREB phosphorylation.
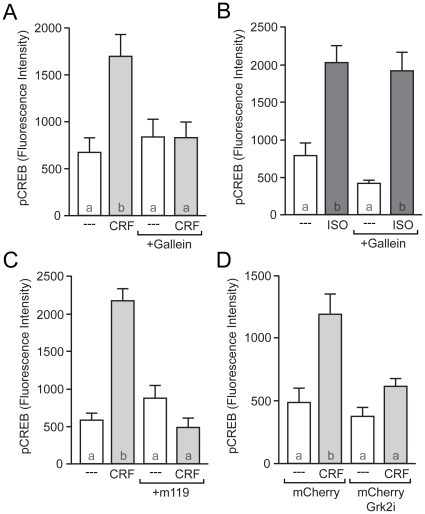
To test for a Gβγ-dependent mechanism in CRF-induced CREB phosphorylation, we utilized the Gβγ-specific inhibitor gallein (75 µM) [37]. In support of a role for Gβγ, CRF failed to induce CREB phosphorylation in the presence of this drug (Figure 5A). To ensure that the effect of gallein was specific to CRF and not dampening all G-protein signaling, we stimulated our neurons with ISO (10 µM) in the presence of gallein. Since ISO leads to CREB phosphorylation via a Gαs pathway in striatal neurons [33], gallein should not affect ISO-dependent CREB phosphorylation. Indeed, this was in fact the case (Figure 5B). To verify our initial results, we blocked CRF-induced CREB phosphorylation with a second Gβγ-specific inhibitor, M119 (5 µM; Figure 5C). Again M119 had no effect on ISO-induced CREB phosphorylation (data not shown; also see [33]).
Figure 5. CRF-induced CREB phosphorylation occurs via a Gβγ-dependent mechanism.
(A) The Gβγ-specific blocker gallein (75 µM) eliminated CRF-induced CREB phosphorylation (F = 6.13). (B) In contrast, gallein had no effect on CREB phosphorylation induced by the β-adrenergic receptor agonist, isoproterenol (F = 16.83). (C) An additional Gβγ blocker (M119; 5 µM) also eliminated CRF-induced CREB phosphorylation (F = 27.96). (D) Neurons transfected mCherry and the Gβγ inhibitory peptide Grk2i failed to exhibit CRF-induced CREB phosphorylation (F = 11.26).
To complement these pharmacological data, we utilized a genetic approach to further characterize the involvement of Gβγ in CRF-induced CREB phosphorylation. Neurons were transfected with either a construct expressing mCherry or co-transfected with the mCherry construct plus a construct expressing the peptide motif of G-protein regulated kinase that scavenges Gβγ (Grk2i), which has previously been shown to specifically block downstream Gβγ signaling [38]. Neurons that were transfected with mCherry alone demonstrated robust CRF-induced CREB phosphorylation. However, neurons that were transfected with Grk2i failed to show CRF-induced CREB phosphorylation (Figure 5D). Together, these pharmacological and genetic data support the hypothesis that CRF-induced CREB phosphorylation is mediated via a Gβγ-dependent signaling mechanism.
CRFR1 Couples to Gαs in Striatal Neurons
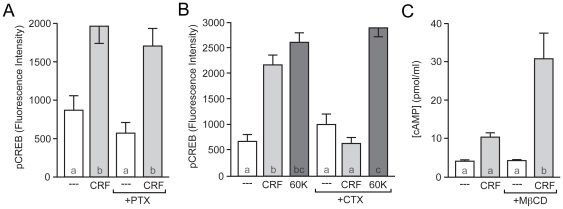
To identify the Gα subunit to which CRFR1 and Gβγ couple in this paradigm, we utilized toxins that specifically target functional classes of Gα. Since functional Gβγ subunits in neurons are classically thought to associate with Gαi/o, we first utilized pertussis toxin (PTX) to specifically inhibit these Gα subunits. PTX prevents the exchange of GDP (inactive state) for GTP (active state), thus hindering the G-protein trimer (Gαβγ) from dissociating from the GPCR, and initiating downstream signaling. Overnight (18 hr) pretreatment of striatal neurons with PTX (0.5 µng/µL) did not alter CRF-mediated CREB phosphorylation (Figure 6A).
Figure 6. CRFR1 couples to Gαs in striatal neurons.
(A) Pre-treatment with pertussis toxin (PTX; 500 ng/mL) did not affect CRF-induced pCREB (F = 11.10). (B) CTX blocked CRF-induced CREB phosphorylation, but had no effect on MAPK-dependent CREB phosphorylation induced by depolarization (60 K; F = 35.15). (C) CRF (4 µM) increased cAMP concentrations in the presence of the cholesterol chelator methyl-β-cyclodextrin (MβCD; 10 mM).
Next, we targeted the Gαs family, which includes Gαs and Gαolf in the striatum [39] (hereafter termed Gαs), with cholera toxin (CTX). CTX blocks inactivation of Gαs by preventing the hydrolysis of GTP (active state) to GDP (inactive state). A short term (2 hr) pre-treatment with CTX induced a transient increase in pCREB on its own (NS: 397.7±93.5; CTX: 2809.7±104.5), confirming that Gαs can signal to CREB in this paradigm. However, to test the effect of disrupting Gαs signaling on CRF-induced CREB phosphorylation, we used long-term CTX treatment, which allows the pCREB signal to reset, as well as prevents Gαs from re-associating with either the Gβγ subunits or the GPCR, precluding agonist-induced reactivation of this signaling pathway. Indeed, overnight pretreatment with CTX resulted in a complete block of CRF-induced CREB phosphorylation (Figure 6B). Importantly, MAPK-mediated CREB phosphorylation following a 60 mM K+ depolarization [36] was unaffected by this treatment (Figure 6B), indicating signaling downstream of CRFR1 was still intact.
Since AC is the primary downstream effector of Gαs, and since CRFR1 couples to Gαs in striatal neurons (Figure 6), we wondered why we failed to observe CRF-induced cAMP accumulation (Figure 3). We hypothesized that CRFR1 is in fact organized into discrete membrane lipid microdomains [40], which (1) facilitate Gβγ activation of downstream MAPK signaling and (2) prevents Gαs from accessing/stimulating AC. Therefore, we hypothesized that disrupting lipid microdomains would allow Gαs to activate AC following CRF stimulation. To test this hypothesis we pre-incubated our striatal neurons with the cholesterol chelator methyl-β-cyclodextrin (MβCD; 10 mM), commonly used to disrupt lipid microdomains [41], [42]. We found that unlike control conditions, 4 µM CRF induced a significant increase in cAMP formation in the presence of MβCD (Figure 6C), although notably, not to the levels seen with isoproterenol (see discussion). These data are consistent with the hypothesis that CRF is liberating Gαs subunits in order to mediate Gβγ-mediated CREB phosphorylation.
Discussion
CRF Increases CREB Phosphorylation via a Novel Signaling Pathway
CRF signaling in neurons was initially characterized as being mediated via the Gαs-coupled CRFR1, and subsequent downstream activation of AC, cAMP and PKA. However, given the recent evidence for the promiscuity of CRF coupling to intracellular signaling pathways, and the profound influence of CRF on NAc processes via CREB, our experiments were designed to determine the specific signaling pathway by which CRF-induced CREB phosphorylation in striatal neurons. Using a combination of pharmacological and genetic approaches, we report a novel neuronal signaling pathway whereby CRF binds to a Gαs-coupled CRFR1, leading to a Gβγ- and MAPK-dependent increase in CREB phosphorylation. Notably, these data are consistent with previous findings showing that stress-facilitation of drug reward depends on CRFR1 and CREB activation in NAc [11], as well as the finding that CRFR1 is the predominant CRF receptor expressed within this brain region [27]–[30].
These data demonstrating CRF signaling through a non-canonical pathway are consistent with numerous reports of CRFRs coupling to diverse intracellular signaling pathways across distinct neuronal populations. CRF has been shown to activate CREB in cerebellar and hippocampal neurons [43], as well as MAPKs in several brain regions [44]. Separate from CREB/MAPK signaling, CRF also affects the activity of various other kinases, second messengers, ion channels and neurotransmitter receptors [45]–[51]. This diversity in cellular effects is not surprising when one considers the ability of CRF to influence a variety of natural and pathological behaviors [17], [52]–[54]. However, this report is unique among papers describing CRF signaling in neurons in that it describes a novel upstream signaling activator at the level of the G-protein subunit (Gβγ).
Although this is the first report demonstrating that Gβγ leads to CREB activation, and that CRF and CRFR1 signal via Gβγ in neurons, there have been reports of CRF acting through Gβγ-dependent mechanisms in cell lines expressing recombinant protein. These include findings that CRF effects on T-type calcium channels [55] and intracellular calcium signaling [56] depend on Gβγ function, as well as a study that found that Gβγ is important for CRFR desensitization [57]. There is also a precedent for activation of Gβγ leading to downstream MAPK activation in non-neuronal cell lines [58]–[60]. One group has shown indirect evidence for Gβγ mediated CREB activation in cerebellar granule neurons [61] by demonstrating (1) that Gβγ subunits mediate GABAB receptor-induced ERK activation, and (2) that GABAB-mediated CREB phosphorylation is sensitive to MEK/ERK inhibition. Importantly, the authors did not demonstrate in their pCREB assay that GABAB-induced CREB phosphorylation is sensitive to Gβγ inhibition. Hence, our approach is unique in that it is the first report using a direct assay of CREB phosphorylation to demonstrate that agonist-induced increases in pCREB are sensitive to inhibition of Gβγ signaling. Given the diversity of Gβγ signaling [58], [60] and the plethora of CRF effects in numerous brain regions and peripheral tissues [25], future studies can test for a role of Gβγ signaling in CRF function throughout the brain and other tissue.
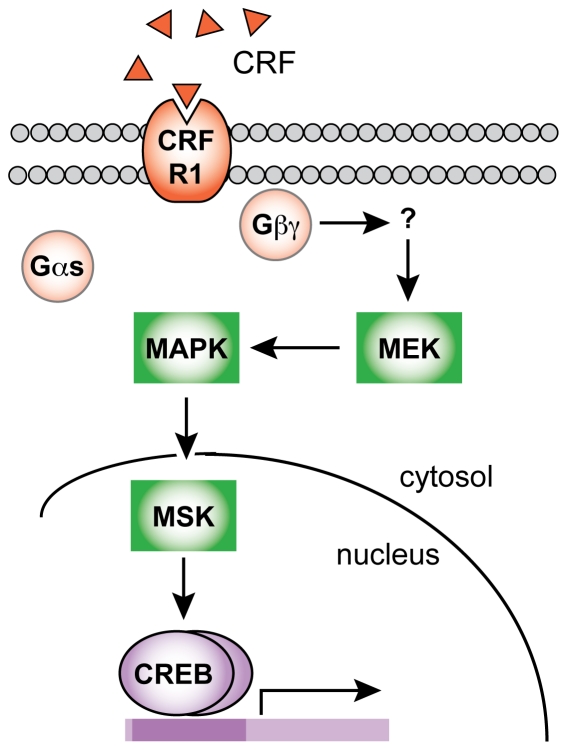
The mechanism by which Gβγ leads to MAPK signaling in striatal neurons remains elusive [59], [60]. One hypothesis is that an intermediate signaling moiety couples Gβγ to MEK (Figure 7). We assayed for the involvement of signaling molecules that have previously been shown to lead to downstream MAPK/CREB activation, are known to be activated by Gβγ, or both. Pharmacological inhibitors/blockers of L-type calcium channels, CaMK, AKT/PKB, RTKs, ROCK, Ras, Raf, and PI3K all failed to block CRF-induced CREB phosphorylation. Furthermore, we ruled out any contribution of calcium to CRF induced CREB phosphorylation in these neurons.
Figure 7. Proposed mechanism of CRF-induced CREB phosphorylation in striatal neurons.
CRF binds to Gαs-coupled CRFR1, leading to a Gβγ-dependent activation of MEK and MAPK, resulting in downstream CREB phosphorylation.
Alternatively, Gβγ could physically interact with and activate MEK to initiate MAPK signaling. Gβγ has been shown to interact with and activate a variety of downstream signaling molecules, including AC and ion channels [60]. However, MEK/MAPK are not among those known be physically regulated by Gβγ activation. Interestingly, Gβγ has been shown to traffic to cytoplasmic sites distinct from the plasma membrane, including the Golgi complex and the endoplasmic reticulum [62], [63]. It is unknown whether receptor-mediated activation of Gβγ subunits can transduce cytoplasmic signaling via translocation into the cytoplasm. However, the hypothesis that Gβγ activated at the membrane can translocate to the cytoplasm to lead to MEK activation cannot be ruled out. Although beyond the scope of this study, future investigations in both neuronal and non-neuronal cells will be needed to determine the exact mechanism by which Gβγ leads to downstream MEK/MAPK activation.
Independent of the mechanism by which Gβγ activates downstream signaling in this paradigm, we hypothesize that the Gβγ subunits released from CRFR1 in response to ligand binding are unique in their ability to elicit downstream CREB phosphorylation, insofar as dissociation of Gβγ from any GPCR is insufficient for this effect. For instance, ISO-mediated activation of β-adrenergic receptors does not result in Gβγ-mediated CREB phosphorylation (Figure 5B). One hypothesis is that CRFR1-coupled Gβγ subunits have a unique molecular property (i.e. post-translational modification) that facilitates activation of downstream signaling pathways. However, our data with the cholesterol chelator MβCD (Figure 6C) suggest an alternative hypothesis whereby the sub-cellular localization of CRFR1 in discrete lipid microdomains allows its dissociated Gβγ subunits privileged access to the downstream signaling machinery necessary for CREB phosphorylation, while preventing CRFR1-associated Gαs from accessing AC. Indeed, an emerging theme in neuronal intracellular signaling is the hypothesis that both membrane-associated and intracellular signaling proteins are organized into discrete structural and functional microdomains that facilitate efficacious intracellular signaling [40]. There is precedent for the organizational influence of scaffolding proteins and microdomains in neuronal ion channel and GPCR-mediated CREB phosphorylation. For example, the privileged ability of L-type calcium channels to mediate downstream CREB phosphorylation, at the exclusion of other calcium influx mechanisms, is due to the specific sub-cellular localization of these L-channels [64]. Our lab has further shown that caveolin proteins (CAVs) are necessary for the functional isolation of distinct GPCR signaling pathways in both hippocampal and striatal neurons [40], [65], [66]. Future experiments will test the hypothesis that functional microdomains and scaffolding proteins, such as CAVs, mediate signaling specificity in this paradigm.
CRF Signaling in Striatal Neurons
The interaction between stress and drug addiction is well established. Stress both pre-disposes individuals to drug-addiction, as well as triggers relapse in previously abstinent addicts [1]–[3]. Animal models of stress and drug addiction mirror these results from clinical populations. Stress enhances locomotor responses to drugs of abuse, and enhances acquisition of self-administration [53]. Stress has also been shown to re-activate extinguished CPP for cocaine [4].
CRF is the molecular mediator of many of these effects of stress on drug taking behavior [67]. Acute administration of drugs of abuse activates both hypothalamic and extra-hypothalamic CRF, while CRF administration mimics the effects of stress on drug taking behaviors in animal models. Since the NAc is thought to be the site of many of the neuro-plastic changes that underlie drug addiction, and since CRF has been shown to influence NAc-dependent behaviors and neurons [11], [17], [18], [68], we hypothesize that CRF action in NAc neurons underlies some of the effects of stress on addictive behaviors. It must be noted that NAc is not the sole site of CRF influence on addiction. In the context of addictive processes, CRF has been shown to influence several regions of the mesolimbic dopamine system including the VTA and amygdala [67].
Here we show that CRF can, in fact, directly influence NAc neurons by activating CREB, a transcription factor that is important for the responses to stress [69], as well as drug reward [12]. Our data are consistent with a report showing that stress-facilitation of drug reward depends on CRFR1 and CREB [11]. It has been known for quite some time that many of the plastic changes in NAc neurons required for drug addiction are dependent on transcription of genes and synthesis of new proteins, and that the transcription factor CREB plays a key role [13].
This work, which identifies a novel mechanism of CRF signal initiation at the level of the Gβγ subunit in striatal neurons, adds to a growing literature describing the diverse intracellular signaling pathways that can be activated by CRF. However, it remains to be determined whether CRF-induced Gβγ signaling is unique to the striatum, or whether it represents an essential mechanism of CRF signaling across diverse brain regions. In terms of CRF action in the striatum, our working hypothesis is that stress-induced release of CRF into NAc could lead to CREB activation via the pathway identified here, thus “priming” these neurons for CRE-dependent transcription following a robust drug-induced stimulus. Consequently, CRF may shift the transcriptional balance towards the expression of genes that are required for the plastic changes that characterize addiction, thereby facilitating addictive behaviors. Future work will be aimed at determining the extent by which CRF activation of this Gβγ pathway underlies the physiological and behavioral effects of CRF in vivo.
Conclusion
CRF was first described as the neuropeptide that mediated the HPA response to stress. Since then, CRF has been shown to modulate a wide variety of brain regions outside of the traditional HPA axis, including the NAc. Here we report a novel molecular signaling pathway in striatal neurons in which CRF leads to a Gβγ- and MAPK-dependent increase in CREB phosphorylation. This pathway not only sheds light on CRF signaling by characterizing a novel, neuronal CRF initiated signaling pathway, but also reveals novel potential targets for altering the effects of CRF on a variety of behaviors.
Materials and Methods
Neuronal Cell Culture
Striatal neurons were cultured from 1- to 2-d-old rat pups as previously described [65], [70], using a protocol approved by the Animal Care and Use Committee at the University of Minnesota. Chemicals and drugs were obtained from Sigma (St. Louis, MO) or Tocris (Ellisville, MO) unless otherwise noted. Following decapitation, the striatum was isolated in cold HBSS containing 20% fetal bovine serum (FBS; HyClone; Logan, UT), 4.2 mM NaHCO3, and 1 mM HEPES, pH 7.35 at 300 mOsm. Tissue was washed before a 5 min digestion in trypsin solution (type XI; 10 mg/mL) containing 137 mM NaCl, 5 mM KCl, 7 mM Na2HPO4, 25 mM HEPES, and 1500 U of DNase at pH 7.2 and 300 mOsm. For dorsal striatum and central striatum enriched cultures, a coronal section was made at the level of the striatum. The dorsal and ventral portions were identified using the anterior commissure, and then transected using a tungsten needle. Following additional washes, the tissue was dissociated, and the cell suspension was pelleted twice before being plated (6×104 cells per well) on Matrigel (BD Biosciences; San Jose, CA)-treated 10 mm coverslips. Cells were incubated at RT for 15 min. One mL of MEM (Invitrogen; Carlsbad, CA) containing 28 mM glucose, 2.4 mM NaHCO3, 0.0013 mM transferring (Calbiochem; La Jolla, CA), 2 mM glutamine, 0.0042 mM insulin, 1% B-27 Supplement (Invitrogen), and 10% FBS was added to each well. Forty-eight hrs later, cells received 1 mL of identical media containing 4 µM cytosine 1-B-D-arabinofuranoside (to inhibit glial mitosis) and 5% FBS. To limit our analysis to GABAergic medium spiny neurons, we excluded large cholinergic interneurons from our analysis based on size.
Drugs
Also see Table S1. Drugs were obtained from Tocris unless noted. Tetrodotoxin (TTX; 1 µM), D(-)-2-amino-5-phosphonopentanoic acid (AP-5; 25 µM), corticotropin releasing factor (CRF; 40 nM), astressin (100 nM), CP154526 (100 nM), K41498 (10 nM), antisauvagine-30 (100 nM); stressin-1 (STR; 70 nM), H89 (2 µM), forskolin (12.5 µM), PKI, 14–22 amide myristoylated (1 µM), KT5720 (1 µM), cAMPS-Rp, triethylammonium salt (10 µM), SQ22536 (90 µM), isoproterenol (10 µM), IBMX (75 µM), KN-93 (2 µM), nifedipine (5 µM), U0126 (10 µM), PD98059 (25 µM), K252a (100 nM), 10-DEBC (5 µM), H-1152 (500 nM), GW-5074 (1 µM), Gallein (75 µM), pertussis toxin (PTX; 500 ng/mL), cholera toxin (CTX; 500 ng/mL; Sigma), M119 (5 µM; gift from Dr. Kirill Martemyanov), BAPTA-AM (5 µM; Molecular Probes; Eugene, OR), methyl-β-cyclodextrin (MβCD; 10 mM; Sigma).
Immunocytochemistry
Protocols followed are those described previously, using a well-characterized commercially available monoclonal antibody directed against the Ser133 phosphorylated version of CREB (see below) [65], [66], [70]–[72]. Briefly, cultured striatal neurons (8–10 d.i.v.) were incubated in a Tyrode's solution containing TTX (1 µM) and AP-5 (25 µM) at room temperature for 1.5 hr. Unless noted otherwise, cell stimulations were performed as follows: vehicle (15 min); agonist stimulations were 15 min, antagonist exposure was 30 min prior to agonist stimulation, except PTX and CTX (18 hr pre-treatment), and concurrently with agonist stimulation. Cells were then fixed for 10 min using ice-cold 4% paraformaldehyde (Electron Microscopy Sciences; Ft. Washington, PA) in PBS containing 4 mM EGTA. Following wash, cells were permeabalized in 0.1% Triton X-100 (VWR Scientific; West Chester, PA) for 5 min. Following an additional wash, cells were incubated at 37°C in block solution (1% IgG-Free BSA and 2% Goat Serum (Jackson ImmunoResearch; West Grove, PA) in PBS) for 30 min. Primary antibody incubation consisted of a 1 hr incubation at 37°C in block solution containing a monoclonal antibody directed against the Ser-133 phosphorylated form of CREB (pCREB 10E9, 1∶1000; Upstate Biotechnology, Lake Placid, NY) and to identify individual cell morphology, a polyclonal antibody targeting microtubule-associated protein 2 (MAP2, 1∶1000; Upstate). For CREB staining (Figure S1), a rabbit monoclonal antibody directed against total CREB (CREB 48H2, 1∶1000; Cell Signaling, Boston, MA) and a mouse monoclonal antibody directed against α-tubulin (236–10501; Invitrogen) were used. Cells were then washed before being incubated in block solution containing FITC- and CY5-conjugated secondary antibodies (1∶200; Jackson ImmunoResearch). Following a final wash, cells were mounted using FluorSave (Calbiochem). Nuclear fluorescent intensities for pCREB or CREB (approximately 25 cells per group) were acquired using a Leica DM5500Q confocal system. Data were quantified with Leica LAS AF (version 1.9.0; Leica).
The confocal excitation and detection settings for each experiment were determined using coverslips stimulated for 5 min with 60 mM potassium (60 K) to establish a “ceiling” for pCREB intensity. A baseline was determined via the vehicle (NS) group, so that each assay has an internal control. Inter-coverslip variability was accounted for by subjecting 2–3 coverslips to each treatment. Data were acquired in random order by a blind observer. Neurons were readily discriminated from glia via size and morphology (see Figure 1 and Figure S1) and selected randomly using α-tubulin or MAP2 fluorescence, allowing the experimenter to remain blind to the CREB or pCREB signal. Images were captured through the approximate midline of each neuron. To analyze pCREB fluorescence intensity, the α-tubulin or MAP2 staining was used to draw a region of interested (ROI) of interest outlining the nucleus of each neuron. The ROI was then transferred to the CREB or pCREB image, and average fluorescence intensities within the nucleus were noted. All images were background subtracted from an area devoid of neuronal α-tubulin or MAP2 staining, with each experiment being performed at least three times to verify results.
cAMP Assay
We measured cAMP concentrations in cultured striatal neurons (8–10 d.i.v.) using a Parameter cAMP kit (R&D Systems; Minneapolis, MN) with a mean minimum detectable dose of 1.50 pmol/mL (manufacturer protocol). In all cAMP assays, striatal neurons were incubated in a Tyrode's solution containing TTX (1 µM) and APV (25 µM) for 1.5 hours, and then switched into an identical solution containing IBMX (75 µM). When indicated, methyl-β-cyclodextrin (MβCD; 10 mM) was included with IBMX. For MβCD experiments, 4 µM CRF was used for 15 min (Figure 6C). Stimulations were performed in the presence of the above compounds for the time-points indicated in “Results” with isoproterenol (10 µM) or CRF. Immediately following stimulation, neurons were washed with ice-cold PBS and then lysed with 215 µL ice-cold lysis buffer. Samples were stored overnight at -20°C before being processed according to manufacturer instructions. A Bio-Rad microplate reader (model 680) was used to measure concentrations of cAMP. Lysate from individual coverslips were placed in separate wells (n∼4 wells/group). Each experiment was performed in triplicate to verify results.
Neuronal Transfection
Cultured striatal neurons were transfected at 7–8 d.i.v. with 1 ug/DNA/coversip using a calcium-phosphate method that results in >95% of the transfected cells being neurons [70].
Statistics
Experiments were analyzed using ANOVAs and Bonferroni's Multiple Comparison post hoc test, or nonlinear curve fits using Prism 4.03 (GraphPad Software, La Jolla, CA). Statistically different groups are denoted by different alphabetical characters in corresponding bar graphs. P-values <0.05 were considered a priori as significant. Data are presented as mean ± SEM.
Supporting Information
CRF does not change total CREB. (A) Immunolabeled confocal images of cultured striatal neurons from 1- to 2-day old rat pups with α-tubulin (green) and total CREB (red). Neurons stimulated with CRF (40 nM) for 15 min showed no change in total CREB staining relative to vehicle stimulated control neurons (NS; Scale Bar = 20 µm). (B) Quantification of immunostaining revealed no effect of CRF on total CREB staining (P = 0.67, Student's two-tailed T-test).
(TIF)
Acknowledgments
We thank Rachel Penrod for expert assistance in dissecting dorsal and ventral striatum, Dr. Brooke Kelley for scientific input, and Dr. Robert Meisel for suggestions on previous versions of manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by National Institutes of Health (NIH) grant NS41302 to PGM. CMS was supported by the training grant DA07234. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sarnyai Z. Neurobiology of stress and cocaine addiction. Studies on corticotropin-releasing factor in rats, monkeys, and humans. Ann N Y Acad Sci. 1998;851:371–387. doi: 10.1111/j.1749-6632.1998.tb09011.x. [DOI] [PubMed] [Google Scholar]
- 2.Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA. Stress, vulnerability and adult alcohol relapse. J Stud Alcohol. 1995;56:538–545. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- 3.Cleck JN, Blendy JA. Making a bad thing worse: adverse effects of stress on drug addiction. J Clin Invest. 2008;118:454–461. doi: 10.1172/JCI33946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- 5.Goeders NE, Guerin GF. Non-contingent electric footshock facilitates the acquisition of intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1994;114:63–70. doi: 10.1007/BF02245445. [DOI] [PubMed] [Google Scholar]
- 6.Haney M, Maccari S, Le Moal M, Simon H, Piazza PV. Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res. 1995;698:46–52. doi: 10.1016/0006-8993(95)00788-r. [DOI] [PubMed] [Google Scholar]
- 7.Miczek KA, Mutschler NH. Activational effects of social stress on IV cocaine self-administration in rats. Psychopharmacology (Berl) 1996;128:256–264. doi: 10.1007/s002130050133. [DOI] [PubMed] [Google Scholar]
- 8.Ramsey NF, Van Ree JM. Emotional but not physical stress enhances intravenous cocaine self-administration in drug-naive rats. Brain Res. 1993;608:216–222. doi: 10.1016/0006-8993(93)91461-z. [DOI] [PubMed] [Google Scholar]
- 9.Shaham Y, Stewart J. Exposure to mild stress enhances the reinforcing efficacy of intravenous heroin self-administration in rats. Psychopharmacology (Berl) 1994;114:523–527. doi: 10.1007/BF02249346. [DOI] [PubMed] [Google Scholar]
- 10.Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- 11.Kreibich AS, Briand L, Cleck JN, Ecke L, Rice KC, et al. Stress-induced potentiation of cocaine reward: a role for CRF R1 and CREB. Neuropsychopharmacology. 2009;34:2609–2617. doi: 10.1038/npp.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlezon WA, Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, et al. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- 13.Nestler EJ. Molecular mechanisms of drug addiction. Neuropharmacology. 2004;47(Suppl 1):24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 14.Walters CL, Cleck JN, Kuo YC, Blendy JA. Mu-opioid receptor and CREB activation are required for nicotine reward. Neuron. 2005;46:933–943. doi: 10.1016/j.neuron.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Dinieri JA, Nemeth CL, Parsegian A, Carle T, Gurevich VV, et al. Altered sensitivity to rewarding and aversive drugs in mice with inducible disruption of cAMP response element-binding protein function within the nucleus accumbens. J Neurosci. 2009;29:1855–1859. doi: 10.1523/JNEUROSCI.5104-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunzell DH, Mineur YS, Neve RL, Picciotto MR. Nucleus accumbens CREB activity is necessary for nicotine conditioned place preference. Neuropsychopharmacology. 2009;34:1993–2001. doi: 10.1038/npp.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim MM, Liu Y, Ryabinin AE, Bai Y, Wang Z, et al. CRF receptors in the nucleus accumbens modulate partner preference in prairie voles. Horm Behav. 2007;51:508–515. doi: 10.1016/j.yhbeh.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pecina S, Schulkin J, Berridge KC. Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: paradoxical positive incentive effects in stress? BMC Biol. 2006;4:8. doi: 10.1186/1741-7007-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behan DP, Khongsaly O, Owens MJ, Chung HD, Nemeroff CB, et al. Corticotropin-releasing factor (CRF), CRF-binding protein (CRF-BP), and CRF/CRF-BP complex in Alzheimer's disease and control postmortem human brain. J Neurochem. 1997;68:2053–2060. doi: 10.1046/j.1471-4159.1997.68052053.x. [DOI] [PubMed] [Google Scholar]
- 20.Merchenthaler I. Corticotropin releasing factor (CRF)-like immunoreactivity in the rat central nervous system. Extrahypothalamic distribution. Peptides. 1984;5(Suppl 1):53–69. doi: 10.1016/0196-9781(84)90265-1. [DOI] [PubMed] [Google Scholar]
- 21.De Souza EB, Whitehouse PJ, Folstein SE, Price DL, Vale WW. Corticotropin-releasing hormone (CRH) is decreased in the basal ganglia in Huntington's disease. Brain Res. 1987;437:355–359. doi: 10.1016/0006-8993(87)91651-9. [DOI] [PubMed] [Google Scholar]
- 22.Guan X, Wang L, Chen CL, Guan Y, Li S. Roles of two subtypes of corticotrophin-releasing factor receptor in the corticostriatal long-term potentiation under cocaine withdrawal condition. J Neurochem. 2010;115:795–803. doi: 10.1111/j.1471-4159.2010.06981.x. [DOI] [PubMed] [Google Scholar]
- 23.Chen FM, Bilezikjian LM, Perrin MH, Rivier J, Vale W. Corticotropin releasing factor receptor-mediated stimulation of adenylate cyclase activity in the rat brain. Brain Res. 1986;381:49–57. doi: 10.1016/0006-8993(86)90688-8. [DOI] [PubMed] [Google Scholar]
- 24.Battaglia G, Webster EL, De Souza EB. Characterization of corticotropin-releasing factor receptor-mediated adenylate cyclase activity in the rat central nervous system. Synapse. 1987;1:572–581. doi: 10.1002/syn.890010610. [DOI] [PubMed] [Google Scholar]
- 25.Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260–286. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
- 26.Perrin MH, Sutton SW, Cervini LA, Rivier JE, Vale WW. Comparison of an agonist, urocortin, and an antagonist, astressin, as radioligands for characterization of corticotropin-releasing factor receptors. J Pharmacol Exp Ther. 1999;288:729–734. [PubMed] [Google Scholar]
- 27.Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iredale PA, Alvaro JD, Lee Y, Terwilliger R, Chen YL, et al. Role of corticotropin-releasing factor receptor-1 in opiate withdrawal. J Neurochem. 2000;74:199–208. doi: 10.1046/j.1471-4159.2000.0740199.x. [DOI] [PubMed] [Google Scholar]
- 29.De Souza EB. Corticotropin-releasing factor receptors in the rat central nervous system: characterization and regional distribution. J Neurosci. 1987;7:88–100. doi: 10.1523/JNEUROSCI.07-01-00088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Primus RJ, Yevich E, Baltazar C, Gallager DW. Autoradiographic localization of CRF1 and CRF2 binding sites in adult rat brain. Neuropsychopharmacology. 1997;17:308–316. doi: 10.1016/S0893-133X(97)00071-7. [DOI] [PubMed] [Google Scholar]
- 31.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lochner A, Moolman JA. The many faces of H89: a review. Cardiovasc Drug Rev. 2006;24:261–274. doi: 10.1111/j.1527-3466.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- 33.Meitzen J, Luoma JI, Stern CM, Mermelstein PG. beta1-adrenergic receptors activate two distinct signaling pathways in striatal neurons. J Neurochem. 2010 doi: 10.1111/j.1471-4159.2010.07137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 35.Deisseroth K, Mermelstein PG, Xia H, Tsien RW. Signaling from synapse to nucleus: the logic behind the mechanisms. Curr Opin Neurobiol. 2003;13:354–365. doi: 10.1016/s0959-4388(03)00076-x. [DOI] [PubMed] [Google Scholar]
- 36.Wu GY, Deisseroth K, Tsien RW. Activity-dependent CREB phosphorylation: convergence of a fast, sensitive calmodulin kinase pathway and a slow, less sensitive mitogen-activated protein kinase pathway. Proc Natl Acad Sci U S A. 2001;98:2808–2813. doi: 10.1073/pnas.051634198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lehmann DM, Seneviratne AM, Smrcka AV. Small molecule disruption of G protein beta gamma subunit signaling inhibits neutrophil chemotaxis and inflammation. Mol Pharmacol. 2008;73:410–418. doi: 10.1124/mol.107.041780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koch WJ, Hawes BE, Inglese J, Luttrell LM, Lefkowitz RJ. Cellular expression of the carboxyl terminus of a G protein-coupled receptor kinase attenuates G beta gamma-mediated signaling. J Biol Chem. 1994;269:6193–6197. [PubMed] [Google Scholar]
- 39.Hara M, Fukui R, Hieda E, Kuroiwa M, Bateup HS, et al. Role of adrenoceptors in the regulation of dopamine/DARPP-32 signaling in neostriatal neurons. J Neurochem. 2010;113:1046–1059. doi: 10.1111/j.1471-4159.2010.06668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stern CM, Mermelstein PG. Caveolin regulation of neuronal intracellular signaling. Cell Mol Life Sci. 2010;67:3785–3795. doi: 10.1007/s00018-010-0447-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butchbach ME, Tian G, Guo H, Lin CL. Association of excitatory amino acid transporters, especially EAAT2, with cholesterol-rich lipid raft microdomains: importance for excitatory amino acid transporter localization and function. J Biol Chem. 2004;279:34388–34396. doi: 10.1074/jbc.M403938200. [DOI] [PubMed] [Google Scholar]
- 42.Li X, Galli T, Leu S, Wade JB, Weinman EJ, et al. Na+-H+ exchanger 3 (NHE3) is present in lipid rafts in the rabbit ileal brush border: a role for rafts in trafficking and rapid stimulation of NHE3. J Physiol. 2001;537:537–552. doi: 10.1111/j.1469-7793.2001.00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bayatti N, Zschocke J, Behl C. Brain region-specific neuroprotective action and signaling of corticotropin-releasing hormone in primary neurons. Endocrinology. 2003;144:4051–4060. doi: 10.1210/en.2003-0168. [DOI] [PubMed] [Google Scholar]
- 44.Refojo D, Echenique C, Muller MB, Reul JM, Deussing JM, et al. Corticotropin-releasing hormone activates ERK1/2 MAPK in specific brain areas. Proc Natl Acad Sci U S A. 2005;102:6183–6188. doi: 10.1073/pnas.0502070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blank T, Nijholt I, Grammatopoulos DK, Randeva HS, Hillhouse EW, et al. Corticotropin-releasing factor receptors couple to multiple G-proteins to activate diverse intracellular signaling pathways in mouse hippocampus: role in neuronal excitability and associative learning. J Neurosci. 2003;23:700–707. doi: 10.1523/JNEUROSCI.23-02-00700.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheng H, Zhang Y, Sun J, Gao L, Ma B, et al. Corticotropin-releasing hormone (CRH) depresses n-methyl-D-aspartate receptor-mediated current in cultured rat hippocampal neurons via CRH receptor type 1. Endocrinology. 2008;149:1389–1398. doi: 10.1210/en.2007-1378. [DOI] [PubMed] [Google Scholar]
- 47.Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol. 2008;586:2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beckstead MJ, Gantz SC, Ford CP, Stenzel-Poore MP, Phillips PE, et al. CRF enhancement of GIRK channel-mediated transmission in dopamine neurons. Neuropsychopharmacology. 2009;34:1926–1935. doi: 10.1038/npp.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, et al. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- 50.Riegel AC, Williams JT. CRF facilitates calcium release from intracellular stores in midbrain dopamine neurons. Neuron. 2008;57:559–570. doi: 10.1016/j.neuron.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magalhaes AC, Holmes KD, Dale LB, Comps-Agrar L, Lee D, et al. CRF receptor 1 regulates anxiety behavior via sensitization of 5-HT2 receptor signaling. Nat Neurosci. 2010;13:622–629. doi: 10.1038/nn.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bale TL. Sensitivity to stress: dysregulation of CRF pathways and disease development. Horm Behav. 2005;48:1–10. doi: 10.1016/j.yhbeh.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 53.Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol Rev. 2001;53:209–243. [PubMed] [Google Scholar]
- 54.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 55.Tao J, Hildebrand ME, Liao P, Liang MC, Tan G, et al. Activation of corticotropin-releasing factor receptor 1 selectively inhibits CaV3.2 T-type calcium channels. Mol Pharmacol. 2008;73:1596–1609. doi: 10.1124/mol.107.043612. [DOI] [PubMed] [Google Scholar]
- 56.Gutknecht E, Van der Linden I, Van Kolen K, Verhoeven KF, Vauquelin G, et al. Molecular mechanisms of corticotropin-releasing factor receptor-induced calcium signaling. Mol Pharmacol. 2009;75:648–657. doi: 10.1124/mol.108.050427. [DOI] [PubMed] [Google Scholar]
- 57.Teli T, Markovic D, Levine MA, Hillhouse EW, Grammatopoulos DK. Regulation of corticotropin-releasing hormone receptor type 1alpha signaling: structural determinants for G protein-coupled receptor kinase-mediated phosphorylation and agonist-mediated desensitization. Mol Endocrinol. 2005;19:474–490. doi: 10.1210/me.2004-0275. [DOI] [PubMed] [Google Scholar]
- 58.Lopez-Ilasaca M, Crespo P, Pellici PG, Gutkind JS, Wetzker R. Linkage of G protein-coupled receptors to the MAPK signaling pathway through PI 3-kinase gamma. Science. 1997;275:394–397. doi: 10.1126/science.275.5298.394. [DOI] [PubMed] [Google Scholar]
- 59.Dupre DJ, Robitaille M, Rebois RV, Hebert TE. The role of Gβγ subunits in the organization, assembly, and function of GPCR signaling complexes. Annu Rev Pharmacol Toxicol. 2009;49:31–56. doi: 10.1146/annurev-pharmtox-061008-103038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smrcka AV. G protein βγ subunits: central mediators of G protein-coupled receptor signaling. Cell Mol Life Sci. 2008;65:2191–2214. doi: 10.1007/s00018-008-8006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tu H, Rondard P, Xu C, Bertaso F, Cao F, et al. Dominant role of GABAB2 and Gβγ for GABAB receptor-mediated-ERK1/2/CREB pathway in cerebellar neurons. Cell Signal. 2007;19:1996–2002. doi: 10.1016/j.cellsig.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 62.Akgoz M, Kalyanaraman V, Gautam N. G protein βγ complex translocation from plasma membrane to Golgi complex is influenced by receptor gamma subunit interaction. Cell Signal. 2006;18:1758–1768. doi: 10.1016/j.cellsig.2006.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saini DK, Kalyanaraman V, Chisari M, Gautam N. A family of G protein βγ subunits translocate reversibly from the plasma membrane to endomembranes on receptor activation. J Biol Chem. 2007;282:24099–24108. doi: 10.1074/jbc.M701191200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weick JP, Groth RD, Isaksen AL, Mermelstein PG. Interactions with PDZ proteins are required for L-type calcium channels to activate cAMP response element-binding protein-dependent gene expression. J Neurosci. 2003;23:3446–3456. doi: 10.1523/JNEUROSCI.23-08-03446.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grove-Strawser D, Boulware MI, Mermelstein PG. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience. 2010;170:1045–1055. doi: 10.1016/j.neuroscience.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci. 2007;27:9941–9950. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holahan MR, Kalin NH, Kelley AE. Microinfusion of corticotropin-releasing factor into the nucleus accumbens shell results in increased behavioral arousal and oral motor activity. Psychopharmacology (Berl) 1997;130:189–196. doi: 10.1007/s002130050228. [DOI] [PubMed] [Google Scholar]
- 69.Briand LA, Blendy JA. Molecular and genetic substrates linking stress and addiction. Brain Res. 2010;1314:219–234. doi: 10.1016/j.brainres.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Groth RD, Weick JP, Bradley KC, Luoma JI, Aravamudan B, et al. D1 dopamine receptor activation of NFAT-mediated striatal gene expression. Eur J Neurosci. 2008;27:31–42. doi: 10.1111/j.1460-9568.2007.05980.x. [DOI] [PubMed] [Google Scholar]
- 71.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, et al. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pearce LR, Alton GR, Richter DT, Kath JC, Lingardo L, et al. Characterization of PF-4708671, a novel and highly specific inhibitor of p70 ribosomal S6 kinase (S6K1). Biochem J. 2010;431:245–255. doi: 10.1042/BJ20101024. [DOI] [PubMed] [Google Scholar]
- 73.Perrin MH, Donaldson CJ, Chen R, Lewis KA, Vale WW. Cloning and functional expression of a rat brain corticotropin releasing factor (CRF) receptor. Endocrinology. 1993;133:3058–3061. doi: 10.1210/endo.133.6.8243338. [DOI] [PubMed] [Google Scholar]
- 74.Facci L, Stevens DA, Pangallo M, Franceschini D, Skaper SD, et al. Corticotropin-releasing factor (CRF) and related peptides confer neuroprotection via type 1 CRF receptors. Neuropharmacology. 2003;45:623–636. doi: 10.1016/s0028-3908(03)00211-9. [DOI] [PubMed] [Google Scholar]
- 75.Ruhmann A, Bonk I, Lin CR, Rosenfeld MG, Spiess J. Structural requirements for peptidic antagonists of the corticotropin-releasing factor receptor (CRFR): development of CRFR2beta-selective antisauvagine-30. Proc Natl Acad Sci U S A. 1998;95:15264–15269. doi: 10.1073/pnas.95.26.15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lawrence AJ, Krstew EV, Dautzenberg FM, Ruhmann A. The highly selective CRF(2) receptor antagonist K41498 binds to presynaptic CRF(2) receptors in rat brain. Br J Pharmacol. 2002;136:896–904. doi: 10.1038/sj.bjp.0704783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Glass DB, Lundquist LJ, Katz BM, Walsh DA. Protein kinase inhibitor-(6-22)-amide peptide analogs with standard and nonstandard amino acid substitutions for phenylalanine 10. Inhibition of cAMP-dependent protein kinase. J Biol Chem. 1989;264:14579–14584. [PubMed] [Google Scholar]
- 78.Zheng XL, Matsubara S, Diao C, Hollenberg MD, Wong NC. Activation of apolipoprotein AI gene expression by protein kinase A and kinase C through transcription factor, Sp1. J Biol Chem. 2000;275:31747–31754. doi: 10.1074/jbc.M000621200. [DOI] [PubMed] [Google Scholar]
- 79.Farrow B, Rychahou P, Murillo C, O'Connor KL, Iwamura T, et al. Inhibition of pancreatic cancer cell growth and induction of apoptosis with novel therapies directed against protein kinase A. Surgery. 2003;134:197–205. doi: 10.1067/msy.2003.220. [DOI] [PubMed] [Google Scholar]
- 80.Fu Y, Han J, Ishola T, Scerbo M, Adwanikar H, et al. PKA and ERK, but not PKC, in the amygdala contribute to pain-related synaptic plasticity and behavior. Mol Pain. 2008;4:26. doi: 10.1186/1744-8069-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harris DN, Asaad MM, Phillips MB, Goldenberg HJ, Antonaccio MJ. Inhibition of adenylate cyclase in human blood platelets by 9-substituted adenine derivatives. J Cyclic Nucleotide Res. 1979;5:125–134. [PubMed] [Google Scholar]
- 82.Freitag A, Wessler I, Racke K. Phosphodiesterase inhibitors suppress alpha2-adrenoceptor-mediated 5-hydroxytryptamine release from tracheae of newborn rabbits. Eur J Pharmacol. 1998;354:67–71. doi: 10.1016/s0014-2999(98)00439-7. [DOI] [PubMed] [Google Scholar]
- 83.Thimmaiah KN, Easton JB, Germain GS, Morton CL, Kamath S, et al. Identification of N10-substituted phenoxazines as potent and specific inhibitors of Akt signaling. J Biol Chem. 2005;280:31924–31935. doi: 10.1074/jbc.M507057200. [DOI] [PubMed] [Google Scholar]
- 84.Groth RD, Mermelstein PG. Brain-derived neurotrophic factor activation of NFAT (nuclear factor of activated T-cells)-dependent transcription: a role for the transcription factor NFATc4 in neurotrophin-mediated gene expression. J Neurosci. 2003;23:8125–8134. doi: 10.1523/JNEUROSCI.23-22-08125.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wasylyk C, Zheng H, Castell C, Debussche L, Multon MC, et al. Inhibition of the Ras-Net (Elk-3) pathway by a novel pyrazole that affects microtubules. Cancer Res. 2008;68:1275–1283. doi: 10.1158/0008-5472.CAN-07-2674. [DOI] [PubMed] [Google Scholar]
- 86.Tamura M, Nakao H, Yoshizaki H, Shiratsuchi M, Shigyo H, et al. Development of specific Rho-kinase inhibitors and their clinical application. Biochim Biophys Acta. 2005;1754:245–252. doi: 10.1016/j.bbapap.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 87.Yu JZ, Dave RH, Allen JA, Sarma T, Rasenick MM. Cytosolic Gα acts as an intracellular messenger to increase microtubule dynamics and promote neurite outgrowth. J Biol Chem. 2009;284:10462–10472. doi: 10.1074/jbc.M809166200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kralisch S, Klein J, Lossner U, Bluher M, Paschke R, et al. Hormonal regulation of the novel adipocytokine visfatin in 3T3-L1 adipocytes. J Endocrinol. 2005;185:R1–8. doi: 10.1677/joe.1.06211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CRF does not change total CREB. (A) Immunolabeled confocal images of cultured striatal neurons from 1- to 2-day old rat pups with α-tubulin (green) and total CREB (red). Neurons stimulated with CRF (40 nM) for 15 min showed no change in total CREB staining relative to vehicle stimulated control neurons (NS; Scale Bar = 20 µm). (B) Quantification of immunostaining revealed no effect of CRF on total CREB staining (P = 0.67, Student's two-tailed T-test).
(TIF)